Introduction
Oxidative stress occurs when the body is subjected
to various harmful stimuli, leading to overproduction in
vivo of active molecules, including reactive oxygen species
(ROS) and reactive nitrogen species. When the amount of oxidation
exceeds the removal of oxide material, the oxidation and
antioxidant systems are imbalanced, which leads to tissue damage.
The interaction between inflammation and ROS is a principal
mechanism of multiple renal diseases (1–3). The
kidney is an organ with high perfusion, rich blood flow and oxygen
supply, and is therefore predisposed towards the production of ROS
(4–7). The inhibition of ROS and ROS-mediated
oxidative damage is an important consideration in clinical practice
for kidney disease.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
and its cytoplasmic adapter protein Kelch-like ECH-associated
protein 1 (Keap1) are the central regulators of the cellular
antioxidant response. Nrf2 is able to interact with other elements
of the antioxidant response, inducing the expression of antioxidant
proteins and phase II detoxification enzymes, and serves an
important role in cellular defense. The Keap1-Nrf2/antioxidant
responsive element (ARE) signaling pathway serves a range of
protective functions against cellular stress, apoptosis and
inflammation (8). However, the
function of the Keap1-Nrf2/ARE pathway in renal antioxidation
remains unclear.
Catechins are polyphenol compounds extracted from
green tea, and are important natural antioxidants produced by
plants (9).
Epigallocatechin-3-gallate (EGCG) is the most potent antioxidant
among the catechins (10). The
present study investigated the protective effect of EGCG on
oxidative injury in mouse renal tubular epithelial cells
(MRTECs).
Materials and methods
Materials
MRTECs were purchased from the cell bank of the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). Dulbecco's modified Eagle's medium (DMEM) with high glucose
and fetal bovine serum were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The EGCG was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The Nrf2 (cat no.
bs-1074R) and γ-GCS (cat no. YSRIBIO-068461) rabbit anti-rat
immunoglobulin (Ig)G primary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). The streptavidin
peroxidase immunocytochemical staining kit and
3,3′-diaminobenzidine(DAB) staining reagent were purchased from
ZSGB-Bio (Beijing, China). The TRIzol total RNA extracting solution
was purchased from Shanghai Sangon Biological Engineering
Technology and Services Co., Ltd (Shanghai, China). The
PrimeScript™ RT Reagent kit was purchased from Takara Biotechnology
Co., Ltd. (Dalian, China). The RT-qPCR SYBR Premix Ex Taq™ kit was
purchased from Takara Biotechnology Co., Ltd. The RT-qPCR primers
were designed and synthesized by Takara Biotechnology Co., Ltd.
Culturing of MRTECs and grouping
MRTECs were digested with 0.25% trypsin to form a
single cell suspension and cultured in DMEM containing 100 ml/l
fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin
dual anti-solution, at 37°C in an atmosphere containing 5%
CO2. The medium was replaced every 24 h and 0.25%
trypsin was used for digestion every 3 days; passaging was
performed at a ratio of 1:3. The passaged cells were inoculated
uniformly and, when the cells had reached the sub-integration
state, serum-free medium was added for a 12-h synchronization,
followed by the addition of 1% fetal bovine serum-containing medium
for the experiments. The cells were seeded on coverslips, 6-well
plates or flasks. When the cells had achieved ~80% fusion, the
serum-free medium was replaced and, according to the experimental
group, different dosages of drugs were added. Each experiment was
performed with ≥3 different generations of cells.
The cultured cells were divided into the untreated
normal group (N); the control group incubated with 250 µmol/l
H2O2 for 6 h (C); and the EGCG groups
incubated with 250 µmol/l H2O2, and
co-cultured with EGCG at concentrations of 5 mg/l (T1), 10 mg/l
(T2) and 20 mg/l (T3).
Detection of cell viability
The MTT chromogenic assay was used to assess the
cell viability of each group. The cells were transfected into
96-well plates, and cultured for 72 h. A total of 50 µl MTT (5 g/l)
was added to each well and incubated for 4 h. Subsequently, the
supernatant was discarded and 100 µl dimethyl sulfoxide was added
for the detection of the absorbance at 570 nm (A570) using an
automatic microplate reader. The number of viable cells was
proportional to the value of A570: Live cell rate=A570 of the
experimental group/A570 of the control group. Trypan blue staining
was performed on the EGCG groups prior to the assay and the living
cell rates were observed to be >90%, indicating that EGCG was
non-toxic to the cells.
