Introduction
Endometrial cancer is the most common female genital
cancer in western countries, and the fourth most common cancer in
women. Although the incidence of endometrial cancer is relatively
low in Asian countries, it has increased by 6–7 fold in recent
years (1). Several genetic
abnormalities have been associated with endometrial cancer,
including alterations in various tumor suppressor genes and
oncogenes, such as phosphatase and tensin homolog, tumor protein
p53, RAS and human epidermal growth factor receptor 2/neu (2). Silencing of tumor suppressor genes by
DNA methylation may play a critical role in the pathogenesis of
various human cancers, including endometrial cancer. In addition,
silencing by DNA methylation of various genes involved in cell
cycle control, apoptosis, angiogenesis and mismatch repair may
contribute to endometrial carcinogenesis (2).
Runt domain transcription factors, also known
previously as polyomavirus enhancer binding protein 2/core binding
factors (PEBP2/CBFs), are targets of the transforming growth factor
(TGF)-β signaling pathway and important regulators of human
development and tumorigenesis (3).
The runt-related transcription factor (RUNX) family includes three
isoforms: RUNX1, RUNX2, and RUNX3. RUNX1 and RUNX2 are associated
with abnormalities in blood and bone formation, whereas loss of
RUNX3 function has recently been reported to be associated with
carcinogenesis in various cancers (4–10).
Indeed, TGF-β/RUNX3 signaling serves an important role in tumor
suppression (3). The RUNX3 gene is
located on chromosome 1p36, and deletion of RUNX3 from the short
arm of chromosome 1 has been reported frequently in various types
of tumors, including endometrial cancer, suggesting that one or
more tumor suppressor genes exist on the short arm of chromosome
1.
Loss of function of RUNX3 by promoter
hypermethylation, protein mislocalization, and hemizygous deletion
has been reported in several cancers, including gastric cancer,
hepatocellular carcinoma, breast cancer, and lung cancer (5–8).
RUNX3 has been reported to function as a tumor suppressor in
gastric cancer, and loss of RUNX3 expression has been reported in
~45–60% of gastric cancer cell lines and gastric cancer tissues
through hypermethylation of its promoter (5). Previous studies of the relationship
between RUNX3 and endometrial cancer are limited, and results
showing the frequency of methylation and loss of RUNX3 expression
in endometrial carcinoma have not been consistent (9,10).
In addition, the relationship between hypermethylation of the RUNX3
promoter and loss of expression has not been determined, and the
correlation between loss of expression and clinicopathological
factors is unclear.
Therefore, the present study aimed to investigate
RUNX3 protein expression and the methylation status of the RUNX3
promoter in endometrial cancer cell lines, normal endometrial
tissues and endometrial cancer tissues, in order to determine the
role of RUNX3 in endometrial carcinogenesis and its relationship
with clinicopathological factors.
Materials and methods
Cell lines and culture
HEC-1α cells were purchased from the American Type
Culture Collection (Manassas, VA, USA), and Ishikawa cells were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
HEC-1α cells were cultured in a 5% CO2 incubator at 37°C
using McCoy's 5a medium (Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany) and Ishikawa cells were cultured in a 5% CO2
incubator at 37°C using Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum.
Endometrial cancer tissues and normal
endometrial tissues
A total of 53 endometrial carcinoma tissues and
eight normal endometrial tissues were obtained during surgery or
endometrial curettage at Soonchunhyang University Cheonan Hospital,
(Cheonan, Korea) between January 1998 and December 2006. All
endometrial cancers exhibited an endometrioid adenocarcinoma
histology. Tumor stage was assessed according to the International
Federation of Gynecology and Obstetrics staging system and
differentiation grade was assessed according to the World Health
Organization classification 1–3 system. The clinicopathological
characteristics of the patients are described in Table I. Written informed consent was
obtained from all patients, and the study was approved by the
Institutional Review Board of Soonchunhyang University Cheonan
Hospital.
 | Table I.Expression of RUNX3 according to
clinicopathological factors. |
Table I.
Expression of RUNX3 according to
clinicopathological factors.
|
|
| RUNX3 expression
score |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | Number of cases | 0 | +1 | +2 | +3 | Total |
|---|
| Age, years
(n=53) |
|
|
|
|
|
|
|
<60 | 28 | 12 | 13 | 3 | 0 | 16 |
|
>60 | 25 | 15 | 9 | 1 | 0 | 10 |
| Tumor grade
(n=53) |
|
|
|
|
|
|
| G1-2 | 39 | 16 | 20 | 3 | 0 | 23a |
| G3 | 14 | 11 | 2 | 1 | 0 | 3 |
| FIGO stage
(n=48) |
|
|
|
|
|
|
| I | 35 | 11 | 20 | 4 | 0 | 24a |
| III | 13 | 12 | 1 | 0 | 0 | 1 |
| Myometrial invasion
(n=48) |
|
|
|
|
|
|
|
<50% | 21 | 6 | 12 | 3 | 0 | 15 |
|
>50% | 27 | 17 | 9 | 1 | 0 | 10 |
| Lymph node
(n=48) |
|
|
|
|
|
|
| Yes | 4 | 0 | 0 | 0 | 0 | 0 |
| No | 44 | 23 | 21 | 4 | 0 | 25 |
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was performed in order to detect the
expression of RUNX3 mRNA in the endometrial cancer cell lines, two
normal endometrium and cancer tissues. Total RNA was extracted
using a QIAamp RNA kit (Qiagen, Inc., Valencia, CA, USA). Reverse
transcription was performed using 5 µg total RNA with the
Superscript II First-Strand Synthesis system (Thermo Fisher
Scientific, Inc.) using oligo(dT) primers. The PCR components were
as follows: 0.5 µl forward primer (10 pmol), 0.5 µl reverse primer
(10 pmol), 1 µl cDNA, 2 µl 2.5 mM dNTPs, 3 µl of 5XQ buffer, 1 U
Taq DNA polymerase (Qiagen, Inc.) and dH2O to reach a
final volume of 30 µl. The primer pair for the human RUNX3 forward,
5′-AGACAGCCTGGGCTGGTAAA-3′ and reverse, 5′-TCAGATGAGTGCAGCAGGTG-3′;
and GAPDH forward, 5′-CTTAGCACCCCTGGCCAAG-3′ and reverse,
5′-GATGTTCTGGAGAGCCCCG-3′. The PCR conditions were as follows:
Initial denaturation for 15 min at 95°C; 35 cycles of denaturation
at 95°C for 30 sec, annealing at 63°C for 30 sec and extension at
72°C for 30 sec; and a final extension for 5 min at 72°C. PCR was
carried out using a thermal cycler (PTC-200; MJ Research, Inc.,
Waltham, MA, USA). PCR products were evaluated by electrophoresis
on ethidium bromide-stained 2% agarose gels and by digital
capillary electrophoresis.
