Introduction
It is reported that up to 70% of patients with
severe sepsis exhibit symptoms of encephalopathy, including
consciousness disturbance, impaired cognitive function, personality
changes, and lack of concentration or somnolence (1–3).
Although some patients can be resolved during hospitalization,
sepsis-associated encephalopathy can cause long-term consequences,
including prolonged length of hospital stay, long-term cognitive
and functional decline, and increased morbidity and mortality
(1–3). Various potential mechanisms,
including oxidative stress, inflammation, neurotransmission
disturbance, mitochondrial dysfunction, and cell death, have been
proposed to be involved in the pathogenesis of sepsis-induced
cognitive impairment (2–4), yet the precise mechanism remains
largely to be determined.
β2-microglobulin, a component of major
histocompatibility complex class I (MHCI) molecules, is a low
molecular weight protein (11,800 Da) and is considered to be a
surrogate marker of putative middle-molecule uremic toxins
(5). Notably, increased
β2-microglobulin in the systemic milieu is implicated in
age-related decline in adult neurogenesis, and impairments in
synaptic plasticity and cognitive function observed during ageing,
as antigen processing 1 (Tap1)-deficient mice with reduced
cell surface expression of MHC I mitigated these abnormalities
(6,7). Moreover, it is reported that
increased systemic soluble β2-microglobulin levels are associated
with cognitive impairments associated with chronic hemodialysis
(8). In the brain,
β2-microglobulin can act independently of their canonical immune
function to regulate neuronal signaling and activity-dependent
changes in synaptic connectivity (9,10).
These findings are in line with previous studies demonstrating that
higher β2-microglobulin levels are observed in the cerebrospinal
fluid of patients with Alzheimer's disease or human
immunodeficiency virus-associated dementia (11,12).
However, the functional role of β2-microglobulin in mediating
sepsis-induced cognitive impairments has not yet been
investigated.
In the present study, we therefore hypothesized that
β2-microglobulin negatively regulates memory and learning in a
mouse model of sepsis induced by cecal ligation and puncture (CLP).
Moreover, we explore the underlying molecular mechanisms.
Materials and methods
Animals
Thirty male wild-type C57BL/6 mice (22–25 g) were
purchased from Nanjing University of Chinese Medicine and forty-two
male transporter associated with antigen processing 1
(Tap1−/−) mutant mice (22–26 g) were from the
Jackson Laboratory (Ben Harbor, ME, USA). All studies were approved
by the Institutional Animal Care and Use Committee of Nanjing
University of Chinese Medicine, China and all experimental
procedures and protocols used in the present study were performed
in accordance with the Guidelines for the Care and Use of
Laboratory Animals from the National Institutes of Health. In the
present study, only male mice were used. The animals were housed
under a 12-h light/dark cycle in a temperature-controlled room of
22–24°C and 40–50% relative humidity with free access to food and
water.
Animal model of sepsis
The sepsis was established by CLP as we previously
described (4,13). Each mouse was anesthetized with 2%
sodium pentobarbital in saline (40 mg/kg, intraperitoneally;
Sigma-Aldrich, St. Louis, MO, USA). The cecum was isolated
carefully and then ligated with 4.0 silk below the ileocecal
junction, approximately 0.6 cm from the distal end. The cecum was
then perforated twice with a sterile 22-gauge needle and was gently
squeezed to extrude the fecal contents into the peritoneal cavity.
The cecum was then returned to the peritoneal cavity and the
laparotomy was closed with 4.0 silk sutures. For the animals that
served as sham controls, the cecum was exposed in the same manner
as CLP, but was neither ligated nor punctured. All mice received
subcutaneous normal saline resuscitation (20 ml/kg of body weight),
and antibiotic therapy (ertapenem, 20 mg/kg; Merck Research
Laboratory, USA) begun immediately after the surgery and once daily
for a total of 3 days. All mice were returned to their cages with
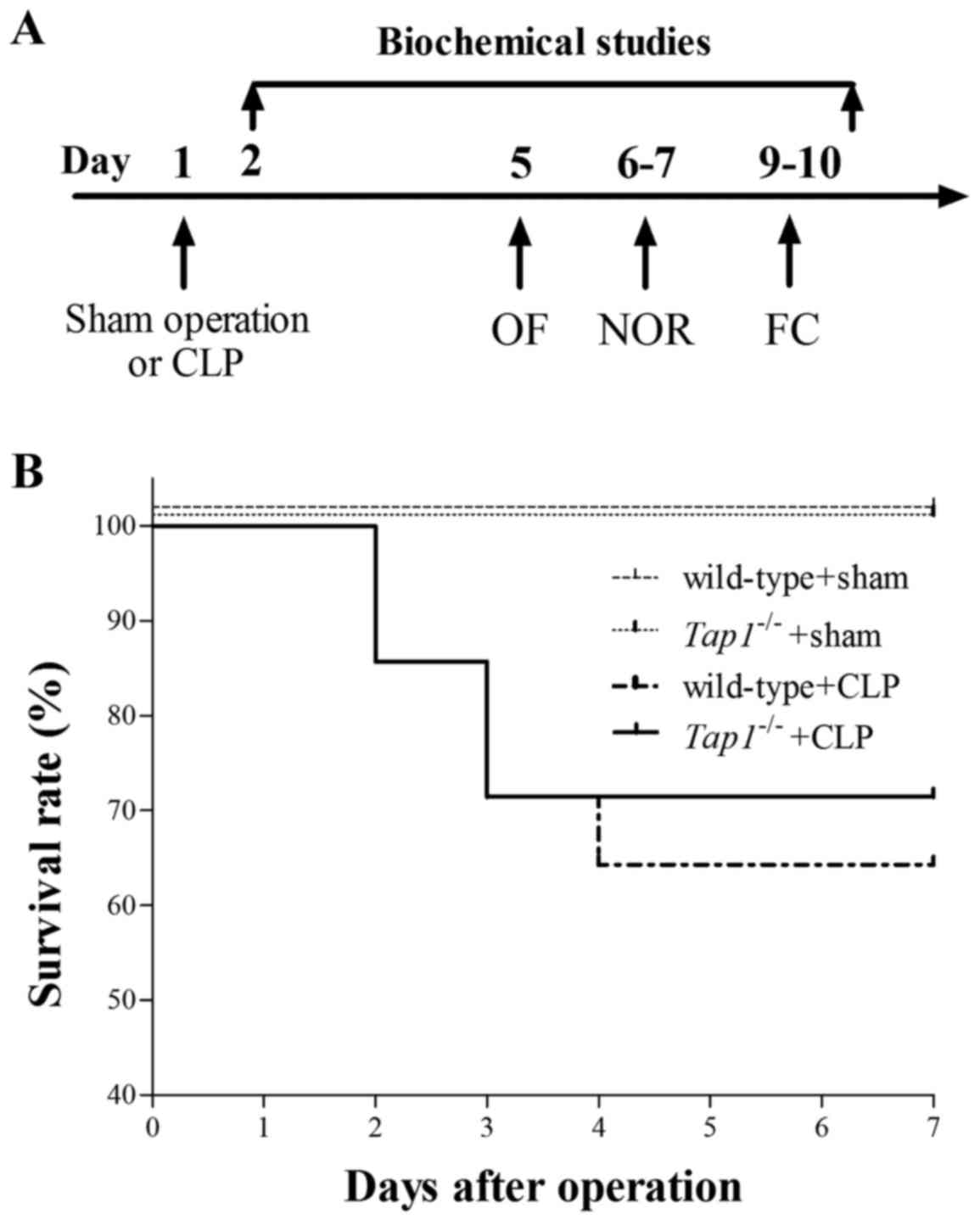
free access to food and water. The flow chart for the experimental
protocol was summarized in Fig.
