Introduction
In total of ~0.1% of the population are affected by
systemic lupus erythematosus (SLE), which is a chronic autoimmune
disease involving multiple systems, with women having a nine times
higher risk of developing the disease, compared with men (1). SLE is a complex disease, which has a
range of manifestations and the combinations of which can lead to
variable levels of disease severity. Autoreactive B and T
lymphocytes have been the focus of the majority of studies
performed to investigate the pathophysiology of this disease
(2).
It is a general agreement that clinically relevant
nephritis occurs in 60% of patients with lupus at some point during
the duration of the disease (3).
It is important that diagnosis is made early and that the treatment
of renal disease occurs early, as early response to therapy is
associated with more favorable results (4).
Current understanding of the correlation between
gene messenger RNAs (mRNAs) and human disease has changed following
the identification of microRNAs (miRNAs) at the turn of the 21st
century, which marked a new era of cell biology and has extended to
those sequences in the residual ~90% of eukaryotic genomes that
produce non-coding RNAs (5).
miRNAs function as meta-controllers of gene expression and are
pivotal for the cellular alterations required for development
(5).
miRNAs are implicated in the pathogenesis of renal
fibrosis and chronic kidney diseases. Certain miRNAs exhibit
antifibrotic effects and others have profibrotic effects. miR-29
and miR-200 are reduced, whereas miR-21, miR-377, miR-205, miR-141
and miR-192 are elevated in animal models and patients with renal
fibrosis, contributing to hypertensive nephrosclerosis, IgA
nephropathy, obstructive nephropathy and diabetic nephropathy
(6–10). Additionally, the suppression of
miR-192 alleviates renal fibrosis in mice with diabetes (11), and it has been demonstrated that
miRNAs serve as crucial mediators in renal fibrosis and may be
promising targets for the prevention of end-stage renal disease
(12). Few studies have focused on
miRNAs in lupus nephritis. In a previous study, 66 miRNAs
differentially expressed in renal biopsies were identified from
patients with lupus nephritis, compared with normal subjects. In
another study, there were differences in the intrarenal expression
levels of miR-146a, miR-198 and miR-638 between patients with lupus
nephritis and normal subjects (13). There have been no reports on the
potential of miRNAs as markers of any specific histologic
presentation or their possible effect in renal fibrosis in lupus
nephritis (14).
Patients with renal failure may have significantly
decreased circulating miRNAs, compared with patients with marginal
renal impairment or normal renal function, as shown by microarrays.
The underlying mechanism may be associated with the substantial
buildup of RNase in patients with renal failure, which elevates
degraded circulating miRNAs, however, this remains to be elucidated
(15). This was demonstrated by
the fact that miR-1233-3p and miR-130b were downregulated,
respectively, and that the total RNA level was significantly
reduced in late stage lupus nephritis of the validation group,
which were consistent with the results reported by Neal et
al, who determined the serum levels of five specific miRNAs in
patients who suffered from renal failure (16).
It has been previously shown that miR-130b is
differentially expressed in lens epithelial cells collected from
individuals with lupus nephritis, and the dysregulation of PTEN has
also been reported to be involved in the molecular mechanism of
mesangial cell apoptosis (17,18).
By searching an online miRNA database, the present study identified
PTEN as a virtual target of miR-133b. It was then confirmed that
PTEN was a target of miR-130b, and the involvement of miR-130b and
PTEN in the development of lupus nephritis was confirmed.
Materials and methods
Sample collection
Tissue samples from 28 patients with SLE and lupus
nephritis (33±4-years old) and 31 healthy patients (37±6-years-old)
were collected between September 2013 and September 2014 at the
Department of Rheumatism and Immunology, Tai'an Central Hospital
(Shenyang, China). All patients were assessed by biopsy to confirm
fulfillment to the 1982 American College of Hematology revised
criteria for SLE and lupus nephritis (19). Written consent was signed by all
patients, glomerular filtration rate was estimated using the
modification of diet in renal disease formula (20), and clinical and demographic data of
the patients were carefully recorded. The protocol of the present
study was approved by the Ethics Committee of the Hospital of
Tai'an Central Hospital.
Target prediction and functional
analysis
By scanning the most commonly used target gene
prediction databases, including miRDB (http://www.mirdb.org/), miRanda (http://www.microrna.org/microrna/home.do) and
TargetScan (www.targetscan.org), the putative
target genes of the miR-130b were pooled from the three databases
in total. Experimental validation on most of the target genes was
performed. Gene ontology (GO) term analysis were performed to gain
a full insight of the functional relevance on these target genes.
GO term analysis indicated that these target genes were enriched in
critical biological processes, including ‘cell proliferation’ and
‘regulation of focal adhesion kinase activity’.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR) analysis of PTEN
mRNA and miR-130b in tissue samples and mesangial cells
TRIzol extraction reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used to extract the total RNA from
tissue samples and mesangial cells (obtained from the tissue
samples) according to manufacturer's protocol. An A260/A280 value
between 1.8 and 2.0 was accepted, which ensured that the RNA
samples were without DNA or proteins. A TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to synthesize the cDNA (PTEN) from RNA with a
mixture of 2 µg total RNA, 1 µg of miRNA-specific primers (25 µM)
and ddH2O (RNase-free) to a final volume of 10 µl. The
mixture was then denatured for 10 min at 70°C and placed on ice,
followed by the addition of the reaction buffer comprising (11 µl
ddH2O (RNase-free), 4 µl dNTP mix, 4 µl 5X RT buffer and
1 µl ReverTra Ace (100 U/µl; Toboyo Co., Ltd., Osaka, Japan), and
then maintained for 60 min at 42°C, followed by 10 min at 90°C.
