Introduction
Tendons are responsible for transmitting tensile
forces from skeletal muscle to bone (1). They are composed of fibroblasts and
extracellular matrix (ECM) components, including type I collagen
(Col I), proteoglycans (PGs) and water (2). Previous findings have demonstrated
that Col I fibrils are highly aligned and significantly contribute
to transmission of mechanical forces (3). Furthermore, Col I molecules are
produced by fibroblasts and organized into fibrillar structures via
a self-assembly process known as fibrillogenesis (4). During this process, PGs regulate the
diameter, length and organization of collagen fibrils (5,6).
In the tensile region of the tendon, the predominant
PG is decorin (80–90% of total PGs), followed by a small presence
of biglycan (7), which are class I
prototype members of the small leucine-rich proteoglycan (SLRP)
family (8). Decorin was reported
to be made up of a 40 kDa protein core and chondroitin/dermatan
sulfate glycosaminglycans, and binds to Col I to regulate collagen
fiber assembly (9,10). Furthermore, variations in decorin
content has been reported to affect tendons; Reese et al
(5) reported that during
polymerization of Col I, decorin significantly increased the
modulus and tensile strength of collagen gels via modification of
collagen fibril organization. Conversely, knockout studies have
demonstrated that mice with a decorin deficiency exhibit decreased
strength and stiffness (11,12).
Therefore, decorin may serve critical roles in tendon structure and
function.
Additionally, it was identified that decorin is
synthesized by stromal fibroblasts and degraded by matrix
metalloproteinases (MMPs) (13,14).
When tendons transmit mechanical force, fibroblasts detect this and
convert mechanical signals into cellular biological events,
including secretion of MMPs, via mechanotransduction mechanisms
(15). MMPs and their specific
inhibitors, tissue inhibitor of metalloproteinases (TIMPs), play
major roles in the degradation and remodeling of ECM components
(16). A balance of MMPs and TIMPs
is required for maintaining healthy remodeling; impaired balance
may result in degradation and disorder (17). Furthermore, a previous study
demonstrated that MMP-2 may cleave and degrade decorin (18). Although four TIMP molecules have
been identified, TIMP-2 is the most common and inhibits the
activities of all MMPs, with a high affinity for MMP-2 (19).
Despite extensive research on decorin (5,7,9) thus
far, how various mechanical loading conditions affect this molecule
in tendons remains to be elucidated. The present study aimed to
observe the effect of varying exercise intensity, characterized by
distinct loading patterns during treadmill running, on alternations
of decorin content at two time intervals (4 and 8 weeks) to assess
decorin metabolism in the rat Achilles tendon.
Materials and methods
Experimental animals and exercise
protocols
The animal ethics committee of Nanfang Hospital,
Southern Medical University (Guangzhou, China) approved all
experimental protocols using rats including treadmill running and
collection of tendon samples (NFYY-2012-056).
Male Wistar rats (age, 12 weeks; weight, 200–250 g;
n=36) were randomly divided into three groups: Control (CON, n=12),
moderate treadmill running (MTR, n=12) and strenuous treadmill
running (STR, n=12). All the animals were housed in a 12-h
light/dark cycle at 22±1°C with free access to food and water.
The running protocol used has been described
previously (20). The animals were
accustomed to treadmill running for one week at a speed of 10 m/min
for 30 min per day, 5 days per week. Subsequently, rats in the MTR
and STR groups regularly ran for 4 or 8 weeks as follows: MTR:
Speed of 19.3 m/min with 5° incline for 60 min per day, 5 days per
week; STR: speed of 26.8 m/min with 10° incline for 60 min per day,
5 days per week. Animals in CON group were allowed to move freely
in cages. All experiments were conducted in accordance with the
institutional guidelines for the care and use of experimental
animals.
Following the treadmill running protocol, rats were
sacrificed by CO2 asphyxiation followed by cervical
dislocation. The gastrocnemius and soleus muscles were dissected
free of all soft tissues including the plantaris muscle tendon
unit. The two merging tendons were isolated by amputation:
Proximally at the distal end of the gastrocnemius soleus muscle
belly, and distally at the calcaneus insertion. One side of the
Achilles tendon of each animal was frozen in liquid nitrogen and
stored at −80°C. The contralateral Achilles tendon was fixed in 10%
buffered formalin for immunohistochemistry.