1A.
Behavioral and cognitive tests
All behavioral tests were performed at 8:00 a.m.
−11:00 a.m. in a sound-isolated room and subsequently analyzed by
using a video-tracking system (Shanghai Mobile Datum Information
Technology Company, Shanghai, China). All behavioral data were
recorded by the same investigator who was blinded to the animal
grouping as described in our previous studies (13,14).
Open field tests
On day 5 after operation, mice were gently placed in
the center of a white plastic chamber (40×40×40 cm) for 5 min while
exploratory behavior was automatically recorded by a video tracking
system. The total distance and total time traveled in the open
field arena were recorded. After each test, the arena was cleaned
with 75% alcohol to avoid olfactory cues.
Novel object recognition test
Novel object recognition test was conducted on days
6–7 after operation to evaluate retention or intact memory as
previously detailed (15). This
test consisted of two trials. In the training trial, two familiar
objects were presented. The testing trial included one familiar
object and one novel object present in the respective zones of the
open field, with 60-min intervals between trials, during which the
animals were placed back to their home cages. The time spent with
each object was recorded, and the cognitive outcomes were
determined by the ‘discrimination index’ for the testing trial,
which was calculated using the formula: % discrimination index=time
spent in novel object zone ×100/(time spent in familiar object zone
+ time spent in novel object zone).
Fear conditioning test
The fear conditioning tests were performed on days 9
and 10 after operation as previously described (13). In the training section, each mouse
was allowed to explore the fear conditioning test chamber for 3 min
before the onset of a 30-sec tone (70 db, 3 kHz), followed by a
2-sec footshock (0.7 mA). Then, the mice remained in the chamber
for another 30 sec and were then returned to their home cages.
After 24 h, the animals were placed in the same chamber in which
they were trained and were observed for 5 min without tone or
footshock presentation. The auditory-cued fear test was performed 2
h later. The mice were placed in an altered chamber (i.e., a
different shaped chamber, odor, no grid floor) and allowed to
explore for 3 min. After that, the tone was delivered, and their
freezing behavior was scored for an additional 3 min. Freezing
behavior was defined as the absence of all visible movement,
excluding respiration. Cognitive impairment was assessed by
measuring the amount of time the mouse demonstrated ‘freezing
behavior’, which is defined as a completely immobile posture except
for respiratory efforts.
Measurement of plasma level of
β2-microglobulin
The plasma levels of β2-microglobulin were
determined at a laboratory by standard antibody-based multiplex
immunoassays on the basis of the principles of immunoassay as
described by the manufacturers in our institution (XieHe, Beijing,
China).
Western blot analysis
Mouse hippocampi were dissected after perfusion of
animals, snap frozen and lysed in RIPA lysis buffer (500 mM Tris,
pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS,
and complete protease inhibitors; Roche, Basel, Switzerland).
Tissue lysates were mixed with 4×NuPage LDS loading buffer and
loaded on a 4–12% SDS polyacrylamide gradient gel (both Invitrogen,
Carlsbad, CA, USA) and subsequently transferred onto a
nitrocellulose membrane. Membranes were blocked with 5% skim milk
in Tris-buffered saline tween for 1 h and then incubated with
anti-β2-microglobulin (1:2,000; cat. no. ab75853; Abcam, Cambridge,
UK), anti-BDNF (1:1,500; Santa Cruz Biotechnology Inc., Dallas, TX,
USA), anti-PSD95 (1:1,000), and anti-GADPH (1:5,000; both Cell
Signaling Technology, Boston, MA, USA) overnight at 4°C temperature
room. Horseradish peroxidaseconjugated secondary antibodies and an
enhanced chemiluminescence (ECL) kit (GE Healthcare, Uppsala,
Sweden) were used to detect protein signals. The bands were
detected with Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific, Rockford, IL, USA) and semiquantified with image J
software (version 1.50i; National Institutes of Health, Bethesda,
MD, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The hippocampus was then separated, weighed and
placed in a homogenizer. The tissue was homogenized with 1 ml
ice-cold physiological saline per 100 mg brain tissue. Hypothermal
centrifugation was performed at 5,000 × g for 10 min and the
supernatant was obtained. Standard curves for all cytokines (in
duplicates) were generated using the reference cytokine
concentrations supplied. The quantifications of tumor necrosis
factor α (TNF-α), interleukin (IL)-1β, IL-6, and BDNF were done by
the instructions of the manufacturers (JianCheng Biotechnology,
Nanjing, China). The readings were normalized to the amount of
standard protein.
Immunohistochemistry
The brains were histologically analyzed using
paraffin-embedded sections. Microglia in the hippocampus were
evaluated by immunohistochemical staining 24 h and 10 days after
operation. The sections were deparaffinized, washed and incubated
with IBA1 antibody (1:1,000; Abcam) and biotinylated secondary
antibody. The IBA1-positive cells in the mouse hippocampus were
counted manually in five randomly selected areas by an investigator
who was blinded to the animal grouping. Six brains from each group
were used for immunohistochemistry analysis and six brain sections
of 5 µm thickness were examined in each brain.
