Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic inflammatory disease of the airways. It is characterized by
progressive and incompletely reversible airflow limitation
(1). COPD severely affects the
quality of life of patients and results in an economic burden to
them, their families, healthcare providers and society (2). Worldwide, COPD ranks as the third
most common cause of death and the fifth most common cause of
chronic disability (1,2). Chronic cigarette smoking, exposure to
noxious gases/particles and aging are the most common risk factors
for the occurrence and development of COPD (3). However, in rural areas and in
populations with low socioeconomic status, nutritional status and
use of indoor biofuels are equally important risk factors (3,4).
Inflammation and the inflammatory mediators released
from immune cells accumulate in the lungs of patients with COPD.
This action can contribute to the development of airway remodeling,
which includes thickening of bronchial smooth muscle and deposition
of the extracellular matrix (5,6).
Pulmonary inflammation can lead to airway stenosis, increased
airway resistance and reduced lung function in patients with COPD
(5,6). Furthermore, there is increasing
evidence indicating that chronic inflammation in patients with COPD
is also a systemic inflammatory response (7,8).
Autophagy is the regulated destructive mechanism of
the cell that disassembles unnecessary or dysfunctional components.
This physiological mechanism identified primarily in
polymorphonuclear phagocytes and mononuclear phagocytes, is
increased during chronic inflammation. However, excessive
activation of autophagy has been demonstrated to promote apoptosis
and inflammation (9). Previous
studies indicated that autophagy is involved in the pathogenesis of
COPD (9–11). Using a mouse model of COPD induced
by smoke inhalation, the authors of the present study previously
demonstrated that autophagy was associated with inflammation as
well as the development of emphysema and airway resistance
(12). The authors have also
previously demonstrated that expression of autophagy-associated
proteins increased in a human bronchial epithelial cell line
(Beas-2B) treated with cigarette smoke condensate and that
14,15-epoxyeicosatrienoic can inhibit the inflammatory response
through suppression of autophagy induced by cigarette smoke
(13). Autophagy involves a
pathway of cell metabolism that leads to phagocytic cell engulfing
its own cytoplasmic proteins or organelles which form vesicles that
fuse with lysosomes to create autophagosomes, which can be observed
by electron microscopy (14).
Autophagy can be classified into macro-autophagy, micro-autophagy
and molecular chaperone-mediated autophagy (15).
To the best of our knowledge, no studies have
evaluated autophagy and expression of pro-inflammatory cytokines in
the peripheral blood mononuclear cells (PBMCs) of patients with
COPD. The present study compared the levels of autophagy of PBMCs
in patients with COPD with those of healthy controls and assessed
the association between autophagy and the clinical parameters of
COPD.
Materials and methods
Ethical approval of the study
protocol
The study protocol complied with the Declaration of
Helsinki and Good Clinical Practice guidelines. The study protocol
was approved by the Ethics Committee of Beijing Friendship Hospital
(approval no. 2017-P2-014-01). All individuals who participated in
this study provided written informed consent.
Chemicals and reagents
Ficoll separation medium was purchased from Tianjin
HaoYang Biological Products Technology Co., Ltd. (Tianjin, China).
Radioimmunorecipitation assay (RIPA) lysis buffer was obtained from
Beyotime Institute of Biotechnology (Shanghai, China).
Bicinchoninic acid (BCA) Protein Assay kit, enhanced
chemiluminescent (ECL) substrate and PageRuler™ Prestained Protein
Ladder were purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Nitrocellulose membranes were obtained from Applygen
Technologies, Inc. (Beijing, China). A ChemiDoc™ MP system for
detecting the antibody-antigen reactivity of western blotting was
purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). The
clinical spirometer used purchased from Jaeger; Vyaire Medical Inc.
(Würzburg, Germany). Enzyme-linked immunosorbent assay (ELISA) kits
for human interleukin (IL)-6 (cat. no. EH08-96), IL-8 (cat. no.
EH07-96) and tumor necrosis factor (TNF)-α (cat. no. EH02-96) were
obtained from Beijing Bio-Biotech Co., Ltd. (Beijing, China). The
primary antibodies against microtubule-associated proteins 1A/1B
light chain 3A (LC3I/II; cat. no. ab62721; 1:1,000),
ubiquitin-binding protein p62 (p62; cat. no. ab56416; 1:1,000) and
beclin-1 (cat. no. ab207612, 1:2,000) were purchased from Abcam
(Cambridge, MA, USA). GAPDH (cat. no. 60004-1-lg; 1:10,000) was
obtained from ProteinTech Group, Inc. (Chicago, IL, USA).
Peroxidase-conjugated goat anti-mouse (cat. no. ZB-2305; 1:5,000)
and goat anti-rabbit (cat. no. ZB-2301; 1:5,000) secondary
antibodies were purchased from OriGene Technologies, Inc. (Beijing,
China).
Recruitment of patients and healthy
controls
A control group of healthy people (n=20; male) and
patients with stable COPD (n=20; male) were recruited at the
Beijing Friendship Hospital, Capital Medical University (Beijing,
China) between February and July 2017.
Inclusion criteria for the control group were
healthy adults without chronic respiratory disease, cancer,
hematologic disease, hypertension, arrhythmia, ischemic heart
disease or diabetes mellitus. Patients with COPD were included if
their disease was stable. Exclusion criteria were diagnosis of COPD
with other chronic respiratory diseases, including asthma,
bronchiectasis, interstitial lung disease. The study population was
free from ischemic heart disease, kidney disease, cancer or
autoimmune disease. Lung-function tests were undertaken by a single
spirometry technician experienced in carrying out such tests. The
diagnostic criteria for stable COPD were in accordance with current
recommendations set by the Global Initiative for Chronic
Obstructive Lung Disease (16).