Immunohistochemistry for decorin and
biglycan
Immunohistochemistry for decorin and biglycan was
performed as described previously (21). Formalin-fixed tendon samples were
dehydrated in ethanol and embedded in paraffin. Sections (4 µm)
were cut and deparaffinized with xylene and varying concentrations
of alcohol. Endogenous peroxidase activity was blocked by the
addition of 3% hydrogen peroxide for 20 min at room temperature.
Antigen retrieval was performed with citric acid (pH 6.0) by high
pressure method. Following blocking with 5% normal bovine serum
(Merck KGaA, Darmstadt, Germany) for 20 min at room temperature,
the sections were incubated with specific primary antibodies at 4°C
overnight. The primary antibodies used were anti-rat decorin
(1:100; cat. no. ab175404) and anti-rat biglycan (1:100; cat. no.
ab49701), purchased from Abcam (Cambridge, MA, USA). Following
this, sections were incubated with mouse anti-rabbit
IgG-horseradish peroxidase (HRP) secondary antibodies (1:200; cat.
no. sc2357; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1
h at room temperature. Subsequently, sections were developed with
3,3′-diaminobenzidine tetrahydrochloride (Dako; Agilent
Technologies GmbH, Waldbronn, Germany) and counter-stained in
haematoxylin. Primary antibodies were replaced with blocking
solution in the controls. For good reproducibility and
comparability, all incubation times and conditions were strictly
controlled. The sections were examined under a light microscope
(Nikon H600L Microscope and image analysis system; Nikon
Corporation, Tokyo, Japan). Sections were imaged using Image-Pro
Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Achilles tendon tissues for RT-qPCR were crushed
with a masher. Subsequently, total RNA was extracted using
TRIzol® reagent (Takara Biotechnology Co., Ltd., Dalian,
China) in accordance with the manufacturer's protocol. Following
this, RNA was reverse transcribed into cDNA using a transcription
RT Kit (Takara Biotechnology Co., Ltd.), following the
manufacturer's protocol. qPCR with SYBR®-Green detection
chemistry was performed using an Applied Biosystems 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc. Waltham, MA, USA). Expression of the target gene was
normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The
thermocycling conditions used were as follows: Initialization for
10 min at 95°C, followed by 45 cycles of denaturation at 95°C for
10 sec, annealing at 55°C for 15 sec, and extension at 72°C for 30
sec. PCR primer sequences (BioTeke Corporation, Beijing, China) are
presented in Table I. Relative
gene expression of the MTR and STR groups to the CON group were
calculated according to the 2−ΔΔCq method (22).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Forward | Reverse |
|---|
| GAPDH |
5′-GGCACAGTCAAGGCTGAGAATG −3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
| Decorin |
5′-ATGATTGTCATAGAACTGGGC-3′ |
5′-TTGTTGTTATGAAGGTAGAC-3′ |
| Biglycan |
5′-TCTACATCTCCAAGAACCACCTGG-3′ |
5′-GCTCTGGGCTCCTACTCCTT-3′ |
| MMP-2 |
5′-GGAAGCATCAAATCGGACTG-3′ |
5′-GGGCGGGAGAAAGTAGCA-3′ |
| TIMP-2 |
5′-CCAAAGCAGTGAGCGAGAA-3′ |
5′-CCCAGGGCACAATAAAGTC-3′ |
Western blotting
Achilles tendon tissue samples were homogenized in
radioimmunoprecipitation assay lysis buffer (50 mM Tris-Cl, pH 8.0;
150 mM NaCl; 0.1% SDS and 1% Igepal CA-630), 0.5% sodium
deoxycholate, a cocktail of protease inhibitors and 0.5 mM
phenylmethylsulfonyl fluoride (all from Sigma-Aldrich; Merck KGaA).