Detection of Nrf2 and γ-GCS mRNA
expression in MRTECs using RT-qPCR
TRIzol was used to extract the total RNA and reverse
transcription was performed to synthesize cDNA. The primer
sequences for the RT-qPCR were as follows: The upstream and
downstream primers of Nrf2 were 5′-TTGATTGACATCCTTTGG-3′ and
5′-GTTCCTTCTGGAGTTGCT-3′, respectively, with an amplified product
of 142 bp; the upstream and downstream primers of γ-GCS were
5′-ATCTACCACGCAGTCAAG-3′ and 5′-CCGCCATTCAGTAACAAC-3′,
respectively, with an amplified product of 119 bp; and the upstream
and downstream primers of GAPDH-F were 5′-GCACCGTCAAGGCTGAGAAC-3′
and 5′-ATGGTGGTGAAGACGCCAGT3′, respectively, with an amplified
product of 142 bp. The PCR composition, reaction conditions (94°C
for 2 min for 1 cycle, 94°C for 40 sec, 50–65°C for 40 sec and 72°C
for 1 min for 25–35 cycles, and 72°C for 5 min for 1 cycle) and
dissolution profile conditions were in accordance with the
manufacturer's protocol.
The SYBR-Green 1 fluorescent dye embedment method
was used to prepare the standard curves of the target genes, Nrf2
and γ-GCS, and the housekeeping gene GAPDH. The standard curves
were used to quantifying the target gene and housekeeping gene
levels in the samples. Through the correction of the housekeeping
gene, the relative gene expression of Nrf2 and γ-GCS was measured
in each group (11).
Detection of Nrf2 and γ-GCS protein
expression in MRTECs using immunocytochemical analysis
The coverslips containing cultured cells were
prepared according to the streptavidin-biotin complex test kit
protocol. The primary antibodies were rabbit anti-Nrf2 and γ-GCS
polyclonal antibodies (both 1:200), which was incubated at 4°C
overnight. After washing with PBS for 5 min 3 times, the secondary
antibody was added, which was a biotinylated goat anti-rabbit IgG
(1:200; Agrisera AB, Vännäs, Sweden; cat no. AS09608), which was
incubated at 37°C for 30 min prior to washing with PBS as before.
DAB staining was subsequently performed, and the DAB developer was
added and incubated for 3 min at room temperature and then the
reaction was stopped by subjecting cells to running water for 1
min. Cells were then stained with hematoxylin for 15 sec. In group
N, PBS was used to replace the primary antibody and all subsequent
steps were the same. The existence of black/dark grey particles
inside the nucleus and/or cytoplasm was considered to be positive.
The optical density values were calculated using MetaMorph image
analysis software.
Detection of Nrf2 and γ-GCS protein
expression in MRTECs using western blot analysis
Total Nrf2 nucleoprotein and γ-GCS cytoplasmic
protein was extracted using radioimmunoprecipiation assay lysis
buffer (BioTeke Corporation, Beijing, China), and the assay was
performed according to the manufacturer's protocol. 3% SDS-PAGE 3%
gel and 10% separation gel was used, and 10 µl protein was loaded
per lane. The protein was then transfered to nitrocellulose
membrane and blocked with 5% skimmed milk powder at 4°C overnight.
The primary antibodies (rabbit anti-Nrf2 and γ-GCS polyclonal
antibody) were diluted 1:1,000, incubated at 4°C overnight and the
reaction was stopped with washing solution, then the goat
anti-rabbit IgG secondary antibody was added (1:2,000) at room
temperature for 1 h and stopped with washing solution as before.
Staining was performed using the illuminating kit (BestBio,
ShangHai; http://bestbio.biogo.net).
Quantitative analysis was performed using Fluor Chem software
(version 2.0; ProteinSimple; Bio-Techne, Minneapolis, MN, USA) and
β-actin (1:200; cat no. A5441; Sigma-Aldrich; Merck KGaA) was used
as the internal reference for the analysis, and was incubated at
room temperature for 1 h.
Statistical analysis
All of the experiments were repeated ≥3 times and
the results are expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
was used for the comparisons of overall and intergroup differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of different EGCG doses on
H2O2-induced MRTEC cytoactivity
Subsequent to 6 h treatment, in the control group,
the cytoactivity of MRTECs was reduced. Among the treatment groups,
at an EGCG concentration of 5 mg/l, the cytoactivity was
significantly increased compared with the control group
(P<0.01); at an EGCG concentration of 10 or 20 mg/l,
cytoactivity was significantly increased compared with the control
group (P<0.01). EGCG protected against the
H2O2-mediated decrease in cytoactivity in a
dose-dependent manner (Table
I).
 | Table I.Effects of EGCG dose on the
cytoactivity of H2O2-induced mouse renal
tubular epithelial cells 6 h post-treatment. |
Table I.
