Introduction
Sjogren's syndrome (SS) is a chronic inflammatory
autoimmune disease that usually results from a functional decline
caused by lymphocyte invasion into salivary and lacrimal glands,
and is associated with a dry mouth, dry eyes and other symptoms
(1,2). In the United States, SS has been
listed as the second most common human autoimmune disease (3). Worldwide, >80% of patients with SS
suffer from oral dryness, diminished taste, angular cheilitis and
oral bacterial infections (4).
Although SS syndrome has been previously investigated, an
understanding of its pathogenesis remains incomplete (5,6). At
present, the treatment of SS syndrome primarily depends on
traditional Chinese medicinal therapy, in which the effects occur
slowly, and its effectiveness and safety require further
investigation (7).
Aquaporins (AQPs) are a family of transmembrane
proteins that selectively allow the passage of water molecules. In
1988, the first member of the AQP family was identified and termed
‘AQP1’ by Denker et al (8).
A total of 10 AQPs with tissue-specific distributions were
identified later, among which AQP5 was the only protein that was
confirmed to be expressed in the salivary gland acinar cells with a
high density in the lacrimal and submandibular glands (9–11).
Previous studies demonstrated that the distribution of AQP5 was
consistent with the sites of SS syndrome, and AQP5 expression in
salivary gland cells increased with stimulation by interferon-α and
increased the level of glandular secretion (12–14).
Motegi et al (15) have demonstrated that
hypomethylation of the CpG island in the AQP5 promoter region
increases AQP5 mRNA expression, thereby increasing AQP5 protein
expression in the human submandibular gland and subsequent
glandular secretion. Reports have indicated that procaine was
investigated at the clinical stage as a candidate for anticancer
treatment due to its specific inhibition of DNA methyltransferase
(DNMT) activity (16,17). This mechanism indicates that
procaine may induce AQP5 expression by inhibiting the activity of
DNMT1. In the present study, human immortalized submandibular gland
ductal cells (NS-SV-DC), lacking AQP5 protein expression, were used
as cell models to investigate the effect of procaine on SS
syndrome.
Materials and methods
Materials and reagents
PcDNA3.1 (+) expression vector and simian virus 40 T
antigen (SV40T) template plasmid (Promega Corporation, Madison, WI,
USA); DH5α competent cells (Takara Bio, Inc., Otsu, Japan); Takar
Ex Taq® enzyme and T4 DNA ligase (Takara Bio, Inc.);
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) primers for GAPDH and AQP5 (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA); mouse monoclonal
immunoglobulin (Ig)G1 antibodies against AQP5, β-actin and DNMT1
(cat. nos. sc-514022, sc-8432 and sc-271729, respectively; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA); TOP10F' competent cells
(Invitrogen; Thermo Fisher Scientific, Inc.).
Plasmid Extraction kit and Agarose Gel
Electrophoresis kit (Tiangen Biotech Co., Ltd., Beijing, China);
Serum-free Keratinocyte Medium (SFKM; Gibco; Thermo Fisher
Scientific, Inc.); MTT solution (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany); TRIzol™ reagent kit and TA cloning
system (Invitrogen; Thermo Fisher Scientific, Inc.); Advantage cDNA
PCR kit (Clontech Laboratories, Inc., Mountainview, CA, USA).
Mem-PER Eukaryotic Membrane Protein Extraction reagent kit (Pierce;
Thermo Fisher Scientific, Inc.); Transwell-COL culture chamber
(Corning Incorporated, Corning, NY, USA); EpiQuik™ DNA
Methyltransferase Activity assay kit (Epigentek Group, Inc.,
Farmingdale, NY, USA); Wizard® DNA Purification kit and
Luciferase Assay System (Promega Corporation); EZ DNA Methylation
kit (Zymo Research Corp., Irvine, CA, USA).
Establishment of immortalized NS-SV-DC or acinar
(NS-SV-AC) phenotype. Primers were designed according to the SV40T
sequence as follows: SV40T-forward, 5′-GGAATTCATGGATAAAGTTTTAAACAGAGGAATCTTTGCA-3′
(EcoR I, underlined sequence); and SV40T-reverse,
5′-CCTCGAGTTATGTTTCAGGTTCAGGGGGAGGTGTGGGAGGTT-3′
(Xho I, underlined sequence).
The reaction conditions of the PCR amplification
were as follows: 5 min of pre-denaturation at 94°C; 30 sec of
denaturation at 94°C, 30 sec of annealing at 58°C and 2.5 min of
extension at 72°C for 30 cycles; and 10 min of extension at 72°C.
The PCR product was analyzed using an agarose gel electrophoresis
kit (cat. nos. DP209-03, Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's instructions.
For the construction and identification of vectors,
the pcDNA3.1 (+) expression vector and the PCR product were
digested at 37°C for 2 h with EcoR I and Xho I
restriction endonucleases. Following ligation with T4 DNA ligase
overnight at 4°C, the ligated product was added to DH5α competent
cells for transformation (Takara Bio, Inc.) according to the
manufacturer's protocols and incubated at 37°C for 3 days. Then,
the plasmid was extracted from DH5α competent cells and
subsequently digested using EcoR I and Xho I
restriction endonucleases. The linearized product obtained by
enzyme digestion was stored at −20°C. Simultaneously, the plasmid
was performed by sequencing identification by Sangon Biotech Co.,
Ltd. (Shanghai, China).
Establishment of cell lines
The characteristics of immortalized NS-SV-DC and
NS-SV-AC cell clones have already been described previously
(18,19). The cryopreserved human
submandibular gland cells from a submandibular gland with no
histopathological disorders were resuscitated and cultured in an
incubator with SFKM medium and 0.5% CO2 at 37°C. When
the cell confluence reached 90%, cells were transfected with the
pcDNA3.1-SV40T plasmid using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols,
then the cells were cultured for 24 h and diluted. Following
culture for about 12 passages, the cells with unaltered phenotype
observed by inverted-phase contrast microscope (Olympus
Corporation, Tokyo, Japan, magnification, ×100) were collected for
the subculture of human NS-SV-AC and NS-SV-DC monoclonal cells, as
previously described (18,19). The submandibular gland tissue was
obtained from a 32-year old healthy female who had given written
informed consent at October 2013 in The Affiliated Hospital of
Hebei University (Baoding, China). The present study was approved
by the Medical Ethics Committee of The Affiliated Hospital of Hebei
University (Baoding, China).