Immunohistochemical staining
The Endometrial cancer tissue were embedded in
paraffin following fixation in 10% neutral buffered formalin
overnight at room temperature and sectioned at 4 µm.
Immunohistochemical staining was performed to identify protein
expression within the endometrial cancer tissues. To facilitate
antigen retrieval, the sections were pretreated using heat-mediated
antigen retrieval with sodium citrate buffer (0.01 M, pH 6.0) by
boiling in a microwave for 20 min, cooling at room temperature for
at least 1 h, and rinsing with distilled water three times for 3
min each. To block endogenous peroxidase, the sections were
incubated in 3% hydrogen peroxide in methanol at room temperature
for 20 min. Next, sections were washed three times with distilled
water, soaked in PBS pH 7.4 for 3 min, and then incubated with
rabbit polyclonal antibody against human RUNX3 (39301; 1:100;
Active Motif, Inc., Carlsbad, CA, USA) at room temperature for 1 h.
Sections were washed with PBS three times for 3 min each and then
were incubated with secondary antibody EnVision HRP-Labelled
Polymer anti-rabbit (K4002; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) for 30 min at room temperature. Thereafter, the
sections were washed three times with PBS and reacted with the
chromogen diaminobenzidine, followed by washing with distilled
water. Sections were then counterstained with hematoxylin and
mounted with DPX mounting medium. The slides were evaluated by a
trained pathologist using an optical light microscope. Depending on
the % of stained cells, quantification was performed according to
the following classification: Staining intensity 0, no stained
cancer cells; +1, positive in <33% of cancer cells; +2, positive
in 33–66% of cancer cells; and +3, positive in >66% of cancer
cells.
Genomic DNA extraction from tissues
and cell lines
A G-spin Genomic DNA Extraction kit (Intron
Biotechnology, Inc., Seongnam, Korea) was used for DNA extraction
from cell lines and tumor tissues. To obtain DNA from paraffin
blocks containing tumor tissues, microdissection was conducted as
follows. First, 5–7 µm sections were cut from the paraffin blocks.
Following deparaffinization, the blocks were hydrated through a
graded series of alcohols to distilled water. Next, the sections
were dried at room temperature. The tumor tissues were then scraped
with a 24-gauge needle from dried tissue under a microscope at
magnification, ×40. The collected tissue was placed in 50 µl of
lysis buffer (500 mM KCl, 150 mM Tris-HCl pH 8.0, 15 mM
MgCl2 and 1.5% Tween-20) containing proteinase K (0.5
mg/l). Samples were mixed well by vortexing and incubated for 24 h
at 55°C. Subsequently, DNA was extracted using the G-spin Genomic
DNA Extraction kit and bisulfite modification, which converts
unmethylated CpG cytosine to uracil, was carried out using an
Epitech Bisulfite kit (Qiagen, Inc.).
Methylation specific PCR (MS-PCR)
In order to determine the methylation status of the
RUNX3 promoter, MS-PCR was performed. Primers specific for
methylated DNA (M; forward, 5′-TATTCGTTAGGGTTCGTTCGT-3′ and
reverse, 5′-AAACAACCACGAAAAACGAC-3′) and unmethylated DNA (U;
forward, 5′-AAGTGGGAAAGTAGAAGTGGTG-3′ and reverse,
5′-CCAAACAAACTACAAACAACCA-3′) were used. The PCR conditions were as
follows: Initial denaturation for 15 min at 95°C; 35 cycles of
denaturation at 95°C for 15 sec, annealing at 58°C for 50 sec and
extension at 72°C for 60 sec, and a final extension for 5 min at
72°C. PCR was carried out in a thermal cycler (PTC-200; MJ
Research, Inc.). PCR products were then subjected to capillary
electrophoresis or electrophoresis on ethidium bromide-stained 2%
agarose gels. The results were verified using a digital image
analysis system. The positive control DNA template was a
universally methylated DNA (S7821), and the negative control DNA
template was a universally unmethylated DNA (S7822; both EMD
Millipore, Billerica, MA, USA).
5-Aza-2′-deoxycytidine (ADC)
treatment
Because methylation is a reversible modification,
the DNA methyltransferase inhibitor ADC (Sigma-Aldrich; Merck KGaA)
was used to examine whether gene expression was inhibited by
promoter methylation. HEC1-α cells were treated with ADC at
different concentrations (0, 0.5, 1 or 5 µM) for 3 days. Cells were
then harvested for analysis of RUNX3 mRNA expression.