Isolation of PBMCs
Ethylenediamine tetraacetic acid Vacutainer™ tubes
(BD Biosciences, Franklin Lakes, NJ, USA) were used to collect
venous blood samples (10 ml) from fasting participants in the early
morning. Aliquots of the supernatant were collected by
centrifugation at 800 × g and 25°C for 15 min and subsequently
stored at −80°C. The remaining blood was mixed and added slowly
dropwise to a centrifuge tube containing 10 ml of Ficoll separation
medium. PBMCs were isolated according to manufacturer's protocol
and stored at −80°C.
Western blotting
PBMCs were lysed with RIPA lysis buffer and protein
concentrations determined with the BCA Protein Assay kit according
to the manufacturer's protocol. Subsequently, 20 µg of protein/well
were separated using 12% SDS-PAGE. The proteins were subsequently
transferred to nitrocellulose membranes at 300 mA for 60 min. The
nitrocellulose membranes were blocked with Tris-buffered saline
containing 0.1% Tween 20 (TBST) and 5% non-fat milk powder for 2 h
at room temperature. The nitrocellulose membranes were incubated
with primary antibodies overnight at 4°C on a shaker set at a slow
speed. The nitrocellulose membranes were washed thrice with TBST
and incubated with secondary antibodies for 1 h at room
temperature. After washing thrice, ECL substrate was added to the
nitrocellulose membrane. The signal was detected using Image Lab™
software (version 5.1; Bio-Rad Laboratories, Inc.). Band density
was quantified with ImageJ software (version 1.51; National
Institutes of Health, Bethesda, MD, USA).
ELISA
Serum levels of IL-6, IL-8 and TNF-α were measured
by ELISA according to manufacturer's protocol. All samples were
measured in duplicate.
Statistical analyses
All statistical analyses were carried out using
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Normally
distributed data are presented as the mean ± standard deviation.