Statistical analysis
Statistical analysis was performed using the SPSS
16.0 software for Windows (SPSS, Inc., Chicago, IL, USA). Data are
expressed as mean ± S.E.M. Means between two groups were compared
with independent student's t-test. Comparisons of means from
multiple groups were assessed by one-way analysis of variance
(ANOVA) followed by a Tukey test. The 7-day survival rate was
compared by the log-rank test by a researcher who was blinded to
the group assignments. Bivariate relationships were evaluated using
Pearson correlation coefficients. P<0.05 was considered to
indicate a statistically significant difference.
Results
Tap1-/- mice did not confer increased
survival rate after CLP
There were five animals in the wild type and four
animals in the Tap1−/− mice subjected to CLP died
within 7 days after operation. Deficient in
Tap1−/− that result in lower expression of
β2-microglobulin did not increase the survival rate after sepsis
development (P>0.05, Fig.
1C).
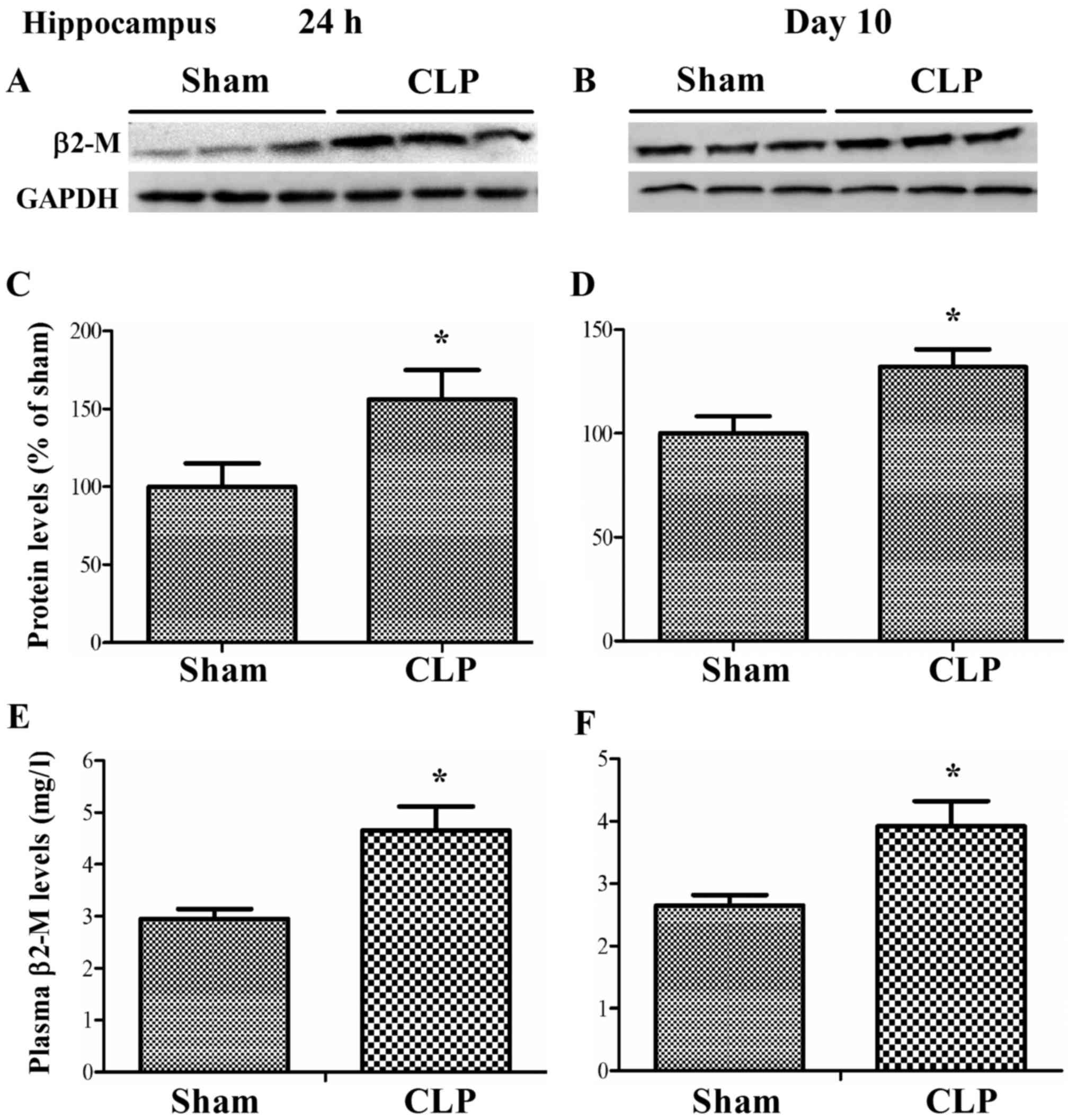
Sepsis increased plasma and hippocapal
levels of β2-microglobulin
As shown in Fig. 2,
CLP caused a sustained increase of β2-microglobulin expression in
the hippocampus in wild-type mice at 24 h and 10 days after CLP (24
h: t=−2.313, P=0.049; 10 days: t=−2.667, P=0.029, independent t
test). Likewise, plasma levels of β2-microglobulin also
significantly increased in CLP mice as compared with sham mice at
24 h and 10 days after CLP (24 h: t=−3.357, P=0.007; 10 days:
t=−2.87, P=0.017, independent t test).
Tap1-/- mice protected sepsis-induced
cognitive impairment
Next, we determined whether β2-microglobulin is
functionally involved in sepsis-induced cognitive impairment after
CLP. Wild-type and Tap1−/− mice that had
previously been subjected to CLP or sham operation were analyzed
sequentially by open-field, novel object recognition test, and fear
conditioning tests.
