Introduction
MicroRNAs (miRs) are groups of highly conserved,
small noncoding RNAs that regulate gene expression by binding to
the 3′-untranslated region (3′-UTR) of mRNAs (1) to control mRNA stability or
degradation at the post-transcriptional level. MiRs are small, but
important modulators that are involved in various physiological and
pathological processes (2), such
as energy homeostasis (3), lipid
metabolism, adipogenesis (4) and
diabetes. MiRs have been identified as oncogenes or tumor
suppressors in cancer (5). Among
them, miR-141 has previously been demonstrated to function as a
tumor suppressor in various types of cancer including colorectal
(6), pancreatic (7), gastric (8) and head and neck squamous cell
carcinoma (9).
Diabetes is a metabolic disease characterized by
resistance to insulin action in the liver and other metabolic
tissues, and by increased blood glucose levels (10–12).
Type 1 diabetes (T1D) and type 2 diabetes (T2D) are the most common
forms (13,14). T1D occurs due to lack of pancreatic
β cell function and autoimmune β cell destruction induced insulin
deficiency (15), whereas T2D
begins with insulin resistance and defects in insulin sensitivity,
and pancreatic β cell dysfunction (16,17).
Therefore, understanding the mechanisms underlying pancreatic β
cell function may aid the development of novel therapeutic
strategies for T2D. However, whether miR-141 is involved in
diabetes remains to be elucidated. Pioglitazone is an agonist of
peroxisome proliferator-activated receptor-γ and an antidiabetic
agent (18). Pioglitazone is used
to improve insulin production and increase insulin sensitivity
(19,20). However, whether there are other
mechanisms by which pioglitazone suppresses diabetes, remains to be
determined.
Materials and methods
Reagents and cell culture
The miR-141 mimic, miR-141 inhibitor and the
scrambled negative control were purchased from Guangzhou RiboBio
Co., Ltd., (Guangzhou, China). The following miRNA sequences were
used: miR-141 mimic, 5-UAACACUGUCUGGUAAAGAUGG-3, miR-141 inhibitor,
5-CCAUCUUUACCAGACAGUGUUA-3 scrambled negative control
5-CAGUACUUUUGUGUAGUAC-3. The antibodies for forkhead box A2 (FOXA2
cat. no. ab108422) and β-actin (cat. no. ab8226) were obtained from
Abcam (Cambridge, UK). Pioglitazone was purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). Pancreatic
INS-1 β-cells (American Type Culture Collection Manassas, VA, USA)
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) at
a 37°C incubator and 5% CO2 with 100 U/ml penicillin and
streptomycin (Chinese Academy of Medical Sciences, Beijing, China).
MIN-6 pseudoislets (National Infrastructure of Cell Line Resource,
Beijing, China) were cultured by plating 6×105 cells
into 100-mm Petri dishes and cultured subsequently for 15 days.
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for transfection according
to the manufacturer's protocol. A total of 250 pmol miR-141 mimic,
miR-141 inhibitor or the scrambled negative control and 2 µl
Lipofectamine® 2000 were used per well with a density of
2.5×105 cells (6-well plates). The short hairpin
(sh)FOXA2 was purchased from Sigma-Aldrich; Merck KGaA and
transfected into the cells using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.).
Patients
The present study was approved by the Research
Ethics Committee of Beijing Tian Tan Hospital, (Beijing, China;
reference no. Eth-2014-057). A total of 50 participants (25 male
and 25 female) with uncomplicated T2D, aged 60–65 years, were
deemed eligible and were enrolled between January 2014 and June
2016. Patients were given written information regarding the
objectives of the present study and written informed consent was
obtained from all patients. All participants were at a stable
weight and did not regularly engage in vigorous physical exercise.
A total of 3 ml peripheral venous blood samples for all
participants were collected using EDTA-coated tubes. A total of 1
ml serum samples were taken in the morning following a period of
overnight fasting. The glucose oxidase method was used for the
determination of blood sugar concentration.
Animals
The animal experiment was approved by the
Institutional Animal Care and Use Committee of Beijing Tian Tan
Hospital. A total of 40 C57BLKS/J db/db mice or 40 C57BL/6 mice (8
weeks old; 20–25 g; male) were purchased from The Jackson
Laboratory (Bar Harbor, ME USA). All animals were housed on a 12-h
light-dark cycle, at 21±1°C with a humidity of 55–65% and free
access to food and water. C57BL/6J mice were fed a standard feed
(D01060501; 10% kcal from fat) or a high-fat diet (HFD; D01060502,
58% kcal from fat; Research Diets, animal center of Beijing Tian
Tan Hospital) for 12 weeks. Pioglitazone (10 mg/kg/day) was mixed
with the food and orally fed to db/db mice or HFD mice for 10 days.
The pancreatic islets were isolated following 12 weeks of the diet
and the RNA was isolated. Blood was collected to quantify the
glucose levels once a week during treatment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For miR extraction, 2×105 cells or 2 µg
tissues samples were lysed in RNAiso for miRNA (miRCURY™ RNA
Isolation kit, Takara Biotechnology Co., Ltd., Dalian, China).
Next, 2 µg total RNA or miRs in each group were used for RT to
obtain the first strand cDNA by using the PrimeScript Reverse
Transcriptase (Takara Biotechnology Co., Ltd.). The following
temperature protocol was used for reverse transcription: 25°C for
10 min, 42°C for 30 min and 85°C for 3 min.
The reactions were performed on an ABI Prism
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR-Green (LightCycler® 480
SYBR-Green I Master, Product No. 04707516001; Roche Diagnostics
GmbH, Mannheim, German). The relative gene expression was
calculated by 2−ΔΔCt (21) and the expression of endogenous
GAPDH mRNA or U6 was used to quantify the amplification. The
experiments were repeated at least 3 times, independently. The
primers used were as follows: GAPDH forward (F),
5′-GAGAAGTATGACAACAGCCTC-3′ and reverse (R),
5′-ATGGACTGTGGTCATGAGTC-3′; FOXA2 F, 5′-CACCATCAGCCCCACAAAAT-3′ and
R, 5′-GGGTAGTGCATGACCTGTTCG-3′; U6 F, 5′-CTCGCTTCGGCAGCACA-3′ and
R, 5′-AACGCTTCACGAATTTGCGT-3′; -miR-141 F,
5′-CGCTAACACTGTCTGGTAAAG-3′ and R, 5′-GTGCAGGGTCCGAGGT-3′. Cycling
parameters were as follows: 95°C for 5 min; 40 cycles of 95°C for
15 sec and 60°C for 1 min.
