Introduction
Hypoxic encephalopathy caused by sudden cardiac
death (1) is a critical clinical
disease, and it is an important reason for the high rate of
disability following cardiopulmonary resuscitation (CPR). Early
identification, effective prevention, treatment of ischemic
encephalopathy and improving the success rate of cerebral
resuscitation are important issues that may not be ignored.
At present, studies have demonstrated that there are
various reasons for the low success rate of treatment for hypoxic
encephalopathy. During and subsequent to cardiopulmonary
resuscitation, high levels of circulating cytokines, the activation
of blood coagulation and platelets, the presence of endotoxins in
plasma, and the alteration of soluble E-selectin and P-selectin
have been described (2). A
considerable number of studies have demonstrated that induced
pluripotent stem cell-derived mesenchymal stem cells (IPSC-MSCs)
have a marked therapeutic effect on hypoxic encephalopathy
(3). IPSC-MSCs have exerted
substantial protective effects and improvements on the survival
rate of acute lung injury in animal experiments (4–6). In
addition, intervention with IPSC-MSCs may reduce neutrophil
infiltration in the lung tissue of mice with ventilator-associated
pneumonia and improve the survival rate (7,8). All
of the above studies have demonstrated that IPSC-MSCs exert an
immune regulatory effect and an inflammatory response that balances
multiple aspects of the immune inflammatory network of the body;
however, the details of the function of IPSC-MSCs and the mechanism
of action remain unclear (9,10).
The role of macrophage differentiation, different phenotypes and
functional status in inflammatory and neoplastic diseases has
attracted much attention. Previous studies have demonstrated that
the Notch-1 signaling pathway is associated with the
differentiation, proliferation and function of a number of types of
immune cells (11,12). However, the mechanisms underlying
the way in which IPSC-MSCs exert their benefits are not well
understood.
IPSC-MSCs may improve the recovery of the brain
following CPR; however, the mechanism underlying the role of
IPSC-MSCs in immune regulation, whether they are able to alter the
direction of macrophage polarization, and whether they may improve
the prognosis of cerebral resuscitation remains unknown. Further
experimental studies are required to examine the mechanism
underlying the way in which IPSC-MSCs exert their anti-inflammatory
effect, and which signaling pathway results in the induction of M2
type macrophages. In the present study, Raw 264.7 cells were used
to perform oxygen and glucose deprivation (OGD) to replicate the
model of cerebral ischemia. Intervention by IPSC-MSCs was performed
in the OGD model and the results demonstrated that IPSC-MSCs were
able to regulate the polarization of macrophages via the neurogenic
locus notch homolog protein 1 (Notch-1) signaling pathway. In
addition, the results of the present study demonstrated that
IPSC-MSCs were able to regulate the polarization of macrophages,
which may be accomplished by inhibiting the Notch-1 signaling
pathway.
Materials and methods
Materials
Cells, cell culture media, serum and cell culture
supplements were purchased from the American Type Culture
Collection (Manassas, VA, USA), EMD Millipore (Billerica, MA, USA),
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
PAA Laboratories (GE Healthcare, Chicago, IL, USA), respectively,
unless otherwise stated. Antibodies against inducible nitric oxide
synthase (iNOS; cat. no. ab178945), Hes1 (cat. no. ab108937),
interleukin (IL)-10 (ab189392), arginase-1 (Arg1; cat. no.
ab124917) and β-actin (cat. no. ab8226) were purchased from Abcam
(Cambridge, UK). Antibodies against tumor necrosis factor (TNF)-α
(cat. no. 11948P) and Notch 1 (cat. no. 4380P) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against allophycocyanin (APC)-cluster of differentiation (CD)197
(cat. no. 120107), fluorescein isothiocyanate (FITC)-F4/80 (cat.
no. 123107), and phycoerythrin (PE)-CD206 (cat. no. 141705) were
purchased from BioLegend, Inc. (San Diego, CA, USA).
Horseradish-peroxidase conjugated secondary antibodies for western
blot (WB) analysis were purchased from Abcam (cat. no. ab150157).
Recombinant mouse vascular endothelial growth factor (VEGF) was
purchased from R&D Systems Europe, Ltd. (Abingdon, UK).
IPSC-MSCs and Raw 264.7 cells
co-culture
Naive IPSC-MSCs and Raw 264.7 cells were cultured in
collagen-coated dishes with High Glucose Dulbecco's modified
Eagle's medium (DMEM; 4.5 g/l glucose; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin at 37°C in a humidified 5% CO2
atmosphere. Medium was changed every 2–3 days. By 3–4 days of
incubation, cells had reached 70–80% confluence and were seeded
into 6-well flat bottom microtitre plates at a cell density of
1×106 cells/well. IPSC-MSCs (1×106
cells/well) were inoculated onto the upper membrane (Transwell
insert) and Raw 264.7 cells (1×106 cells/well) were
inoculated into the lower chamber of a conventional double cell
co-culture system. The cells were divided into three groups,
including Raw 264.7 cells (1×106 cells/well; control
group), Raw 264.7 cells (1×106 cells/well; OGD group)
and IPSC-MSCs (1×106 cells/well) + Raw 264.7 cells
(1×106 cells/well; OGD + IPSC-MSCs group).