Cell survival
To evaluate the biological function of RUNX3, the
viability of HEC1α cells was analyzed following treatment with ADC
using an MTT assay (Sigma-Aldrich; Merck KGaA), according to the
manufacturer's instructions. Cell survival was calculated as the
optical density value at 545 nm ×100.
Statistical analysis
All statistical analyses were carried out with SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA) using
χ2, students-t test and one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
As described in Table
I, the average age of the patients was 59.3±11.8 years, and the
histological grades were grade 1 or 2 in 39 patients (73.6%) and
grade 3 in 14 patients (26.4%). A total of 48 patients underwent
staging surgery, including hysterectomy, bilateral salpingo
oophorectomy, pelvic lymphadenectomy, multiple peritoneal biopsy,
and peritoneal washing cytology. According to the surgical
pathology reports, clinicopathological factors were as follows:
Stage I in 35 patients (72.9%); stage III in 13 patients (27.1%);
myometrial invasion, less than half in 21 patients (43.7%); and
myometrial invasion, more than half in 27 patients (56.3%).
RUNX3 mRNA expression and methylation
of the promoter region of the RUNX3 gene in endometrial cancer cell
lines
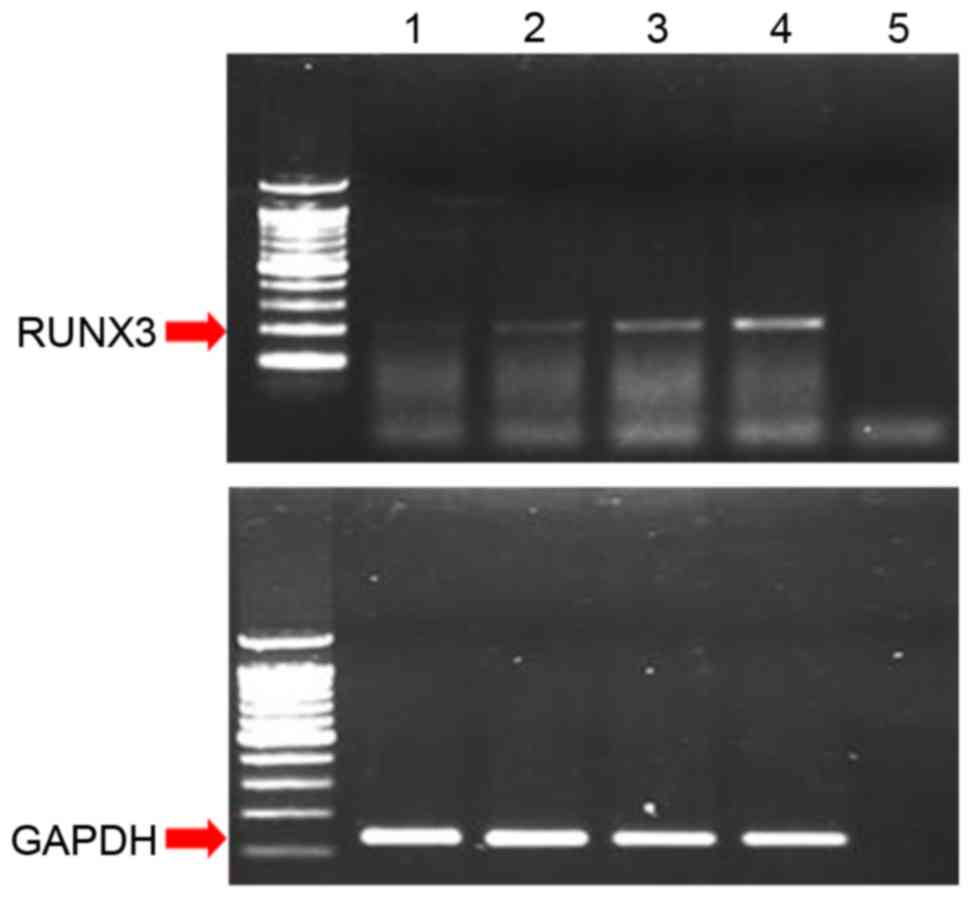
RUNX3 mRNA was expressed in Ishikawa cells but not
in HEC1-α cells (Fig. 1A). In
addition, results from MS-PCR revealed that the RUNX3 promoter
region was methylated in HEC1-α cells but not in Ishikawa cells
(Fig. 1B). RUNX3 mRNA expression
was absent in two cases of fresh endometrial cancer tissue but was
present in two cases of fresh normal endometrial tissues (Fig. 1A).