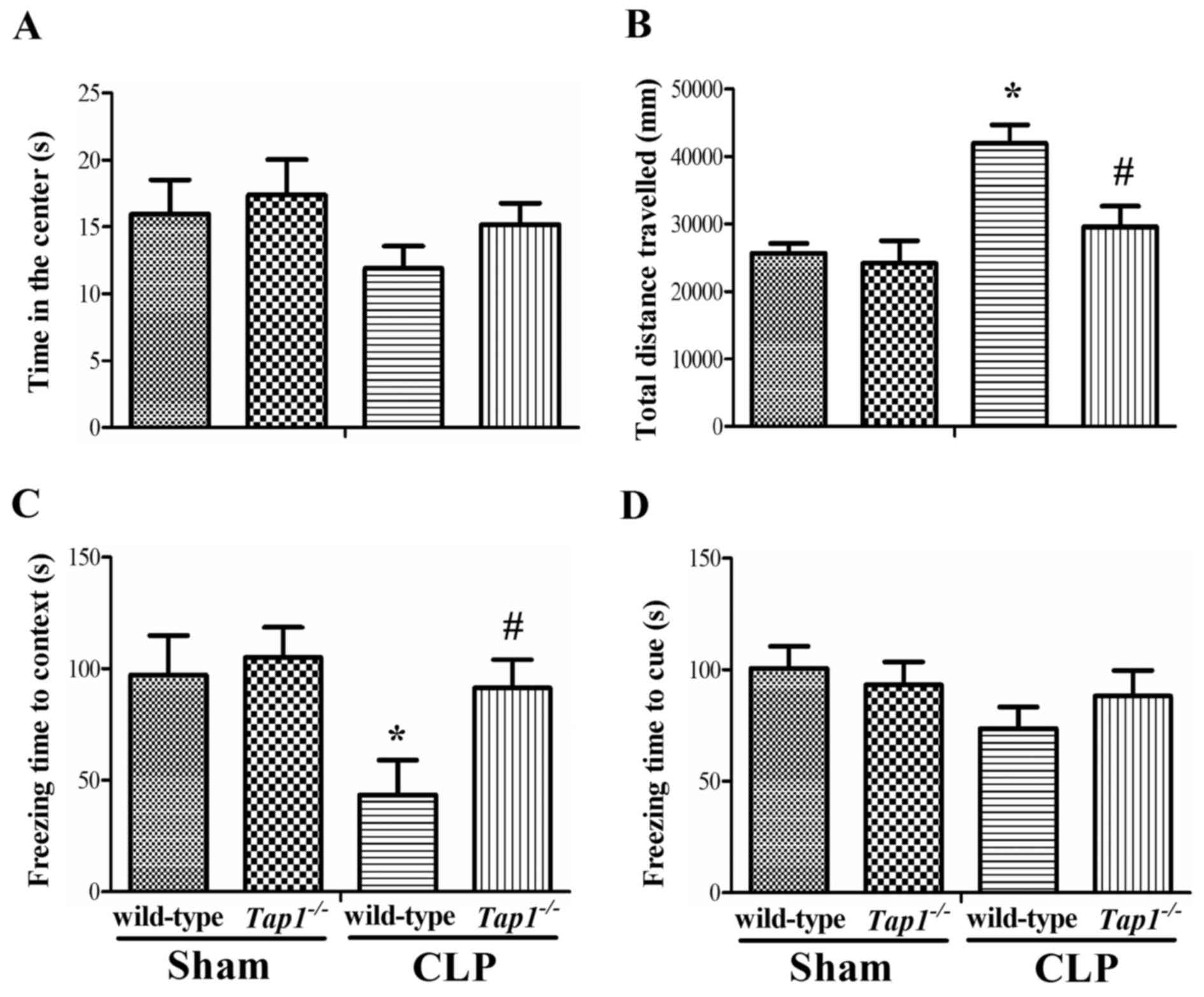
As shown in Fig.
3A, there was no significant difference in time spent in the
center among the four groups (one-way ANOVA; F (3, 40)=1.014,
P=0.396). However, we found that wild-type mice had significantly
increased total distance traveled in the open field arena as
compared with Tap1−/− mice subjected to CLP
(one-way ANOVA; F (3, 40)=5.742, P<0.001), suggesting sepsis
induced anxiety-like behavior.
The fear conditioning test was performed to assess
whether β2-microglobulin deficiency could improve the ability of
mice to learn and remember an association between environmental
cues and aversive experiences. The wild-type mice exhibited
decreased freezing time in the contextual fear conditioning
compared with Tap1−/− mice after CLP (F (3,
40)=3.066, P=0.039, Fig.
3C), suggesting increased β2-microglobulin level induced by
sepsis might contribute to memory dysfunction. However, there was
no significant difference in the freezing time in the
hippocampal-independent cued test among groups (F (3,
40)=1.188, P=0.326, Fig.
3D).
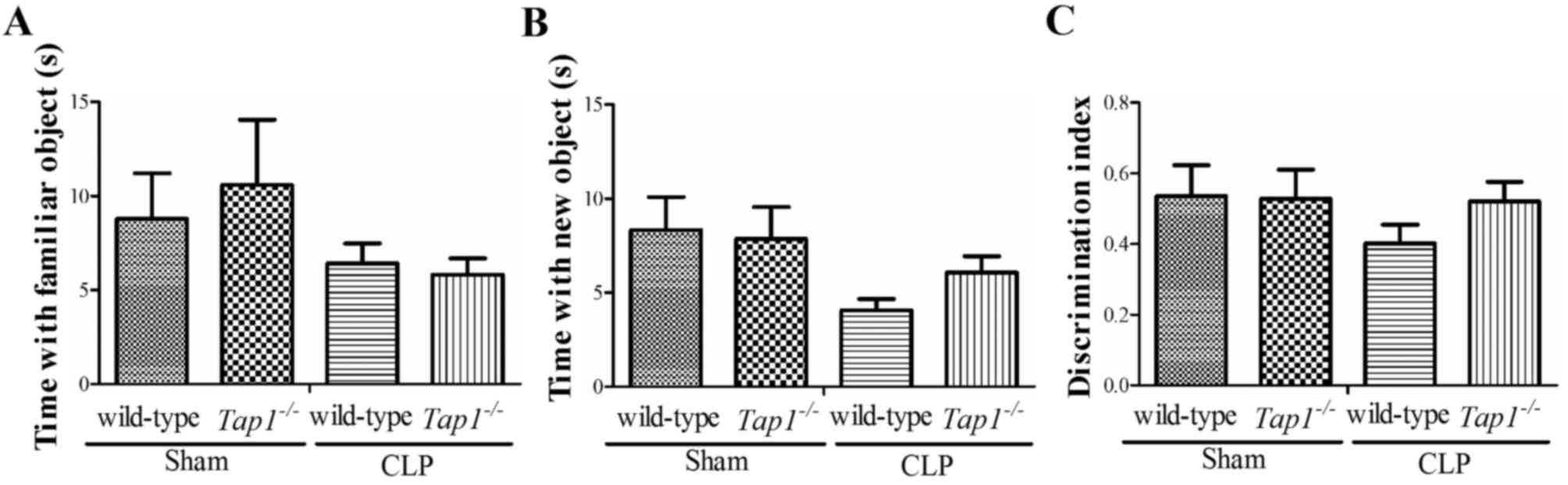
The social interaction test is used to assess
working memory. There was a general tendency of the
Tap1−/− mice to spend more time with the novel
object as compared with wild-type mice subjected to CLP (familiar,
F (3, 40)=0.849; novel, F (3, 40)=1.827;
discrimination index, F (3, 40)=0.703, P=0.556; Fig. 4), although this phenomenon did not
reach the level of statistical significance and also was not
influenced by CLP.