Western blot analysis
Total protein was extracted from the cells and lysed
in 0.5 ml cell lysis buffer (Total Protein Extraction kit; ProMab
Biotechnologies, Inc., Richmond, CA, USA) at 4°C for 45 min.
Following centrifugation at 13,000 × g for 15 min at 4°C, the
concentration of the supernatant was determined using a
bicinchoninic acid protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). An equal amount of protein (25 µg/lane) was
resolved on 10% SDS-PAGE gels and then transferred onto
polyvinylidene fluoride membranes. Following blocking with 5%
non-fat milk for 30 min at room temperature, the membranes were
incubated with the primary antibodies overnight at 4°C: FOXA2
(1:1,000) and β-actin (1:2,000). Horseradish peroxidase
(HRP)-conjugated antibodies goat anti-mouse immunoglobulin
(Ig)G-HRP, (cat. no. sc-2005; 1:3,000; goat) or anti-rabbit IgG-HRP
(cat. no. sc-2004; 1:3,000; goat) at room temperature were used as
secondary antibodies. An enhanced chemiluminescence detection kit
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used to for
signal detection. The films were quantified using ImageJ software
(version 1.8.0; National Institutes of Health, Bethesda, MD, USA).
At least three independent repeats of the experiments were
performed.
MTT assay
Cells were seeded at density of 3×105
cells/well into 6-well plates and cultured, with or without
pioglitazone (0.5 µM). The control cells well treated with PBS. The
MTT kit was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. Cells were incubated with 0.5 mg/ml MTT at 37°C and cultured
for an additional 4 h, and then 50 µl dimethyl sulfoxide was added
into each well to stop the reaction. The absorbance was measured at
540 nm using a Synergy HT microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Luciferase reporter assay
TargetScan Human version 7.0 (www.targetscan.org) predicted that FOXA2 was a
potential target of miR-141. The wild-type (WT) or mutant (MUT;
without miR-141 binding site) human FOXA2 3′UTR sequences were
synthesized using Quik Change Multi Site-Directed Mutagenesis kit
(Agilent Technologies, Inc., Santa Clara, CA, USA) and separately
cloned into the pGL-3 luciferase reporter plasmid (Promega
Corporation, Madison, WI, USA). The recombinant plasmids were