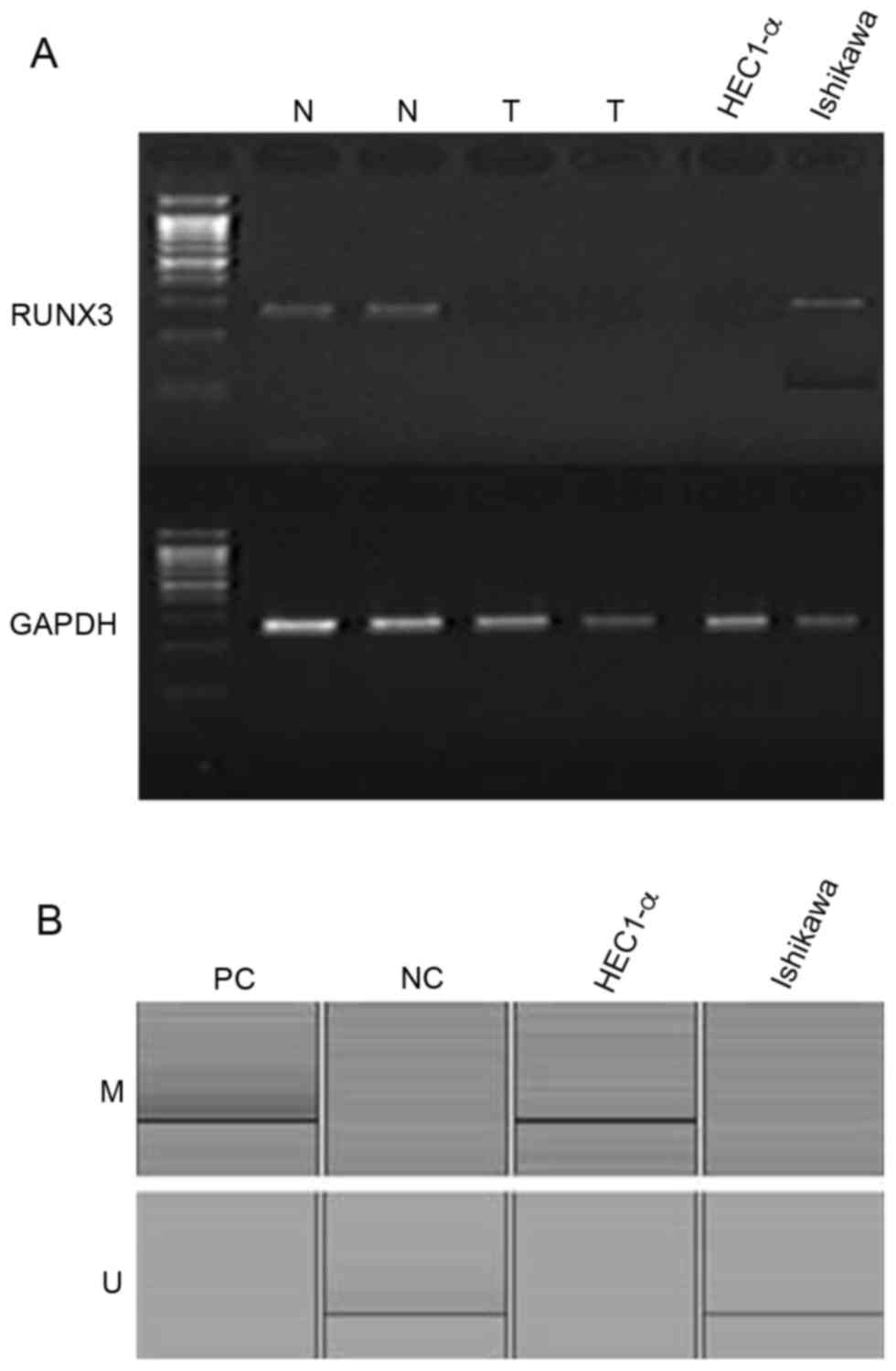 | Figure 1.(A) Expression patterns of RUNX3 mRNA
in two normal endometrial tissues, two endometrial cancer tissues,
and two endometrial cancer cell lines, as determined by
reverse-transcription-PCR. GAPDH was used as a standard reference
(B) Methylation-specific PCR for the two endometrial cancer cell
lines, HEC1-α and Ishikawa. RUNX3, runt-related transcription
factor 3; PCR, polymerase chain reaction; N, normal endometrial
tissue; T, endometrial cancer tissue; M, methylated; U,
unmethylated; PC, positive control; NC, negative control. |
Loss of RUNX3 expression is correlated
with clinicopathological factors
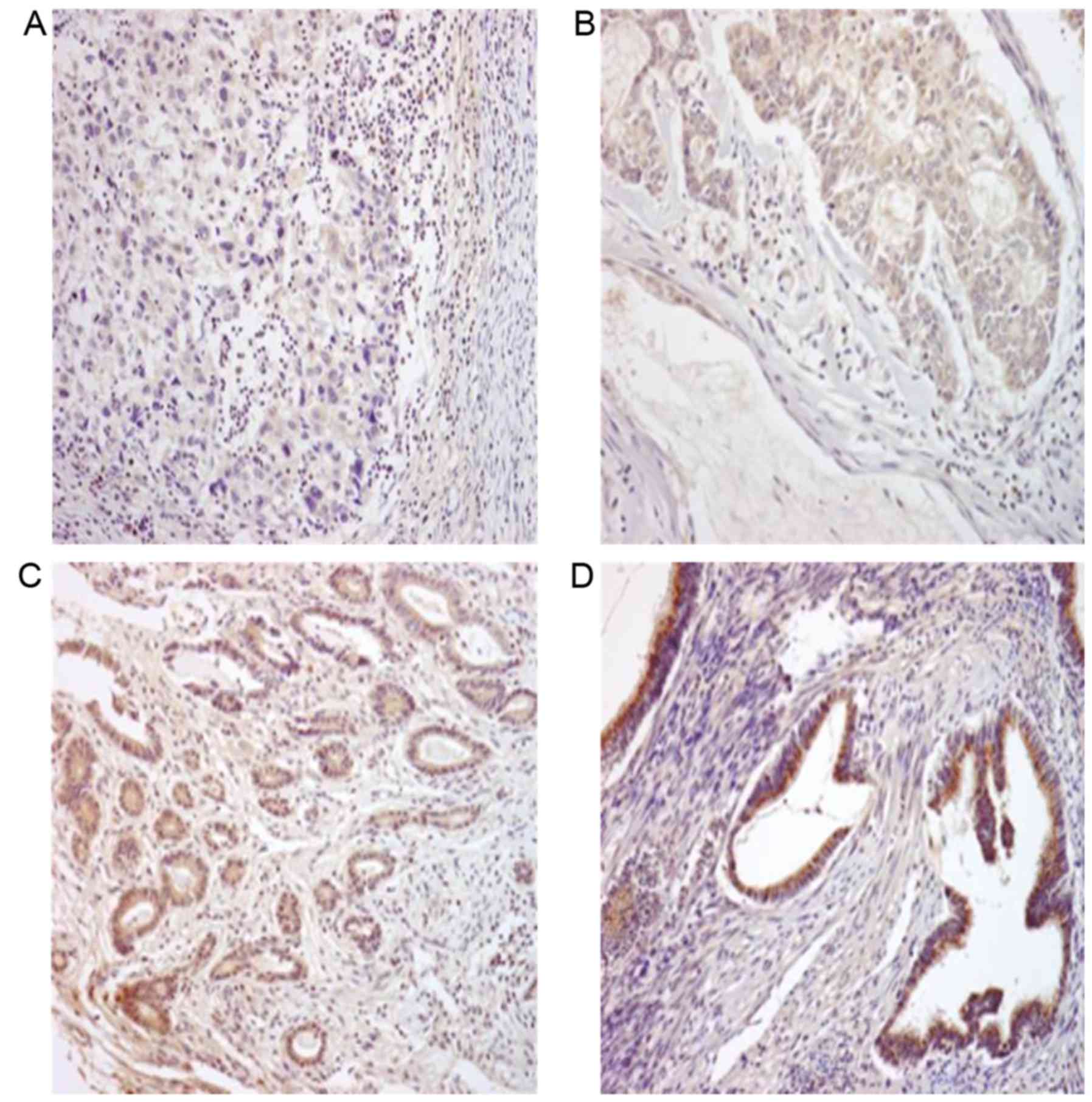
Immunohistochemical staining of tumor sections from
53 cases of endometrioid adenocarcinoma revealed that RUNX3 protein
was expressed in 26 cases (49.1%). Normal endometrial tissues
surrounding the endometrial cancer exhibited strong RUNX3 protein
expression (+3) and were selected as the positive control group
(data not shown). RUNX3 protein expression was observed in the
cytoplasm and was widely spread evenly throughout the samples
(Fig. 2). Among 39 cases with
grade 1~2 tumors, 16 cases (41.0%) demonstrated negative staining,
20 cases (51.3%) demonstrated +1 expression and only 3 cases (7.7%)
demonstrated +2 expression, among 14 cases with grade 3 tumors, 11
cases (78.5%) demonstrated negative staining, 2 cases (14.2%)
demonstrated +1 expression and only 1 case (7.1%) demonstrated +2
expression (Table I). Of the 53
patients, 48 underwent surgery, including total hysterectomy,
bilateral salpingo oophorectomy, multiple peritoneal biopsy, pelvic
lymphadenectomy, and peritoneal washing cytology. Therefore, more
detailed staging information, as well as myometrial and lymph node
invasion status, was available for these samples (Table I). Among 35 cases with stage I
tumors, 11 cases (31.4%) demonstrated negative staining, 20 cases
(57.1%) demonstrated +1 expression and 4 cases (11.4%) demonstrated