Detection of cell growth
Following 3–4 passages, the NS-SV-DC cells were
seeded into 96-well plates (1×104/well) and incubated
for 12 h at 37°C until adhered to the bottom, and the supernatant
was discarded. A volume of 200 µl SFKM medium containing different
concentrations of procaine (0, 500 nM, and 1 and 2 µM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into the
96-well plates. A total of 20 µl MTT solution (5 mg/ml) was added
to the cells on the 1st, 2rd, 4th and 7th day of incubation. The
cells incubated with MTT solution was continued for 4 h at 37°C and
then formazan crystals were dissolved with 200 µl dimethyl
sulfoxide at 37°C for 10 min. The cell number was calculated by
measuring optical density at 450 nm (Synergy 4; BioTek Instruments,
Inc., Winooski, VT, USA).
Primer design and RT-qPCR
According to the whole gene sequence of AQP5 and
GAPDH (Seq ID nos. K09867 and K00134, respectively) described by
the Kyoto encyclopedia of Genes and Genomes (http://www.kegg.jp/), primers were designed
respectively as follows: AQP5 upstream primer
5′-CAAGGCCGTGTTCGCAGAGTTCT-3′, AQP5 downstream primer
5′-TCTTCCGCTCTTCCCGCTGCTCC-3′, amplified product 739 bp; GAPDH
upstream primer 5′-ACGCATTTGGCTGTATTGGG-3′, GAPDH downstream primer
5′-TGATTTTGGAGGGATCTCGC-3′, amplified product 280 bp.
The NS-SV-DC cells were seeded into 96-well plates
(1×104/well) for 12 h and cultured with SFKM medium
containing procaine (2 µM). Following 0, 48, 72, 96 and 120 h
culture, the cells were collected. The total RNA was extracted by
TRIzol reagent kit, with normal human submandibular gland cells
used as a positive control and untreated NS-SV-AC cells served as a
negative control. cDNA synthesis was performed by Advantage cDNA
PCR kit according to the manufacturer's instructions. The reaction
of RT-qPCR primers (AQP5: Hs00387048 m1; Applied Biosystems; Thermo
Fisher Scientific, Inc.) was performed using the synthesized AQP5
cDNA as template with GAPDH as the internal reference. RT-qPCR
measurements were performed on CFX96 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with a SYBR® Premix Ex Taq kit
(Takara Bio, Inc.). The temperature process was 95°C for 30 sec
followed by 40 cycles of amplification (95°C for 5 sec, 60°C for 30
sec, and 72°C for 30 sec). The results were analyzed using
2−ΔΔCq method (20).
Detection of protein expression by
western blotting
The NS-SV-DC cells treated with procaine (2 µM) at
different times (0, 48, 72, 96 and 120 h) were collected and rough
extraction of cell membranes was performed using Eukaryotic
Membrane Protein Extraction Reagent kit. Following protein
quantification with BCA protein assay reagent kit (Invitrogen;
Thermo Fisher Scientific, Inc.), SDS-PAGE electrophoresis was
performed using a total of 30 µg proteins separated on 12% SDS-PAGE
gels. The protein aggregations were collected, transferred to a
polyvinylidene fluoride membrane (END Millipore, Billerica, MA,
USA), blocked with 3% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) and kept overnight at 4°C. Following blocking, the membranes
were incubated with mouse monoclonal IgG1 AQP5 antibody (dilution:
1:100) for 1 h at 37°C, washed three times with PBS-Tween-20 (PBST,
0.1% Tween) and subsequently incubated with horseradish peroxidase
(HRP)-conjugated antibodies (dilution: 1:1,000; cat. no. sc-516102,
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, Inc.) for 1 h
at 37°C, washed with PBST and detected by an Enhanced
Chemiluminescence assay kit (Thermo Fisher Scientific, Inc.). The
protein expression of DNMTs was analyzed by the same method with
mouse monoclonal IgG1 DNMT1 (1:100), DNMT3A (dilution: 1:100; cat.
no. sc-373905, Santa Cruz Biotechnology, Inc.) and DNMT3B
(dilution: 1:100; cat. no. sc-70984, Santa Cruz Biotechnology,
Inc.) antibodies and corresponding HRP-conjugated antibodies
(dilution: 1:1,000; cat. no. sc-516102). β-actin protein was used
as internal reference and detected as aforementioned with mouse
monoclonal β-actin antibody (dilution: 1:100; cat. no. sc-8432,
Santa Cruz Biotechnology, Inc.) and corresponding HRP-conjugated
antibodies (dilution: 1:1,000; cat. no. sc-516102). Images were
captured using a ChemiDoc™ XRS+ image analyzer (Bio-Rad
Laboratories, Inc.) and quantification of the protein results was
performed using ImageJ 1.47i software (National Institutes of
Health, Bethesda, MD, USA).
Determination of glandular secretion
rate
The glandular secretion rate was determined as
described previously (21).
NS-SV-DC cells were seeded into the upper chamber of the
Transwell-Col culture chamber, which had a diameter of 24.5 mm, and
cells were cultured with 500 or 2 µM procaine. The non-treated
cells were used as controls. Following 48 h, the medium in the
upper chamber was replaced with 0.4 ml hypertonic medium (400 mOsm,
i.e. 100 mM sucrose), while in the lower chamber fresh isotonic
medium (300 mOsm) was added. Following 4 h, the liquid in the upper
chamber was collected and measured by determining the alteration in
volume in the upper chamber to determine the secretion rate with a
calibrated pipette.
Analysis of DNMT activity
DNA was extracted from the NS-SV-DC cells treated
with procaine (500 nM and 2 µM) at different times (0, 6, 12, 24
and 48 h) using a Wizard DNA Isolation kit (cat. no. A1120, Promega
Corporation), and DNA methyltransferase activity was determined
with an EpiQuik™ DNA Methyltransferase Activity Assay
kit.
Analysis of CpG methylation in AQP5
gene
The genomic DNA was extracted from NS-SV-DC cells
treated with procaine for 48 h using Promega's Wizard®
DNA Purification kit. The non-treated cells were used as controls.
The extracted DNA was modified by bisulfite using the EZ DNA
Methylation kit for PCR amplification (upstream primer,
5′-GGGAATTTCGGTTTGGGAGA-3′; downstream primer,
5′-CCCGTCCGAACCACGTAAC-3′). The amplification conditions were as
follows: 1 min pre-denaturation at 94°C; 30 sec denaturation at
94°C and 2 min annealing at 68°C, repeated 35 cycles; and 3 min of
extension at 72°C. The amplified fragment was inserted into the
pCR2.1-TOPO plasmid vector (Invitrogen; Thermo Fisher Scientific,
Inc.) by TA cloning system and transformed into TOP10 F' competent
cells (One Shot™ TOP10F' Chemically Competent E. coli,
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols, followed by plasmid extraction. A total
of six monoclonal strains were selected from the experimental group
and the control group, respectively, for plasmid sequencing by
Sangon Biotech Co. Ltd.