termed pGL3-FOXA2-WT and pGL3-FOXA2-MUT. These plasmids were
co-transfected with 50 nm miR-141 mimic or inhibitor or their
negative control using Lipofectamine® 2000. Cell lysates
were prepared and luciferase assays were performed 48 h after
transfection. Luciferase activity was normalized to Renilla
luciferase activity.
Fasting blood glucose (FBG)
quantification
The mice were fasted for 6 h and 0.5 ml blood
samples were collected from the orbital venous plexus. FBG
concentration was immediately quantified using a blood glucose
meter and strips (Roche Accu-Check; Roche Diagnostics, Basel,
Switzerland).
Statistical analysis
SPSS version 19.0 (IBM Corporation, Armonk, NY, USA)
was used to perform the statistical analysis. Data were expressed
as the mean ± standard deviation. The difference between groups was
performed by analysis of variance followed by the
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-141 is upregulated
in diabetic mice and humans
Due to the suppressive function of miR-141 in
hepatocarcinogenesis (22–24), it was hypothesized that miR-141 may
also have a role in T2D. RT-qPCR was used to examine the miR-141
plasma level in 50 patients with diabetes. When compared with the
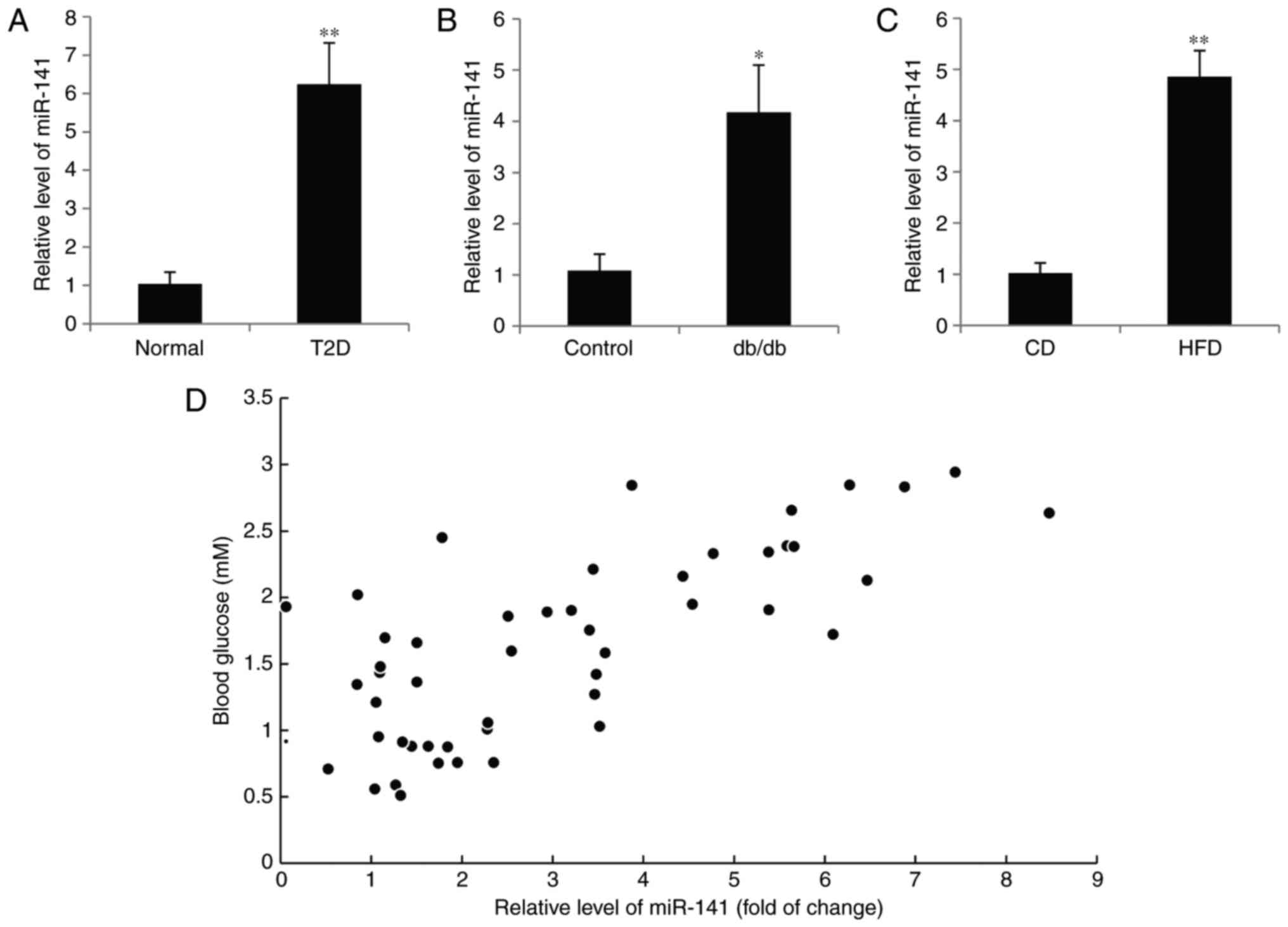
blood of normal subjects, miR-141 expression was significantly
increased in the diabetic patients (P<0.01; Fig. 1A). It is of note that the
expression of miR-141 was also upregulated in the pancreatic islets
of db/db mice compared with the control mice (P<0.05; Fig. 1B). C57BL/6 mice were fed with a HFD
for 16 weeks and miR-141 expression was significantly increased
compared with mice fed a standard diet (P<0.01; Fig. 1C). In addition, a positive
correlation was also observed in 50 patients with diabetes between
the miR-141 expression and blood glucose concentration. The mean
level of miR-141 in the normal subjects was used as the control for
relative level of miR-141 in the diabetic patients. (P<0.05;
r=0.5226; Fig. 1D).
FOXA2 is a direct target gene of
miR-141 in INS-1 β cells
Bioinformatics analysis using TargetScan revealed
that FOXA2 was a potential target of miR-141. As presented in
Fig. 2A, at the 3′-UTR region of
FOXA2, there are 6 consecutive complementary nucleotides of
miR-141. In order to test whether FOXA2 was a direct target for