Effects of EGCG dose on the
cytoactivity of H2O2-induced mouse renal
tubular epithelial cells 6 h post-treatment.
|
|
|
| EGCG treatment |
|---|
|
|
|
|
|
|---|
| Group | N | C | T1 | T2 | T3 |
|---|
| Cell survival rate,
% | 93.51±2.27 |
56.54±5.42a |
77.87±5.86a,b |
82.41±5.66a,b |
90.22±2.48a,b |
Effects of EGCG on Nrf2 and γ-GCS mRNA
expression in MRTECs following oxidative stress
The standard curves of Nrf2, γ-GCS and GAPDH were
prepared, and the correlation coefficients were 0.998, 0.990 and
0.995, respectively. The linearities were good, and the fusion
curves demonstrated that the specificity was good.
The standard curve was used to quantify the
expression of Nrf2, γ-GCS and GAPDH in the samples, and it was
observed that, compared with the N group, the Nrf2 and γ-GCS mRNA
in the C group was increased; compared with the C group, the
expression of Nrf2 and γ-GCS mRNA was increased in the T1 group
(P<0.05), and in the T1 and T3 groups (P<0.01), indicating
that EGCG was able to upregulate the gene expression of Nrf2 and
γ-GCS in MRTECs in a dose-dependent manner (Table II; Fig. 1).
 | Table II.Effects of EGCG doses on relative
expression of Nrf2 and γ-GCS mRNA in mouse renal tubular epithelial
cells (n=10). |
Table II.
Effects of EGCG doses on relative
expression of Nrf2 and γ-GCS mRNA in mouse renal tubular epithelial
cells (n=10).
|
|
|
| EGCG treatment |
|---|
|
|
|
|
|
|---|