The statistical difference of the levels of autophagy and
pro-inflammatory cytokines between patients with COPD and healthy
controls was performed using the independent sample Student's
t-test. Correlation between the levels of autophagy, serum levels
of pro-inflammatory cytokines and pulmonary function was determined
using Pearson's or Spearman's tests, for normally and not normally
distributed data, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of the study
cohort
The study cohort was divided into 20 healthy
controls and 20 patients with COPD. Clinical characteristics of
this cohort, including age, body mass index, blood tests and lung
function parameters are summarized in Table I. Neutrophil counts and lung
function were significantly different between patients with COPD
and healthy subjects.
 | Table I.Clinical characteristics of the study
cohort. |
Table I.
Clinical characteristics of the study
cohort.
| Characteristic | Controls
(n=20) | Patients with COPD
(n=20) | P-value |
|---|
| Age (years) | 67.05±8.96 | 62.25±5.30 | 0.076 |
| Height (cm) | 169.05±5.86 | 168.5±5.38 | 0.576 |
| Weight (kg) | 70.0±6.59 | 68.4±9.76 | 0.307 |
| BMI
(kg/m2) | 24.49±1.87 | 24.04±2.69 | 0.467 |
| WBC
(×109/l) | 6.88±1.86 | 7.08±1.76 | 0.739 |
| NEU (%) | 61.62±9.20 | 68.54±8.09 | 0.018 |
| FEV1
(%) | 98.02±3.71 | 43.41±14.51 | <0.01 |
| FEV1/FVC
(%) | 88.05±5.19 | 59.98±8.40 | <0.01 |
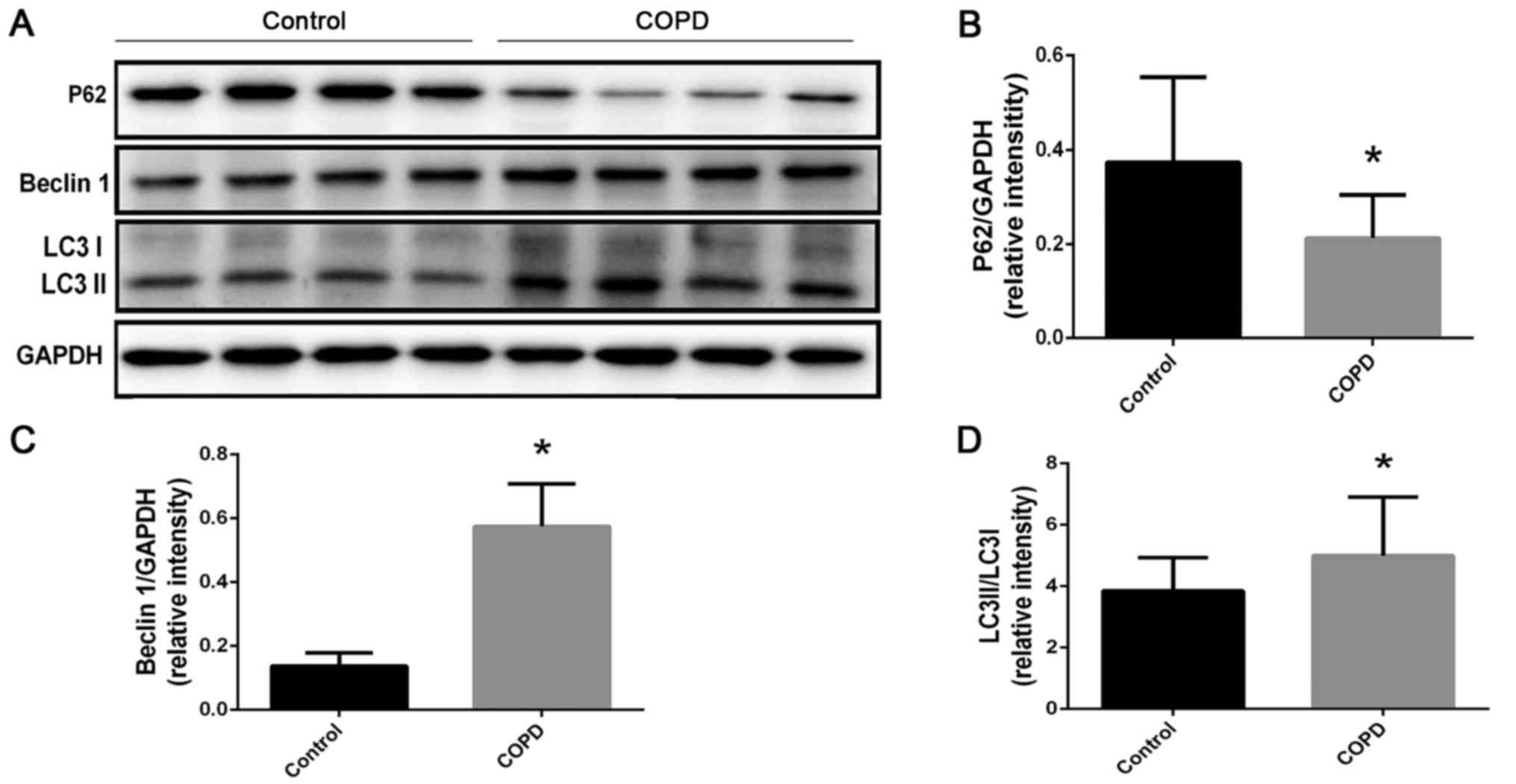
Autophagy levels of PBMCs were
increased in patients with COPD
Western blotting (Fig.
1) demonstrated that expression of the autophagy-associated
protein p62 was reduced compared with the control group (Fig. 1B; P=0.0011). Furthermore, beclin-1
expression (Fig. 1C; P<0.0001)
and LC3II/I ratio (Fig. 1D;
P=0.0249) were increased compared with the control group.
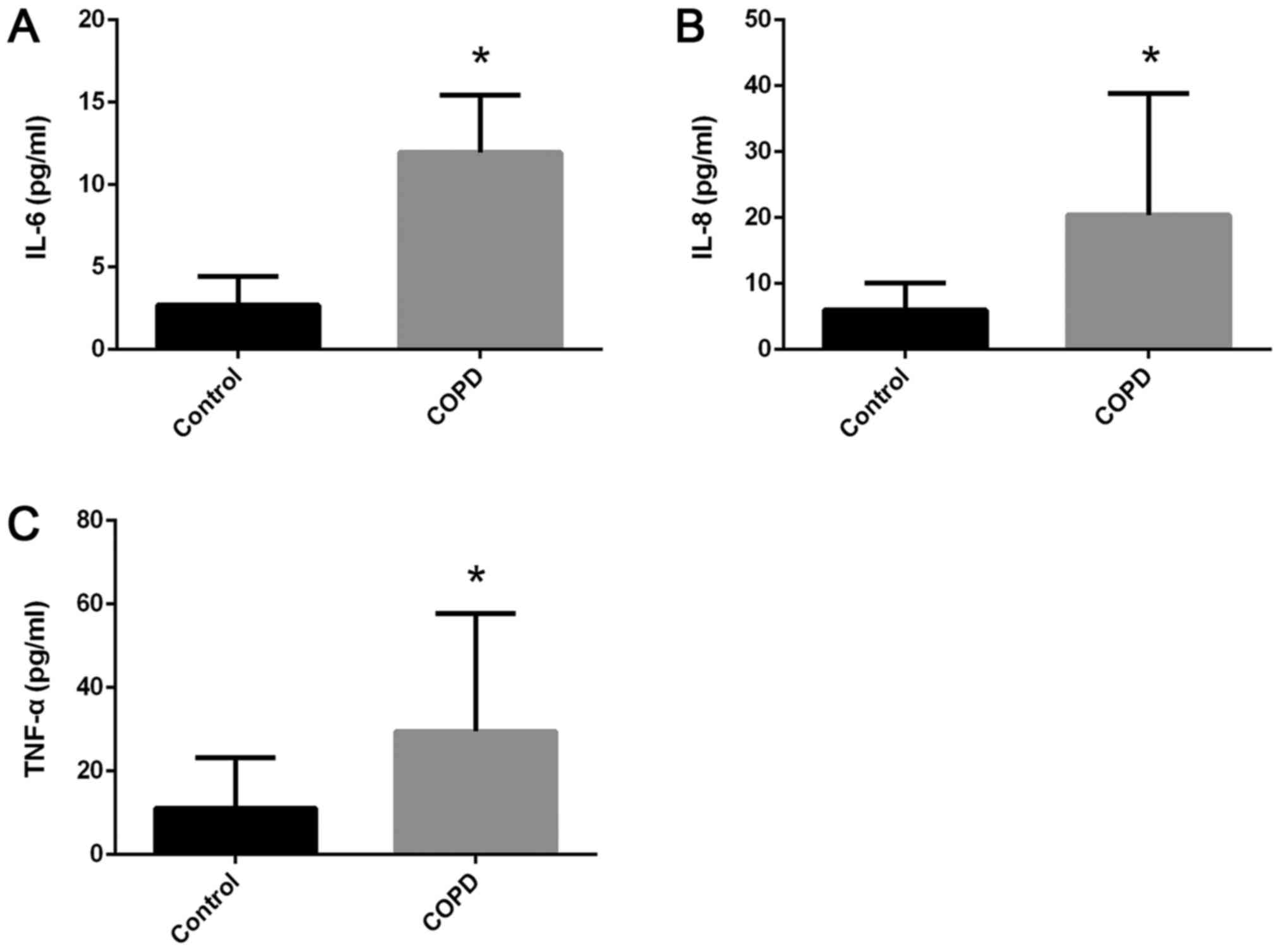
Cytokine levels were increased in
patients with COPD
ELISA results (Fig.