miR-141, a series of experiments was performed as follows: The
3′-UTR of FOXA2 was cloned by PCR and inserted into the pGL3
reporter plasmid to quantify luciferase activity. In the INS-1
cells transfected with miR-141 mimic, the PGL3-FOXA2 reporter
activity was significantly inhibited, whereas in the cells
transfected with an miR-141 inhibitor, the relative reporter
activity was increased (P<0.01; Fig. 2B). To further investigate the
specific binding of miR-141 at the predicted FOXA2 seed sequences,
a mutant reporter plasmid was produced using a 3-nucleotide
mutation at the center of the 6 seed sequences, as presented in
Fig. 2C, the mutant reporter
exhibited no response to the miR-141 mimic or miR-141 inhibitor
transfection. The aforementioned data demonstrated that miR-141 was
able to bind to the 3′-UTR of FOXA2 and the 3 nucleotides were
crucial for the binding of miR-141. In addition, the transfection
of with the miR-141 mimic reduced the FOXA2 protein expression,
whereas following transfection with the miR-141 inhibitor, the
FOXA2 protein level was increased (Fig. 2D).
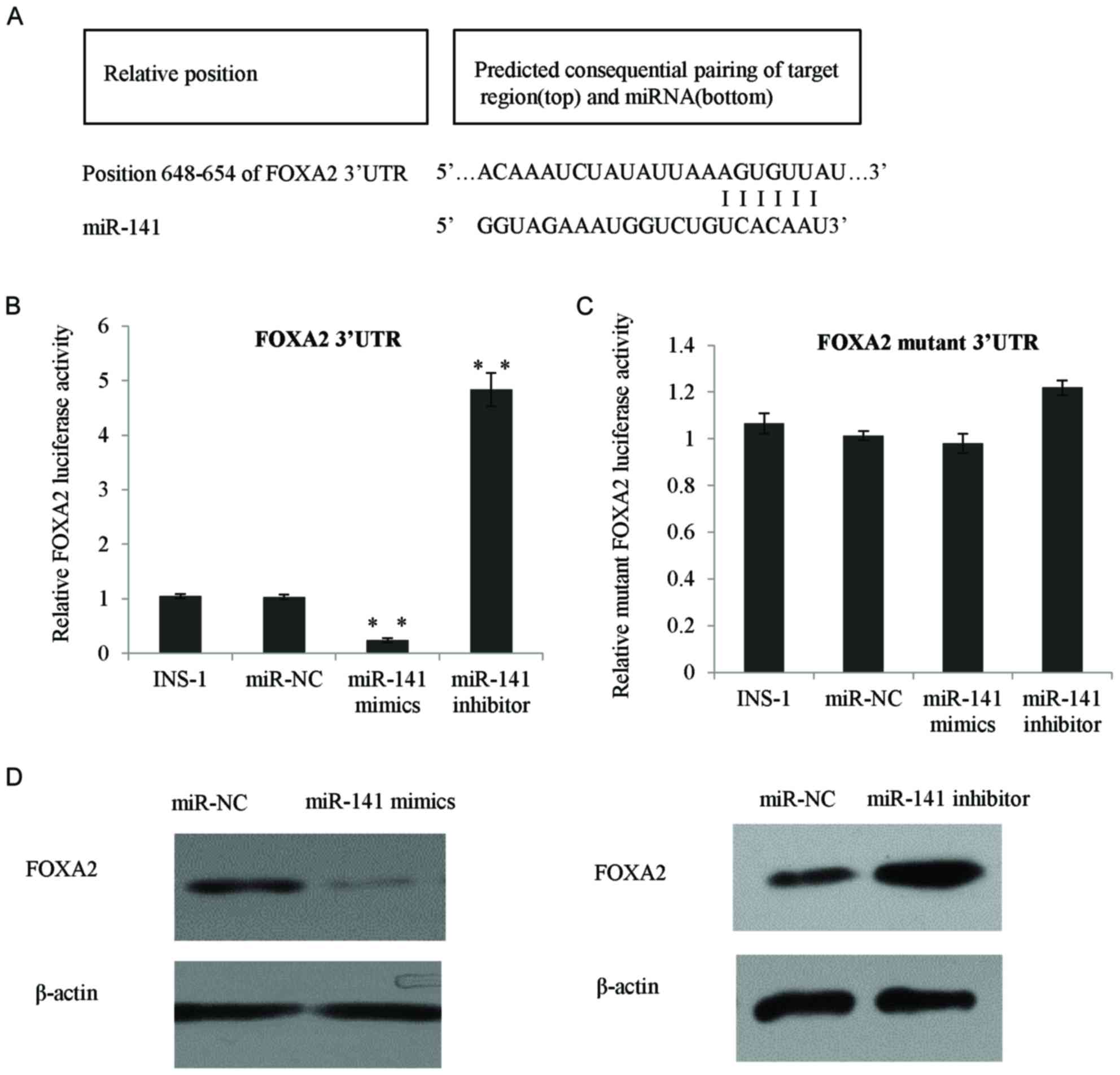 | Figure 2.FOXA2 was a direct target gene of
miR-141 in INS-1 β cells. (A) Bioinformatics analysis indicated the
potential miR-141 binding sites in the 3′-UTR region of FOXA2. (B)
Luciferase reporter assay was performed in INS-1 β cells by
co-transfection of the FOXA2 luciferase reporter vector and the
miR-141 mimic, miR-141 inhibitor, or with the NC. (C) Then, the
luciferase reporter assay was performed in INS-1 β cells with the
FOXA2 luciferase mutant reporter vector, co-transfected with the
NC, miR-141 mimic or miR-141 inhibitor. Luciferase activity was
tested 24 h following transfection. (D) FOXA2 protein expression
was detected using western blotting in INS-1 β cells transfected
NC, miR-141 mimic and miR-141 inhibitor, respectively. **P<0.01.
NC, normal control; 3′-UTR, 3′-untranslated region; miR, microRNA;
FOXA2, forkhead box A2. |
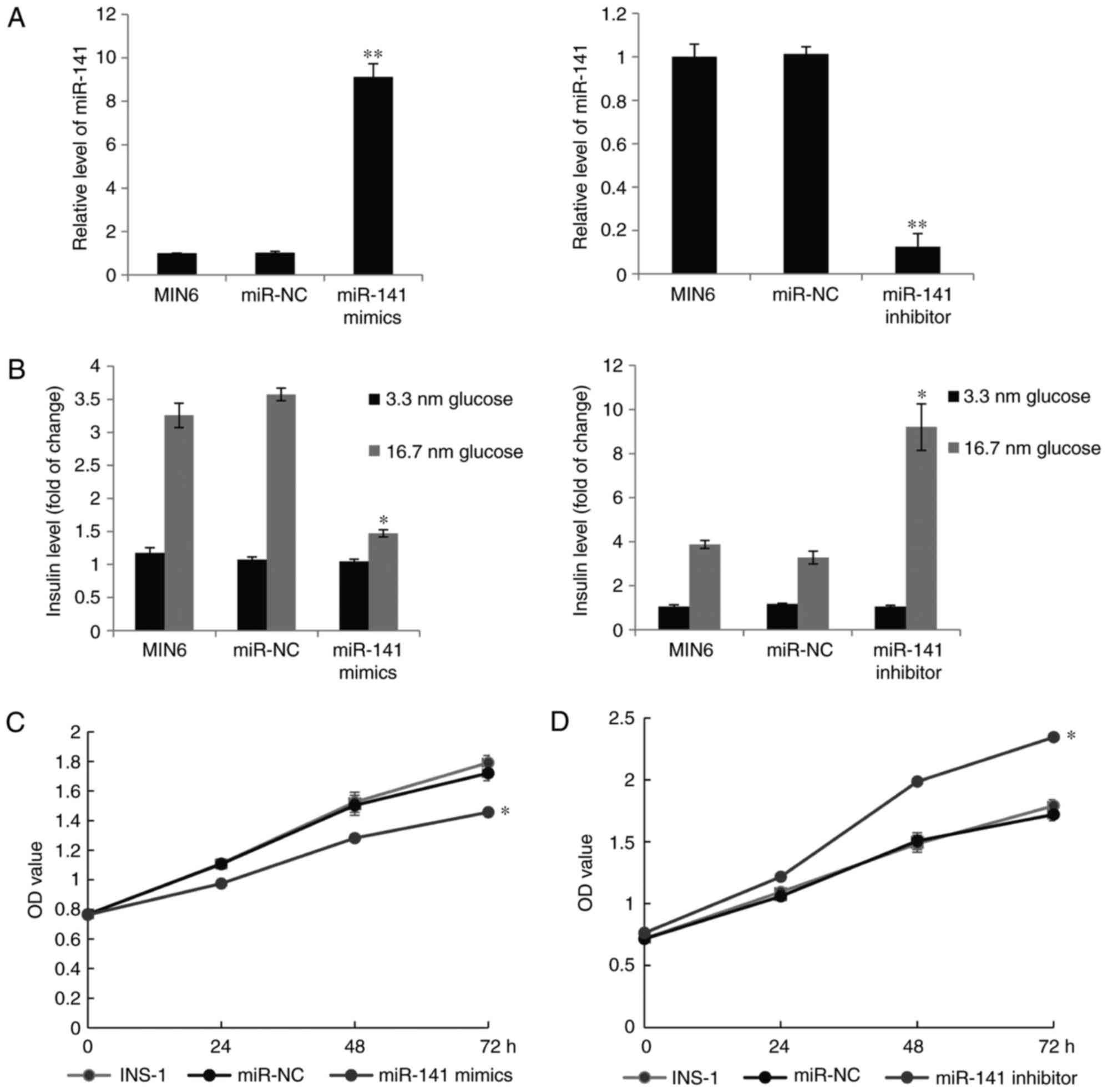
Upregulation of miR-141 results in
impaired glucose-stimulated insulin secretion and INS-1 β cells
proliferation
FOXA2 has been previously identified as a master
regulator in pancreatic development and is involved in regulating
both glucose-sensing apparatus and insulin release (25,26).