| Group | N | C | T1 | T2 | T3 |
|---|
| Nrf2 | 0.0018±0.0002 |
0.0036±0.0004a |
0.0042±0.0005a,b |
0.0063±0.0005a,c |
0.0067±0.0006a,c |
| γ-GCS | 0.0023±0.002 |
0.0046±0.0004a |
0.0049±0.0004a,b |
0.0071±0.0005a,c |
0.0074±0.0005a,c |
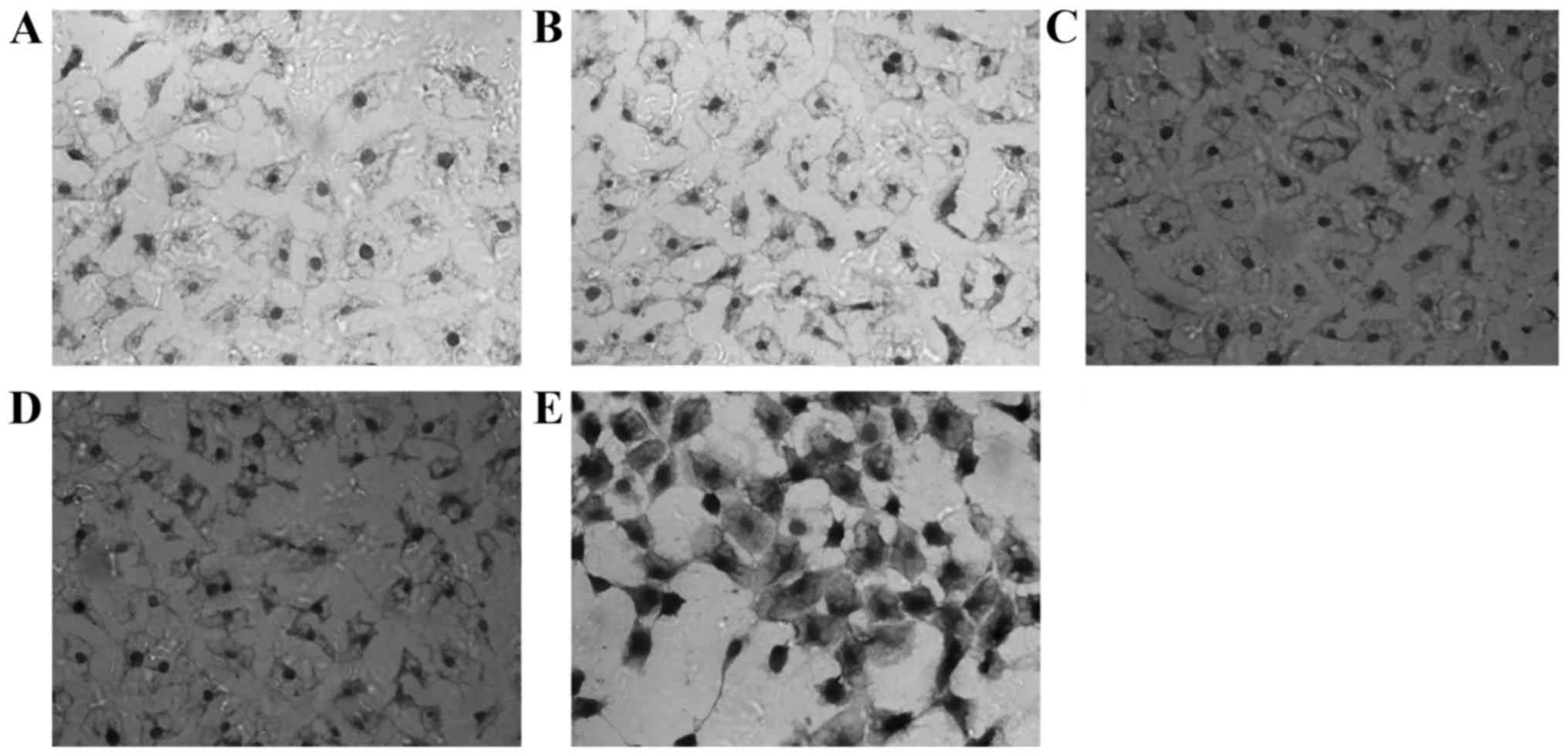
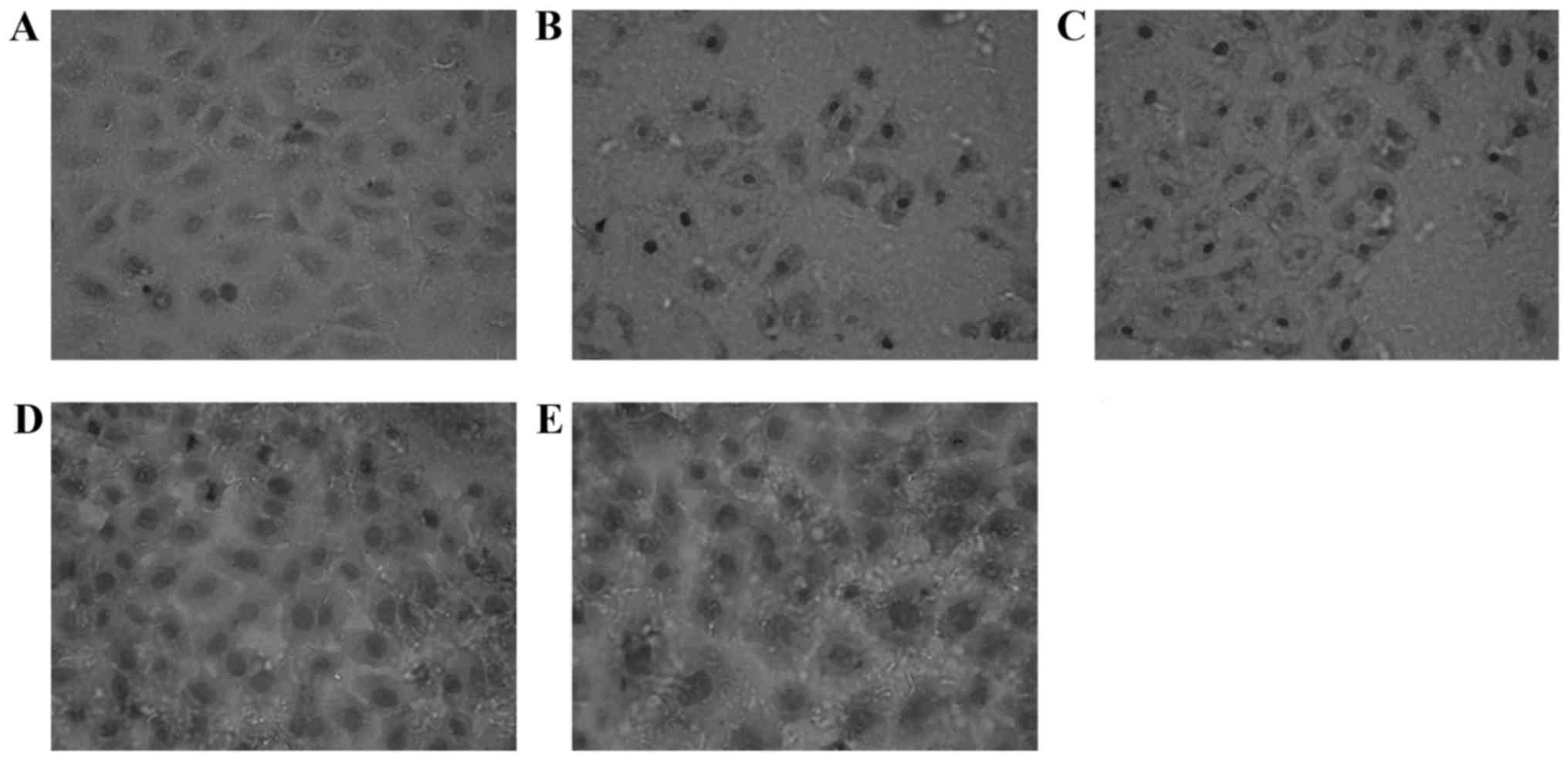
Impact of EGCG on the expression of
Nrf2 and γ-GCS protein in MRTECs following oxidative stress
Nrf2-positive expression is exhibited as brown
particles in the MRTECs. When stimulated with 250 µmol/l
H2O2 for 6 h, the Nrf2-positive expression
increased. At increasing concentrations of EGCG, the expression of
Nrf2 in the nucleus increased markedly, exhibited by deeper
staining, most notably in the T3 group (Fig. 2). The γ-GCS-positive expression is
exhibited as brown particles, and also located in the cytoplasm of
normal MRTECs. When stimulated with H2O2 for
6 h, the γ-GCS-positive expression increased. At increasing
concentrations of EGCG, the expression of γ-GCS in the nucleus
increased markedly, exhibited by deeper staining, most notably in
the T3 group (Fig. 3). Compared
with the normal control group, the average optical density values
in the control group and each treatment group were significantly
increased (P<0.01); compared with the H2O2
control group, the average optical density values in the T1
(P<0.05), and the T2 and T3 groups (P<0.01) significantly
increased (Table III).