Invitrogen Platinum SYBR-Green qPCR SuperMix-UDG (Thermo Fisher
Scientific, Inc.) was used to amplify the cDNA according to the
manufacturer's protocol with a mixture of 1.5 µl forward primer (10
µl), 25 µl SYBR mix, 1.5 µl reverse primer (10 µM), 1 µl cDNA and
21 µl ddH2O. Sequences of primers were: miR-130b
forward, 5′GGGCAGTGCAATGATGAAA3′, reverse, 5′GTGCGTGTCGTGGAGTCG3′;
PTEN forward, 5′-TTTGAAGACCATAACCCACCAC-3′, reverse,
5′-ATTACACCAGTTCGTCCCTTTC-3′ and GAPDH forward,
5′-AGCCTCAAGATCATCAGCAATG-3′ and reverse,
5′-TGTGGTCATGAGTCCTTCCACG-3′. Fluorescence detection and qPCR were
performed using the Qiagen Rotor-Gene Q (Qiagen GmbH, Hilden,
Germany). The reaction settings were as follows: 10 min at 50°C, 10
min at 95°C, 15 sec at 95°C, 45 sec at 60°C for 40 cycles. The
2−ΔΔCq method (21) was
used to calculate the relative mRNA expression of PTEN and miR-130b
according to the expression of GAPDH for human renal biopsies.
Three assessments were performed in triplicate.
Cell culture and transfection
DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) was used for incubation of
the mesangial cells at 37°C in 5% CO2 until use. When
confluence reached 30–50% and the cells were serum-deprived for 24
h, Lipofectamine® RNAiMAX Transfection reagent (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to transfect
the cells with miR-130b mimics or PTEN small interfering (si)RNA
(Sunbio, Shanghai, China) or inhibitors according to protocols
provided by the manufacturers. Sequence of PTEN siRNA was as
follows: 5′-GGCGUAUACAGGAACAAUATT-3′. The medium was replaced with
DMEM/F12 culture medium containing 2% reduced FBS 6 h following
transfection. The experiments were repeated three times
independently.
Cell proliferation assay
An MTT assay was used to assess the cell
proliferation, as described previously. The mesangial cells were
incubated in a 96-well plate at a final density of 5×104
cells per well for 1 day at 37°C. Subsequently, 200 µl of MTT
solution was used to treat the mesangial cells for 60 min at 37°C,
following which the MTT solution was carefully removed and the
cells were washed with 100 µl PBS. Finally, the cells were treated
with 200 µl dimethyl sulfoxide solution for 120 min on a plate
shaker at room temperature. A microplate reader (Synergy HT;
BioTek, Instruments, Inc., Winooski, VT, USA) was used to measure
the proliferation of the mesangial cells based on the absorbance at
575 nm. All tests were performed in triplicate.
Luciferase assay
The 3′-untranslated region (UTR) of PTEN containing
the binding site of miR-130b was amplified by PCR and inserted into
the psiCHECK-2 reporter vector (Promega Corporation, Madison, WI,
USA). The mutagenesis was performed for the same site and
introduced into the control vector (Ambion; Thermo Fisher
Scientific, Inc.). For transfection, the cells were co-transfected
with wild-type/mutant type vector and miR-130b mimic/negative
control using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) with a mixture of 1×106 cells, 1 µg
Renilla luciferase expression construct, pRL-TK (Promega
Corporation), 1 µg of psiCHECK-2 reporter vector (Promega
Corporation) or psiCHECK-2-mut plasmid and 50 pmol of miR-130b
mimic or control. Renilla luciferase activity was used as an
internal control. After incubation at 37°C for 48 h, the Dual
Luciferase assay reagent (Promega Corporation) was used to measure
the luciferase activity in accordance with the manufacturer's
protocol. All results were calculated as fold differences relative
to Renilla luciferase activity. Each experiment was
performed at least three times.
Western blot analysis
For analysis of the mRNA expression of PTEN and
miR-130b, ice-cold lysis buffer containing 1% NP-40, 0.1% sodium
dodecyl sulfate, 50 mM Tris-HCl (pH 7.4) and 150 mM NaCl, including
protease inhibitors (Roche Diagnostics, Basel, Switzerland) was
used to lyse the mesangial cells according to the manufacturer's
protocol. The lysates were centrifuged at 14,000 × g at 4°C for 15
min. A Bradford protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to determine the concentration of
protein. To separate 35 µg protein, 8% SDS-PAGE was used, and the
protein then was transferred onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA) for 60 min (120 V).
Tris-buffered saline containing 0.1% Tween-20 (TBST) and 5% non-fat
dry milk was used to block the membrane to avoid unspecific
binding. Primary antibodies, including anti-β-actin (1:5,000;
A5441; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and anti-PTEN
(1:1,000; SAB4300337; Sigma-Aldrich; Merck KGaA) were used to treat
the membrane. Subsequently, HRP-conjugated secondary antibody
(1:10,000; A6154; Sigma-Aldrich; Merck KGaA) was used for
incubation of the membrane. Quantity One 1-D Analysis Software
(v4.6.9; Bio-Rad Laboratories, Inc.) was used to quantify the band
intensity.
Analysis of apoptosis
Apoptosis was performed using propidium
iodide/annexin V staining with an apoptosis detection kit (Nanjing
KeyGEN Biotech Co., Ltd., Nanjing, China). The mesangial cells were
maintained at room temperature for 15 min in the dark, following
which flow cytometry (BD Biosciences, San Jose, CA, USA) was used
to assess the specimens. Annexin V immunofluorescence is shown on
the X-axis and plasma membrane integrity is shown on the Y-axis.
Analyses were repeated three times.
Statistical analysis
All data are shown as the mean ± standard error of
the mean. Each experiment was performed at least three times to
ensure the reproducibility of each test. One-way analysis of
variance or a Student's t-test was used to analyze differences to
determine statistical significance between groups. Pearson's linear
correlation analysis was used to analyze the correlation between
two variables. The predictive accuracy of renal miR-130b levels was
assessed using ROC analysis. P<0.05 was considered to indicate a
statistically significant difference. SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA) was employed for statistical analysis. All tests
were repeated three times.