It was hypothesized that miR-141 may have a role in insulin
secretion and β-cell proliferation. In order to verify the effects
of miR-141 on glucose-stimulated insulin secretion, the cultured
MIN6 pseudoislets were transfected with an miR-141 mimic, miR-141
inhibitor or scrambled negative control RNA. Insulin secretion was
measured at 3.3 and 16.7 mM glucose concentration 48 h after the
transfection. As presented in Fig.
3A, the expression of miR-141 was increased in the miR-141
mimic transfection group and the increase in miR-141 led to a
significantly impaired insulin secretion at 16.7 mM glucose
(P<0.05; Fig. 3B). Transfection
with the miR-141 inhibitor, resulted in the opposite effect.
Additionally, the effect of miR-141 was also tested on INS-1 β cell
viability. Results from MTT assays (Fig. 3C) confirmed that overexpression of
miR-141 reduced cell viability while knockdown of miR-141
significantly increased INS-1 β cell proliferation (P<0.05;
Fig. 3D).
Expression of miR-141 is corrected by
treatment with pioglitazone
INS-1 β cells were treated with 0, 0.01, 0.1, 1, or
10 µM pioglitazone for 24 h, the miR-141 expression level was
quantified as presented in Fig.
4A, 10 µM pioglitazone resulted in the lowest miR-141 level
(Fig. 4A). Furthermore, the INS-1
β cells were treated with l µM pioglitazone for 0, 3, 6, 12 and 24
h, the expression of miR-141 was also reduced (Fig. 4B). It is of note that the function
of pioglitazone in animal models was also observed. Using db/db
mice at the age of 8 weeks, or C57BL/6 mice on a HFD for 16 weeks,
the mice were treated with the insulin-sensitizing pioglitazone for
4 weeks and the blood glucose level was measured once a week. As
exhibited in Fig. 4C, the glucose
level was decreased in the groups treated with pioglitazone.
Furthermore, reduced pancreatic islet miR-141 expression was
observed compared with the control groups (Fig. 4D).
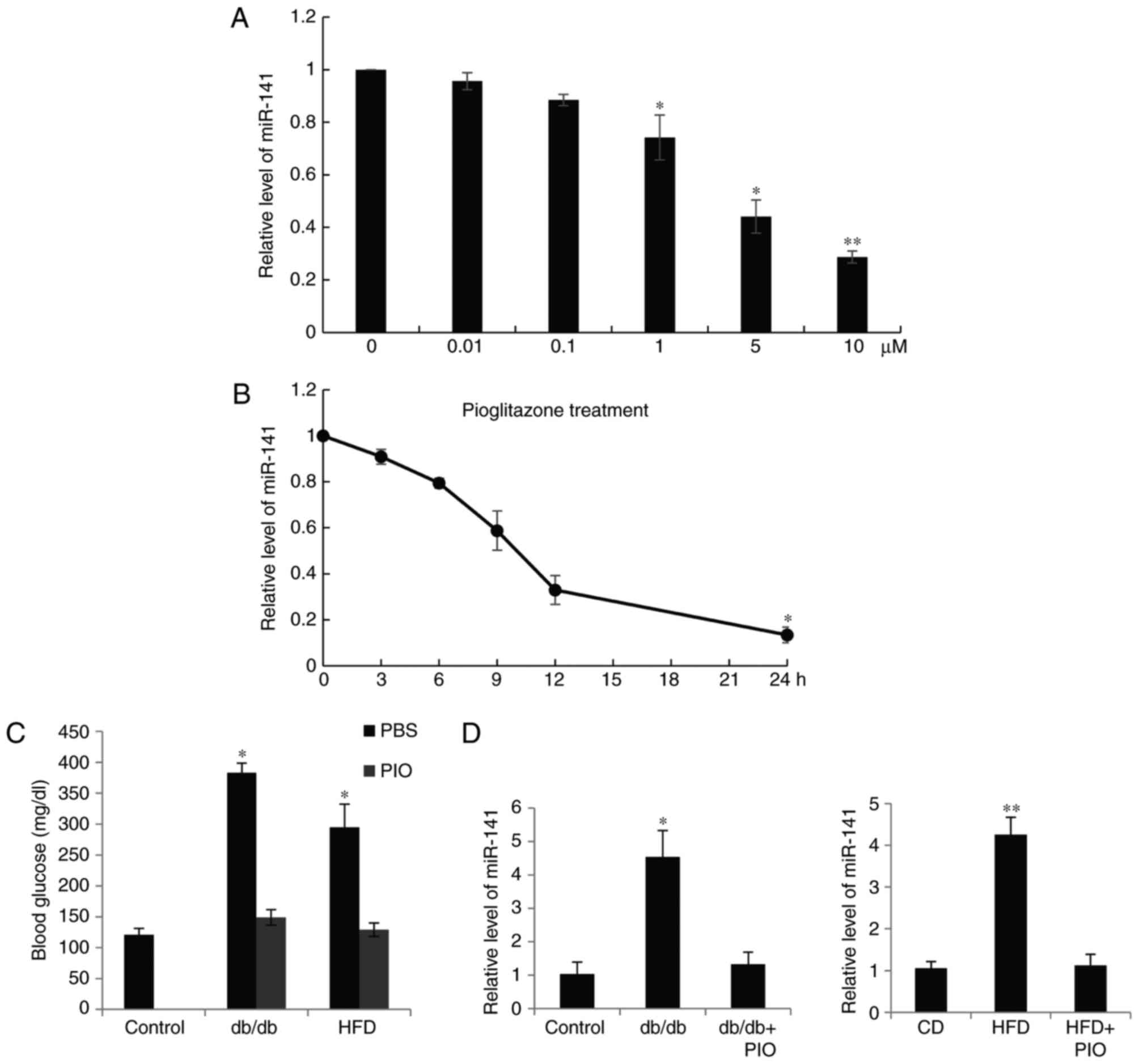 | Figure 4.Expression of miR-141 was altered by
treatment with PIO. (A) INS-1 β cells were treated with (A) 0,
0.01, 0.1, 1, 5 and 10 µM PIO for 24 h and (B) 1 µM PIO for 0, 3,
6, 12 and 24 h. The expression of miR-141 was quantified and all
the data was presented as the mean ± standard deviation. (C)
Fasting blood glucose levels were measured in diabetic model db/db
mice, or C57BL/6 mice fed with a HFD, followed by treatment with
PIO. (D) Relative hepatic levels of miR-141 were measured by
reverse transcriptase-quantitative-quantitative polymerase chain
reaction in the mice. *P<0.05, **P<0.01. HFD, high fat diet;
PIO, pioglitazone; CD, control diet; miR, microRNA. |
miR-141 regulates glucose-stimulated
insulin secretion and proliferation through FOXA2
It has been demonstrated that miR-141 may regulate
glucose-stimulated insulin secretion and INS-1 β cell proliferation
and FOXA2 has been identified as a direct target gene of miR-141.