+2 expression, among 13 cases with stage III tumors, only 1 case
(7.6%) demonstrated +1 expression (Table I).
The RUNX3 expression pattern was compared to age,
stage, grade, myometrial invasion and lymph node metastasis of the
patients. The statistical analysis revealed that the higher
histological grades were significantly associated with lower RUNX3
protein expression (P<0.05; Table
I) and that loss of RUNX3 protein expression was correlated
with advanced stage (P<0.05; Table
I).
Methylation of the RUNX3 promoter is
not correlated with clinicopathological factors
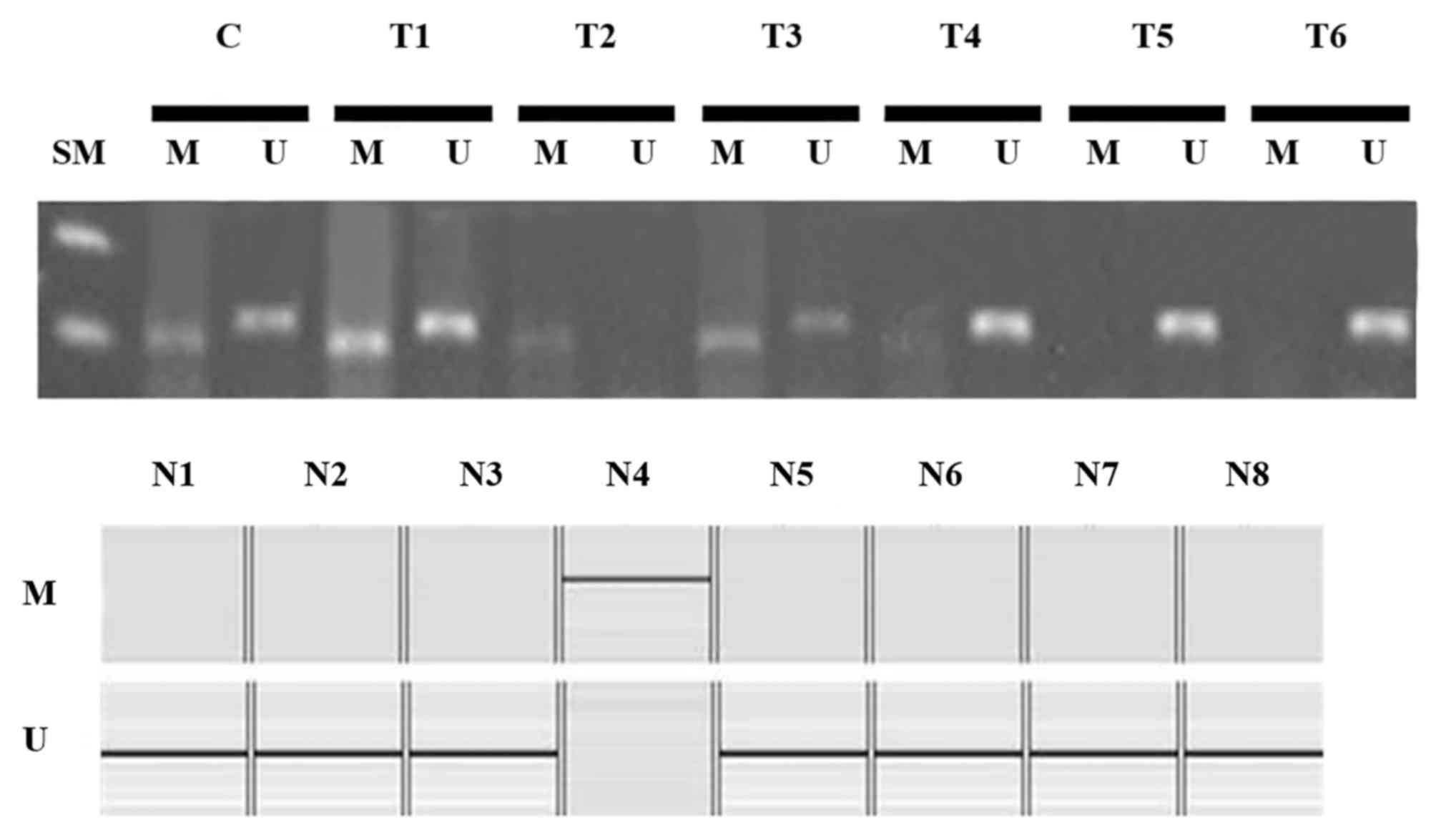
Methylation of the RUNX3 promoter region was
observed in 33 of 53 cases (62.3%) of endometrial cancer and in one
of eight cases (12.5%) of normal endometrial tissues (Fig. 3). Methylation was more frequently
observed in tumor tissues than in normal tissues (P<0.05).
However, there was no significant correlation between RUNX3
promoter methylation and clinicopathological prognostic factors
(Table II).
 | Table II.Expression and methylation of RUNX3
according to clinicopathological factors. |
Table II.
Expression and methylation of RUNX3
according to clinicopathological factors.
| Clinicopathological
factors | Number of
cases | RUNX3
expression | RUNX3
methylation |
|---|
| Age, years
(n=53) |
| 26 (49.1%) | 33 (62.3%) |
|
<60 | 28 | 16 | 19 |
|
>60 | 25 | 10 | 14 |
| Tumor grade
(n=53) |
|
|
|
|
G1-2 | 39 | 23a | 25 |
| G3 | 14 | 3 | 8 |
| FIGO stage
(n=48) |
|
|
|
| I | 35 | 24a | 25 |
|
III | 13 | 1 | 8 |
| Myometrial invasion
(n=48) |
|
|
|
|
<50% | 21 | 15 | 16 |
|
>50% | 27 | 10 | 17 |
| Lymph node
(n=48) |
|
|
|
|
Yes | 4 | 0 | 2 |
| No | 44 | 25 | 31 |
Effects of ADC treatment on RUNX3
expression
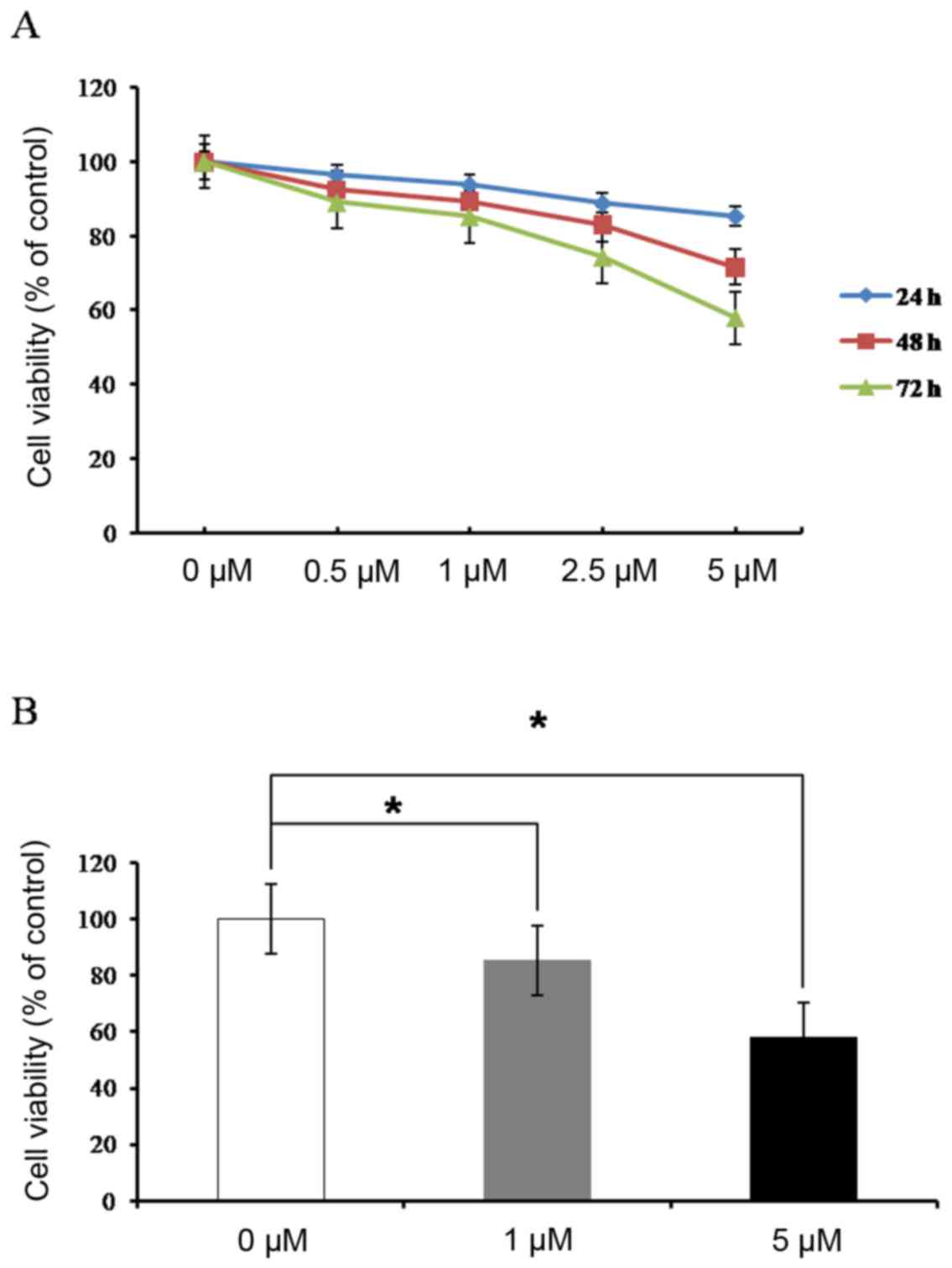
RUNX3 mRNA expression was increased following 72 h
of treatment with increasing concentrations of ADC in HEC1-α cells
(Fig. 4), which were demonstrated
in the present study to be negative for RUNX3 protein expression