Detection of luciferase activity
The AQP5 promoter fragment (575 bp) containing the
transcription initiation site was amplified according to the
genomic DNA sequence (GenBank no. AH006636, https://www.ncbi.nlm.nih.gov/nuccore/). The
amplification primers were as follows: Upstream primer
5′-CTCGAGAAGGGGAACCCCGGCCGGGAGAG-3′, underlined Xhol site;
downstream primer 5′-AAGCTTGTCCGGGCCACGTGACCCAGG-3′, underlined
HindIII site. The amplified fragment was inserted into the
PGL3 Basic vector (Promega Corporation) containing luciferase
reporter gene to perform transformation, coating, sequencing and
double-enzyme digestion. Subsequently, the human luciferase
reporter plasmid of AQP5 promoter pC3-Luc was successfully
constructed. PLANTCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)
was used to analyze the AQP5 promoter sequence. The AQP5 promoter
fragment contains three Sp1 binding sites: Sp1-1, Sp1-2 and Sp1-3
sites. In order to further investigate the effect of methylation on
the expression of AQP5 protein, methylation of the above three
sites was performed with different methylation modification. All
CGs (Sp1-1: 1st CG; Sp1-2: 23rd and 24th CGs; 31st CG and Sp1-3:
33rd CG) in the pD1-Luc plasmid were methylated; all CGs in pD2-Luc
plasmid were methylated except for the 24th CG; the 1st, 23rd and
31st CGs in pD3-Luc plasmids were methylated; and the 1st, 23rd,
24th and 31st CGs in pD4-Luc plasmids were methylated. The four
constructed plasmids were transfected into untreated NS-SV-DC cells
using Lipofectamine® 2000 (Sigma-Aldrich; Merck KGaA)
according to the manufacturer's protocols. Following incubation for
24 h at 37°C after transfection, the luciferase activity was
detected by Luciferase Assay System. The luciferase activity of
each sample was normalized to the amount of protein in the cell
lysate which was measured with BCA protein assay reagent kit
(Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
GraphPad Prism 7.0 (GraphPad software, Inc., La
Jolla, CA, USA) was used for data analysis and the data were
presented as mean ± standard deviation. Quantitative data between
groups were compared and analyzed using one- and two-way analysis
of variance and Dunnett's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of procaine on cell growth
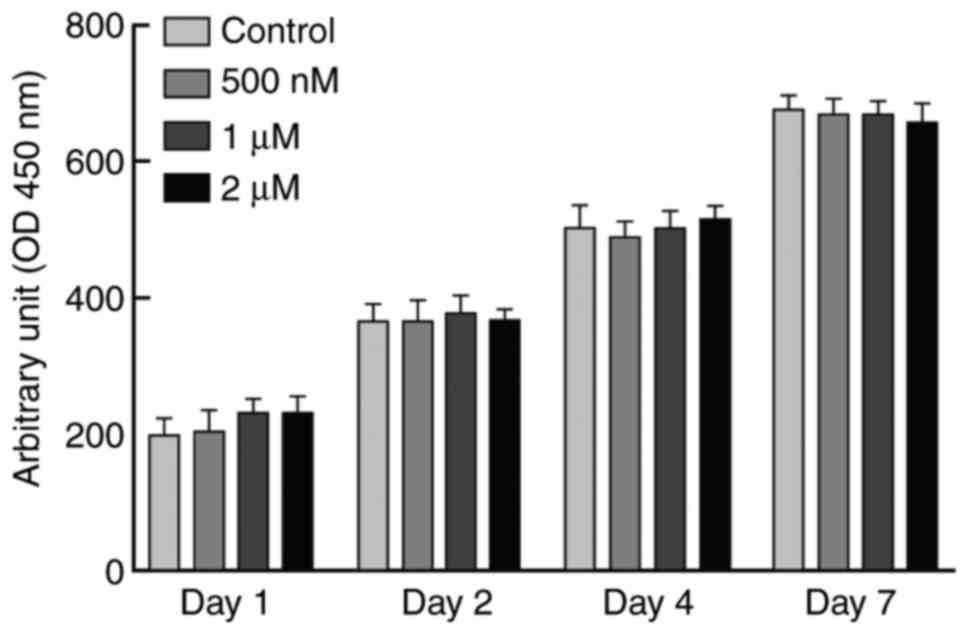
Cell numbers were measured by MTT reagent at 1st,
2rd, 4th and 7th day of incubation to test the effects of different
concentrations of procaine (0, 500 nM, and 1 and 2 µM) on the
growth of NS-SV-DC cells (Fig. 1).
The results demonstrated that 2 µM procaine had no significant
impact on cell growth, which indicated that this concentration of
procaine is nontoxic or of low toxicity to cells (Fig. 1). Therefore, 2 µM procaine was used
for subsequent experiments.
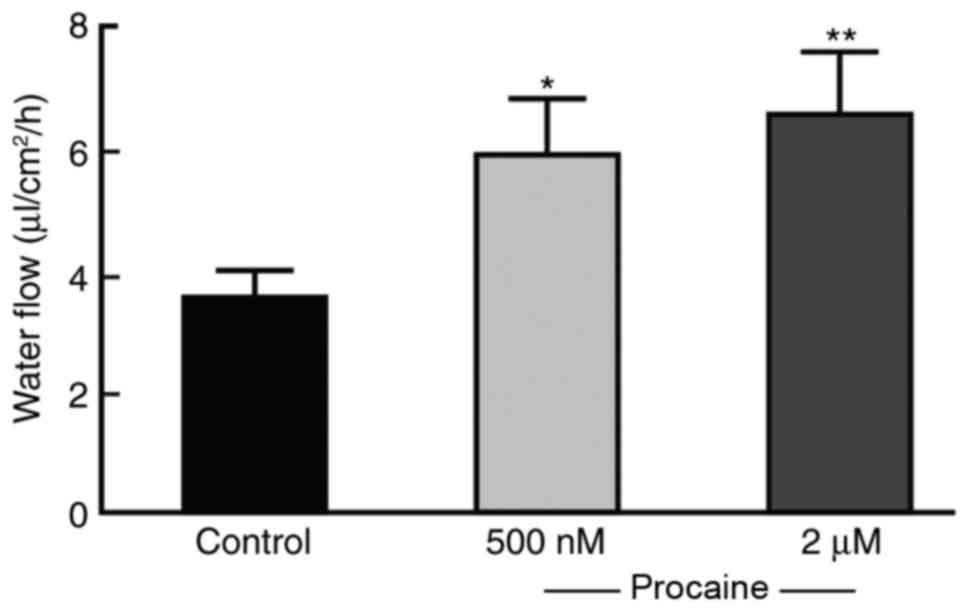
Effect of procaine on fluid secretion
of NS-SV-DC cells
Although procaine is widely used as a clinical drug,
to the best of our knowledge, there have been no previous reports
of procaine use for SS syndrome treatment. Therefore, it was
important to confirm whether procaine may promote fluid secretion
by NS-SV-DC cells. AQP is considered to mediate the passive
transmembrane transport of free water. In the present study,
Transwell culture plates were used to simulate the osmotic pressure
gradient model with the osmotic pressure of the cell culture higher
compared with the lower chamber. Following incubation for 4 h, the
volume of liquid was quantified and the cell capacity of fluid
secretion was calculated by the alteration in volume in the upper
chamber (Fig. 2). The liquid
transport capacity of the untreated NS-SV-DC cells was ~3.8
µl/cm2/h, while the cell transport capacity of NS-SV-DC
cells treated with 500 or 2 µM procaine was 5.87 and 7.0
µl/cm2/h, respectively, which was significantly
increased compared with the control group (Fig. 2), indicating that procaine promoted
fluid secretion by NS-SV-DC cells.