Tap1-/- Mice decrease sepsis-induced
inflammatory response in the hippocampus
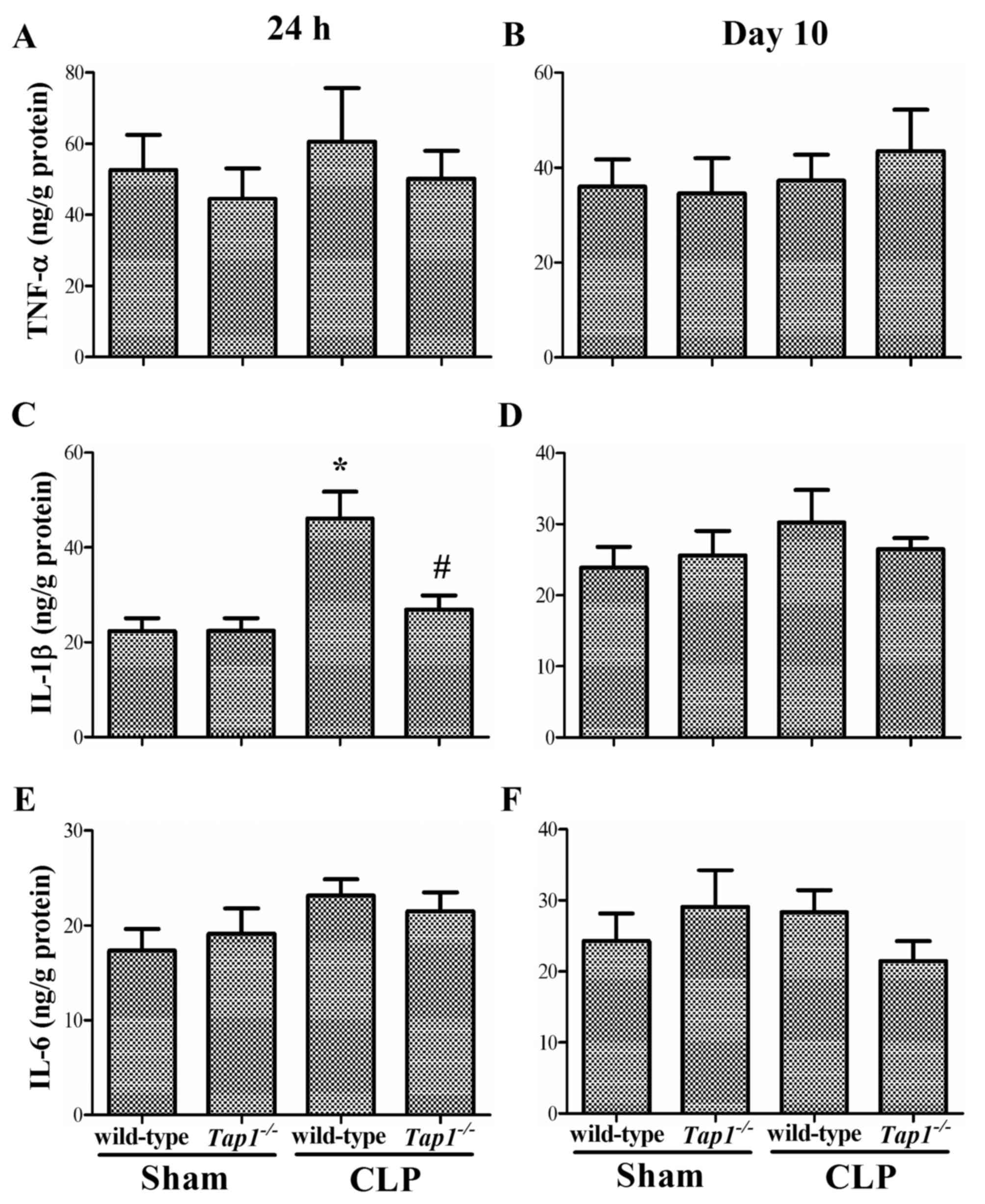
We determined proinflamatory cytokines including
TNF-α, IL-1β, and IL-6 in the hippocampus. Wild type mice subjected
to CLP had significantly increased levels of IL-1β in the
hippocampus (F (3, 20)=9.364, P<0.001, Fig. 5C), while Tap1−/−
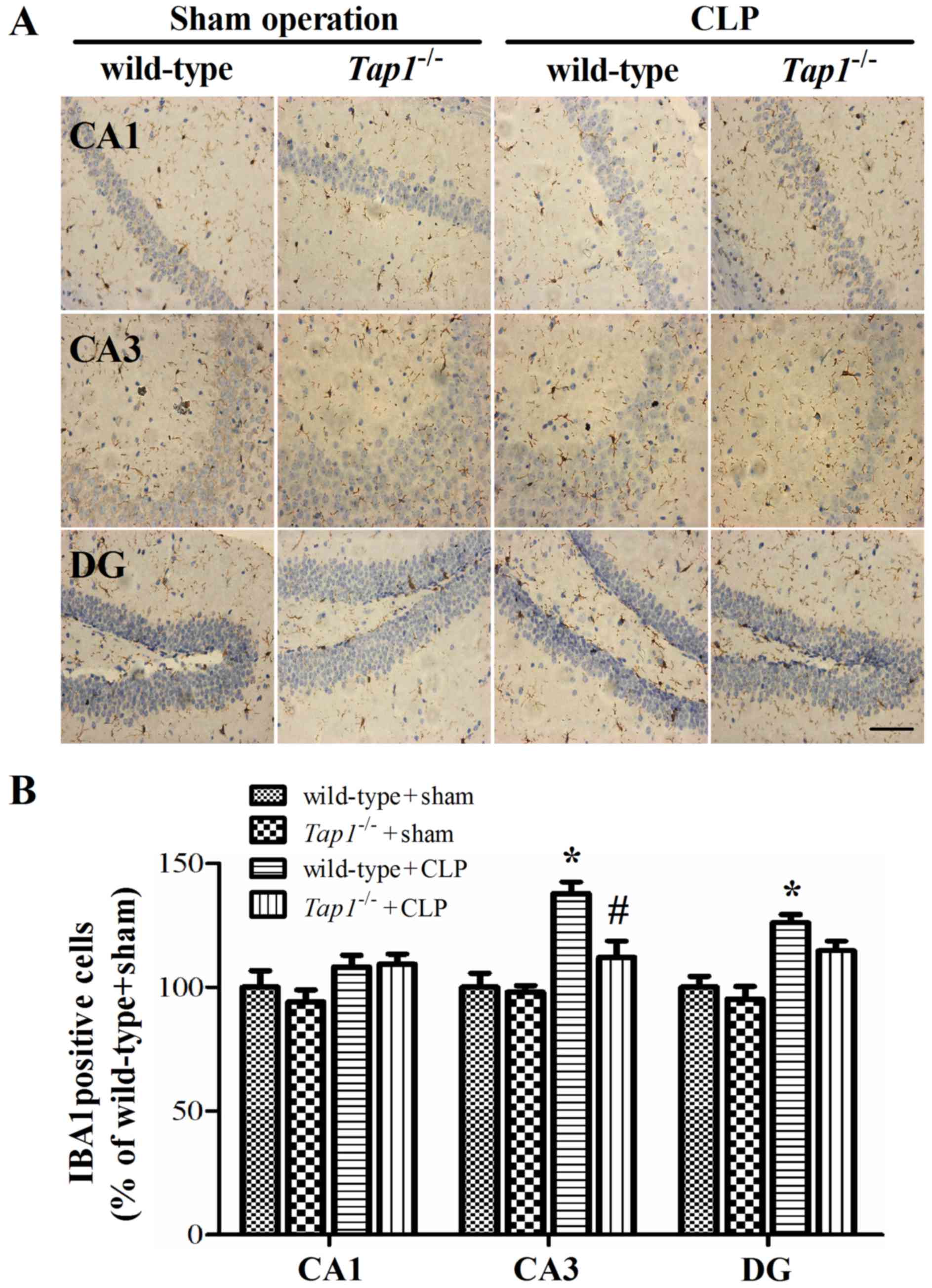
mice did not show a similar increase. Supporting the ELISA results,
CLP induced significantly microglia activation in wild-type mice,
which was significantly attenuated in Tap1−/−
mice at 24 h (CA1: F (3, 20)=1.91, P=0.160; CA3:
(F(3, 20)=12.612, P<0.001; DG: (F (3,
20)=11.087, P<0.001, Fig.
6) but not 10 days (data not shown) after CLP, indicating that
β2-microglobulin might contribute to the neuroinflammatory reaction