and positive for methylation of the RUNX3 promoter (Fig. 1). This result confirmed that
silencing of RUNX3 in HEC1-α cells occurred by methylation of the
RUNX3 promoter region.
Effects of ADC treatment on cell
growth
Cell viability was evaluated by MTT assay in HEC1-α
cells following treatment with ADC (0, 0.5, 1, 2.5 and 5 µM) for 72
h. The results demonstrated that ADC treatment inhibited cell
growth in a concentration and time-dependent manner (Fig. 5).
Discussion
RUNX3, a target of TGF-β and bone morphogenic
protein signaling, serves an important role in development and
tumorigenesis in mammals (3).
RUNX3 is located on chromosome 1p36, which is frequently deleted in
various types of tumors and is associated with failure of the TGF-β
pathway (3), suggesting a
mechanism to explain the effects of RUNX3 on the carcinogenesis of
malignant tumors. In the present study, the expression of RUNX3 was
examined in endometrial carcinoma. The results demonstrated that
RUNX3 expression was downregulated due to promoter methylation in
approximately half of endometrial tumors. Thus, these data provide
important insights into the role of RUNX3 and DNA methylation in
endometrial carcinogenesis.
Li et al (5)
reported that loss of RUNX3 expression was observed in 61% of
gastric cancer tissues, including 40% of early cancer tissues and
90% of the advanced cancer tissues. In addition, consistent with
the present study, they demonstrated that loss of RUNX3 expression
was caused by hypermethylation of the promoter region or hemizygous
deletion of the RUNX3 gene (5).
Kim et al (9) reported that
hypermethylation occurred in 2.5% of cervical cancer samples and
12.5% of endometrial cancer samples. Yoshizaki et al
(10) reported loss of RUNX3
protein expression in 12 of 21 endometrial cancer tissues (57%) by
immunohistochemistry and methylation of the RUNX3 promoter region
in 18 of 21 endometrial cancer tissues (86%) and two of nine normal
endometrial samples (22%). This latter study also demonstrated that