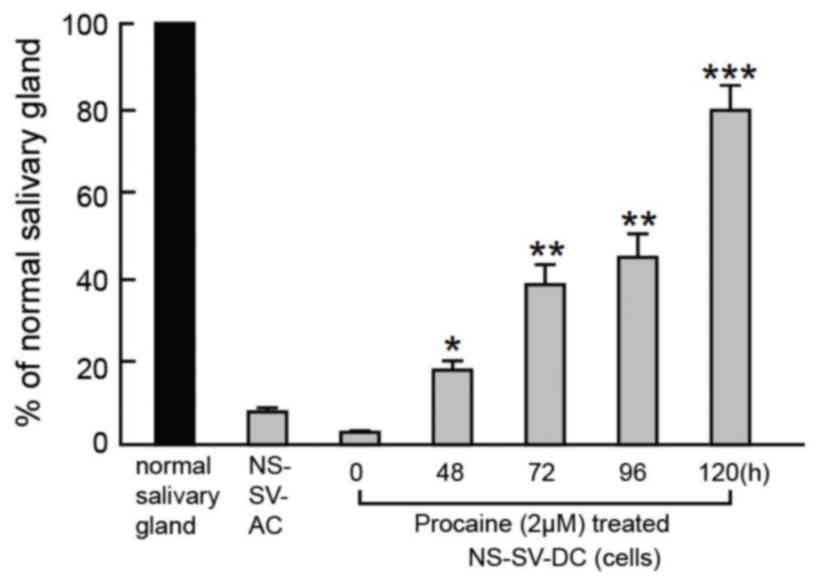
Effect of procaine on AQP5
The mRNA expression of AQP5 in NS-SV-DC cells was
measured by RT-qPCR at 0, 48, 72, 96 and 120 h (Fig. 3). The AQP5 mRNA content of the
untreated NS-SV-DC cells was ~10% of that in normal submandibular
gland cells. The AQP5 mRNA content of NS-SV-DC cells treated with
procaine was significantly increased with the extension of
processing time and the mRNA content was close to that in the
normal submandibular gland cells following 120 h incubation
(Fig. 3).
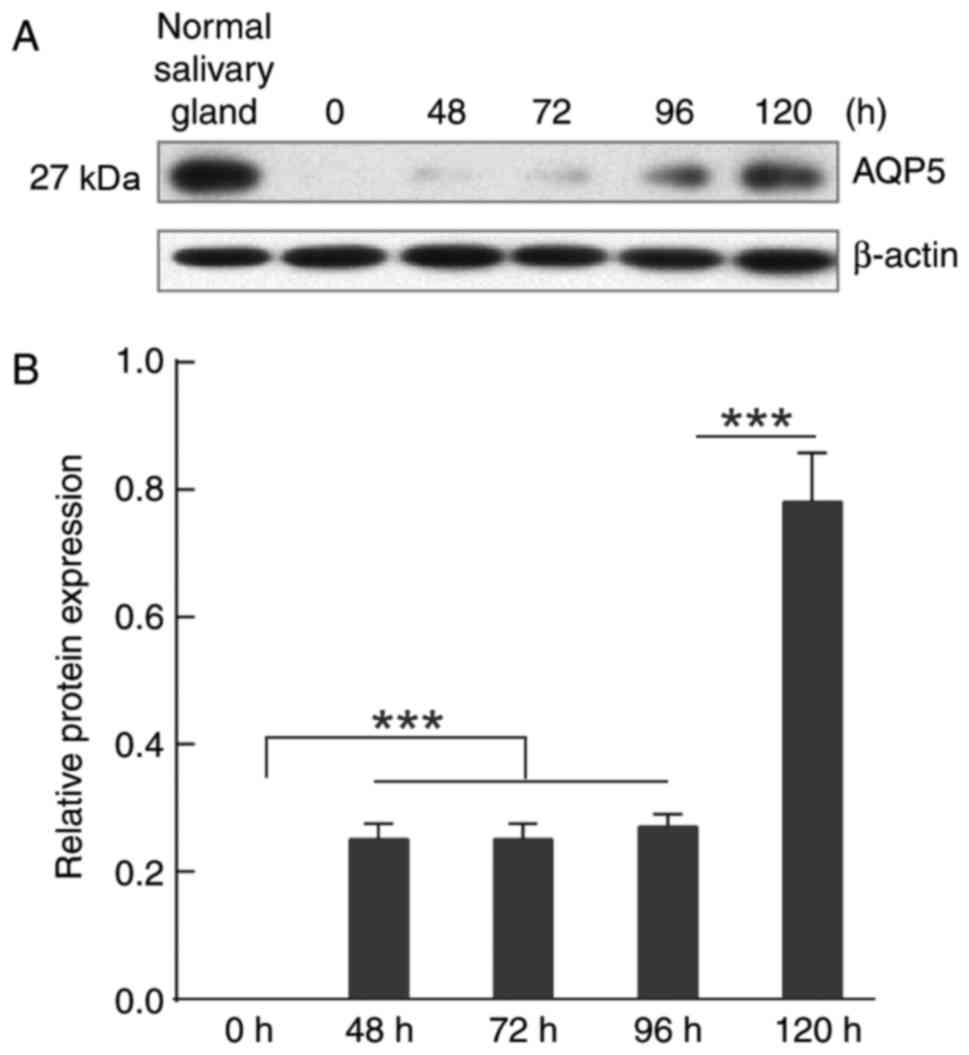
The protein expression of AQP5 was determined by
western blotting (Fig. 4), with
untreated cells at the different time points serving as controls.