Results
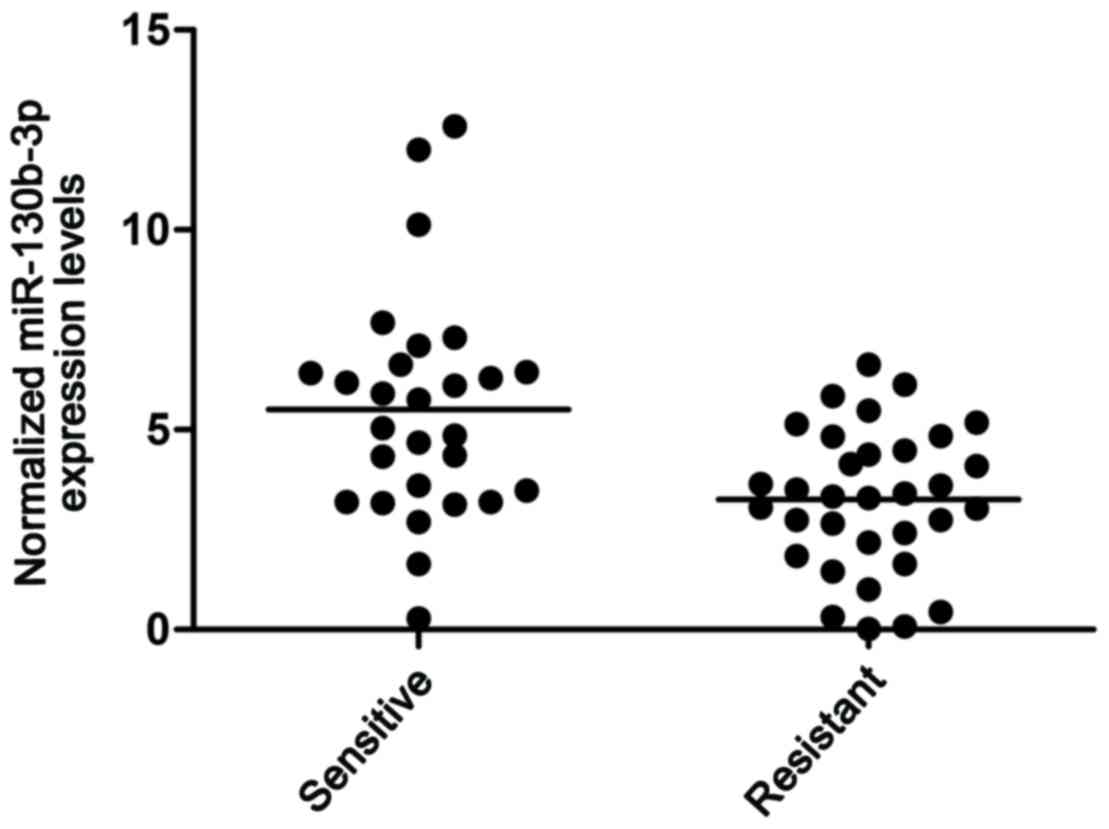
miR-130 is upregulated in patients
with lupus nephritis
In the present study, kidney tissue samples were
collected from patients with SLE. These samples were then divided
into two groups based on whether they had nephritis or not: Lupus
nephritis(+) and lupus nephritis(−), respectively. RT-qPCR analysis
was then performed, and the results showed that the expression
level of miR-130b was higher in the lupus nephritis(+) group
(Fig. 1). These results indicated
that miR-130b is a risk factor for lupus nephritis in patients.
PTEN is virtual target of
miR-130b
There is a series of literature on miR-130b, and it
has been reported that miR-130b is involved in several diseases.
The present study was performed to understand the association
between miR-130b levels and lupus nephritis. An online miRNA target
prediction tool was used to identify the regulatory gene of
miR-130b, which identified PTEN as a candidate target gene of
miR-130b with the ‘seed sequence’ in the 3′UTR (Fig. 2) and three existing binding sites.
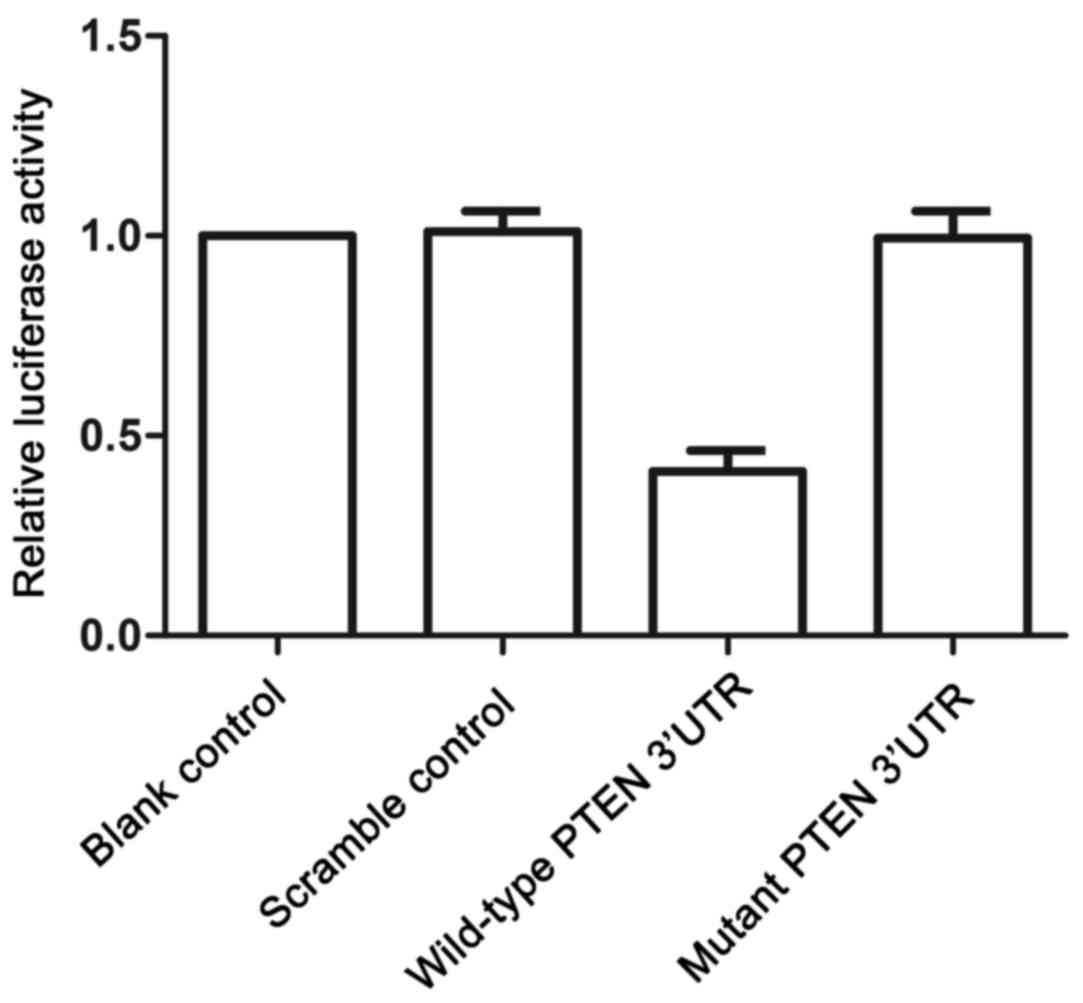