 | Table III.Effects of EGCG on expression of Nrf2
and γ-GCS protein in mouse renal tubular epithelial cells measured
by immunocytochemical assay (OD value). |
Table III.
Effects of EGCG on expression of Nrf2
and γ-GCS protein in mouse renal tubular epithelial cells measured
by immunocytochemical assay (OD value).
|
|
|
| EGCG treatment |
|---|
|
|
|
|
|
|---|
| Group | N | C | T1 | T2 | T3 |
|---|
| Nrf2 | 0.291±0.022 |
0.421±0.023a |
0.441±0.030a,b |
0.523±0.024a,c |
0.545±0.031a,c |
| γ-GCS | 0.340±0.028 |
0.482±0.023a |
0.507±0.017a,b | 0.548±
0.029a,c |
0.596±0.028a,c |
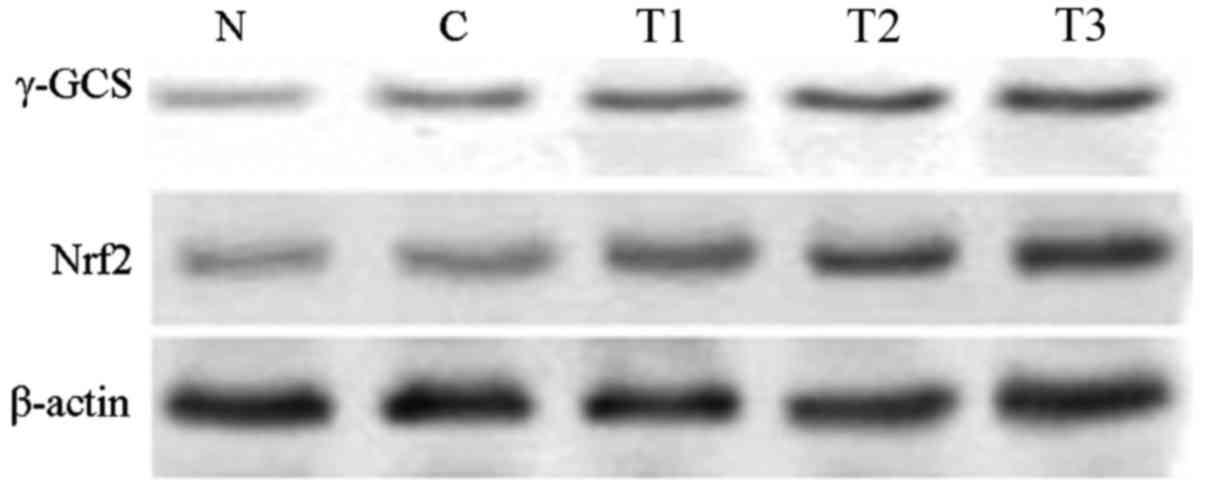
The western blotting analysis results demonstrated
that, compared with the normal group, Nrf2 and γ-GCS protein
expression in the H2O2 control group
significantly increased (P<0.01). Compared with the
H2O2 control group, Nrf2 and γ-GCS protein
expression increased in the T1 (P<0.05), and the T2 and T3
groups (P<0.01), suggesting that EGCG was able to increase Nrf2
and γ-GCS protein expression in MRTECs in a dose-dependent manner
(Table IV; Fig. 4).
 | Table IV.Effects of EGCG on expression of Nrf2
and γ-GCS protein in mouse renal tubular epithelial cells measured
by quantified western blotting. |
Table IV.