Consistent with the alteration of AQP5 mRNA expression, the AQP5
protein expression increased as the processing time increased. The
protein content ratios of AQP5/β-actin at the different time points
(0, 48, 72, 96 and 120 h) were 0, 0.25, 0.25, 0.27 and 0.78,
respectively, which revealed that procaine significantly
upregulated the expression of AQP5 at 120 h compared with other
durations (Fig. 4).
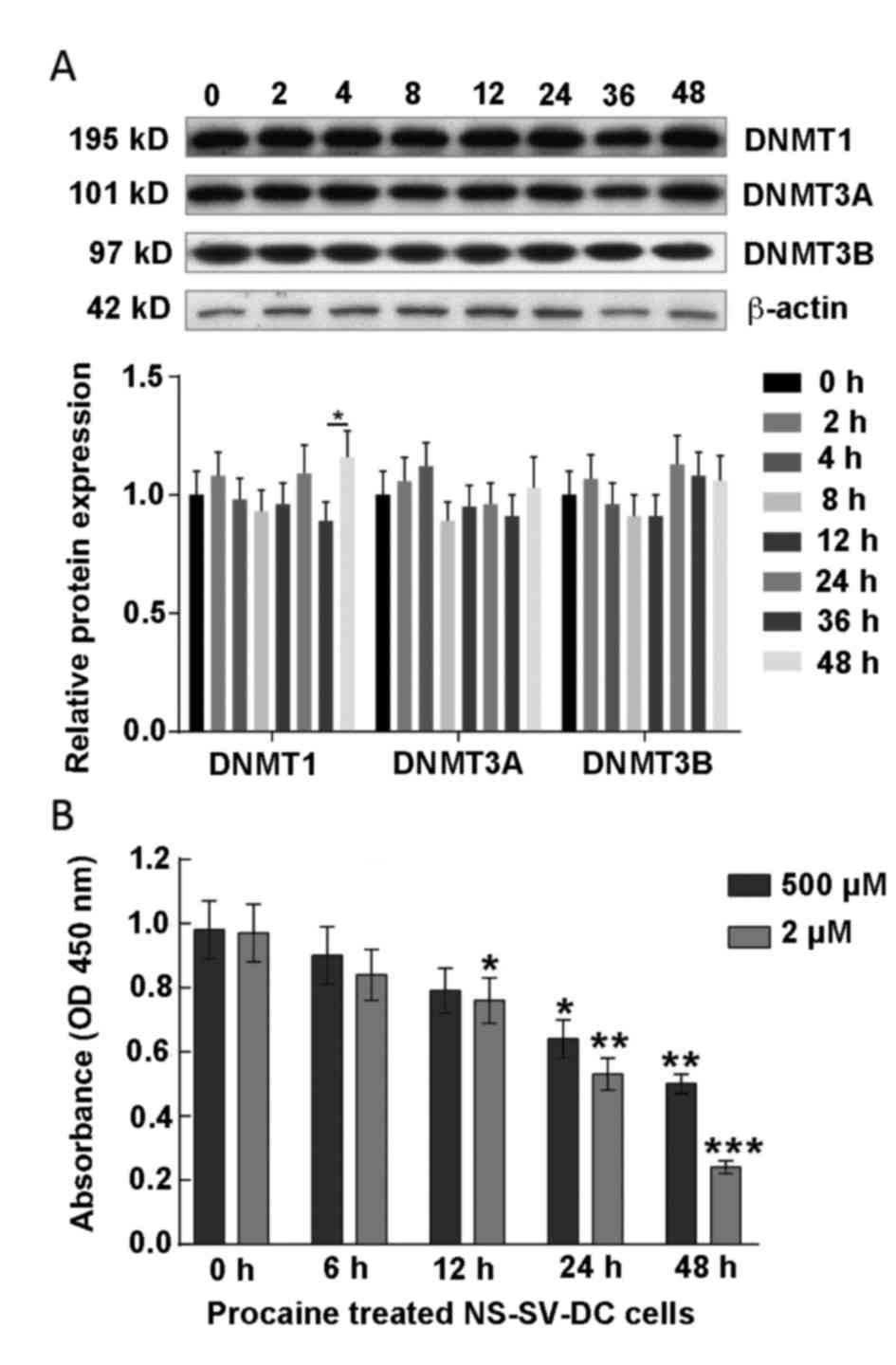
Content and activity of DNMT1 in the
cells treated with procaine
Procaine, which specifically inhibits the activity
of DNMT1, has been investigated up to clinical stage II as a
potential anticancer drug. A study has confirmed that
hypomethylation of the CpG island in the AQP5 promoter region
promoted AQP5 protein expression in NS-SV-DC cells (15). In order to study the effect of
procaine on DNMTs in the present study, the expression of DNMTs
(DNMT1/3A/3B) in NS-SV-DC cells was detected by western blotting,
presented in Fig. 5A. The results
demonstrated that there were no significant alterations in the
expression of DNMTs with increasing time of procaine treatment,
which indicated that procaine did not influence the expression of
DNMTs protein in NS-SV-DC cells.
The effect of procaine on the activity of DNMT1 was
further investigated using an EpiQuik™ DNA
Methyltransferase Activity assay kit, which demonstrated that
procaine significantly reduced the activity of DNMT1
methyltransferase (Fig. 5B).
Effect of procaine on CpG island
demethylation in AQP5 of NS-SV-DC cells
In this experiment, the CpG island methylation in
AQP5 was analyzed by methylation-specific PCR. The extracted DNA
was initially treated with bisulfite to convert unmethylated C to
U, followed by primer design with specific PCR. The target fragment
was obtained and sequenced. Through analysis of the AQP5 promoter
sequence by PLANTCARE, three Sp1 binding sites in CpG island of
AQP5 were identified, Sp1-1, Sp1-2 and Sp1-3, respectively
(Fig. 6A).