Following ultraphonic oscillation, the samples were centrifuged at
12,000 × g for 15 min at 4°C to obtain the supernatant. Protein
concentration was measured by Bicinchoninic Acid assay (Thermo
Fisher Scientific, Inc.) and a NanoDrop 1000 Spectrophotometer
(Thermo Fisher Scientific, Inc.). A total of 20 µg protein was
separated by 12% SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Subsequently, proteins were electrophoretically
transferred onto polyvinylidene difluoride membranes (Invitrogen;
Thermo Fisher Scientific, Inc.) and blocked with 3% bovine serum
albumin and non-fat milk. The expression of decorin was detected
incubation the membranes for 12 h at 4°C with an anti-rat decorin
antibody (1:100; cat. no. ab175404; Abcam), with gentle shaking.
Following washing with 0.2% TBS with Tween-20 buffer, membranes was
incubated with a mouse anti-rabbit IgG-HRP secondary antibodies
(1:1,000; cat. no. sc2357; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Protein detection was performed by Enhanced
Chemiluminescence (Luminata™ Crescendo Western HRP Substrate; EMD
Millipore, Billerica, MA, USA) using a Molecular Imager®
ChemiDoc™ XRS system (Bio-Rad Laboratories, Inc.). The resulting
bands were assessed by densitometric quantitative analysis of
proteins using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA) and normalized to GAPDH
levels.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using one-way analysis of
variance followed by Tukey's post hoc test using SPSS software
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Immunohistochemistry
Immunostaining for decorin was performed in Achilles
tendon sections of rats in CON (Fig.
1A), MTR (Fig. 1B) and STR
(Fig. 1C) groups after 4 weeks,
and in CON (Fig. 1D), MTR
(Fig. 1E) and STR (Fig. 1F) groups after 8 weeks. At 4 weeks,
decorin expression was markedly increased in the MTR group
(0.21±0.009) compared with the CON group (0.16±0.011), and was
markedly reduced in STR group (0.11±0.013) compared with the CON or
MTR groups. At 8 weeks, a similar pattern of expression was
observed. The MTR group (0.23±0.017) exhibited markedly increased
expression of decorin compared with the CON group (0.17±0.008).
However, the STR group (0.08±0.010) exhibited significantly reduced
expression of decorin compared with the CON or MTR groups.
Furthermore, in the MTR group, decorin expression was significantly
increased at 8 weeks compared with at 4 weeks. Conversely, in the
STR group, decorin expression was significantly reduced at 8 weeks
compared with at 4 weeks (Fig.
1G).
Immunostaining for biglycan was performed in
Achilles tendon sections of rats in CON (Fig. 2A), MTR (Fig. 2B) and STR (Fig. 2C) groups after 4 weeks, and in CON
(Fig. 2D), MTR (Fig. 2E) and STR (Fig. 2F) groups after 8 weeks. At 4 weeks,
although markedly reduced expression levels of biglycan were
observed in the MTR group (0.10±0.015) compared with the CON group
(0.13±0.008), expression of biglycan was markedly increased in the
STR group (0.18±0.007) compared with the CON and MTR groups. At 8
weeks, the MTR group (0.07±0.009) had markedly reduced expression
levels of biglycan compared with the CON group (0.11±0.012).
However, the STR group (0.21±0.005) had significantly increased
biglycan expression compared with the CON or MTR groups.
Furthermore, in the MTR group, biglycan expression was
significantly reduced at 8 weeks compared with at 4 weeks.
Conversely, in the STR group, biglycan expression was significantly
increased at 8 weeks compared with at 4 weeks (Fig. 2G).