after sepsis development. However, we did not detect any difference
in hippocapal levels of TNF-α and IL-6 among groups ((TNF-α, 24 h:
(F (3, 20)=0.387, P=0.764, Fig. 5A; 10 days: (F (3,
20)=0.317, P=0.813), Fig.
5B; IL-1β, 10 days: (F (3, 20)=0.652, P=0.591),
Fig. 5D; IL-6, 24 h: (F (3,
20)=1.368, P=0.281, Fig.
5E); 10 days: (F (3, 20)=0.876, P=0.470, Fig. 5).
Tap1-/- mice protected sepsis-induced
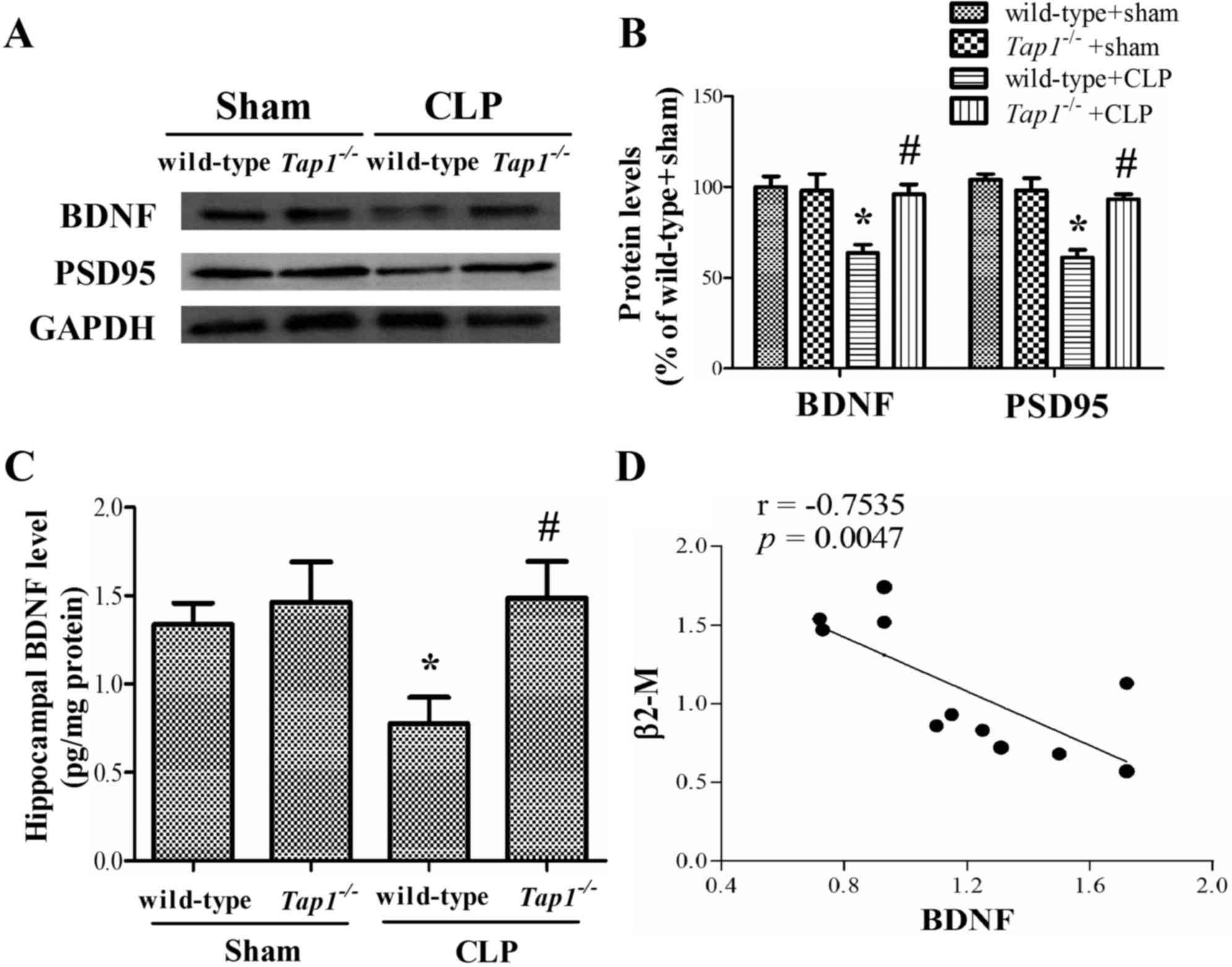
BDNF and PSD-95 loss in the hippocampus
We further analyzed changes of synaptic related
proteins in the hippocampus, known to be critical for spatial
memory formation. Wild-type mice subjected to CLP had lower
hippocampal levels of BDNF and PSD-95 levels (BDNF: (F (3,
17)=3.454, P=0.046; PSD-95: (F (3, 17)=4.75,
P=0.014), whereas the reduction of the BDNF and PSD-95 was not
observed in Tap1−/− mice with the same stimulus.
