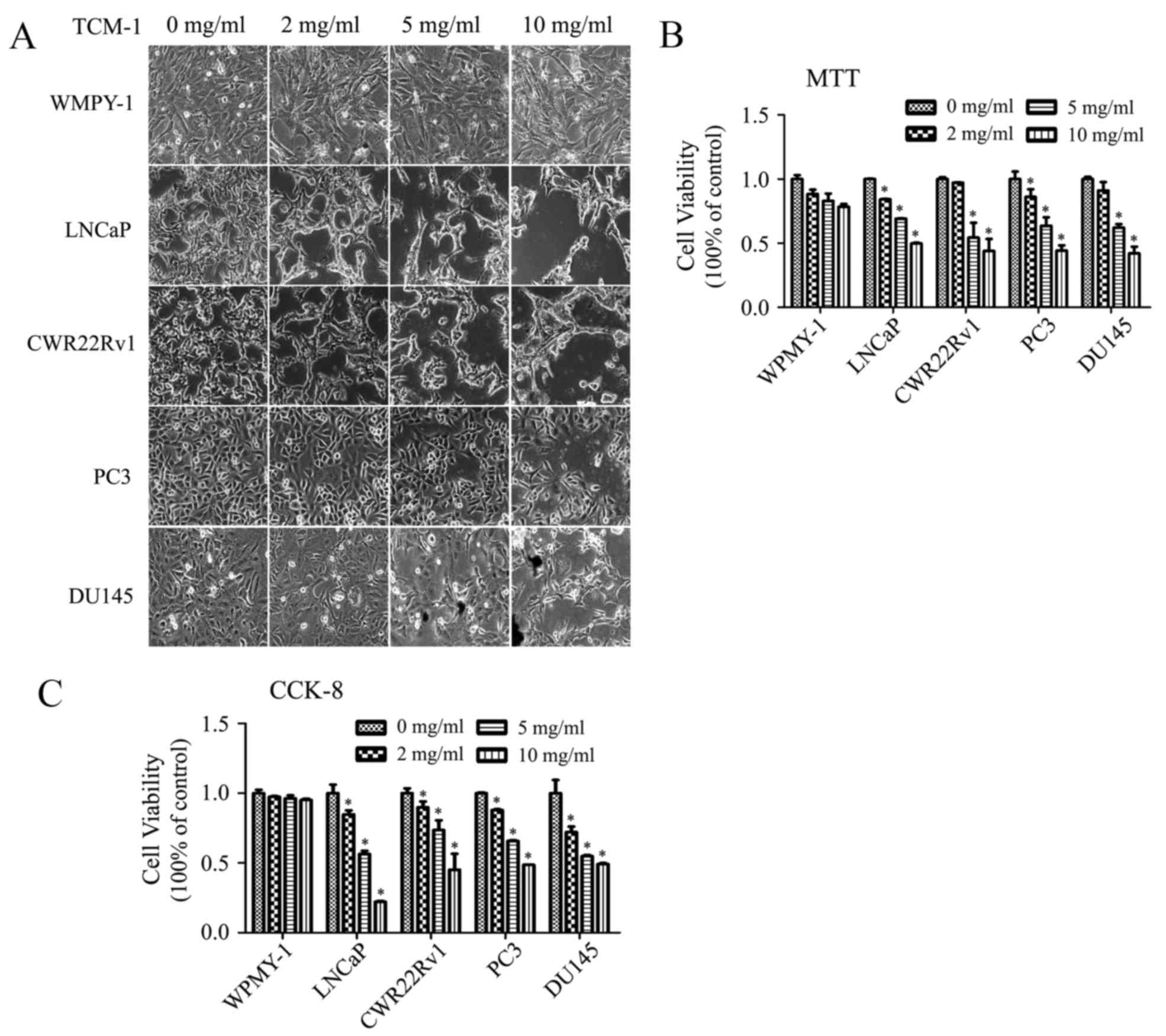
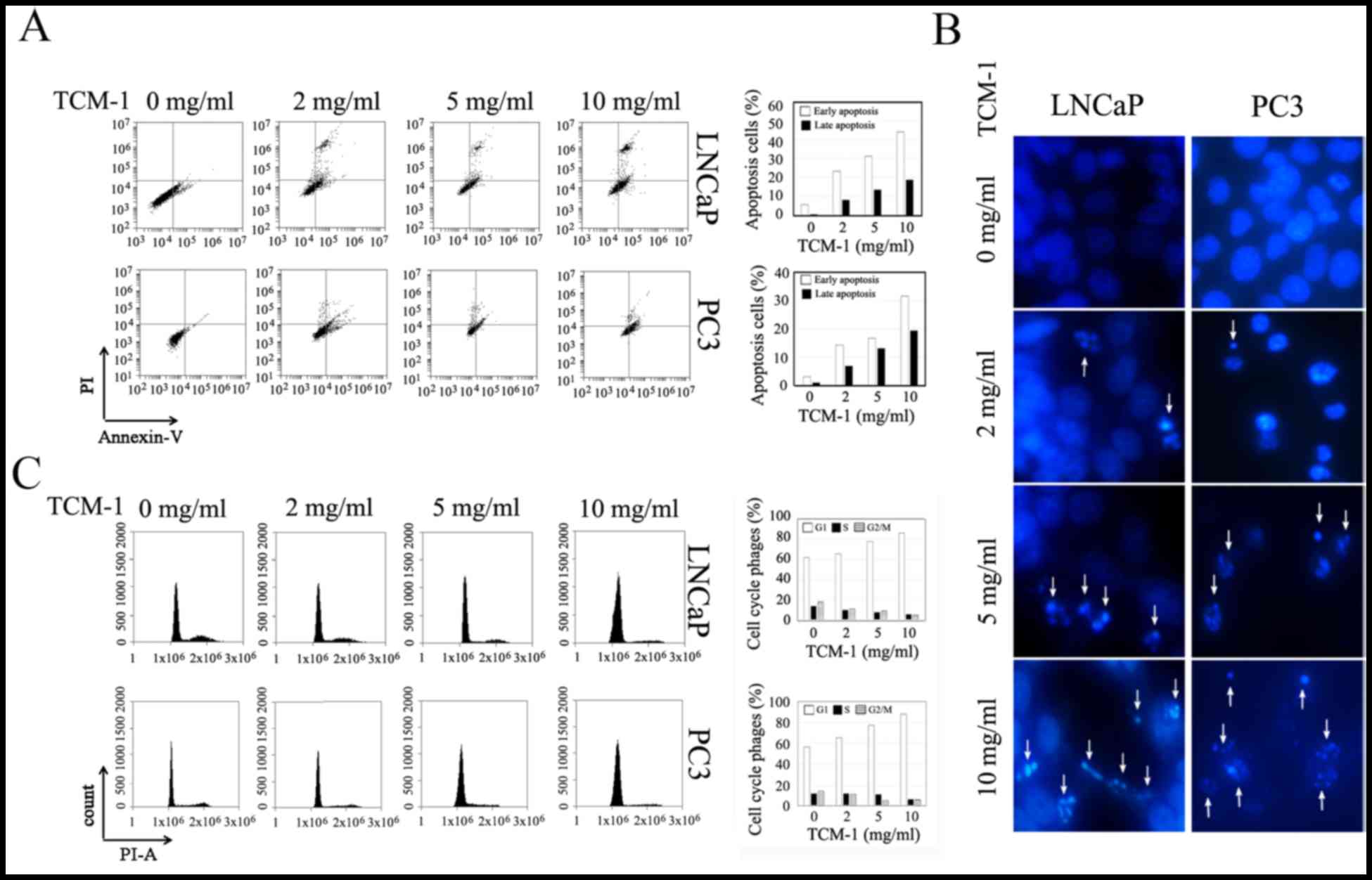
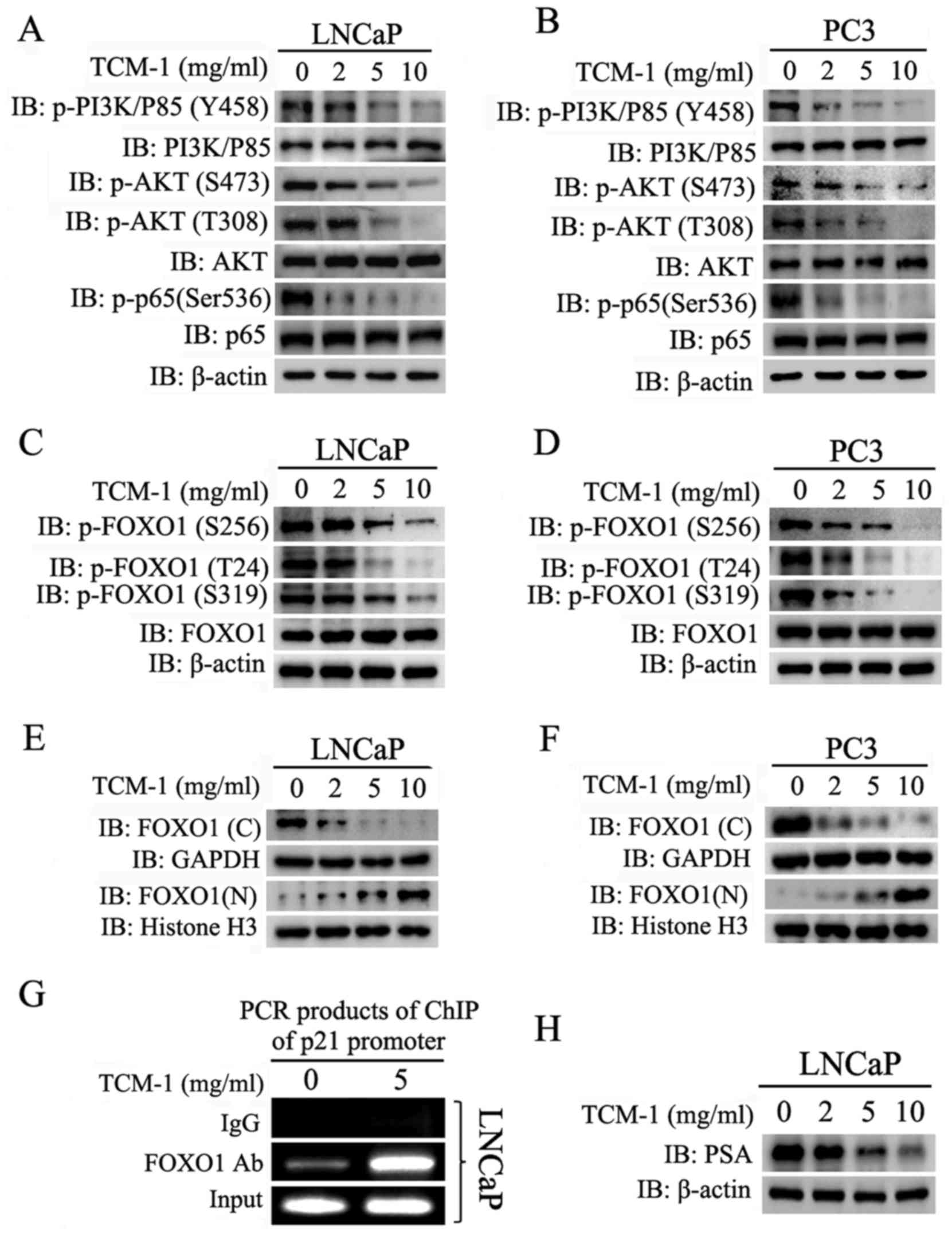
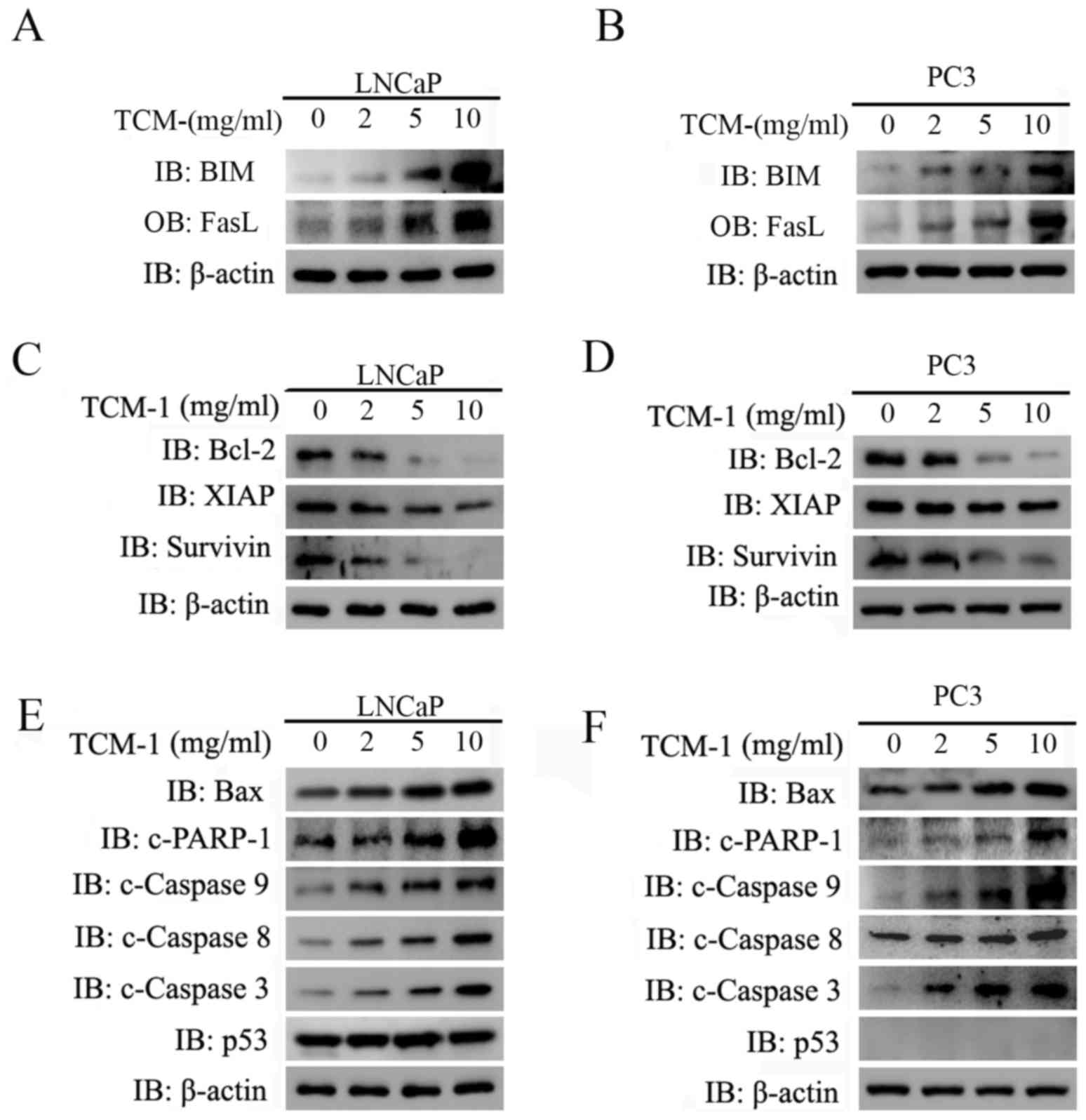
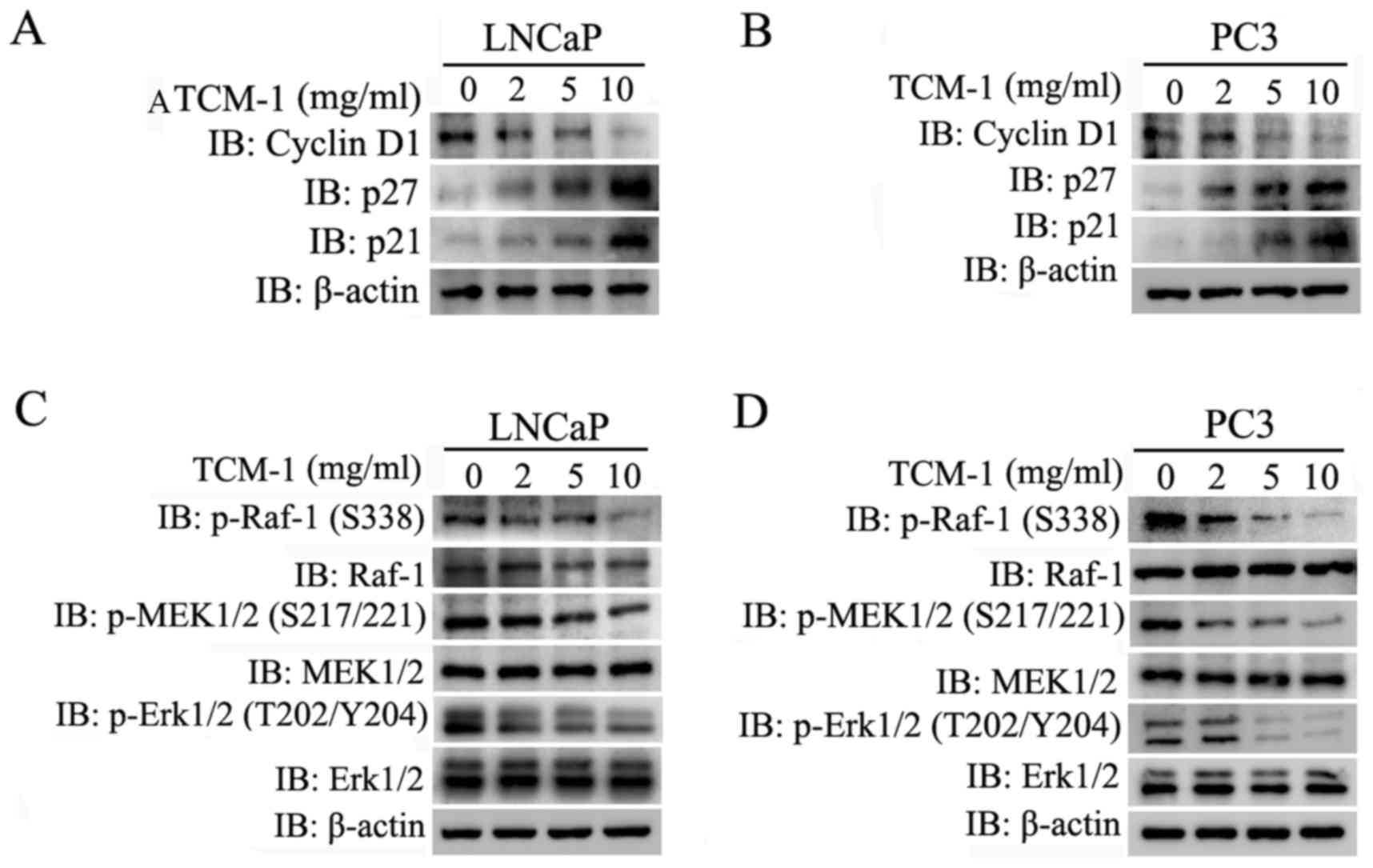
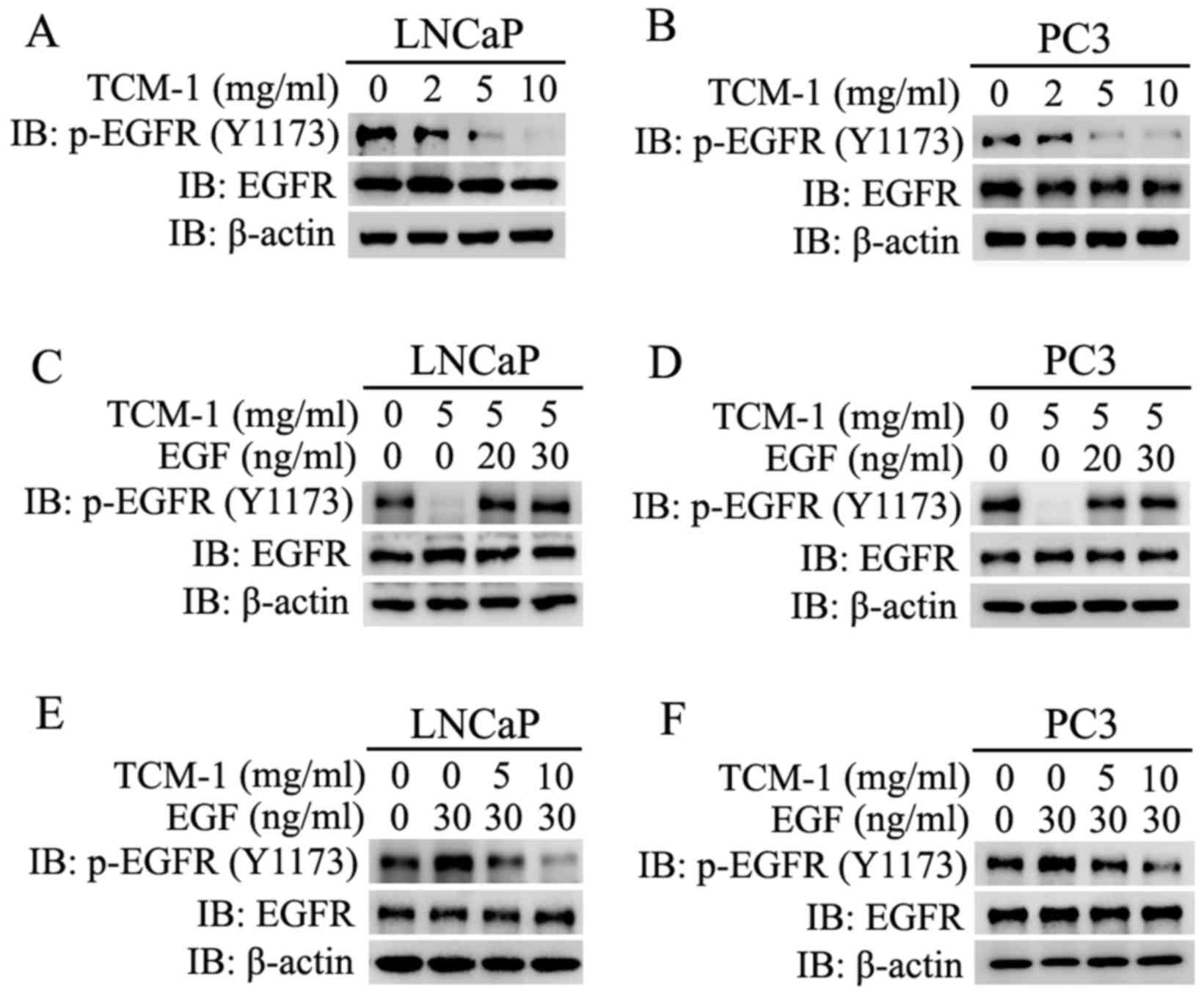
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrylak DP: Chemotherapy for advanced
hormone refractory prostate cancer. Urology. 54 Suppl 6A:S30–S35.