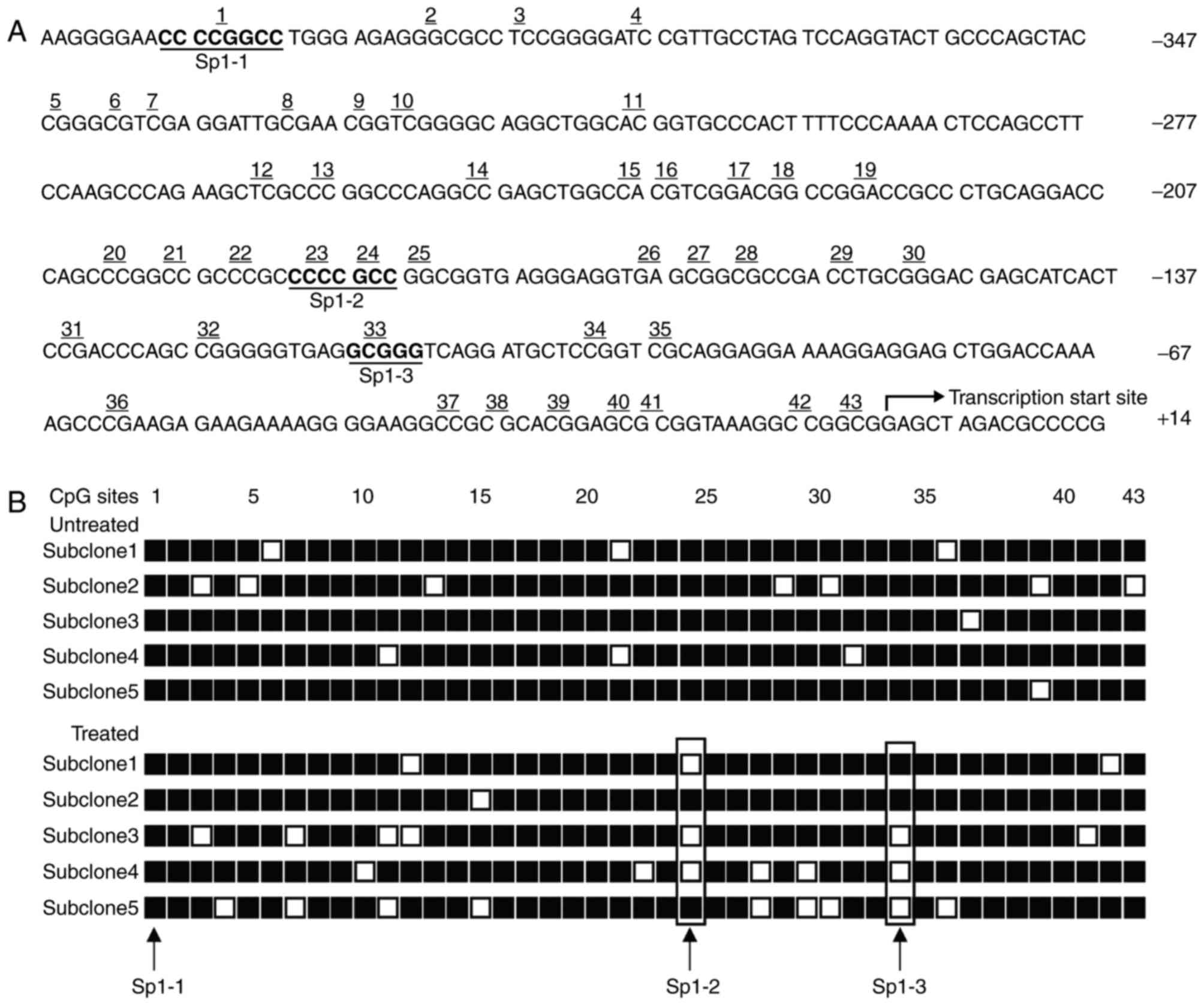 | Figure 6.Bisulfite sequencing of the CpG
islands in the AQP5 promoter. (A) The CpG island of the AQP5
promoter (GenBank/EMBL Data Bank no. U46566) was analyzed. This
sequence spans 578 bp between positions −406 to +172 relative to
the transcription start site, including 43 CGs upstream of the
transcriptional start site. Three CG-containing Sp1-binding sites
within this sequence are indicated as underlined and in bold,
corresponding to the 1st, 23rd, 24th and 33rd CGs within this
island. (B) DNA from the control NS-SV-DC and procaine (2
µM)-treated NS-SV-DC cells was treated with bisulfite, and the AQP5
promoter was PCR amplified. The PCR product was ligated into
pCR2.1-TOPO using the TA cloning system. Five subclones from the
control cells and procaine-treated cells were selected and
sequenced. Demethylation was observed at the CGs in the second Sp1
(Sp1-2) and the third Sp1 (Sp1-3) sites, as indicated by the boxes.
AQP5, aquaporin-5; NS-SV-DC, normal human salivary gland ductal
cells; PCR, polymerase chain reaction; □, unmethylated cytosines;
■, methylated cytosines. |
The analysis of CpG islands in the experiment and
control groups is presented in Fig.
6B. The three Sp1 binding sites in the NS-SV-DC cells not
treated with procaine were highly methylated, while the treated
cells demonstrated marked demethylation at the Sp1-2 and Sp1-3
sites, which demonstrated that hypermethylation of these two sites
may inhibit the expression of the AQP5 gene.
Effect of demethylation at different
sites on the expression of AQP5
In order to determine whether procaine may promote
the expression of AQP5 via CpG island demethylation at Sp1-2 and
Sp1-3 sites, four fluorescent protein reporter plasmids (pD1-Luc,
pD2-Luc, pD3-Luc and pD4-Luc) were constructed (Fig. 7A), and transfected into NS-SV-DC
cells. Following incubation for 24 h, the luciferase content was
measured (Fig. 7B). The results
demonstrated that the luciferase activity in the NS-SV-DC cells
transfected with the pD2-Luc, pD3-Luc and pD4-Luc plasmids was
significantly elevated compared with the empty vector group
(P<0.01, P<0.001; Fig. 7B),
which indicated that procaine may lead to CpG demethylation of the
23rd and 24th and 33rd CG sites in the AQP5 promoter by inhibiting
the activity of DNMT1, subsequently resulting in the upregulation
of AQP5 expression.