Effects of EGCG on expression of Nrf2
and γ-GCS protein in mouse renal tubular epithelial cells measured
by quantified western blotting.
|
|
|
| EGCG |
|---|
|
|
|
|
|
|---|
| Group | N | C | T1 | T2 | T3 |
|---|
| Nrf2 | 0.044±0.005 |
0.083±0.005a |
0.091±0.006a,b |
0.116±0.007a,c |
0.121±0.006a,c |
| γ-GCS | 0.051±0.006 |
0.095±0.006a |
0.099±0.007a,b |
0.126±0.005a,c |
0.132±0.0.006a,c |
Discussion
Studies have demonstrated that catechins exhibit
therapeutic effects in immune nephritis (C-BSA nephritis and Masugi
nephritis), diabetic nephropathy, renal dysfunction, chronic renal
failure, and antibiotic- and heavy metal-induced acute kidney
disease (12–16). Catechins are efficient scavengers
of ROS, and enhance the activity and complexation of antioxidant
enzymes (17,18). Although H2O2
is not a free radical, it is a highly-reactive ROS and may promote
the generation of free radicals, thereby causing lipid peroxidation
of biological membranes, and resulting in necrosis and apoptosis
(19,20). In the present study,
H2O2 was used in MRTECs to replicate
oxidative stress. The potential protective mechanism of EGCG
following oxidative stress was investigated. The results of the
present study demonstrated that oxidative stress led to a decrease
in cell viability and an increase in apoptosis, while EGCG was able
to ameliorate the H2O2-mediated cell damage,
increasing cell viability and decreasing apoptosis. Following the
EGCG intervention, the antioxidant capacity of the MRTECs was
increased, as was the capacity of the cells to protect against and
repair H2O2-mediated damage.
The receptor Nrf2 regulates the expression of
certain target genes under conditions of oxidative stress. Studies
have demonstrated that, when exposed to oxidative and/or
electrophilic stress, overexpression of Nrf2 is able to upregulate
the expression of target genes, and Nrf2 is more effective at
activating gene expression compared with Nrf1 (21). In addition, Nrf2-knockout mice
exhibit decreased expression of target genes (22). These previous results indicated
that the Nrf2 serves an important role in the regulation of
antioxidative genes and the expression of oxidative stress-induced
genes.
The metabolism of foreign compounds or the
pathological production of active may directly or indirectly
interfere with the physiological functions of biological
macromolecules, including DNA, proteins and lipids, and therefore
serve an important role in the pathology of cancer,
neurodegenerative diseases, atherosclerosis and aging. In order to
combat this, cells have evolved defensive capabilities against
toxic substances; for example, detoxifying enzymes and antioxidant
stress protein genes are synergistically induced following exposure
to nucleophilic substances and ROS (23,24).
This antioxidant response involves cis-acting regulatory regions of
target genes, also termed the ARE or electrophile response element
(25,26). Previous studies have demonstrated
that Nrf2 and its cytoplasmic adapter protein Keap1 are the central
regulators of the cellular antioxidant response (27). Experiments have demonstrated that
Nrf2 is able to interact with ARE to regulate the expression of
antioxidant proteins and phase II detoxifying enzymes (28). A number of studies have
demonstrated that the Keap1-Nrf2/ARE pathway exhibits broad
cytoprotective effects in antitumor, neuroprotective and
anti-inflammatory responses (29,30).
These previous results suggested that the Keap1-Nrf2/ARE pathway
served an important role in resisting the cell damage caused by
foreign compounds, drugs and ultraviolet radiation. Nrf2 is highly
expressed in detoxification organs, including the liver and
kidneys, and other organs which are exposed to the outside
environment, including the skin, lungs and digestive tract
(31). The Nrf2-Keap1 system is an
essential part of the mechanisms of resisting environmental and
endogenous stress.
γ-GCS is able to catalyze the reaction between
glutamic acid and cysteine to generate γ-glutamyl cysteine, which
further reacts with glycine to form glutathione (GSH). γ-GCS is the
rate-limiting enzyme in the synthesis of GSH and is the target gene
of Nrf2 regulation. A previous study has demonstrated that
antioxidants and exogenous toxic substances may induce the
overexpression of Nrf2, and upregulate the expression of γ-GCS
(32). Nrf2 is therefore able to
regulate the expression of γ-GCS, consequently affecting the
synthesis of GSH.
Expression of γ-GCS is regulated by a variety of
transcription factors, which are enhanced by ARE, heavy metal
response element, transcription factor AP-1 and nuclear factor-κB.
Previous studies have demonstrated that the majority of antioxidant
genes are enhanced by ARE, and that Nrfs, particularly Nrf2, are
trans-acting factors of ARE, which are able to regulate the
expression of antioxidant genes (33,34).
The present study further investigated the association between the
Nrf2 and γ-GCS signaling pathway, and the effect of EGCG on
oxidative stress-induced tubular epithelial cell injury. The
results of the present study demonstrated that
H2O2-induced oxidative stress in MRTECs led
to increased gene and protein expression of Nrf2, which further
increased following treatment with EGCG. The results of the present
study indicated that the regulation of Nrf2 in MRTECs primarily
occurred at the transcriptional and post-transcriptional
levels.