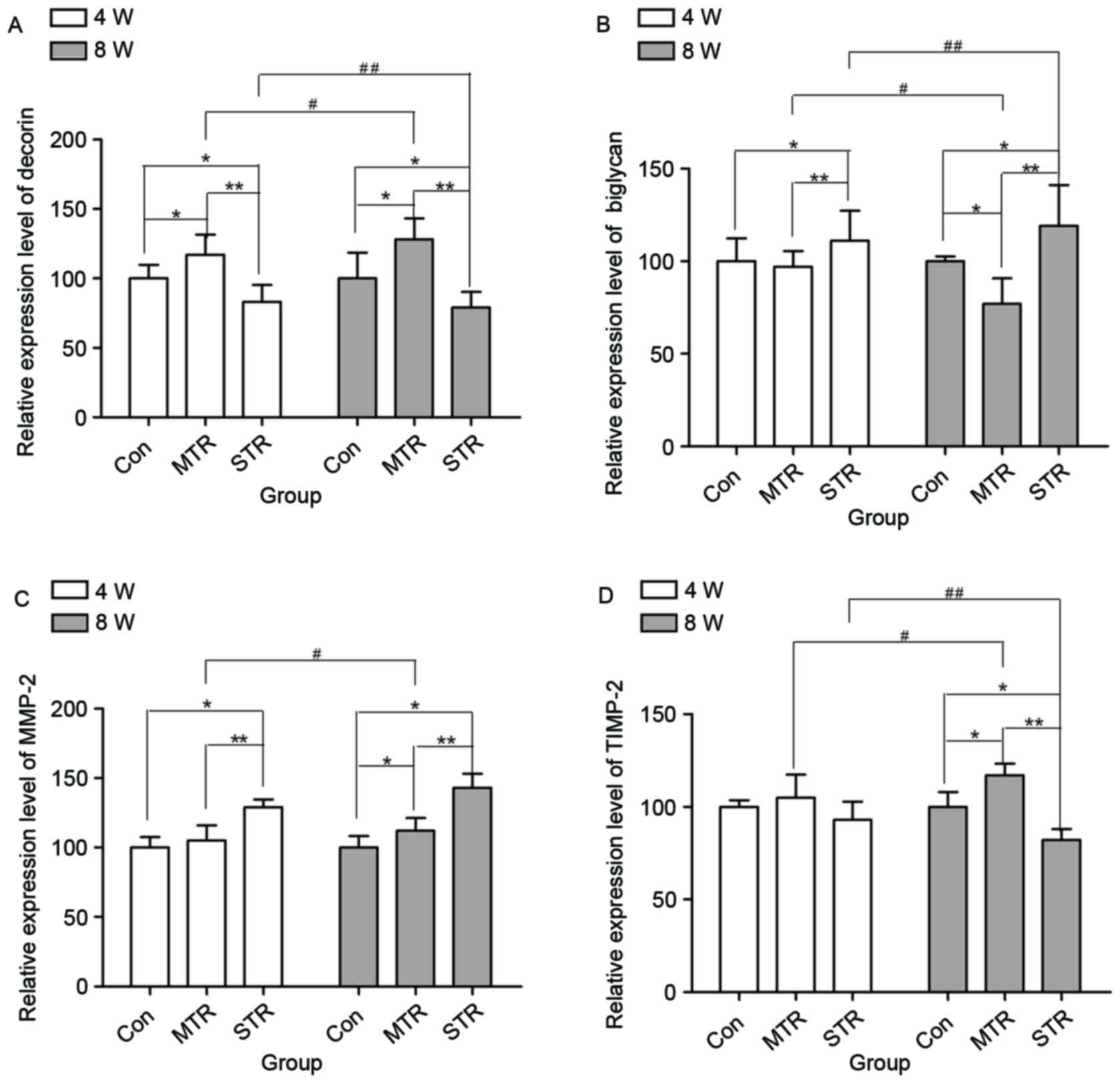
RT-qPCR
mRNA expression levels of decorin (Fig. 3A), biglycan (Fig. 3B), MMP-2 (Fig. 3C) and TIMP-2 (Fig. 3D) were detected in rat Achilles
tendons in the CON, MTR and STR groups after 4 and 8 weeks. At 4
weeks, the expression of decorin was significantly increased in the
MTR group compared with the CON group (P=0.043), and were
significantly decreased in the STR group compared with the CON and
MTR groups (P=0.044 and P=0.028, respectively). Similarly, at 8
weeks, mRNA expression levels of decorin were markedly increased in
the MTR group in comparison with the CON group (P=0.039), and were
markedly decreased in the STR group compared with the CON and MTR
groups (P=0.040 and P=0.022, respectively). Furthermore, in the MTR
group, decorin expression was increased at 8 weeks compared with at
4 weeks (P=0.039). However, in the STR group, decorin expression
was markedly decreased at 8 weeks compared with at 4 weeks
(P=0.036). At 4 weeks, no significant differences in the mRNA
expression of biglycan was recorded in MTR group compared with the
CON group (P=0.081); however, it was significantly increased in the
STR group compared with the CON and MTR groups (P=0.038 and
P=0.021, respectively). Similarly, at 8 weeks, mRNA expression
levels of biglycan were markedly decreased in the MTR group
compared with the CON group (P=0.034), and were markedly increased
in the STR group compared with the CON and MTR groups (P=0.037 and
P=0.018, respectively). Furthermore, in the MTR group, biglycan
expression was significantly decreased at 8 weeks compared with at
4 weeks (P=0.032). However, in the STR group, biglycan expression
levels were increased at 8 weeks compared with at 4 weeks
(P=0.035).