Consistently, ELISA results showed that the sepsis-induced decrease
of hippocapal level of BDNF was more pronounced in wild-type than
in Tap1−/− mice (F (3,28)=3.368, P=0.032, Fig. 7C). In addition, correlation
analysis showed that the expression of BDNF was negatively
correlated with β2-microglobulin expression in the hippocampus
(r=−0.7535, P=0.0047, Fig.
7C).
Discussion
It has been demonstrated that the brain is one of
the first organs affected during sepsis, which leads to
neurological complications, such as sepsis-associated
encephalopathy (2–4). Compared to the widely used
lipopolysaccharide model, the CLP model is clinically relevant
because it has a feature shared with human sepsis-induced cognitive
impairment (4,13,14).
Although many mechanisms, including oxidative stress, endothelial
dysfunction, inflammation, unbalanced neurotransmission,
mitochondrial dysfunction, and cell death have been implicated in
the pathogenesis of sepsis-associated cognitive impairment
(16–20), the precise mechanism remains to be
elucidated. In this study, we showed that β2-microglobulin
deficiency protected mice from sepsis-induced neurobehavioral and
biochemical abnormities, suggesting β2-microglobulin may serve as a
therapeutical target for sepsis-associated cognitive
impairment.
Class I MHC molecules, known to be important for
immune responses to antigen, are also expressed by neurons that
undergo activity-dependent, long-term structural, and synaptic
modifications (9). Accumulating
evidence has demonstrated increased β2-microglobulin level in the
systemic milieu is associated with age-related decline in adult
neurogenesis, and impairments in synaptic plasticity and cognitive
function observed during ageing (6,7). In
dialysis patients, β2-microglobulin is considered to be a potential
marker of inflammation and could be served as a predictor of
mortality (5,21). Moreover, higher serum
β2-microglobulin level may be a result of increased inflammation
such as in cardiovascular diseases in end-stage renal disease
patients (22). Based on the
observation that higher β2-microglobulin level is negatively
associated with cognitive performance, we take advantage of
Tap1−/− mice with reduced cell surface expression
of MHC I to address the functional role of β2-microglobulin in
sepsis-induced cognitive impairment. In our study, the detrimental
effects of β2-microglobulin on cognition were confirmed by
anxiety-like behavior and impaired hippocapal-dependent contextual
memory. Although we did not observe that β2-microglobulin
deficiency protected working memory dysfunction after CLP, our
results collectively support the hypothesis that β2-microglobulin
can negatively affect sepsis-induced cognitive impairment. However,
the mechanisms by which β2-microglobulin negatively induced
cognitive impairments remain unclear. Previous studies suggest that
β2-microglobulin is a marker of low-grade inflammation (21–23).
This notion is supported by our data that analysis of the brain
innate immune system revealed a subtle but sustained activation of
microglia that was detected in wild-type mice but not in
Tap1−/− mice, which was accompanied by a distinct
regulation of cytokine levels including IL-1β. Due to the reported
negative impact of IL-1β on learning and memory function, as well
as on LTP (24,25), the reduced level of IL-1β in
Tap1−/− mice may partly explain the observed
protection.
Given the important role of β2-microglobulin in
regulation of the activity-dependent remodeling and plasticity of
connections in the developing and mature mammalian central nervous
system, we next studied the effects of CLP and β2-microglobulin
deficiency on the regulation of neurotrophic factor BDNF and
synaptic related protein PSD-95, which have key functions for
learning and memory (26–28). It has been demonstrated that LPS
induced significant reduction of BDNF and PSD-95 contribute to
memory deficits (29,30). In line with the findings presented
in this study, wild-type mice with decreased PSD-95 and BDNF
expressions after sepsis development showed impaired memory
performance (31,32). Importantly, β2-microglobulin
deficiency protected from PSD-95 and BDNF loss. In addition, we can
not rule out that other mechanism are also involved in the
detrimental effects of β2-microglobulin in sepsis. Therefore,
future studies are required to elucidate the specific mechanism by
which β2-microglobulin exerts its effects in sepsis-associated
cognitive impairment.
In conclusion, our study suggests that the
detrimental role of the β2-microglobulin in cognitive impairment
after sepsis development, and possibly the usefulness of
β2-microglobulin as a therapeutical target for sepsis-induced
long-term cognitive impairment. However, future studies should
evaluate whether these results can be replicated in more
heterogeneous samples and in different clinical settings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81400876 and 81701884)
and the Great Project of Jiangsu Provincial Commission of Health
and Family Planning (no. YG201409).
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Widmann CN and Heneka MT: Long-term
cerebral consequences of sepsis. Lancet Neurol. 13:630–636. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gofton TE and Young GB: Sepsis-associated
encephalopathy. Nat Rev Neurol. 8:557–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwashyna TJ, Ely EW, Smith DM and Langa
KM: Long-term cognitive impairment and functional disability among
survivors of severe sepsis. JAMA. 304:1787–1794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao R, Kan MQ, Wang SG, Yang RH and Zhang
SG: Disrupted tryptophan metabolism induced cognitive impairment in
a mouse model of sepsis-associated encephalopathy. Inflammation.