loss of RUNX3 protein expression was more frequent in grade 3
tumors than in grade 1 or 2 tumors (10). Similarly, in the present study,
immunohistochemistry analysis revealed loss of RUNX3 protein
expression in 27 (51%) of 53 endometrial cancer tissues. Notably,
although normal endometrial tissues exhibited strong expression of
RUNX3 protein, tumor tissues exhibited primarily weak expression,
with only three cases of moderate expression and no cases of strong
expression. In samples from patients with stage III cancer, only
one of 13 samples exhibited weak RUNX3 expression. Thus, higher
histological grade and stage was significantly associated with
greater loss of RUNX3 protein expression.
Additional studies have reported that loss of RUNX3
expression or increased hypermethylation of the RUNX3 promoter is
associated with survival in patients with gastric, colorectal and
bladder cancers (11–14). Zhang et al (15) examined the methylation of the RUNX3
promoter in epithelial ovarian cancer samples, and no correlation
was observed with clinicopathological factors (15). In the present study, methylation of
the RUNX3 promoter was also not correlated with prognostic factors,
including stage, grade, myometrial invasion and lymph node
metastasis. These results are consistent with a report by Park
et al (16), who revealed
no correlation between methylation of the RUNX3 promoter and
clinicopathological prognostic factors in liver cancer (16). These data suggest that DNA
methylation may occur during the early stages of endometrial
carcinogenesis. Notably, the frequency of methylation observed in
the present study (62.3%, 33/53) was lower than that in the study
by Yoshizaki et al (10),
but higher than that observed in liver, ovarian, cervical, and lung
cancers (6,7,9,15).
Methylation is a reversible phenomenon; treatment
with ADC and trichostatin A has been reported to reactivate RUNX3
mRNA expression in various tumors, including gastric, lung and
liver (5,7,16).