At 4 weeks, no significant differences were observed
in the gene expression levels of MMP-2 in the MTR group compared
with the CON group (P=0.113); however, they were markedly increased
in the STR group compared with the CON and MTR groups (P=0.039 and
P=0.037, respectively). At 8 weeks, the gene expression of MMP-2
was increased in the MTR group compared with the CON group
(P=0.042), and in the STR group compared with the CON and MTR
groups (P=0.024 and P=0.037, respectively). Additionally, in the
MTR group, the expression levels of MMP-2 were significantly
increased at 8 weeks compared with at 4 weeks (P=0.032). However,
in the STR group, no significant differences were observed between
4 and 8 weeks (P=0.092).
At 4 weeks, no significant differences were observed
in mRNA expression levels of TIMP-2 in the MTR group compared with
the CON group (P=2.371), and in the STR group compared with the CON
and MTR groups (P=0.738 and P=0.917, respectively). However, at 8
weeks, mRNA expression levels of TIMP-2 were increased in the MTR
group compared with the CON group (P=0.034); however, were
decreased in the STR group compared with the CON and MTR groups
(P=0.026 and P=0.015, respectively). Additionally, in the MTR
group, the expression levels of TIMP-2 were increased at 8 weeks
compared with at 4 weeks (P=0.037). However, in STR group, TIMP-2
expression levels were decreased at 8 weeks compared with at 4
weeks (P=0.021).
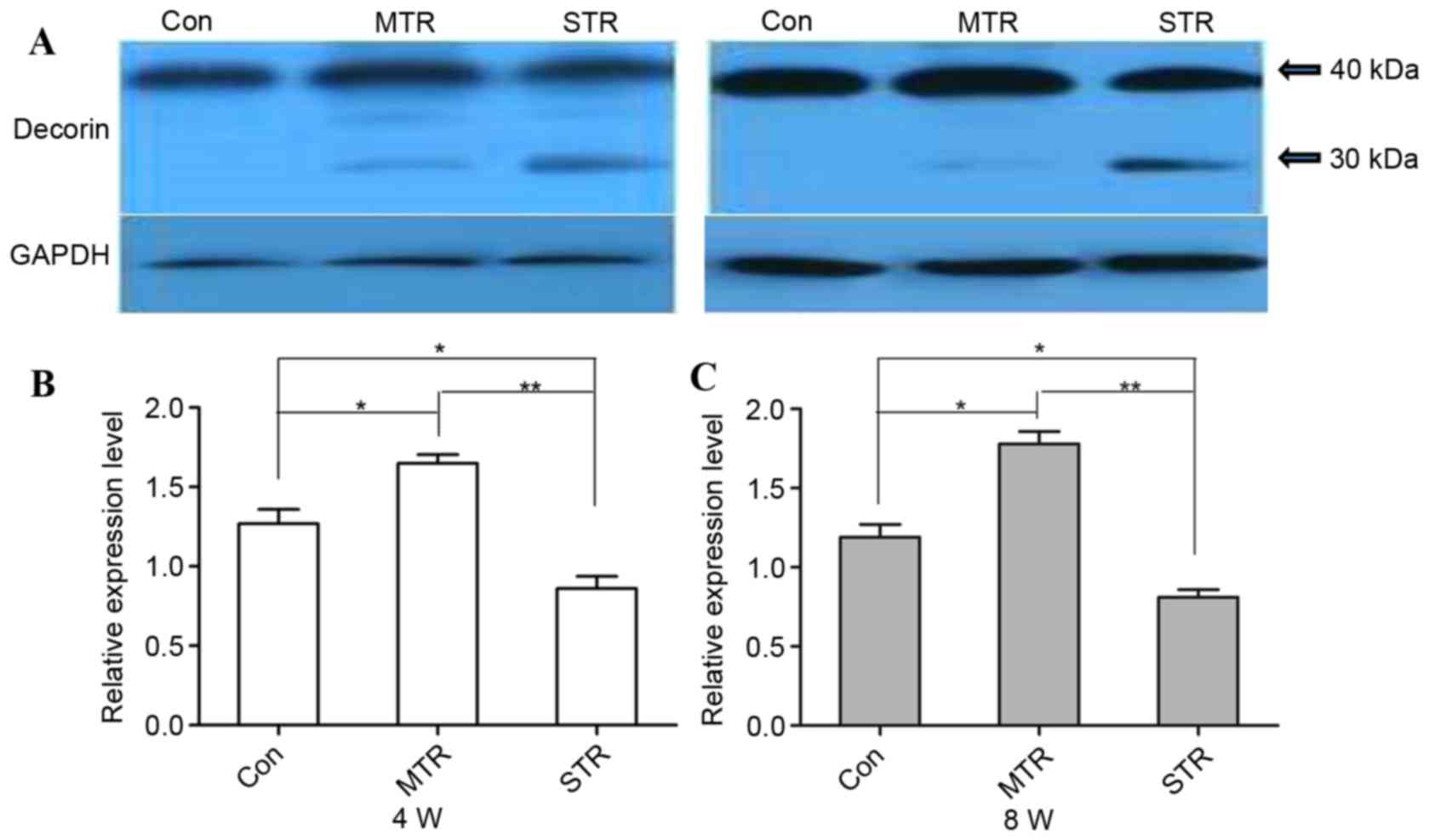
Western blotting
Protein expression levels of decorin were detected
by western blotting (Fig. 4A), and
the results confirmed the observations on mRNA gene expression
levels. At 4 weeks, protein expression levels for decorin were
increased in the MTR group compared with the CON group (P=0.039);
however, were markedly decreased in the STR group compared with the
CON and MTR groups (P=0.032 and P=0.016, respectively; Fig. 4B). Similarly, at 8 weeks, decorin
protein expression levels were increased in the MTR group compared
with the CON group (P=0.034); however, were markedly decreased in
the STR group compared with the CON and MTR group (P=0.031 and