2) exhibited significantly increased serum levels (in pg/ml) of
IL-6 (11.96±3.46 vs. 2.70±1.72; P<0.0001; Fig. 2A), IL-8 (20.38±18.44 vs. 6.0±4.08;
P=0.0016; Fig. 2B), and TNF-α
(29.5±28.18 vs. 11.08±12.07; P=0.0106; Fig. 2C) in patients with COPD compared
with the control group.
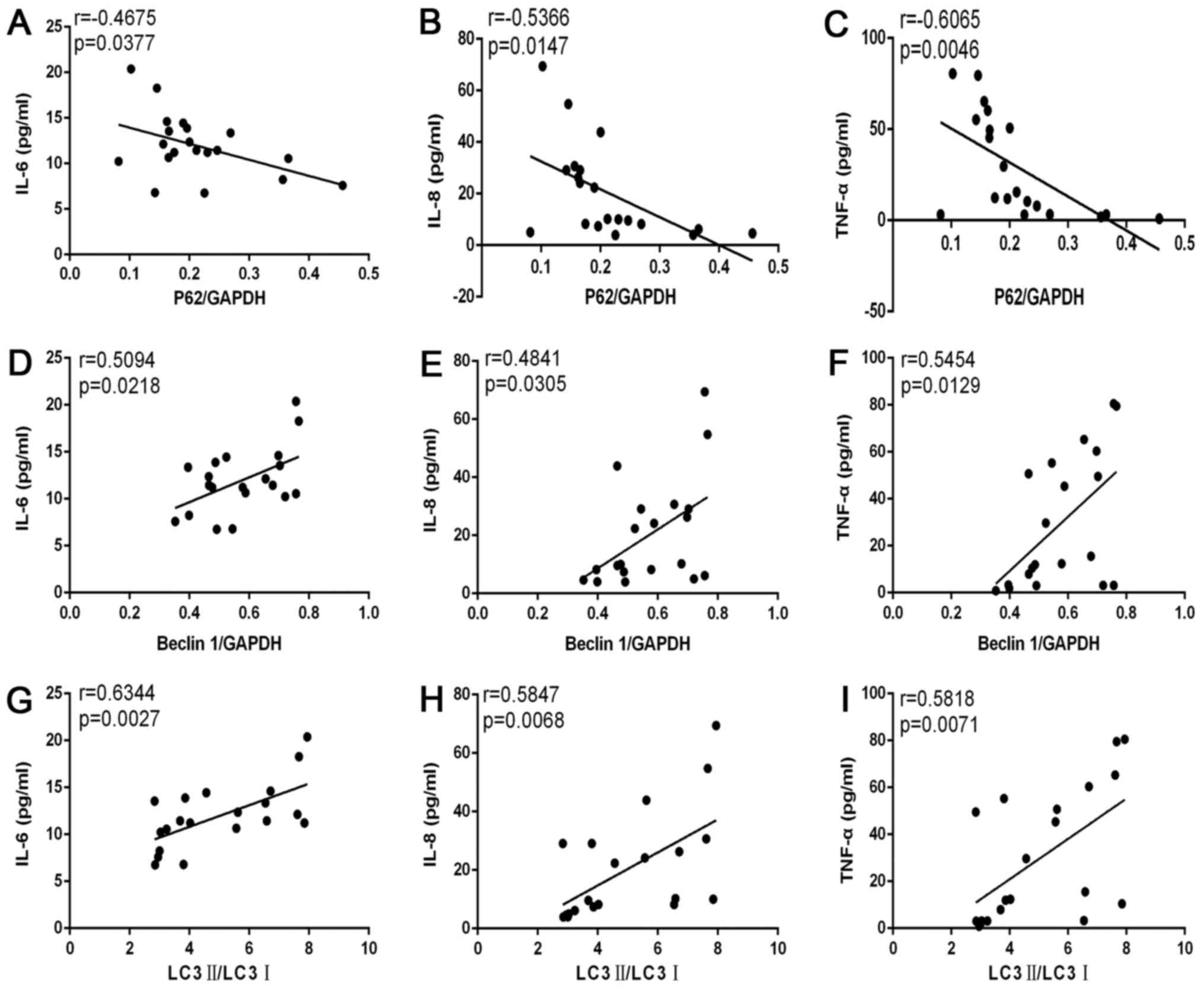
Levels of pro-inflammatory cytokines
were associated with autophagy levels in patients with COPD
Correlation analyses (Fig. 3) demonstrated that levels of IL-6
(r=−0.4675; P=0.0377; Fig. 3A),
IL-8 (r=−0.5366; P=0.0147; Fig.