39:550–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koh ES, Lee K, Kim SH, Kim YO, Jin DC,
Song HC, Choi EJ, Kim YL, Kim YS, Kang SW, et al: Serum
β2-microglobulin predicts mortality in peritoneal dialysis
patients: A prospective cohort study. Am J Nephrol. 42:91–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villeda SA, Luo J, Mosher KI, Zou B,
Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al:
The ageing systemic milieu negatively regulates neurogenesis and
cognitive function. Nature. 477:90–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith LK, He Y, Park JS, Bieri G,
Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, et
al: β2-microglobulin is a systemic pro-aging factor that impairs
cognitive function and neurogenesis. Nat Med. 21:932–937. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murray AM: Cognitive impairment in the
aging dialysis and chronic kidney disease populations: An occult
burden. Adv Chronic Kidney Dis. 15:123–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glynn MW, Elmer BM, Garay PA, Liu XB,
Needleman LA, El-Sabeawy F and McAllister AK: MHCI negatively
regulates synapse density during the establishment of cortical
connections. Nat Neurosci. 14:442–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elmer BM and McAllister AK: Major
histocompatibility complex class I proteins in brain development
and plasticity. Trends Neurosci. 35:660–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carrette O, Demalte I, Scherl A,
Yalkinoglu O, Corthals G, Burkhard P, Hochstrasser DF and Sanchez
JC: A panel of cerebrospinal fluid potential biomarkers for the
diagnosis of Alzheimer's disease. Proteomics. 3:1486–1494. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McArthur JC, Nance-Sproson TE, Griffin DE,
Hoover D, Selnes OA, Miller EN, Margolick JB, Cohen BA, Farzadegan
H and Saah A: The diagnostic utility of elevation in cerebrospinal
fluid beta 2-microglobulin in HIV-1 dementia. Multicenter AIDS
cohort study. Neurology. 42:1707–1712. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao R, Ji MH, Gao DP, Yang RH, Zhang SG,
Yang JJ and Shen JC: Neuroinflammation-induced downregulation of
hippocampacal neuregulin 1-ErbB4 signaling in the parvalbumin
interneurons might contribute to cognitive impairment in a mouse
model of sepsis-associated encephalopathy. Inflammation.
40:387–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao R, Tang YH, Tong JH, Yang JJ, Ji MH
and Zhu SH: Systemic lipopolysaccharide administration-induced
cognitive impairments are reversed by erythropoietin treatment in
mice. Inflammation. 38:1949–1958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melani R, Chelini G, Cenni MC and Berardi
N: Enriched environment effects on remote object recognition
memory. Neuroscience. 352:296–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji MH, Qiu LL, Tang H, Ju LS, Sun XR,
Zhang H, Jia M, Zuo ZY, Shen JC and Yang JJ: Sepsis-induced
selective parvalbumin interneuron phenotype loss and cognitive
impairments may be mediated by NADPH oxidase 2 activation in mice.
J Neuroinflammation. 12:1822015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Dong L, Zhang M, Jia M, Zhang G, Qiu
L, Ji M and Yang J: Class I histone deacetylase inhibitor valproic
acid reverses cognitive deficits in a mouse model of septic
encephalopathy. Neurochem Res. 38:2440–2449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schramm P, Klein KU, Falkenberg L, Berres
M, Closhen D, Werhahn KJ, David M, Werner C and Engelhard K:
Impaired cerebrovascular autoregulation in patients with severe
sepsis and sepsis-associated delirium. Crit Care. 16:R1812012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang J, Lian Y, Xie K, Cai S and Wen P:
Epigenetic modulation of neuronal apoptosis and cognitive functions
in sepsis-associated encephalopathy. Neurol Sci. 35:283–288. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berg RM, Møller K and Bailey DM:
Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow
Metab. 31:1532–1544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuno S, Ishimura E, Kohno K, Fujino-Katoh
Y, Maeno Y, Yamakawa T, Inaba M and Nishizawa Y: Serum
beta2-microglobulin level is a significant predictor of mortality
in maintenance haemodialysis patients. Nephrol Dial Transplant.
24:571–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu HC, Lee LC and Wang WJ: Associations
among serum Beta 2 microglobulin, malnutrition, inflammation, and
advanced cardiovascular event in patients with chronic kidney
disease. J Clin Lab Anal. 31–May;2017.doi: 10.1002/jcla.22056.
View Article : Google Scholar
|
|
23
|
Raikou VD and Kyriaki D: The relationship
between glycemic control, beta2-microglobulin and inflammation in
patients on maintenance dialysis treatment. J Diabetes Metab
Disord. 14:342015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imamura Y, Wang H, Matsumoto N, Muroya T,
Shimazaki J, Ogura H and Shimazu T: Interleukin-1β causes long-term
potentiation deficiency in a mouse model of septic encephalopathy.
Neuroscience. 187:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoshino K, Hayakawa M and Morimoto Y:
Minocycline prevents the impairment of hippocampal long-term
potentiation in the septic mouse. Shock. 48:209–214. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Snigdha S, Prieto GA, Petrosyan A,
Loertscher BM, Dieskau AP, Overman LE and Cotman CW: H3K9me3
inhibition improves memory, promotes spine formation, and increases
BDNF levels in the aged hippocampus. J Neurosci. 36:3611–3622.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Liao Y, Li T, Cui Y, Wang G, Zhao F
and Jin Y: Alterations of synaptic proteins in the hippocampus of
mouse offspring induced by developmental lead exposure. Mol
Neurobiol. 53:6786–6798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu D, Li C, Swanson AM, Villalba RM, Guo
J, Zhang Z, Matheny S, Murakami T, Stephenson JR, Daniel S, et al:
BAI1 regulates spatial learning and synaptic plasticity in the
hippocampus. J Clin Invest. 125:1497–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chao HW, Tsai LY, Lu YL, Lin PY, Huang WH,
Chou HJ, Lu WH, Lin HC, Lee PT and Huang YS: Deletion of CPEB3
enhances hippocampus-dependent memory via increasing expressions of
PSD95 and NMDA receptors. J Neurosci. 33:17008–17022. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim DM and Leem YH: Chronic stress-induced
memory deficits are reversed by regular exercise via AMPK-mediated
BDNF induction. Neuroscience. 324:271–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim E and Sheng M: PDZ domain proteins of
synapses. Nat Rev Neurosci. 5:771–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Şahin TD, Karson A, Balcı F, Yazır Y,
Bayramgürler D and Utkan T: TNF-alpha inhibition prevents cognitive
decline and maintains hippocampal BDNF levels in the unpredictable
chronic mild stress rat model of depression. Behav Brain Res.
292:233–240. 2015. View Article : Google Scholar : PubMed/NCBI
|