The axis of miR-141 targeting FOXA2 was investigated in T2D
progression. As presented in Fig.
5A, overexpression of FOXA2 in MIN6 pseudoislets increased the
effect of the miR-141 inhibitor on glucose-stimulated insulin
secretion. Conversely, cells co-transfected with the miR-141 mimic
and the knockdown of FOXA2 synergistically inhibited
glucose-stimulated insulin secretion (Fig. 5B). Additionally, the INS-1 cells
co-transfected with the miR-141 mimic and FOXA2 plasmid exhibited
an increase in cell proliferation compared with the cells
transfected with miR-141 (Fig.
5C). The INS-1 cells co-transfected with miR-141 inhibitor and
shFOXA2 had reduced cell proliferation potential compared with the
miR-141 inhibitor transfection groups (Fig. 5D). The results of the present study
demonstrated that miR-141 inhibited the proliferation and insulin
secretion of pancreatic β cells by directly targeting FOXA2.
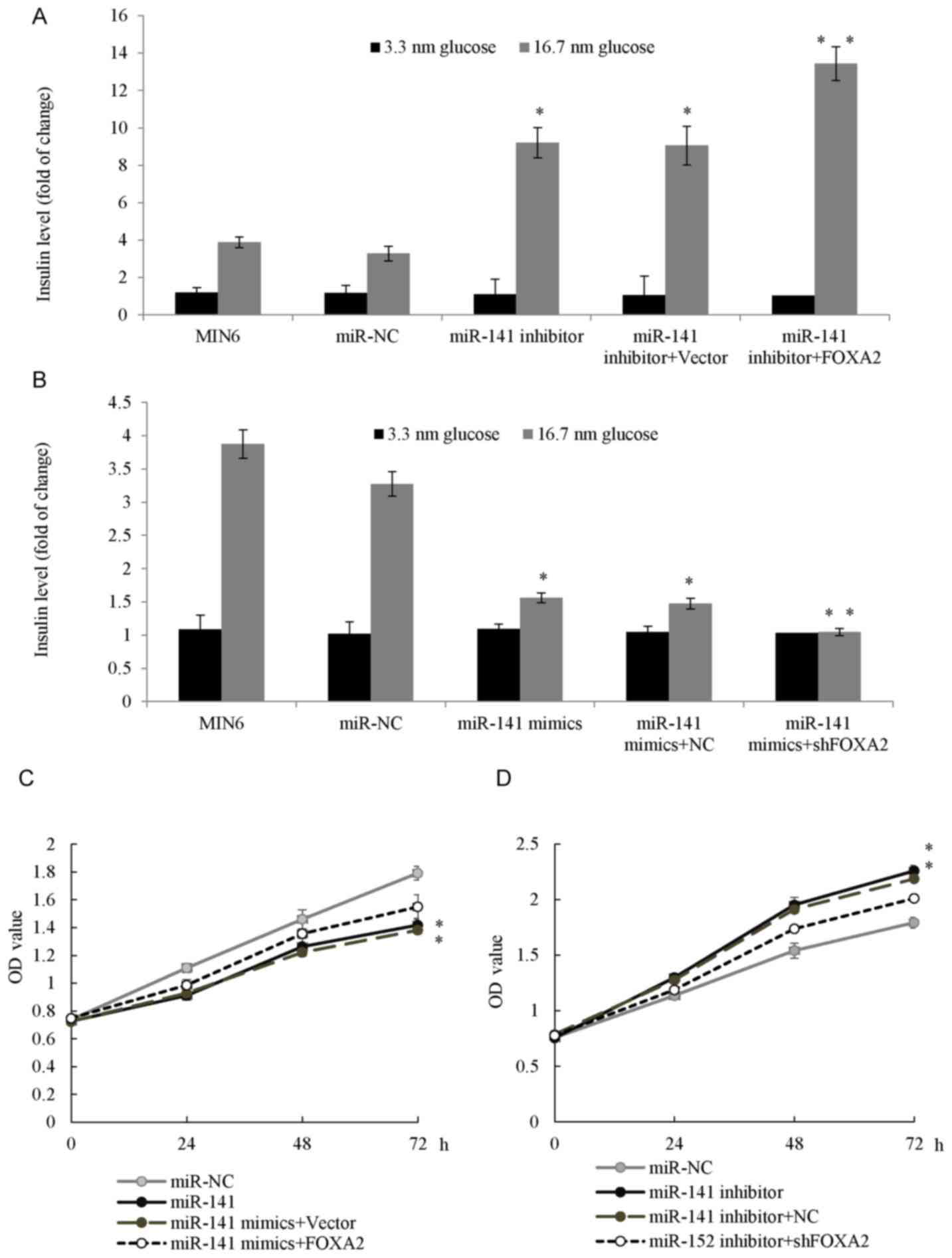 | Figure 5.miR-141 regulated GSIS and cell
proliferation through FOXA2. (A) MIN6 pseudoislets were transfected
with an miR-141 inhibitor, or co-transfected with miR-141 inhibitor
and FOXA2 overexpression plasmid. GSIS was determined in each group
at 3.3 or 16.7 nM glucose. (B) MIN6 pseudoislets were transfected
with the miR-141 mimic, or co-transfected with miR-141 mimic and
shFOXA2. GSIS was determined in each group. (C) INS-1 cells were
transfected with the miR-141 mimic, or co-transfected with the
miR-141 mimic and FOXA2 plasmid and an MTT assay was performed in
each group. (D) INS-1 cells were transfected with the miR-141
inhibitor or co-transfected with the miR-141 inhibitor and shFOXA2,
MTT assay was performed in each group. *P<0.05, **P<0.01 vs.