3B) and TNF-α (r=−0.6065; P=0.0046; Fig. 3C) were negatively correlated with
p62/GAPDH levels. Levels of IL-6 (r=0.5094; P=0.0218; Fig. 3D), IL-8 (r=0.4841; P=0.0305;
Fig. 3E) and TNF-α (r=0.5454;
P=0.0129; Fig. 3F) were positively
correlated with beclin-1/GAPDH levels. Levels of IL-6 (r=0.6344;
P=0.0027; Fig. 3G), IL-8
(r=0.5847; P=0.0068; Fig. 3H) and
TNF-α (r=0.5818; P=0.0071; Fig.
3I) were positively correlated with LC3II/I levels.
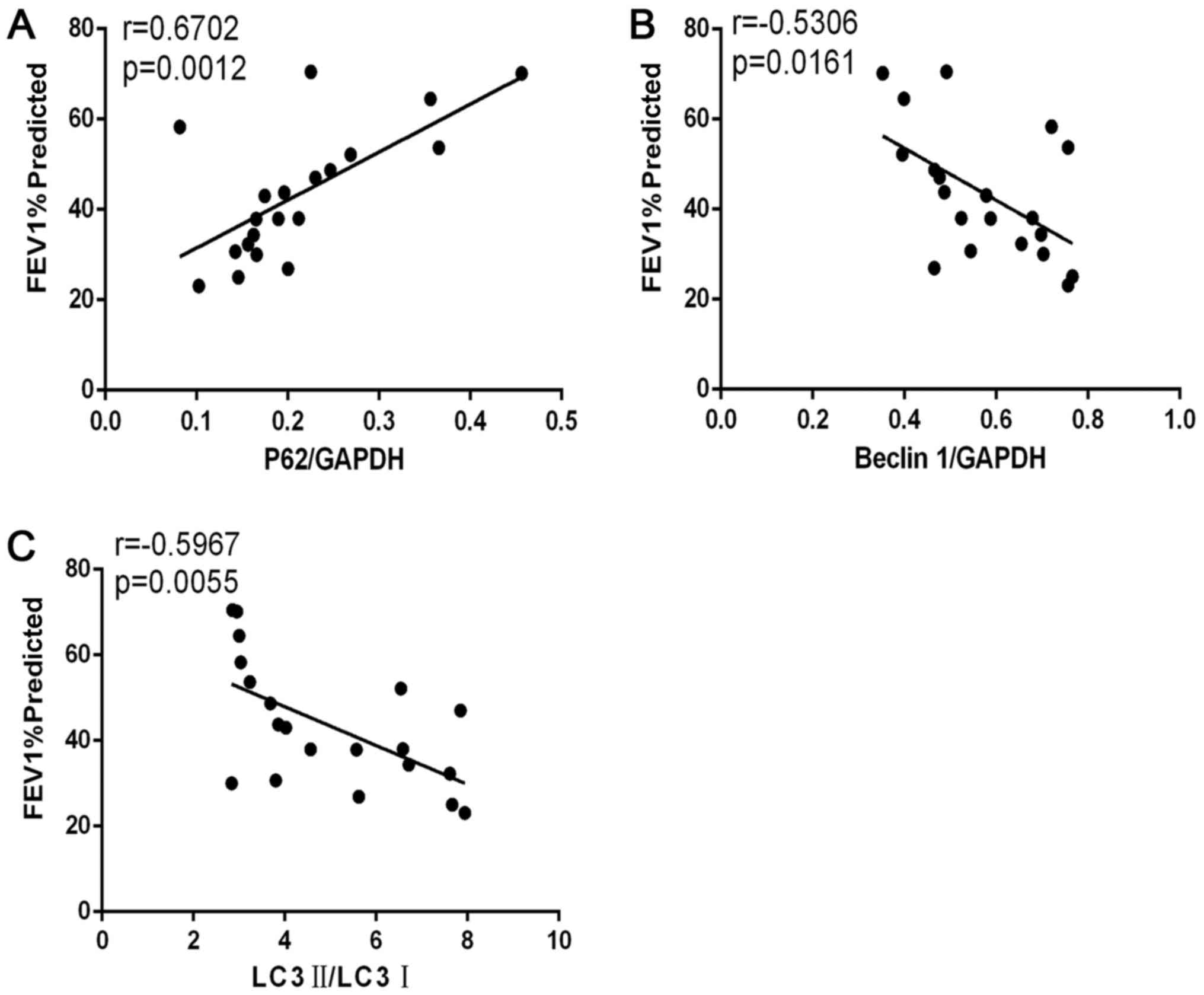
COPD severity according to lung
function was correlated with autophagy
Correlation analyses (Fig. 4) demonstrated that forced
expiratory volume in 1 sec % predicted (FEV1% predicted)
was positively correlated with p62/GAPDH levels in patients with
COPD (r=0.6702; P=0.0012; Fig.
4A). FEV1% predicted was negatively correlated with
beclin-1/GAPDH levels (r=−0.5306; P=0.0161; Fig. 4B) and with LC3II/I levels
(r=−0.5967, P=0.0055; Fig.
4C).