P=0.012, respectively; Fig. 4C).
Decorin from the CON group was visualized as a single band of ~40
kDa, and no decorin fragments were observed in the MTR group.
However, distinct decorin fragments of ~30 kDa were detected in the
STR group.
Discussion
Decorin is regarded as a vital element in the tendon
and regulates collagen fibril organization, thus affecting
structure and function of the tendon. However, whether its
expression is affected by various mechanical loading conditions
remains to be elucidated. The present study examined alterations in
decorin content in rat Achilles tendons at two time intervals (4
and 8 weeks), using a running treadmill model at various speeds and
inclinations to represent moderate and strenuous exercise. These
results indicated that at 4 and 8 weeks, expression levels of
decorin were statistically increased in the MTR group compared with
the CON group, whereas they were significantly decreased in the STR
group compared with the CON and MTR groups. Furthermore, between
the two time points, decorin expression was markedly increased in
the MTR group, whereas it was significantly decreased in the STR
group.
Subsequently, to evaluate the metabolism of decorin,
mRNA expression levels of MMP-2 and TIMP-2 were examined. The data
from the MTR group demonstrated that the balance of MMP-2 and
TIMP-2 expression was maintained at 4 weeks; however, levels were
altered at 8 weeks. This state of equilibrium may be conducive to
the synthesis of decorin, and thus elevate the level of decorin in
the ECM. Taken together, these results suggested that moderate
exercise induced synthesis of decorin via the balance of MMP-2 and
TIMP-2. This was consistent with previous biochemical analyses,
which revealed an increase in decorin expression levels following
moderate exercise (1,2). Decorin is the most abundant SLRP in
the tendon and regulates the specialized assembly of Col I, which
is the primary structural component and contributor to the
transmission of mechanical strength (2). This may explain why moderate exercise
increased tendon stiffness and enhanced tendon tensile
strength.