1999. View Article : Google Scholar
|
|
3
|
Pisters LL: The challenge of locally
advanced prostate cancer. Semin Oncol. 26:202–216. 1999.PubMed/NCBI
|
|
4
|
Richie JP: Anti-androgens and other
hormonal therapies for prostate cancer. Urology. 54 6A Suppl:15–18.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathew MP, Tan E, Saeui CT, Bovonratwet P,
Sklar S, Bhattacharya R and Yarema KJ: Metabolic flx-driven
sialylation alters internalization, recycling and drug sensitivity
of the epidermal growth factor receptor (EGFR) in SW1990 pancreatic
cancer cells. Oncotarget. 7:66491–66511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 Suppl
2:S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and -independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang JY and Hung MC: A new fork for
clinical application: Targeting forkhead transcription factorsin
cancer. Clin Cancer Res. 15:752–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo JP, Tian W, Shu S, Xin Y, Shou C and
Cheng JQ: IKBKE phosphorylation and inhibition of FOXO3a: A
mechanism of IKBKE oncogenic function. PLoS One. 8:e636362013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Brachène Coomans A and Demoulin JB:
FOXO transcription factors in cancer development and therapy. Cell
Mol Life Sci. 73:1159–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marino G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Ting W, Xiao L, Shufei F, Wangxiao
T, Xiaoying W, Xiumei G and Boli Z: Immunoregulation of Shenqi
fuzheng injection combined with chemotherapy in cancer patients: A
systematic review and meta-analysis. Evid Based Complement Alternat
Med. 2017:51215382017.PubMed/NCBI
|
|
14
|
Youns M, Hoheisel JD and Efferth T:
Traditional Chinese medicines (TCMs) for molecular targeted
therapies of tumours. Curr Drug Discov Technol. 7:37–45. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y: Application of traditional Chinese
medicine in the comprehensive treatment of cancer. Chin J Integr
Trad West Med. 17:323–324. 1997.(In Chinese).