To confirm the regulatory association between miR-130b and PTEN, a
luciferase activity reporter assay was performed in mesangial
cells. Only the luciferase activity in the mesangial cells
cotransfected with miR-130b and wild-type PTEN 3′UTR was decreased
significantly (Fig. 3), whereas
mesangial cells cotransfected with miR-130b and mutant PTEN 3′UTR
showed comparable luciferase activity to that in the scramble
control (Fig. 3). These results
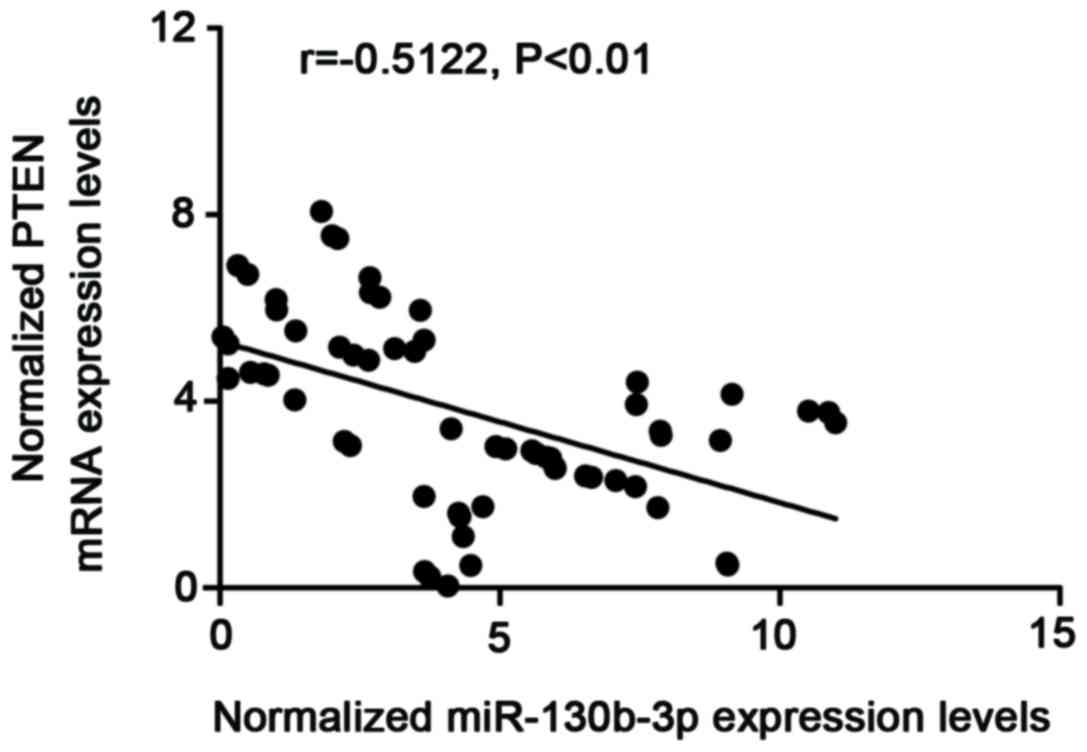
confirmed that PTEN was a target of miR-130b in mesangial cells. To
further examine the regulatory association between miR-130b and
PTEN, the correlation between the expression level of miR-130b and
mRNA expression of PTEN was examined among the blood samples
(n=61), which showed a negative regulatory association (Fig. 4; r=−0.5122; P<0.01).
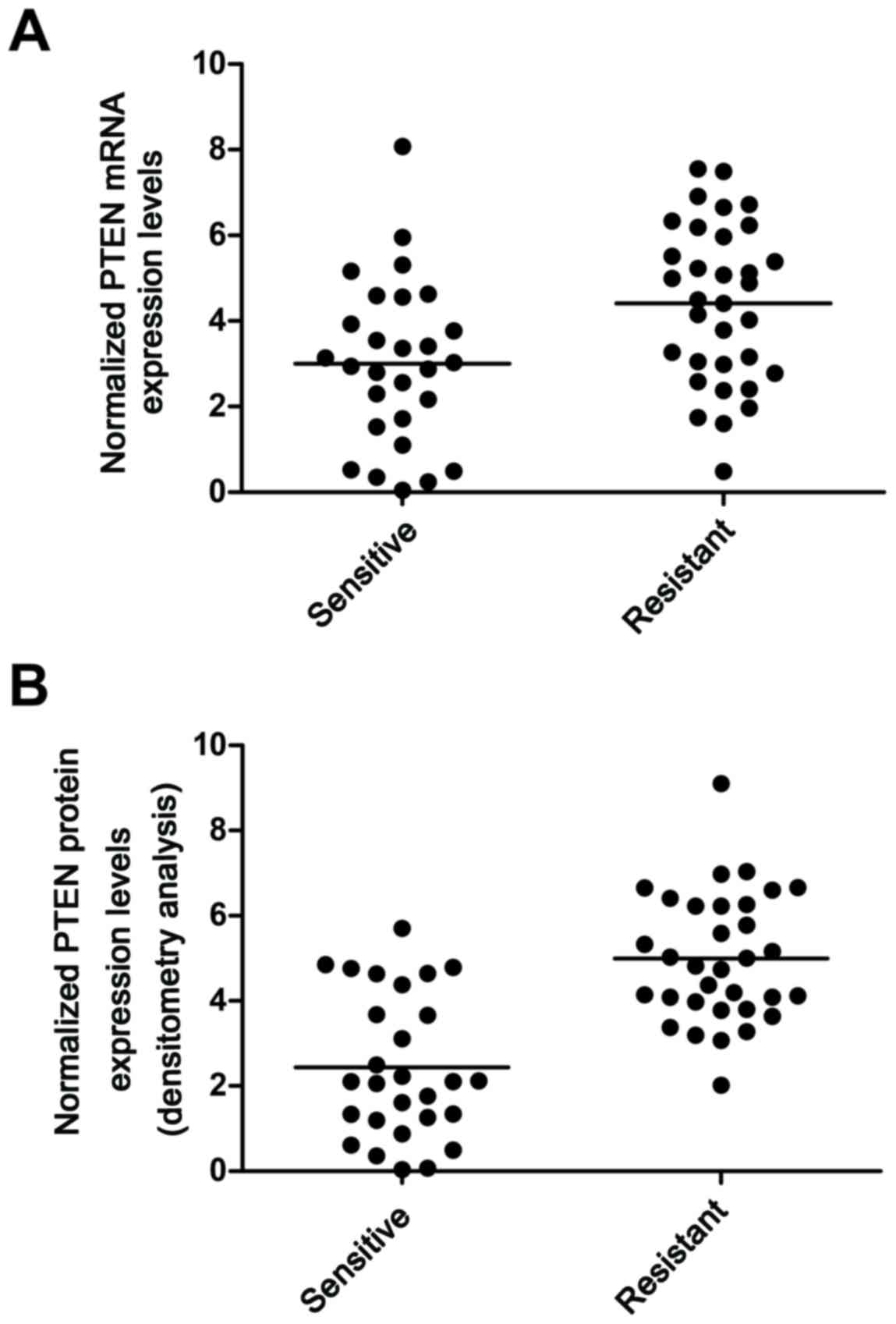
Determination of expression patterns
of miR-130b and PTEN in tissues of different groups
The tissue samples of the lupus nephritis(+) (n=28)
and lupus nephritis(−) (n=33) patients were used to further examine
the effects on the interaction between miR-130b and the PTEN 3′UTR.