ADC is a cytosine analog developed as an inhibitor of DNA
methyltransferase 3B and has been demonstrated to inhibit growth,
survival and tumorigenesis in endometrial cancer cell lines
(17). In the present study, ADC
treatment blocked cell growth and reactivated RUNX3 mRNA expression
in HEC1-α cells in a concentration-dependent manner. These results
suggest that demethylation and subsequent upregulation of RUNX3 may
have resulted in cell growth inhibition in the endometrial cancer
cells and ADC may have a therapeutic role in the management of
endometrial cancer.
The relevance of RUNX3 silencing in the survival of
patients with endometrial cancer could not be investigated in the
present study due to the small number of patients with advanced
stage cancer and good outcome of patients with early stage cancer.
However, the observed association with higher tumor grade and
advanced stage implies that RUNX3 promoter methylation and
silencing may be important in the development and prognosis of this
tumor type. Thus, loss of RUNX3 expression may have applications as
a prognostic marker, and RUNX3 may serve as a potential epigenetic
target in the treatment of patients with endometrial cancer.
Acknowledgements
Authors will like to thank Professor Moo Jun Baek,
Director of Soonchunhyang Medical Science Research Institute for
organizing research equipment.
Funding
The present study was supported by the Soonchunhyang
University Research Fund (grant no. 20180006).
Availability of data and materials
All data generated during the study are included in
this manuscript.
Authors' contributions
DJ the drafted manuscript and carried out part of
the experiments; HK designed and performed immunohistochemical
staining; AR participated in the analysis of clinical data; JS
analyzed RT-qPCR data; SC carried out methylation specific PCR; GN
participated in study design and editing the manuscript; SJ
participated in experimental design, data analysis and
interpretation, and drafting and reviewing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Soonchunhyang University Cheonan Hospital. Informed
consent was provided by all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
SOG Gynecologic Oncology Committee: Annual
report of gynecologic cancer registry program in Korea for 2004
(Jan. 1st, 2004-Dec. 31st, 2004). Korean J Obstet Gynecol.
50:28–78. 2007.
|
|
2
|
Catasus L, Gallardo A and Prat J:
Molecular genetics of endometrial carcinoma. Diagno Histopathol.
15:554–563. 2009. View Article : Google Scholar
|
|
3
|
Ito Y and Miyazono K: RUNX transcription
factors as key targets of TGF-beta superfamily signaling. Curr Opin
Genet Dev. 13:43–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bae SC and Choi JK: Tumor suppressor
activity of RUNX3. Oncogene. 23:4336–4340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue KI, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao WH and Liu WW: Hemizygous deletion
and hypermethylation of the RUNX3 gene in hepatocelluar carcinoma.
World J Gastroenterol. 10:376–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li QL, Kim HR, Kim WJ, Choi JK, Lee YH,
Kim HM, Li LS, Kim H, Chang J, Ito Y, et al: Transcriptional
silencing of the RUNX3 gene by CpG hypermethylation is associated
with lung cancer. Biochem Biophys Res Commun. 314:223–228. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang KT, Han W, Bae JY, Hwang SE, Shin
HJ, Lee JE, Kim SW, Min HJ and Noh DY: Downregulation of the RUNX3
gene by promoter hypermethylation and hemizygous deletion in breast
cancer. J Korean Med Sci. 22 Suppl:S24–S31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW,
Bang YJ and Kang GH: Methylation of RUNX3 in various types of human
cancers and premalignant stages of gastric carcinoma. Lab Invest.
84:479–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshizaki T, Enomoto T, Fujita M, Ueda Y,
Miyatake T, Fujiwara K, Miyake T and Kimura T, Yoshino K and Kimura
T: Frequent inactivation of RUNX3 in endometrial carcinoma. Gynecol
Oncol. 110:439–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim EJ, Kim YJ, Jeong P, Ha YS, Bae SC and
Kim WJ: Methylation of the RUNX3 promoter as a potential prognostic
marker for bladder tumor. J Urol. 180:1141–1145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang
L, Le X, Yao J, Wu TT, Huang S and Xie K: Loss of RUNX3 expression
significantly affects the clinical outcome of gastric cancer
patients and its restoration causes drastic suppression of tumor
growth and metastasis. Cancer Res. 65:4809–4816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogino S, Meyerhardt JA, Kawasaki T, Clark
JW, Ryan DP, Kulke MH, Enzinger PC, Wolpin BM, Loda M and Fuchs CS:
CpG island methylation, response to combination chemotherapy, and
patient survival in advanced microsatellite stable colorectal
carcinoma. Virchows Arch. 450:529–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soong R, Shah N, Peh BK, Chong PY, Ng SS,
Zeps N, Joseph D, Salto-Tellez M, Iacopetta B and Ito Y: The
expression of RUNX3 in colorectal cancer is associated with disease
stage and patient outcome. Br J Cancer. 100:676–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Wei L, Zhang A, Zhang L and Yu H:
RUNX3 gene methylation in epithelial ovarian cancer tissues and
ovarian cancer cell lines. OMICS. 13:307–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park WS, Cho YG, Kim CJ, Song JH, Lee YS,
Kim SY, Nam SW, Lee SH, Yoo NJ and Lee JY: Hypermethylation of the
RUNX3 gene in hepatocellular carcinoma. Exp Mol Med. 37:276–281.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui M, Wen Z, Chen J, Yang Z and Zhang H:
5-Aza-2′-deoxycytidine is a potent inhibitor of DNA
methyltransferase 3B and induces apoptosis in human endometrial
cancer cell lines with the up-regulation of hMLH1. Med Oncol.
27:278–285. 2010. View Article : Google Scholar : PubMed/NCBI
|