The present study additionally investigated the gene
and protein expression of γ-GCS in MRTECs, and the results
suggested that oxidative stress promoted the expression of γ-GCS.
Treatment with EGCG was able to further promote the increase in
γ-GCS expression, indicating that oxidative stress in MRTECs may
upregulate the expression of Nrf2, thereby upregulating its
downstream target gene, γ-GCS.
In the present study, the upregulated expression of
the downstream target gene γ-GCS during oxidative stress, through
upregulation of the transcription factor Nrf2, was demonstrated to
occur via the Keap1-Nrf2/ARE signaling pathway. Following treatment
with EGCG, the expression of γ-GCS and Nrf2 increased further.
Therefore, the results of the present study demonstrated that EGCG
was able to increase the expression of Nrf2 in a dose-dependent
manner, and improve the antioxidant activity of MRTECs. In
conclusion, EGCG exhibited antioxidant effects in oxidative
stress-induced MRTECs in a dose-dependent manner.
Acknowledgements
The present study was supported by the Science and
Technology Research Project of Heilongjiang Province, China (grant
no. 12541324).
References
|
1
|
Rani V, Deep G, Singh RK, Palle K and
Yadav UC: Oxidative stress and metabolic disorders: Pathogenesis
and therapeutic strategies. Life Sci. 148:183–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tamay-Cach F, Quintana-Pérez JC,
Trujillo-Ferrara JG, Cuevas-Hernández RI, Del Valle-Mondragón L,
García-Trejo EM and Arellano-Mendoza MG: A review of the impact of
oxidative stress and some antioxidant therapies on renal damage.
Ren Fail. 38:171–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindblom R, Higgins G, Coughlan M and de
Haan JB: Targeting mitochondria and reactive oxygen species-driven
pathogenesis in diabetic nephropathy. Rev Diabet Stud. 12:134–156.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervellati F, Cervellati C, Romani A,
Cremonini E, Sticozzi C, Belmonte G, Pessina F and Valacchi G:
Hypoxia induces cell damage via oxidative stress in retinal
epithelial cells. Free Radic Res. 48:303–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costa JG, Saraiva N, Guerreiro PS, Louro
H, Silva MJ, Miranda JP, Castro M, Batinic-Haberle I, Fernandes AS
and Oliveira NG: Ochratoxin A-induced cytotoxicity, genotoxicity
and reactive oxygen species in kidney cells: An integrative
approach of complementary endpoints. Food Chem Toxicol. 87:65–76.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi K, Ortega MT, Jeffery B, Riviere JE
and Monteiro-Riviere NA: Oxidative stress response in canine in
vitro liver, kidney and intestinal models with seven potential
dietary ingredients. Toxicol Lett. 241:49–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho YE, Kim HS, Lai C, Stanfill A and
Cashion A: Oxidative stress is associated with weight gain in
recipients at 12-months following kidney transplantation. Clin
Biochem. 49:237–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Qiu J, Wang Z, You W, Wu L, Ji C
and Chen G: Dimethylfumarate alleviates early brain injury and
secondary cognitive deficits after experimental subarachnoid
hemorrhage via activation of Keap1-Nrf2-ARE system. J Neurosurg.
123:915–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka Y, Kume S, Araki H, Nakazawa J,
Chin-Kanasaki M, Araki S, Nakagawa F, Koya D, Haneda M, Maegawa H
and Uzu T: 1-Methylnicotinamide ameliorates lipotoxicity-induced
oxidative stress and cell death in kidney proximal tubular cells.
Free Radic Biol Med. 89:831–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andrich K and Bieschke J: The Effect of
(−)-Epigallo-catechin-(3)-gallate on amyloidogenic proteins
suggests a common mechanism. Adv Exp Med Biol. 863:139–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Navarro E, Serrano-Heras G, Castaño MJ and
Solera J: Real-time PCR detection chemistry. Clin Chim Acta.
439:231–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soung HS, Wang MH, Tseng HC, Fanga HW and
Chang KC: (−)Epigallocatechin-3-gallate decreases the
stress-induced impairment of learning and memory in rats. Neurosci
Lett. 602:27–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai S, Zhong Y, Li Y, Huang J, Zhang J,
Luo G and Liu Z: Blockade of the formation of insoluble
ubiquitinated protein aggregates by EGCG3′ Me in the
alloxan-induced diabetic kidney. PLoS One. 8:e756872013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hyung SJ, DeToma AS, Brender JR, Lee S,
Vivekanandan S, Kochi A, Choi JS, Ramamoorthy A, Ruotolo BT and Lim
MH: Insights into antiamyloidogenic properties of the green tea
extract (−)-epigallocatechin-3-gallate toward metal-associated
amyloid-β species. Proc Natl Acad Sci USA. 110:3743–3748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Liu N, Bian X, Sun G, Du F, Wang
B, Su X and Li D: Epigallocatechin-3-gallate reduces tubular cell
apoptosis in mice with ureteral obstruction. J Surg Res.