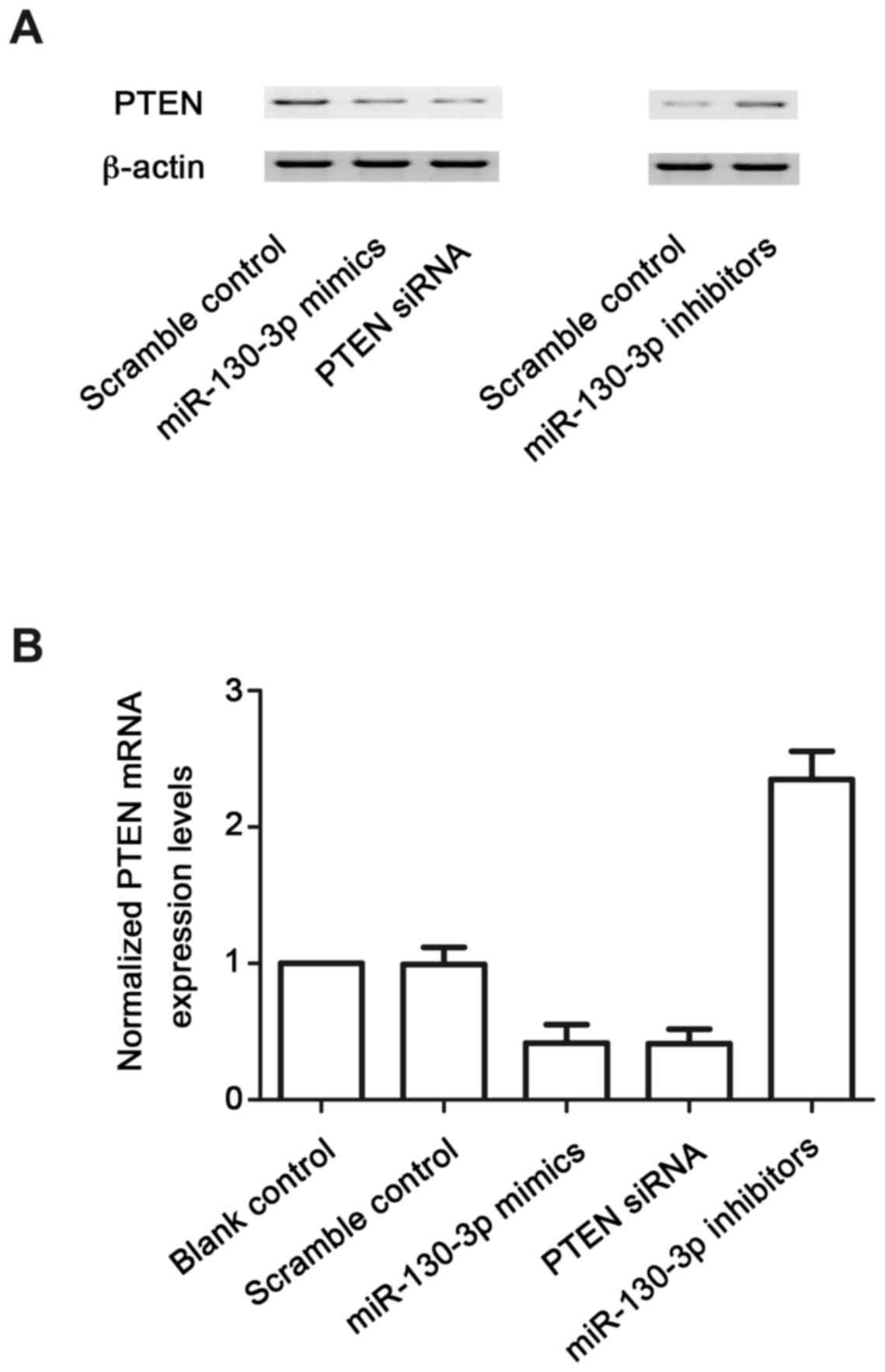
By performing RT-qPCR analysis, it was found that the mRNA
expression of PTEN (Fig. 5A) was
decreased in the lupus nephritis(+) group, compared with that in
the lupus nephritis(−) group. The protein expression of PTEN
(Fig. 5B) was measured using
densitometric analysis, which revealed it was also decreased in the
lupus nephritis(+) group, compared with that in the lupus
nephritis(−) group. To further confirm the hypothesis of the
negative regulatory association between miR-130b and PTEN, the
mesangial cells were transfected with scramble control, miR-130b
mimics, PTEN siRNA and miR-130b inhibitors. The protein (Fig. 6A) and mRNA (Fig. 6B) expression levels of PTEN in the
mesangial cells treated with iR-130b mimics and PTEN siRNA were
lower, compared with those in the scramble control, whereas
mesangial cells treated with miR-130b inhibitors were higher,
compared with those in the scramble control. This confirmed the
negative regulatory association between miR-130b and PTEN.