|
|
16
|
Li P, Lee H, Guo S, Unterman TG, Jenster G
and Bai W: AKT-independent protection of prostate cancer cells from
apoptosis mediated through complex formation between the androgen
receptor and FKHR. Mol Cell Biol. 23:104–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crighton D, Wilkinson S and Ryan KM: DRAM
links autophagy to p53 and programmed cell death. Autophagy.
3:72–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Zhao H, Xu J, Zhang L, Yan L and
Shen Z: Elevated cyclin D1 expression is governed by plasma IGF-1
through Ras/Raf/MEK/ERK pathway in rumen epithelium of goats
supplying a high metabolizable energy diet. J Anim Physiol Anim
Nutr (Berl). 97:1170–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Luca A, Carotenuto A, Rachiglio A,
Gallo M, Maiello MR, Aldinucci D, Pinto A and Normanno N: The role
of the EGFR signaling in tumor microenvironment. J Cell Physiol.
214:559–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howe LR and Brown PH: Targeting the
HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res
(Phila). 4:1149–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaplan AL, Hu JC, Morgentaler A, Mulhall
JP, Schulman CC and Montorsi F: Testosterone therapy in men with
prostate cancer. European Urology. 69:894–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferro M, Lucarelli G, Bruzzese D, Di
Lorenzo G, Perdonà S, Autorino R, Cantiello F, La Rocca R, Busetto
GM, Cimmino A, et al: Low serum total testosterone level as a
predictor of upstaging and upgrading in low-risk prostate cancer
patients meeting the inclusion criteria for active surveillance.
Oncotarget. 8:18424–18434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burstein HJ, Krilov L, Aragon-Ching JB,
Baxter NN, Chiorean EG, Chow WA, De Groot JF, Devine SM, DuBois SG,
El-Deiry WS, et al: Clinical cancer advances 2017: Annual report on
progress against cancer from the american society of clinical
oncology. J Clin Oncol. 35:1341–1367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lastwika KJ, Wilson W III, Li QK, Norris
J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et
al: Control of PD-L1 expression by oncogenic activation of the
AKT-mTOR pathway in non-small cell lung cancer. Cancer Res.
76:227–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Westin JR: Status of PI3K/Akt/mTOR pathway
inhibitors in lymphoma. Clin Lymphoma Myeloma Leuk. 14:335–342.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burgering BM and Kops GJ: Cell cycle and
death control: Longlive forkheads. Trends Biochem Sci. 27:352–360.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Essafi A, de Mattos Fernández S, Hassen
YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH and Lam EW: Direct
transcriptional regulation of Bim by FoxO3a mediates STI571-induced
apoptosis in Bcr-Abl-expressing cells. Oncogene. 24:2317–2329.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meek DW: Regulation of the p53 response
and its relationship to cancer. Biochem J. 469:325–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee H, Kim JS and Kim E: Fucoidan from
seaweed Fucusvesiculosus inhibits migration and invasion of human
lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One.
7:e506242012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bracho-Valdés I, Moreno-Alvarez P,
Valencia-Martínez I, Robles-Molina E, Chávez-Vargas L and
Vázquez-Prado J: mTORC1-and mTORC2-interacting proteins keep their
multifunctional partners focused. IUBMB Life. 63:896–914. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo GF, Jiang WQ, Zhang B, Cai YC, Xu RH,
Chen XX, Wang F and Xia LP: Autophagy-related proteins beclin-1 and
lc3 predict cetuximab efficacy in advanced colorectal cancer. World
J Gastroenterol. 17:4779–4786. 2011. View Article : Google Scholar : PubMed/NCBI
|