In the present study, observations from the STR
group indicated that the balance of MMP-2 and TIMP-2 was impaired
with a bias to MMP-2 at 4 weeks, and this effect increased in a
time-dependent manner (from 4 to 8 weeks). This impaired balance
may result in the degradation of decorin, reducing the expression
levels of decorin in the ECM. In addition, the degraded fragment of
decorin in the STR group was visualized by western blot analysis.
This suggested that strenuous exercise, including STR, causes
degradation of decorin; a key regulator of collagen fibril
assembly. Previous studies have reported that collagen fibrils are
coarse, irregular and haphazardly arranged with decreased strength
and stiffness in the absence of decorin (11,12,23).
In agreement with this, decorin content was significantly decreased
in clinical samples of tendinopathy compared with healthy tendon
samples (24,25). Tendinopathy is hypothesized to be
involved in micro-injury and failed tendon healing due to
repetitive excessive exercise (26). Therefore, decrease of decorin under
strenuous exercise may lead to abnormal fibril structure and
organization, decreasing tendon strength and stiffness, thus
predisposing to micro-injury and tendinopathy.
In addition, biglycan has been reported to be highly
homologous with decorin and bind to collagen to regulate collagen
fiber assembly (6). The present
study demonstrated a converse pattern of alteration in the
expression levels of biglycan between the three groups at the two
time intervals. A potential reason for this is that biglycan and
decorin share common functions and partially compensated for each
other.
In conclusion, the present study demonstrated a
significant intensity-specific influence of treadmill running on
decorin expression levels in rat Achilles tendons. These results
suggested that moderate exercise may induce increased synthesis of
decorin via balance of MMP-2 and TIMP-2, which may increase tendon
stiffness and enhance tendon tensile strength, improving the
structure and function of the tendon. However, strenuous exercise
may result in the degradation of decorin by imbalance of MMP-2 and
TIMP-2 with a bias to MMP-2, which may decrease tendon strength and
stiffness, thus increasing the risk of damage and predispose to
tendinopathy. Therefore, decorin may serve an important role in
tendon pathophysiology. These results suggest decorin as a
potential therapeutic target for the treatment of tendinopathies.
However, further studies are required to validate these
findings.
Acknowledgements
The authors gratefully acknowledge Mr. Zhao from the
Laboratory of Bone and Cartilage Regenerative Medicine in Southern
Medical University (Guangzhou, China) for the technical
assistance.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81371686 and 81572219) and
the Guangdong Natural Science Foundation (grant no.
S20140006946).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
GXN, SYL and SYX designed the study. SYX, SYL and LX
performed the experiments. SYX, SYD and YBH collected the data. SYX
and SFL analyzed the data. GXN, SYL and SYX interpreted the data.
GXN, SYL, LX and SYX wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
committee of Nanfang Hospital, Southern Medical University
(application no. NFYY-2012-056).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoon Hae J, Brooks R, Kim Hwan Y, Terada M
and Halper J: Proteoglycans in chicken gastrocnemius tendons change
with exercise. Arch Biochem Biophys. 412:279–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franchi M, Torricelli P, Giavaresi G and
Fini M: Role of moderate exercising on Achilles tendon collagen
crimping patterns and proteoglycans. Connect Tissue Res.
54:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frizziero A, Fini M, Salamanna F,
Veicsteinas A, Maffulli N and Marini M: Effect of training and
sudden detraining on the patellar tendon and its enthesis in rats.
BMC Musculoskelet Disord. 12:202011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Killian ML, Cavinatto L, Galatz LM and
Thomopoulos S: The role of mechanobiology in tendon healing. J
Shoulder Elbow Surg. 21:228–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reese SP, Underwood CJ and Weiss JA:
Effects of decorin proteoglycan on fibrillogenesis, ultrastructure,
and mechanics of type I collagen gels. Matrix Biol. 32:414–423.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon JH and Halper J: Tendon
proteoglycans: Biochemistry and function. J Musculoskelet Neuronal
Interact. 5:22–34. 2005.PubMed/NCBI
|
|
7
|
Dourte LM, Pathmanathan L, Jawad AF, Iozzo
RV, Mienaltowski MJ, Birk DE and Soslowsky LJ: Influence of decorin
on the mechanical, compositional, and structural properties of the
mouse patellar tendon. J Biomech Eng. 134:0310052012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni GX, Li Z and Zhou YZ: The role of small
leucine-rich proteoglycans in osteoarthritis pathogenesis.