Discussion
COPD is a public health problem. However, at
present, the pathogenesis and therapeutic targets of COPD are not
completely elucidated. Autophagy is common in eukaryotic cells.
Increasingly sophisticated research on the role of autophagy has
revealed that autophagy and COPD are closely associated (13,17–20).
To the best of the authors' knowledge, the present
study is the first to demonstrate that levels of autophagy in PBMCs
in patients with COPD were increased compared with normal controls
and that autophagy levels were negatively correlated with
FEV1% predicted, but positively correlated with
circulating levels of pro-inflammatory cytokines.
Exposure to cigarette smoke is the primary cause of
COPD (13). Furlong et al
(17) demonstrated that exposure
to cigarette smoke can trigger an autophagic cascade by activating
the adenosine monophosphate-activated protein kinase pathway in
mouse ovaries. Gannon et al (18) demonstrated that, in murine
granulosa cells induced by cigarette-smoke exposure, the levels of
autophagy increased and mitochondrial dynamics were impaired. Chen
et al (19) demonstrated
that autophagy is involved in inflammation and that excess mucus
production is induced by ultrafine particles in the airway
epithelium. Zhou et al (20) demonstrated that autophagy serves an
essential part in bronchial mucus production induced by cigarette
smoke by regulating MUC5AC expression. The studies of
Hussain and Sandri (21) and Plant
et al (22) demonstrated
that autophagy can affect the bronchial-muscle function of patients
with COPD. The pathophysiological mechanisms involved in COPD,
including oxidative stress and inflammation, are associated with
autophagy (14,23–25).
Therefore, autophagy serves a role in the pathogenesis of COPD. The
results of our study support the current view that autophagy is
involved in COPD.
In the present study, levels of LC3II/I were
evaluated since in the process of autophagy, the characteristic
microtubule-associated protein light chain LC3-I is converted to
LC3-II and, therefore, LC3II/I levels are expected to increase
(26,27). Beclin-1 is an autophagy-mediated
protein and its expression is known to increase during autophagy
(28). p62 is an intracellular and
multifunctional protein that contains a variety of protein domains
and serves a role in signal transduction and pathogenesis of
multiple diseases, including COPD (29–32).
As a link between LC3 and polyubiquitinated proteins, p62 can
transport damaged organelles or proteins into autophagosomes
through the ubiquitin signaling pathway. Eventually, p62 and its
substrates are degraded together in autophagosomes. Therefore, p62
levels are negatively correlated with autophagy levels, which can
be used as a marker of autophagy (33). In the present study, p62 levels
were reduced and those of LC3II/I and beclin-1 increased, in
patients with COPD, compared with healthy controls.
The present study demonstrated that levels of the
pro-inflammatory cytokines IL-6, IL-8 and TNF-α in the serum of
patients with COPD were increased and were correlated with
autophagy levels. The pathophysiological processes of inflammation,
cytokine production and autophagy are involved in airway remodeling
in COPD (34). One study
demonstrated that the detection of IL-6 levels could be used to
predict the frequency of future COPD exacerbations (35). The aforementioned study supports
the view that inhibition of inflammation induced by inhibition of
the production of pro-inflammatory mediators may prevent/limit the
pulmonary parenchymal, vascular and airway alterations that result
in clinical manifestations of COPD (35,36).
Studies have demonstrated that autophagy may serve
different roles in different diseases, including metabolic disease,
neurodegenerative disease, infections and immunologic disease
(37–39). Vij et al (40) demonstrated that cigarette
smoke-induced impairment of autophagy accelerates lung aging. Li
et al (41) reported that
silymarin, similar to our previous study focusing on
14,15-epoxyeicosatrienoic acid (13), attenuates cigarette smoke
extract-induced inflammation via inhibition of autophagy. The above
studies, together with the present study indicating that autophagy
levels of PBMCs in patients with COPD were positively correlated
with circulating levels of inflammatory cytokines, suggest that
autophagy may regulate the inflammatory response in COPD through
different pathways.
The present study also demonstrated that the
FEV1% predicted was negatively correlated with levels of
autophagy in PBMCs of patients with COPD. This result raises the
possibility that autophagy may be used as a biomarker to evaluate
severity of COPD. There is an association between autophagy,
inflammation and lung function, and a high level of autophagy can
activate the inflammatory response, and chronic inflammation can
lead to airway remodeling and accelerate reductions in lung
function. By contrast, long periods of low levels of oxygen and
hypercapnia due to airway obstruction can aggravate inflammation.
The above phenomena can influence each other (42,43).
The present study had three main limitations. The
study cohort was entirely male and the medication history of
patients was not studied in detail. Furthermore, according to the
FEV1% predicted value, the study cohort contained only
six patients with moderate airflow limitation and no patients with
mild airflow limitation; the remaining patients had severe and very
severe airflow limitation.
In conclusion, the present study demonstrated that
autophagy levels in PBMCs in patients with COPD were increased and
were correlated with FEV1% predicted values and
circulating levels of pro-inflammatory cytokines. Autophagy may
serve a role as a biomarker of severity or as a therapeutic target
for treatment of COPD.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Basic-Clinical Cooperation Program of Capital Medical University
(grant no. 17JL49), National Natural Science Foundation of China
(grant no. 81700038), Beijing Municipal Natural Science Foundation
(grant nos. 7142046 and 7164250) and the Capital Health Research
and Development of Special (grant no. 2018-2-2024).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT and HW conceived and designed the study. GY and
CT provided administrative support. BW and YL provided the study
materials and/or subjects. YW and BX collected and assembled the
data. XH, BW, YL and BX provided technical support, analyzed and
interpreted the data. YW and GY were involved in drafting the
manuscript. CT and HW critically revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol complied with the Declaration of
Helsinki and Good Clinical Practice guidelines. The study protocol
was approved by the Ethics Committee of Beijing Friendship Hospital
(2017-P2-014-01). All individuals who participated in this study
provided written informed consent.