miR-130b and PTEN interfere with the
viability of mesangial cells
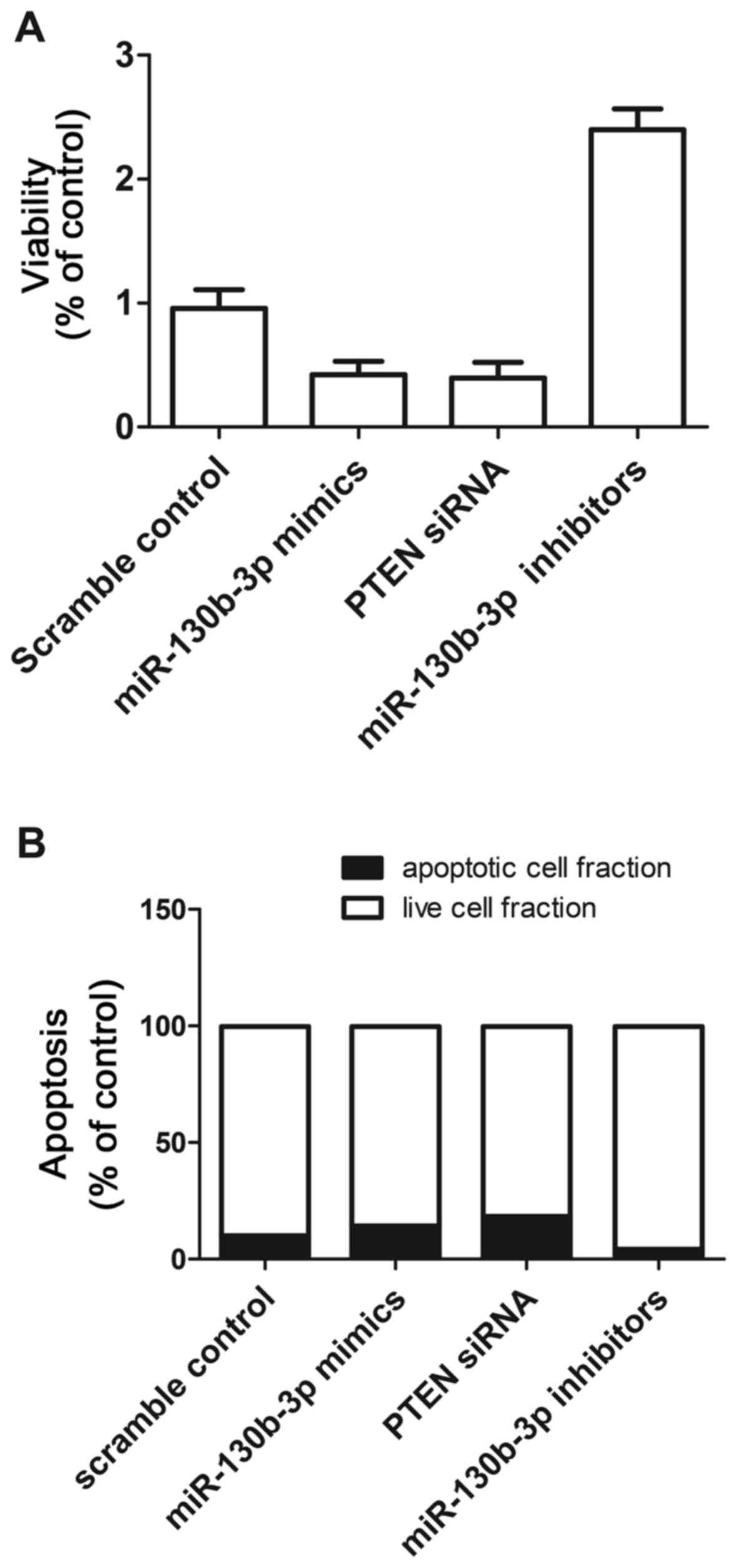
The present study also investigated the relative
viability of mesangial cells when transfected with scramble
control, miR-130b mimics, PTEN siRNA and miR-130b inhibitors. The
mesangial cells transfected with miR-130b inhibitors showed
upregulated viability (Fig. 7A),
compared with that in the scramble control, whereas the mesangial
cells transfected with miR-130b mimics and PTEN siRNA showed
comparably lower viability. This indicated that miR-130b negatively
affected the viability of mesangial cells, whereas PTEN positively
affected the viability of mesangial cells.
miR-130b and PTEN interfere with the
apoptosis of mesangial cells
The present study subsequently investigated the
relative apoptosis of mesangial cells when transfected with
scramble control, miR-130b mimics, PTEN siRNA and miR-130b
inhibitors. When transfected with miR-130b mimics and PTEN siRNA,
the number of surviving mesangial cells was reduced and the number
of apoptotic mesangial cells was increased, compared with numbers
in the scramble control, whereas the mesangial cells transfected
with miR-130b inhibitors showed comparably higher cell survival and
reduced apoptotsis (Fig. 7B).
These results indicated that miR-130b accelerated apoptosis,
whereas PTEN inhibited apoptosis.
Discussion
MiRNAs are highly conserved small noncoding RNAs
involved in numerous biologic processes. MiR-130b was reported to
be associated with numerous diseases, including type 2 diabetes
(22), colorectal cancer (23), and bladder cancer (24). Zhaohui Ni revealed that patients
with early stage LN exhibited a higher level of miR-130b compared
with healthy patients, and suggested that miR-130b may be involved
in LN by regulating the expression of Erbb2 interacting protein
(17). Xiao Han demonstrated that
miR-130b was associated with pathogenesis of LN via regulating
IRF-1 and further inhibited the type I IFN pathway (25). It is important to further
investigate the mechanisms underlying miR-130b, mesangial cells and
proteinuria in the future. A carefully designed investigation
performed on a larger sample size is also necessary and the
inclusion of other kidney conditions is likely to provide more
valuable evidence. In the present study, kidney tissue samples were
collected from patients with SLE, which were divided into two
groups based on whether they had nephritis or not: Lupus
nephritis(+) and lupus nephritis(−). RT-qPCR analysis was
performed, and the results showed that the expression level of
miR-130b was higher in the lupus nephritis(+) group (Fig. 1). In addition, online miRNA target
prediction tools were used to identify the regulatory gene of
miR-130b, and PTEN was identified as the candidate target gene of
miR-130b with the ‘seed sequence’ in the 3′UTR (Fig. 2) and the existence of binding
sites. A luciferase activity reporter assay was also performed on
the mesangial cells, in which luciferase activity was only reduced
in the mesangial cells cotransfected with miR-130b and wild-type
PTEN 3′UTR (Fig. 3). The
luciferase activities in mesangial cells cotransfected with
miR-130b and mutant PTEN 3′UTR were comparable with that of the
scramble control (Fig. 3).