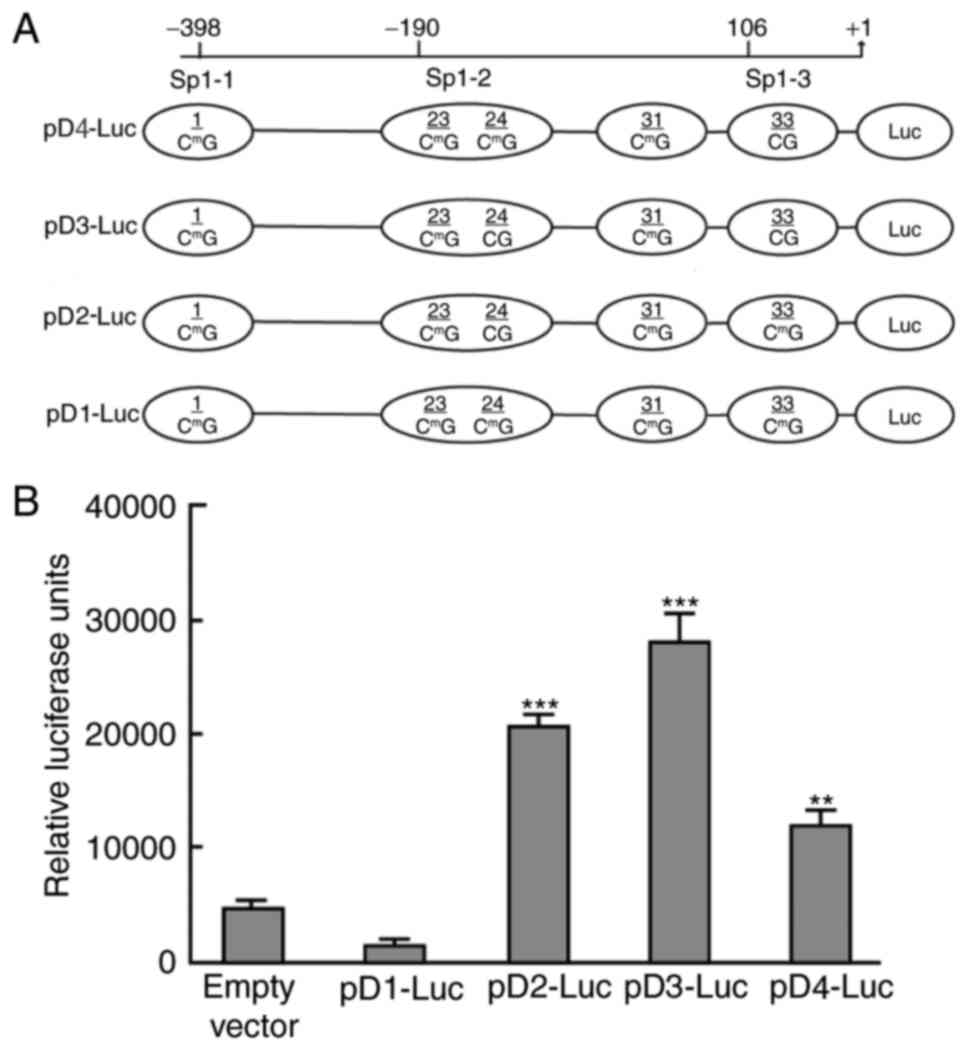 | Figure 7.Analysis of relative luciferase
activity in NS-SV-DC cells. (A) AQP5-promoter constructs used for
the luciferase transfection assay. The human wild-type AQP5
promoter luciferase fusion plasmid, pD1-Luc, contained all
methylated CGs, including the 1st, 23rd, 24th, 31st and 33rd, as
well as the transcription start site. pD2-Luc contains an
unmethylated CG at the 24th position and methylated CGs at the 1st,
23rd, 31st and 33rd positions. pD3-Luc contains unmethylated CGs at
the 24th and 33st positions, and methylated CGs at the 1st, 23rd
and 31rd positions. pD4-Luc contains an unmethylated CG at the 33rd
position and methylated CGs at the 1st, 23rd, 24th and 31st
positions. (B) At 24 h following transfection, NS-SV-DC cells were
harvested for analysis of luciferase activity. The luciferase
activity of each sample was normalized to the amount of protein in
the cell lysate. The experiments were performed at least two times
in triplicate. The luciferase activity of NS-SV-DC cells
transfected with an empty vector served as a control. *P<0.05,
**P<0.01 and ***P<0.001 vs. empty vector. NS-SV-DC, normal
human salivary gland ductal cells; AQP5, aquaporin-5; Luc,
luciferase. |
Discussion
Previous studies have investigated whether AQP5 is
specifically expressed in the salivary gland. It has been
demonstrated that AQP5 was closely associated with salivary
secretion through regulation of AQP5 protein expression, which is
an area of interest in SS syndrome treatment research (22,23).
In the present study, human NS-SV-DC cells lacking
AQP5 gene expression were successfully constructed and it was
confirmed that 500 nM or 2 µM procaine promoted fluid secretion by
these cells. The results of the present study further demonstrated
that procaine upregulated the protein expression of AQP5 in
NS-SV-DC cells, which may promote gland secretion. In human
salivary glands, AQP5 was previously demonstrated to localize to
the apical membranes in acinar cells instead of those in ductal
cells (24). AQP5 stimulates water
to flow into the acinar lumen. Reduced salivary gland secretion was
observed in mice harboring a mutant form of the AQP5 channel
(25). In addition, it has been
reported that AQP3 is localized to the basolateral surface in
acinar cells, where it regulates water movement into those cells
(26). Therefore, it may be
hypothesized that AQP5 and AQP3 may be responsible for the control
of normal fluid outflow in acinar cells. A previous study has
demonstrated that in alveolar epithelial cells (AECs), CpG islands
exist within the promoter region of AQP5. In MLE-12 cells and AECs,
high AQP5 expression and hypomethylation was demonstrated in the
AQP5 promoter. Furthermore, endogenous SP1 was reported to bind to
the hypomethylated Sp1 binding sites in the AQP5 promoter region,
instead of the hypermethylated Sp1 binding sites (27). In the present study, protein
expression of DNMT1 in NS-SV-DC cells was measured, and the results
demonstrated no significant alterations in DNMT1 content between
the experimental and control groups. However, the activity of DNMT1
methyltransferase was reduced following procaine treatment,
indicating that procaine may induce the expression of AQP5 protein
through the demethylation of CpG islands in the AQP5 promoter
region. Previous research has demonstrated that procainamide was a
partial competitive inhibitor of DNMT1 and reduced the affinity of
the enzyme for its two substrates, S-adenosyl-l-methionine and
hemi-methylated DNA (17). In
animal experiments, it was demonstrated that procaine inhibited the
levels of DNMT1 and 5-methylcytosine in the lungs of endotoxemic
animals and simultaneously ameliorated neutrophil infiltration and
the production of hyperoxide (16). In the present study, CpG
bisulfite-sequencing PCR demonstrated that the CpG island
demethylation in AQP5 in the cells treated with procaine was marked
compared with the control group, and that the demethylation sites
were two Sp1 binding sites: Sp1-2 and Sp1-3 sites, which
upregulated the expression of the AQP5 gene when analyzed by a
luciferase reporter assay. Collectively, the results of the current
study demonstrated that procaine may suppress the methylation of
CpG islands in the AQP5 promoter region by inhibiting the activity
of DNMT1 methyltransferase. Hypomethylation of Sp1-2 and Sp1-3
sites in the AQP5 promoter region caused an upregulation of AQP5
expression, which may subsequently lead to the observed increase in
fluid secretion by NS-SV-DC cells following procaine treatment.
Procaine, as an anticancer treatment candidate, has
been widely used in the clinic to inhibit DNMT1 activity. The
present study demonstrated that procaine may promote the secretion
of NS-SV-DC cells by promoting the expression of AQP5, providing
further information concerning its upregulation mechanism and a
potential novel regimen for the treatment of SS syndrome.