the miR-NC group. GSIS, glucose-stimulated insulin secretion; miR,
microRNA; FOXA2, forkhead box A2; NC, negative control; sh, short
hairpin; OD, optical density. |
Discussion
Several miRs have been reported to be associated
with insulin resistance and/or diabetes. For example, increased
expression of miR-429 may downregulate the expression of occludin
and induce impaired intestinal barrier function in diabetes
mellitus mice (27). MiR-593-3p
negatively regulated insulin-regulated glucose metabolism in
hepatocellular carcinoma cell lines such as HepG2 (28). As Chen et al (29) reported, under metabolic stress,
miR-17-92 regulated glucose-stimulated insulin secretion and
pancreatic β-cell adaptation. By inhibiting glycerol kinase,
miR-451 negatively regulated hepatic gluconeogenesis and blood
glucose levels in diabetes (30).
Fu et al (31) reported
that miR-26a was downregulated in two obese mouse models and
regulated insulin signaling and metabolism of glucose. A previous
study revealed that in the diabetic kidney, the renal expression of
miR-141 was reduced in mouse models representing early and advanced
kidney disease, which indicated miR-141 may have a role in diabetes
(32).
In the present study, miR-141 expression was
analyzed in plasma from T2D and non-diabetic donors using RT-qPCR
and an upregulation of miR-141 was detected in elderly diabetic
patients. However, as sufficient plasma was not collected from the
mice, the plasma miR-141 level was not measured in the animal
model, this was a limitation for the present study. The direct
binding of miR-141 to the FOXA2 3′-UTR was confirmed by luciferase
assay. The therapeutic effects of pioglitazone were considered to
be the result of the regulation of multiple pathways (33–35).
The expression of miR-141 was corrected by treatment with
pioglitazone, suggesting that the dysregulation of miR-141 was
associated with the progression of diabetes. INS-1 and MIN6
pseudoislets are glucose-responsive pancreatic β cells, in order to
investigate the role of miR-141 in pancreatic β cell function,
INS-1 cells or MIN6 pseudoislets were transfected with the miR-141
mimic or miR-141 inhibitor. The overexpression of miR-141 inhibited
the proliferation and insulin secretion, whereas knockdown of
miR-141 promoted the proliferation and insulin secretion, which
further supported the notion that miR-141 served a role in
diabetes, which is consistent with the results of the present
study, where the expression of miR-141 was increased in T2D
patients. The present study revealed that upregulation of miR-141
may lead to impaired glucose-stimulated insulin secretion and INS-1
β cell proliferation though targeting FOXA2. However, it is of note
that the MIN6 pseudoislets used in the present study were obtained
from mice and INS-1 b were from rats, and it is preferable to use
the two different cell lines from the same species for more
reliable findings. This is a limitation of the present study. As
Sebastiani et al (36)
previously reported, miR-124a was also increased in T2D human
pancreatic islets and has a role in the control of FOXA2 and
myotrophin. Therefore, it is interesting to investigate the
association between miR-124a and miR-141. To the best of the
authors knowledge, this is the first study to reveal the regulatory
mechanism of pioglitazone/miR-141/FOXA2 axis in pancreatic β cells
proliferation and insulin secretion. The findings of the present
study provide a plausible alternative for the treatment of T2D.
However, the specific function of this axis requires further
investigation in terms of clinical characteristics in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY conceived and designed the present study. LZ
provided technical assistance and analyzed the data. XY and LZ
drafted the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All human tissues are collected under Institutional
Review Committee (IRB) and Health Insurance Portability and
Accountability Act (HIPAA) approved protocols.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wienholds E and Plasterk RH: MicroRNA
function in animal development. FEBS Lett. 579:5911–5922. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rane S, He M, Sayed D, Vashistha H,
Malhotra A, Sadoshima J, Vatner DE, Vatner SF and Abdellatif M:
Downregulation of miR-199a derepresses hypoxia-inducible
factor-1alpha and Sirtuin 1 and recapitulates hypoxia
preconditioning in cardiac myocytes. Circ Res. 104:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie H, Lim B and Lodish HF: MicroRNAs
induced during adipogenesis that accelerate fat cell development
are downregulated in obesity. Diabetes. 58:1050–1057. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu M, Xia M, Chen X, Lin Z, Xu Y, Ma Y and
Su L: MicroRNA-141 regulates Smad interacting protein 1 (SIP1) and
inhibits migration and invasion of colorectal cancer cells. Dig Dis
Sci. 55:2365–2372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Li Q, Xu D, Wang Q, An Y, Du Q,
Zhang J, Zhu Y and Miao Y: hsa-miR-141 downregulates TM4SF1 to
inhibit pancreatic cancer cell invasion and migration. Int J Oncol.
44:459–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen B, Huang T, Jiang J, Lv L, Li H and
Xia S: miR-141 suppresses proliferation and motility of gastric
cancer cells by targeting HDGF. Mol Cell Biochem. 388:211–218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamagawa S, Beder LB, Hotomi M, Gunduz M,
Yata K, Grenman R and Yamanaka N: Role of miR-200c/miR-141 in the
regulation of epithelial-mesenchymal transition and migration in
head and neck squamous cell carcinoma. Int J Mol Med. 33:879–886.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weir GC and Bonner-Weir S: Sleeping islets
and the relationship between β-cell mass and function. Diabetes.
60:2018–2019. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maris M, Ferreira GB, D'Hertog W, Cnop M,
Waelkens E, Overbergh L and Mathieu C: High glucose induces
dysfunction in insulin secretory cells by different pathways: A
proteomic approach. J Proteome Res. 9:6274–6287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chatterjee S and Davies MJ: Current
management of diabetes mellitus and future directions in care.
Postgrad Med J. 91:612–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaul K, Apostolopoulou M and Roden M:
Insulin resistance in type 1 diabetes mellitus. Metabolism.