PTEN is a potent tumor-inhibitor gene present at
chromosome 10q23.31, which was identified in 1997 (26,27).
A phosphatase with double properties against proteins and
phospholipids is encoded by PTEN (28). The signal transduction pathways can
be regulated by PTEN protein via either phosphatase-independent or
dependent mechanisms (29).
Regardless of its possible serine/threonine and tyrosine
phosphatase property, the tumor-inhibitory effect of PTEN
contributes to its lipid phosphatase effect (30). PTEN is considered to be the major
factor negatively regulating class I PI3Ks (31). Additionally, the regulatory effect
triggered by specific miRNAs on the function of important immune
cells, including B and T lymphocytes, in lupus has been
investigated. PTEN regulates normal signaling via the B cell
receptor, and abnormal miR-7 regulation results in
hyper-responsiveness of B cells in SLE (32). In the present study, a microarray
was performed to determine the expression levels of miRNAs in B
cells obtained from patients with active SLE, compared with healthy
subjects. A marked reduction in the expression of miR-1246
expression in B cells was found in patients with active SLE, but
not in patients with inactive SLE or healthy subjects, which
suggested that miR-1246 was involved in active SLE and may offer
potential as a biomarker or promising therapeutic target in active
SLE. In the present study, it was found that the mRNA expression of
PTEN (Fig. 5A) was decreased in
the lupus nephritis(+) group, compared with that in the lupus
nephritis(−) group. The protein expression of PTEN (Fig. 5B) was also measured using
densitometric analysis, which revealed its expression was decreased
in lupus nephritis(+), compared with that in lupus nephritis(−),
and that the expression level of PTEN in mesangial cells treated
with miR-130b mimics and PTEN siRNA were lower, compared with that
in the scramble control. The expression of PTEN in mesangial cells
treated with miR-130b inhibitors was higher than that in the
scramble control, confirming the negative regulatory association
between miR-130b and PTEN.
Apoptosis can occur via mitochondrial and
receptor-mediated pathways. The importance of apoptosis in SLE has
been confirmed, as apoptotic cells result in a high level of
autoantigens, which appear to be processed inappropriately in this
disease (33). Apoptotic
suppression with a pan-caspase inhibitor or with anti-Fas ligand
antibodies results in prevention against renal disorder in models
of lupus nephritis (34,35). It is increasingly accepted that
inflammation and apoptosis can be correlated rather than being
mutually exclusive (36). A
previous observation that C3aRa inhibited apoptosis and
inflammatory cell infiltration is consistent with this and may even
be caused by the same mechanism (37). The first step toward its membrane
correlation and complete activation is the phosphorylation of
PKB/Akt on serine 473, which was found to be substantially elevated
by the inhibition of C3aR, possibly leading to the apoptotic
decrease observed (38).
Additionally, significantly higher levels of phosphorylated PTEN
(serine 380) have been observed in control lupus mice, compared
with that in C3aRa-treated mice; as PTEN negatively regulates PI3K,
and is closely associated with immune cell activation, cell growth
and survival, these findings are likely to be correlated with the
intrinsic pathophysiology of lupus nephritis and the mechanism by
which signaling through C3aR causes disease (39,40).
In the present study, the relative apoptosis was investigated when
of mesangial cells were transfected with scramble control, miR-130b
mimics, PTEN siRNA and miR-130b inhibitors. When transfected with
miR-130b mimics and PTEN siRNA, the number of surviving mesangial
cells was reduced and the number of apoptotic mesangial cells was
increased, compared with those in the scramble controls group,
whereas the mesangial cells transfected with miR-130b inhibitors
showed comparably higher numbers of surviving cells and fewer
apoptotic mesangial cells.
In conclusion, the present study demonstrated that
miR-130b was upregulated in the lupus nephritis group, compared
with that in the control group. PTEN was identified as a virtual
target of miR-130b, and there was a negative regulatory association
between miR-130b and PTEN. miR-130b and PTEN interfered with the
viability and apoptosis of mesangial cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SW planned the study, collected the data, analysed
and interpreted the data and prepared the manuscript. JW collected
the data, prepared the manuscript. FL interpreted the data,
collected the literature.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Hospital of Tai'an Central Hospital. Written
consent was signed by all patients.
Consent for publication
Written consent was signed by all patients for the
publication of any associated data or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Helmick CG, Felson DT, Lawrence RC,
Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD,
Merkel PA, et al: Estimates of the prevalence of arthritis and
other rheumatic conditions in the United States. Part I. Arthritis
Rheum. 58:15–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng SL: Altered T and B lymphocyte
signaling pathways in lupus. Autoimmun Rev. 8:179–183. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogan J and Radhakrishnan J: The treatment
of minimal change disease in adults. J Am Soc Nephrol. 24:702–711.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bertsias G, Gordon C and Boumpas DT:
Clinical trials in systemic lupus erythematosus (SLE): Lessons from
the past as we proceed to the future-the EULAR recommendations for
the management of SLE and the use of end-points in clinical trials.
Lupus. 17:437–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceman S and Saugstad J: MicroRNAs:
Meta-controllers of gene expression in synaptic activity emerge as
genetic and diagnostic markers of human disease. Pharmacol Ther.
130:26–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lorenzen JM, Haller H and Thum T:
MicroRNAs as mediators and therapeutic targets in chronic kidney
disease. Nat Rev Nephrol. 7:286–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farris AB and Colvin RB: Renal
interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol
Hypertens. 21:289–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang G, Kwan BC, Lai FM, Choi PC, Chow KM,
Li PK and Szeto CC: Intrarenal expression of miRNAs in patients
with hypertensive nephrosclerosis. Am J Hypertens. 23:78–84. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Kwan BC, Lai FM, Choi PC, Chow KM,
Li PK and Szeto CC: Intrarenal expression of microRNAs in patients
with IgA nephropathy. Lab Invest. 90:98–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung AC, Huang XR, Meng X and Lan HY:
miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc
Nephrol. 21:1317–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato M, Zhang J, Wang M, Lanting L, Yuan
H, Rossi JJ and Natarajan R: MicroRNA-192 in diabetic kidney
glomeruli and its function in TGF-beta-induced collagen expression
via inhibition of E-box repressors. Proc Natl Acad Sci USA.