197:145–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao CG, Zhou P and Wu YB: Impact and
significance of EGCG on Smad, ERK, and β-catenin pathways in
transdifferentiation of renal tubular epithelial cells. Genet Mol
Res. 14:2551–2560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao L, Forester SC and Lambert JD: The
role of the mitochondrial oxidative stress in the cytotoxic effects
of the green tea catechin, (−)-epigallocatechin-3-gallate, in oral
cells. Mol Nutr Food Res. 58:665–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong RZ, Fang Y, Qin GX, Li HY and Zhou
DW: Tea catechins protect goat skeletal muscle against
H2O2-induced oxidative stress by modulating
expression of phase 2 antioxidant enzymes. J Agric Food Chem.
63:7921–7928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai W, Zheng J, Yao X, Peng B, Liu M,
Huang J, Wang G and Xu Y: Catechin prevents the calcium oxalate
monohydrate induced renal calcium crystallization in NRK-52E cells
and the ethylene glycol induced renal stone formation in rat. BMC
Complement Altern Med. 13:2282013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding X, Wang D, Li L and Ma H:
Dehydroepiandrosterone ameliorates
H2O2-induced Leydig cells oxidation damage
and apoptosis through inhibition of ROS production and activation
of PI3K/Akt pathways. Int J Biochem Cell Biol. 70:126–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schultz MA, Abdel-Mageed AB and Mondal D:
The Nrf1 and Nrf2 balance in oxidative stress regulation and
androgen signaling in prostate cancer cells. Cancers (Basel).
2:1354–1378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furnari MA, Saw CL, Kong AN and Wagner GC:
Altered behavioral development in Nrf2 knockout mice following
early postnatal exposure to valproic acid. Brain Res Bul.
109:132–142. 2014. View Article : Google Scholar
|
|
23
|
Khoi PN, Park JS, Kim JH, Xia Y, Kim NH,
Kim KK and Jung YD: (−)-Epigallocatechin-3-gallate blocks
nicotine-induced matrix metalloproteinase-9 expression and
invasiveness via suppression of NF-κB and AP-1 in endothelial
cells. Int J Oncol. 43:868–876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SY, Jeong YJ, Kim SH, Jung JY and Kim
WJ: Epigallocatechin gallate protects against nitric oxide-induced
apoptosis via scavenging ROS and modulating the Bcl-2 family in
human dental pulp cells. J Toxicol Sci. 38:371–378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng B, Fang Y and Wei SM: Effect and
mechanism of epigallocatechin-3-gallate (EGCG). against the
hydrogen peroxide-induced oxidative damage in human dermal
fibroblasts. J Cosmet Sci. 64:35–44. 2013.PubMed/NCBI
|
|
26
|
Toniolo A, Buccellati C, Pinna C, Gaion
RM, Sala A and Bolego C: Cyclooxygenase-1 and prostacyclin
production by endothelial cells in the presence of mild oxidative
stress. PLoS One. 8:e566832013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deshmukh P, Unni S, Krishnappa G and
Padmanabhan B: The Keap1–Nrf2 pathway: Promising therapeutic target
to counteract ROS-mediated damage in cancers and neurodegenerative
diseases. Biophys Rev. 9:41–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Magesh S, Chen Y and Hu L: Small molecule
modulators of Keap1-Nrf2-ARE pathway as potential preventive and
therapeutic agents. Med Res Rev. 32:687–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitsuishi Y, Motohashi H and Yamamoto M:
The Keap1–Nrf2 system in cancers: Stress response and anabolic
metabolism. Front Oncol. 2:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandberg M, Patil J, D'Angelo B, Weber SG
and Mallard C: NRF2-regulation in brain health and disease:
Implication of cerebral inflammation. Neuropharmacology.
79:298–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tebay LE, Robertson H, Durant ST, Vitale
SR, Penning TM, Dinkova-Kostova AT and Hayes JD: Mechanisms of
activation of the transcription factor Nrf2 by redox stressors,
nutrient cues, and energy status and the pathways through which it
attenuates degenerative disease. Free Radic Biol Med. 88:108–146.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Brien S and Kay NE: Maintenance therapy
for B-chronic lymphocytic leukemia. Clin Adv Hematol Oncol.
9:22–31. 2011.PubMed/NCBI
|
|
33
|
Muthusamy VR, Kannan S, Sadhaasivam K,
Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L and
Rajasekaran NS: Acute exercise stress activates Nrf2/ARE signaling
and promotes antioxidant mechanisms in the myocardium. Free Radic
Biol Med. 52:366–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Q: Role of Nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|