Acknowledgements
The authors would like to give their sincere
appreciation to the reviewers for their helpful comments on this
article.
References
|
1
|
Kono M, Aoyagi S, Okazaki T and Tayama K:
Aortic stenosis in a patient with sjogren's syndrome. Int Heart J.
57:251–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kivity S, Arango MT, Ehrenfeld M, Tehori
O, Shoenfeld Y, Anaya JM and Agmon-Levin N: Infection and
autoimmunity in Sjogren's syndrome: A clinical study and
comprehensive review. J Autoimmun. 51:17–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anand A, Krishna GG, Sibley RK and Kambham
N: Sjogren syndrome and cryoglobulinemic glomerulonephritis. Am J
Kidney Dis. 66:532–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng R, Gong B, Cheng F, Fang X, Yang S
and Tang J: The significance of serological markers and European
League Against Rheumatism SS Disease Activity Index score in
patients with primary Sj (o) gren's syndrome. Chin J Rheumatol.
446–452. 2016.(In Chinese).
|
|
5
|
de Paiva CS and Rocha EM: Sjögren
syndrome: What and where are we looking for? Curr Opin Ophthalmol.
26:517–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mavragani C, Nezos A, Sagalovskiy I,
Seshan S, Kirou KA and Crow MK: Defective regulation of L1
endogenous retroelements in primary sjogren's syndrome and systemic
lupus erythematosus: Role of methylating enzymes. J Autoimmun.
2014.(Epub Ahead of Print). PubMed/NCBI
|
|
7
|
Chen S, Ma H, Wang C, Yang Y, Wang H and
Zhang L: Diagnostic value of a modified dynamic salivary gland
scintigraphy for Sj (o) gren's syndrome. Chin J Nuclear Med Mol
Imag. 36:441–444. 2016.(In Chinese).
|
|
8
|
Denker BM, Smith BL, Kuhajda FP and Agre
P: Identification, purification, and partial characterization of a
novel Mr 28,000 integral membrane protein from erythrocytes and
renal tubules. J Biol Chem. 263:15634–15642. 1988.PubMed/NCBI
|
|
9
|
Hosoi K: Physiological role of aquaporin 5
in salivary glands. Pflugers Arch. 468:519–539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vered M, Allon I, Tunis TS, Buchner A and
Dayan D: Expression of the homeostasis-related markers, maspin,
heat shock proteins 70 & 90, glutathione S-transferase,
aquaporin 5 and NF-κB in young and old labial and palatal salivary
glands. Exp Gerontol. 48:444–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong F, Yang J, Li N, Zhao H and Mao Y:
Identification and characterization of PyAQPs from Pyropia
yezoensis, which are involved in tolerance to abiotic stress.
Journal of Applied Phycology. 1–12. 2017.
|
|
12
|
Yamamura Y, Motegi K, Kani K, Takano H,
Momota Y, Aota K, Yamanoi T and Azuma M: TNF-α inhibits aquaporin 5
expression in human salivary gland acinar cells via suppression of
histone H4 acetylation. J Cell Mol Med. 16:1766–1775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gresz V, Horvath A, Gera I, Nielsen S and
Zelles T: Immunolocalization of AQP5 in resting and stimulated
normal labial glands and in Sjögren's syndrome. Oral Dis.
21:e114–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinfeld S, Cogan E, King LS, Agre P,
Kiss R and Delporte C: Abnormal distribution of aquaporin-5 water
channel protein in salivary glands from Sjogren's syndrome
patients. Lab Invest. 81:143–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motegi K, Azuma M, Tamatani T, Ashida Y
and Sato M: Expression of aquaporin-5 in and fluid secretion from
immortalized human salivary gland ductal cells by treatment with
5-aza-2′-deoxycytidine: A possibility for improvement of xerostomia
in patients with Sjogren's syndrome. Lab Invest. 85:342–353. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shih CC, Liao MH, Hsiao TS, Hii HP, Shen
CH, Chen SJ, Ka SM, Chang YL and Wu CC: Procainamide Inhibits DNA
methylation and alleviates multiple organ dysfunction in rats with
endotoxic shock. PLoS One. 11:e01636902016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee BH, Yegnasubramanian S, Lin X and
Nelson WG: Procainamide is a specific inhibitor of DNA
methyltransferase 1. J Biol Chem. 280:40749–40756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azuma M, Tamatani T, Kasai Y and Sato M:
Immortalization of normal human salivary gland cells with duct-,
myoepithelial-, acinar-, or squamous phenotype by transfection with
SV40 ori-mutant deoxyribonucleic acid. Lab Invest. 69:24–42.
1993.PubMed/NCBI
|
|
19
|
Sato M, Kuroda S, Mansjur KQ, Khaliunaa G,
Nagata K, Horiuchi S, Inubushi T, Yamamura Y, Azuma M and Tanaka E:
Low-intensity pulsed ultrasound rescues insufficient salivary
secretion in autoimmune sialadenitis. Arthritis Res Ther.
17:2782015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delporte C, O'Connell BC, He X, Ambudkar
IS, Agre P and Baum BJ: Adenovirus-mediated expression of
aquaporin-5 in epithelial cells. J Biol Chem. 271:22070–22075.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai Z, Yin H, Cabrera-Pérez J, Guimaro MC,
Afione S, Michael DG, Glenton P, Patel A, Swaim WD, Zheng C, et al:
Aquaporin gene therapy corrects Sjogren's syndrome phenotype in
mice. Proc Natl Acad Sci USA. 113:5694–5699. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu GL, Pu XH, Yu GY and Li TY: Effects of
total glucosides of peony on AQP-5 and its mRNA expression in
submandibular glands of NOD mice with Sjogren's syndrome. Eur Rev
Med Pharmacol Sci. 19:173–178. 2015.PubMed/NCBI
|
|
24
|
Agre P, Brown D and Nielsen S: Aquaporin
water channels: Unanswered questions and unresolved controversies.
Curr Opin Cell Biol. 7:472–483. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma T, Song Y, Gillespie A, Carlson EJ,
Epstein CJ and Verkman AS: Defective secretion of saliva in
transgenic mice lacking aquaporin-5 water channels. J Biol Chem.
274:20071–20074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gresz V, Kwon TH, Hurley PT, Varga G,
Zelles T, Nielsen S, Case RM and Steward MC: Identification and
localization of aquaporin water channels in human salivary glands.
Am J Physiol Gastrointest Liver Physiol. 281:G247–G254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nomura J, Hisatsune A, Miyata T and
Isohama Y: The role of CpG methylation in cell type-specific
expression of the aquaporin-5 gene. Biochem Biophys Res Commun.
353:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|