Consent for publication
Written informed consent was obtained from all
individuals who participated in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnes PJ: Senescence in COPD and its
comorbidities. Annu Rev Physiol. 79:517–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chun P: Role of sirtuins in chronic
obstructive pulmonary disease. Arch Pharm Res. 38:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burney P, Kato B, Janson C, Mannino D,
Studnicka M, Tan W, Bateman E, Koçabas A, Vollmer WM, Gislason T,
et al: Chronic obstructive pulmonary disease mortality and
prevalence: The associations with smoking and poverty: A BOLD
analysis-authors' reply. Thorax. 69:869–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrusca DN, Van Demark M, Gu Y, Justice
MJ, Rogozea A, Hubbard WC and Petrache I: Smoking exposure induces
human lung endothelial cell adaptation to apoptotic stress. Am J
Respir Cell Mol Biol. 50:513–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun J, Bao J, Shi Y, Zhang B, Yuan L, Li
J, Zhang L, Sun M, Zhang L and Sun W: Effect of simvastatin on MMPs
and TIMPs in cigarette smoke-induced rat COPD model. Int J Chron
Obstruct Pulmon Dis. 12:717–724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou Y, Chen X, Liu J, Zhou DB, Kuang X,
Xiao J, Yu Q, Lu X, Li W, Xie B and Chen Q: Serum IL-1β and IL-17
levels in patients with COPD: Associations with clinical
parameters. Int J Chron Obstruct Pulmon Dis. 12:1247–1254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gea J, Pascual S, Casadevall C,
Orozco-Levi M and Barreiro E: Muscle dysfunction in chronic
obstructive pulmonary disease: Update on causes and biological
findings. J Thorac Dis. 7:E418–E438. 2015.PubMed/NCBI
|
|
8
|
Kawayama T, Kinoshita T, Matsunaga K,
Kobayashi A, Hayamizu T, Johnson M and Hoshino T: Responsiveness of
blood and sputum inflammatory cells in Japanese COPD patients,
non-COPD smoking controls, and non-COPD nonsmoking controls. Int J
Chron Obstruct Pulmon Dis. 11:295–303. 2016.PubMed/NCBI
|
|
9
|
Ryter SW, Lam HC, Chen ZH and Choi AM:
Deadly triplex: Smoke, autophagy and apoptosis. Autophagy.
7:436–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryter SW, Lee SJ and Choi AM: Autophagy in
cigarette smoke-induced chronic obstructive pulmonary disease.
Expert Rev Respir Med. 4:573–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryter SW, Chen ZH, Kim HP and Choi AM:
Autophagy in chronic obstructive pulmonary disease: Homeostatic or
pathogenic mechanism. Autophagy. 5:235–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Yu G, Yuan S, Tan C, Lian P, Fu L,
Hou Q, Xu B and Wang H: Cigarette smoke-induced pulmonary
inflammation and autophagy are attenuated in Ephx2-deficient mice.
Inflammation. 40:497–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Yu G, Yuan S, Tan C, Xie J, Ding Y,
Lian P, Fu L, Hou Q, Xu B and Wang H: 14,15-Epoxyeicosatrienoic
acid suppresses cigarette smoke condensate-induced inflammation in
lung epithelial cells by inhibiting autophagy. Am J Physiol Lung
Cell Mol Physiol. 311:L970–L980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pei J, Deng J, Ye Z, Wang J, Gou H, Liu W,
Zhao M, Liao M, Yi L and Chen J: Absence of autophagy promotes
apoptosis by modulating the ROS-dependent RLR signaling pathway in
classical swine fever virus-infected cells. Autophagy.
12:1738–1758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Azad MB and Gibson SB: Methods for
detecting autophagy and determining autophagy-induced cell death.
Can J Physiol Pharmacol. 88:285–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furlong HC, Stämpfli MR, Gannon AM and
Foster WG: Cigarette smoke exposure triggers the autophagic cascade
via activation of the AMPK pathway in mice. Biol Reprod. 93:932015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gannon AM, Stämpfli MR and Foster WG:
Cigarette smoke exposure elicits increased autophagy and
dysregulation of mitochondrial dynamics in murine granulosa cells.
Biol Reprod. 88:632013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ZH, Wu YF, Wang PL, Wu YP, Li ZY,
Zhao Y, Zhou JS, Zhu C, Cao C, Mao YY, et al: Autophagy is
essential for ultrafine particle-induced inflammation and mucus
hyperproduction in airway epithelium. Autophagy. 12:297–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou JS, Zhao Y, Zhou HB, Wang Y, Wu YF,
Li ZY, Xuan NX, Zhang C, Hua W, Ying SM, et al: Autophagy plays an
essential role in cigarette smoke-induced expression of MUC5AC in
airway epithelium. Am J Physiol Lung Cell Mol Physiol.
310:L1042–L1052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussain SN and Sandri M: Role of autophagy
in COPD skeletal muscle dysfunction. J Appl Physiol (1985).