Osteoarthritis Cartilage. 22:896–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunkman AA, Buckley MR, Mienaltowski MJ,
Adams SM, Thomas SJ, Kumar A, Beason DP, Iozzo RV, Birk DE and
Soslowsky LJ: The injury response of aged tendons in the absence of
biglycan and decorin. Matrix Biol. 35:232–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Juneja SC and Veillette C: Defects in
tendon, ligament, and enthesis in response to genetic alterations
in key proteoglycans and glycoproteins: A review. Arthritis.
2013:1548122013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang G, Ezura Y, Chervoneva I, Robinson
PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV and Birk DE:
Decorin regulates assembly of collagen fibrils and acquisition of
biomechanical properties during tendon development. J Cell Biochem.
98:1436–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robinson PS, Huang TF, Kazam E, Iozzo RV,
Birk DE and Soslowsky LJ: Influence of decorin and biglycan on
mechanical properties of multiple tendons in knockout mice. J
Biomech Eng. 127:181–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neill T, Schaefer L and Iozzo RV: Decorin:
A guardian from the matrix. Am J Pathol. 181:380–387. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown S, Melrose J, Caterson B, Roughley
P, Eisenstein SM and Roberts S: A comparative evaluation of the
small leucine-rich proteoglycans of pathological human
intervertebral discs. Eur Spine J. 21 Suppl 2:S154–S159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JH, Thampatty BP, Lin JS and Im HJ:
Mechanoregulation of gene expression in fibroblasts. Gene.
391:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mandal M, Mandal A, Das S, Chakraborti T
and Sajal C: Clinical implications of matrix metalloproteinases.
Mol Cell Biochem. 252:305–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalton S, Cawston TE, Riley GP, Bayley IJ
and Hazleman BL: Human shoulder tendon biopsy samples in organ
culture produce procollagenase and tissue inhibitor of
metalloproteinases. Ann Rheum Dis. 54:571–577. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imai K, Hiramatsu A, Fukushima D,
Pierschbacher MD and Okada Y: Degradation of decorin by matrix
metalloproteinases: Identification of the cleavage sites, kinetic
analyses and transforming growth factor-beta1 release. Biochem J.
322:809–814. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koskinen SO, Heinemeier KM, Olesen JL,
Langberg H and Kjaer M: Physical exercise can influence local
levels of matrix metalloproteinases and their inhibitors in
tendon-related connective tissue. J Appl Physiol (1985).
96:861–864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni GX, Liu SY, Lei L, Li Z, Zhou YZ and
Zhan LQ: Intensity-dependent effect of treadmill running on knee
articular cartilage in a rat model. Biomed Res Int.
2013:1723922013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lui PP, Chan LS, Lee YW, Fu SC and Chan
KM: Sustained expression of proteoglycans and collagen type
III/type I ratio in a calcified tendinopathy model. Rheumatology
(Oxford). 49:231–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danielson KG, Baribault H, Holmes DF,
Graham H, Kadler KE and Iozzo RV: Targeted disruption of decorin
leads to abnormal collagen fibril morphology and skin fragility. J
Cell Biol. 136:729–743. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alfredson H, Lorentzon M, Backman S,
Backman A and Lerner UH: cDNA-arrays and real-time quantitative PCR
techniques in the investigation of chronic Achilles tendinosis. J
Orthop Res. 21:970–975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corps AN, Robinson AH, Movin T, Costa ML,
Hazleman BL and Riley GP: Increased expression of aggrecan and
biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford).
45:291–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rees SG, Dent CM and Caterson B:
Metabolism of proteoglycans in tendon. Scand J Med Sci Sports.
19:470–478. 2009. View Article : Google Scholar : PubMed/NCBI
|