64:1629–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sergienko VA: Insulin resistance and
arterial stiffness in patients with diabetes mellitus type 2 and
cardiovascular autonomic neuropathy. Zh Nevrol Psikhiatr Im S S
Korsakova. 114:11–15. 2014.(In Russian). PubMed/NCBI
|
|
15
|
Polsky S and Ellis SL: Obesity, insulin
resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol
Diabetes Obes. 22:277–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fakhrzadeh H, Sharifi F, Alizadeh M,
Arzaghi SM, Tajallizade-Khoob Y, Tootee A, Alatab S, Mirarefin M,
Badamchizade Z and Kazemi H: Relationship between insulin
resistance and subclinical atherosclerosis in individuals with and
without type 2 diabetes mellitus. J Diabetes Metab Disord.
15:412016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iversen DS, Støy J, Kampmann U, Voss TS,
Madsen LR, Møller N and Ovesen PG: Parity and type 2 diabetes
mellitus: A study of insulin resistance and β-cell function in
women with multiple pregnancies. BMJ Open Diabetes Res Care.
4:e0002372016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ao C, Huo Y, Qi L, Xiong Z, Xue L and Qi
Y: Pioglitazone suppresses the lipopolysaccharide-induced
production of inflammatory factors in mouse macrophages by
inactivating NF-kappaB. Cell Biol Int. 34:723–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan H, Yuan Y, Qian A, Sun Y and Qiao M:
Pioglitazone, a PPARgamma ligand, suppresses NFkappaB activation
through inhibition of IkappaB kinase activation in cerulein-treated
AR42J cells. Biomed Pharmacother. 62:466–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collino M, Aragno M, Mastrocola R,
Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C and
Fantozzi R: Modulation of the oxidative stress and inflammatory
response by PPAR-gamma agonists in the hippocampus of rats exposed
to cerebral ischemia/reperfusion. Eur J Pharmacol. 530:70–80. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin L, Liang H, Wang Y, Yin X, Hu Y, Huang
J, Ren T, Xu H, Zheng L and Chen X: microRNA-141 inhibits cell
proliferation and invasion and promotes apoptosis by targeting
hepatocyte nuclear factor-3β in hepatocellular carcinoma cells. BMC
Cancer. 14:8792014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue J, Niu YF, Huang J, Peng G, Wang LX,
Yang YH and Li YQ: miR-141 suppresses the growth and metastasis of
HCC cells by targeting E2F3. Tumour Biol. 35:12103–12107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Ding Y, Huang J, Wang S, Ni W, Guan
J, Li Q, Zhang Y, Ding Y, Chen B and Chen L: MiR-141 suppresses the
migration and invasion of HCC cells by targeting Tiam1. PLoS One.
9:e883932014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CS, Sund NJ, Vatamaniuk MZ,
Matschinsky FM, Stoffers DA and Kaestner KH: Foxa2 controls Pdx1
gene expression in pancreatic beta-cells in vivo. Diabetes.
51:2546–2551. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baroukh N, Ravier MA, Loder MK, Hill EV,
Bounacer A, Scharfmann R, Rutter GA and Van Obberghen E:
MicroRNA-124a regulates Foxa2 expression and intracellular
signaling in pancreatic beta-cell lines. J Biol Chem.
282:19575–19588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu T, Lu XJ, Li JY, Shan TD, Huang CZ,
Ouyang H, Yang HS, Xu JH, Zhong W, Xia ZS and Chen QK:
Overexpression of miR-429 impairs intestinal barrier function in
diabetic mice by down-regulating occludin expression. Cell Tissue
Res. 366:341–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Tao Z, Zhu Z, Liao H, Zhao Y and
Fan H: MicroRNA-593-3p regulates insulin-promoted glucose
consumption by targeting Slc38a1 and CLIP3. J Mol Endocrinol.
57:211–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Tian L, Wan S, Xie Y, Chen X, Ji
X, Zhao Q, Wang C, Zhang K, Hock JM, et al: MicroRNA-17-92 cluster
regulates pancreatic beta-cell proliferation and adaptation. Mol
Cell Endocrinol. 437:213–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhuo S, Yang M, Zhao Y, Chen X, Zhang F,
Li N, Yao P, Zhu T, Mei H, Wang S, et al: MicroRNA-451 negatively
regulates hepatic glucose production and glucose homeostasis by
targeting glycerol kinase-mediated gluconeogenesis. Diabetes.
65:3276–3288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu X, Dong B, Tian Y, Lefebvre P, Meng Z,
Wang X, Pattou F, Han W, Wang X, Lou F, et al: MicroRNA-26a
regulates insulin sensitivity and metabolism of glucose and lipids.
J Clin Invest. 125:2497–2509. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang B, Koh P, Winbanks C, Coughlan MT,
McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC,
Cooper ME and Kantharidis P: miR-200a prevents renal fibrogenesis
through repression of TGF-β2 expression. Diabetes. 60:280–287.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanatani Y, Usui I, Ishizuka K, Bukhari A,
Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T and Kobayashi
M: Effects of pioglitazone on suppressor of cytokine signaling 3
expression: Potential mechanisms for its effects on insulin
sensitivity and adiponectin expression. Diabetes. 56:795–803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwata M, Haruta T, Usui I, Takata Y,
Takano A, Uno T, Kawahara J, Ueno E, Sasaoka T, Ishibashi O and
Kobayashi M: Pioglitazone ameliorates tumor necrosis
factor-alpha-induced insulin resistance by a mechanism independent
of adipogenic activity of peroxisome proliferator-activated
receptor-gamma. Diabetes. 50:1083–1092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu SH, Jiang T, Yang SS and Yang Y:
Pioglitazone ameliorates intracerebral insulin resistance and
tau-protein hyperphosphorylation in rats with type 2 diabetes. Exp
Clin Endocrinol Diabetes. 121:220–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sebastiani G, Po A, Miele E, Ventriglia G,
Ceccarelli E, Bugliani M, Marselli L, Marchetti P, Gulino A,
Ferretti E and Dotta F: MicroRNA-124a is hyperexpressed in type 2
diabetic human pancreatic islets and negatively regulates insulin
secretion. Acta Diabetol. 52:523–530. 2015. View Article : Google Scholar : PubMed/NCBI
|