114:1273–1281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plant PJ, Brooks D, Faughnan M, Bayley T,
Bain J, Singer L, Correa J, Pearce D, Binnie M and Batt J: Cellular
markers of muscle atrophy in chronic obstructive pulmonary disease.
Am J Respir Cell Mol Biol. 42:461–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bodas M and Vij N: Augmenting autophagy
for prognosis based intervention of COPD-pathophysiology. Respir
Res. 18:832017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh K, Matsuyama S, Drazba JA and
Almasan A: Autophagy-dependent senescence in response to DNA damage
and chronic apoptotic stress. Autophagy. 8:236–251. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bodas M, Patel N, Silverberg D, Walworth K
and Vij N: Master autophagy regulator transcription factor EB
regulates cigarette smoke-induced autophagy impairment and chronic
obstructive pulmonary disease-emphysema pathogenesis. Antioxid
Redox Signal. 27:150–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee
SJ, Ifedigbo E, Parameswaran H, Ryter SW and Choi AM: Autophagy
protein microtubule-associated protein 1 light chain-3B (LC3B)
activates extrinsic apoptosis during cigarette smoke-induced
emphysema. Proc Natl Acad Sci USA. 107:18880–18885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsukahara T, Matsuda Y, Usui Y and Haniu
H: Highly purified, multi-wall carbon nanotubes induce light-chain
3B expression in human lung cells. Biochem Biophys Res Commun.
440:348–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aparicio IM, Espino J, Bejarano I,
Gallardo-Soler A, Campo ML, Salido GM, Pariente JA, Peña FJ and
Tapia JA: Autophagy-related proteins are functionally active in
human spermatozoa and may be involved in the regulation of cell
survival and motility. Sci Rep. 6:336472016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhu WG and Zhao Y: Autophagy
substrate SQSTM1/p62 regulates chromatin ubiquitination during the
DNA damage response. Autophagy. 13:212–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bitto A, Lerner CA, Nacarelli T, Crowe E,
Torres C and Sell C: P62/SQSTM1 at the interface of aging,
autophagy, and disease. Age (Dordr). 36:96262014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taniguchi K, Yamachika S, He F and Karin
M: p62/SQSTM1-Dr. Jekyll and Mr. Hyde that prevents oxidative
stress but promotes liver cancer. FEBS Lett. 590:2375–2397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schläfli AM, Adams O, Galván JA, Gugger M,
Savic S, Bubendorf L, Schmid RA, Becker KF, Tschan MP, Langer R and
Berezowska S: Prognostic value of the autophagy markers LC3 and
p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget.
7:39544–39555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hewitt G, Carroll B, Sarallah R,
Correia-Melo C, Ogrodnik M, Nelson G, Otten EG, Manni D, Antrobus
R, Morgan BA, et al: SQSTM1/p62 mediates crosstalk between
autophagy and the UPS in DNA repair. Autophagy. 12:1917–1930. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuhn C III, Homer RJ, Zhu Z, Ward N,
Flavell RA, Geba GP and Elias JA: Airway hyperresponsiveness and
airway obstruction in transgenic mice. Morphologic correlates in
mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am J
Respir Cell Mol Biol. 22:289–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhowmik A, Seemungal TA, Sapsford RJ and
Wedzicha JA: Relation of sputum inflammatory markers to symptoms
and lung function changes in COPD exacerbations. Thorax.
55:114–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryter SW, Koo JK and Choi AM: Molecular
regulation of autophagy and its implications for metabolic
diseases. Curr Opin Clin Nutr Metab Care. 17:329–337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feizi N, Mehrbod P, Romani B, Soleimanjahi
H, Bamdad T, Feizi A, Jazaeri EO, Targhi HS, Saleh M, Jamali A, et
al: Autophagy induction regulates influenza virus replication in a
time-dependent manner. J Med Microbiol. 66:536–541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Y, Galluzzi L, Zitvogel L and Kroemer
G: Autophagy and cellular immune responses. Immunity. 39:211–227.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong Z, Sanchez-Lopez E and Karin M:
Autophagy, NLRP3 inflammasome and auto-inflammatory/immune
diseases. Clin Exp Rheumatol. 34 4 Suppl 98:S12–S16. 2016.
|
|
40
|
Vij N, Chandramani-Shivalingappa P, Van
Westphal C, Hole R and Bodas M: Cigarette smoke induced
autophagy-impairment accelerates lung aging, COPD-emphysema
exacerbations and pathogenesis. Am J Physiol Cell Physiol.
314:C73–C87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li D, Hu J, Wang T, Zhang X, Liu L, Wang
H, Wu Y, Xu D and Wen F: Silymarin attenuates cigarette smoke
extract-induced inflammation via simultaneous inhibition of
autophagy and ERK/p38 MAPK pathway in human bronchial epithelial
cells. Sci Rep. 6:377512016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Polosukhin VV, Lawson WE, Milstone AP,
Egunova SM, Kulipanov AG, Tchuvakin SG, Massion PP and Blackwell
TS: Association of progressive structural changes in the bronchial
epithelium with subepithelial fibrous remodeling: A potential role
for hypoxia. Virchows Arch. 451:793–803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baek KJ, Cho JY, Rosenthal P, Alexander
LE, Nizet V and Broide DH: Hypoxia potentiates allergen induction
of HIF-1α, chemokines, airway inflammation, TGF-β1, and airway
remodeling in a mouse model. Clin Immunol. 147:27–37. 2013.
View Article : Google Scholar : PubMed/NCBI
|