104:3432–3437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Putta S, Lanting L, Sun G, Lawson G, Kato
M and Natarajan R: Inhibiting microRNA-192 ameliorates renal
fibrosis in diabetic nephropathy. J Am Soc Nephrol. 23:458–469.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai Y, Sui W, Lan H, Yan Q, Huang H and
Huang Y: Comprehensive analysis of microRNA expression patterns in
renal biopsies of lupus nephritis patients. Rheumatol Int.
29:749–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu J, Kwan BC, Lai FM, Tam LS, Li EK, Chow
KM, Wang G, Li PK and Szeto CC: Glomerular and tubulointerstitial
miR-638, miR-198 and miR-146a expression in lupus nephritis.
Nephrology (Carlton). 17:346–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rabinovitch MB, Liberman and Fausto N:
Plasma ribonuclease activity in human uremia. J Lab Clin Med.
53:563–568. 1959.PubMed/NCBI
|
|
16
|
Neal CS, Michael MZ, Pimlott LK, Yong TY,
Li JY and Gleadle JM: Circulating microRNA expression is reduced in
chronic kidney disease. Nephrol Dial Transplant. 26:3794–3802.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Mou S, Wang L, Zhang M, Shao X,
Fang W, Lu R, Qi C, Fan Z, Cao Q, et al: Up-regulation of serum
MiR-130b-3p level is associated with renal damage in early lupus
nephritis. Sci Rep. 5:126442015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan W, Gu Z, Shen B, Jiang J, Meng Y, Da
Z, Liu H, Tao T and Cheng C: PTEN/Akt-p27(kip1) signaling promote
the BM-MSCs senescence and apoptosis in SLE patients. J Cell
Biochem. 116:1583–1594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan EM, Cohen AS, Fries JF, Masi AT,
McShane DJ, Rothfield NF, Schaller JG, Talal N and Winchester RJ:
The 1982 revised criteria for the classification of systemic lupus
erythematosus. Arthritis Rheum. 25:1271–1277. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamb EJ, Webb MC and O'Riordan SE: Using
the modification of diet in renal disease (MDRD) and Cockcroft and
Gault equations to estimate glomerular filtration rate (GFR) in
older people. Age Ageing. 36:689–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv C, Zhou YH, Wu C, Shao Y, Lu CL and
Wang QY: The changes in miR-130b levels in human serum and the
correlation with the severity of diabetic nephropathy. Diabetes
Metab Res Rev. 31:717–724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colangelo T, Fucci A, Votino C, Sabatino
L, Pancione M, Laudanna C, Binaschi M, Bigioni M, Maggi CA, Parente
D, et al: MicroRNA-130b promotes tumor development and is
associated with poor prognosis in colorectal cancer. Neoplasia.
15:1086–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han X, Wang Y, Zhang X, Qin Y, Qu B, Wu L,
Ma J, Zhou Z, Qian J, Dai M, et al: MiR-130b ameliorates murine
lupus nephritis through targeting type I interferon pathway on
resident renal cells. Arthritis Rheumatol. 68:2232–2243. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Govender D and Chetty R: Gene of the
month: PTEN. J Clin Pathol. 65:601–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li DM and Sun H: TEP1, encoded by a
candidate tumor suppressor locus, is a novel protein tyrosine
phosphatase regulated by transforming growth factor beta. Cancer
Res. 57:2124–2129. 1997.PubMed/NCBI
|
|
28
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu WT, Yang Z and Lu NH: Roles of PTEN
(Phosphatase and Tensin Homolog) in gastric cancer development and
progression. Asian Pac J Cancer Prev. 15:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Myers MP and Tonks NK: PTEN: Sometimes
taking it off can be better than putting it on. Am J Hum Genet.
61:1234–1238. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye B, Jiang LL, Xu HT, Zhou DW and Li ZS:
Expression of PI3K/AKT pathway in gastric cancer and its blockade
suppresses tumor growth and metastasis. Int J Immunopathol
Pharmacol. 25:627–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu XN, Ye YX, Niu JW, Li Y, Li X, You X,
Chen H, Zhao LD, Zeng XF, Zhang FC, et al: Defective PTEN
regulation contributes to B cell hyperresponsiveness in systemic
lupus erythematosus. Sci Transl Med. 6:246ra992014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaplan MJ: Apoptosis in systemic lupus
erythematosus. Clin Immunol. 112:210–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seery JP, Cattell V and Watt FM: Cutting
edge: Amelioration of kidney disease in a transgenic mouse model of
lupus nephritis by administration of the caspase inhibitor
carbobenzoxy-valyl-alanyl-aspartyl-(beta-o-methyl)-fluoromethylketone.
J Immunol. 167:2452–2455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakajima A, Hirai H, Kayagaki N, Yoshino
S, Hirose S, Yagita H and Okumura K: Treatment of lupus in NZB/W F1
mice with monoclonal antibody against Fas ligand. J Autoimmun.
14:151–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schaub FJ, Han DK, Liles WC, Adams LD,
Coats SA, Ramachandran RK, Seifert RA, Schwartz SM and Bowen-Pope
DF: Fas/FADD-mediated activation of a specific program of
inflammatory gene expression in vascular smooth muscle cells. Nat
Med. 6:790–796. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lenda DM, Kikawada E, Stanley ER and
Kelley VR: Reduced macrophage recruitment, proliferation, and
activation in colony-stimulating factor-1-deficient mice results in
decreased tubular apoptosis during renal inflammation. J Immunol.
170:3254–3262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scheid MP and Woodgett JR: Unravelling the
activation mechanisms of protein kinase B/Akt. FEBS Lett.
546:108–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deane JA and Fruman DA: Phosphoinositide
3-kinase: Diverse roles in immune cell activation. Annu Rev
Immunol. 22:563–598. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|