OGD
OGD was induced by exposing Raw 264.7 cells to a
calibrated gas mixture of 2% CO2, 5% CO2 and
93% N2 in a 3-gas incubator (Forma; Thermo Fisher
Scientific, Inc.) in PBS for 0.5, 1, 2 and 4 h. The OGD modeling
time was determined by the results of the Cell Counting Kit-8
(CCK-8) assay. Control cells were maintained in normal conditions
(5% CO2, 95% humidified air) in complete high glucose
medium (DMEM 4.5 g/l).
CCK-8 assay
The integrity of cellular function was measured
using a CCK-8 assay (formazan crystals were solubilized with 0.1 N
HCl isopropanol). Raw 264.7 cells exposed to OGD were further
incubated for 0.5, 1, 2 and 4 h with 10 µl CCK-8 at 37°C in a
normoxic chamber. At the end of the incubation period, absorption
was detected at 450 nm, with background subtraction at 630 nm,
using a microplate reader (Stat Fax-2100; Awareness Technology,
Inc., Palm City, FL, USA) (13).
Flow cytometry (FCM)
Cells were harvested, washed in PBS and resuspended
in binding buffer, and a 0.5 ml aliquot was withdrawn for analysis.
Following the addition of annexin V-FITC, APC and PE (BioLegend,
Inc.), the sample was incubated for 30 min in the dark. Stained
cells were analyzed using a BD Biosciences (Franklin Lakes, NJ,
USA) flow cytometer. A total of 1×106 cells were counted
per sample, and the data were processed using Beckman Coulter Cell
Lab Quanta™ SC MPL (AL510171; Beckman Coulter, Inc., Brea, CA,
USA).
The CCK-8 and the cell sorting staining assays for
FCM were performed in parallel in twin cultures that were subjected
to identical conditions. This procedure was adopted to eliminate
variations in the cell population, growth conditions and
experimental procedures.
WB analysis
10X RIPA lysis buffer (Abcam) was used for protein
extraction (cat. no. ab156034) and a BCA protein assay kit was used
for protein determination. A total of 20 µg denatured protein
diluted in 20 µl solution samples were loaded on a 10% SDS-PAGE
gel, and electrophoresis was run at 150 V for 1 h. Proteins were
transferred to a polyvinylidene fluoride membrane (BioRad
Laboratories, Inc., Hercules, CA, USA) using a Trans-Blot semi-dry
transfer. Cell membranes were blocked with 5% skimmed milk in
TBS-Tween 20 at 4°C for 1 h and subsequently incubated with primary
antibodies against TNF-α, iNOS, Notch1, Hes1, IL-10, Arg1 and
β-actin at a dilution of 1:1,000 for 1 h at room temperature.
Following incubation with the primary antibodies, the secondary
antibody (1:1,000) was used and membranes were incubated at room
temperature for 1 h. The membranes were treated with an enhanced
chemiluminescence substrate (Thermo Fisher Scientific, Inc.) for
1–2 min and exposed at different exposure times. β-actin (1:5,000)
was used as the loading control. Blots from 4–6 different
experiments were scanned and band intensities from each blot were
analyzed using Image J software (version 1.8.0_101; National
Institutes of Health, Bethesda, MD, USA) and expressed relative to
the β-actin signal.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from cells using TRIzol
reagent (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. For RT, 1 µg total RNA from each sample
was reverse transcribed using Superscript II Reverse Transcriptase
(Takara Bio, Inc.). Aliquots of diluted cDNA (1:5) were amplified
using TransStart Top Green qPCR SuperMix in a final volume of 20
µl. The RT reaction was performed at 30°C for 10 min, 42°C for 60
min, and 70°C for 10 min. qPCR amplification was performed using a
LabCycler Real-Time PCR system (SensoTech GmbH, Magdeburg-Barleben,
Germany) using SYBR Green dye (Takara Bio, Inc.), and protein
quantification was performed using the 2−ΔΔCq method
(14). The sequences of the
primers are listed in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
|
|
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|
|
|---|
| Symbol | Gene ID | Amplicon size,
bp | Forward | Reverse |
|---|
| Nos2 (iNOS) | 18126 | 127 |
GTTCTCAGCCCAACAATACAAGA |
GTGGACGGGTCGATGTCAC |
| Abl2 (Arg1) | 11352 | 198 |
GAGCCACCGTTTTACATTGTGA |
CTCGCCCACTAGGCAGTTC |
| Notch1 | 18128 | 227 |
ACACCGTGTAAGAATGCTGGA |
GCCTGCTGACATGATTTTCCTG |
| Atcay (Hes1) | 16467 | 157 |
TCCGACGACTTCCTCGACA |
CACCAGGCATGTTTTTGGCG |
| β-actin | 11461 | 154 |
GGCTGTATTCCCCTCCATCG |
CCAGTTGGTAACAATGCCATGT |
Data and statistical analysis
The values are presented as the mean ± standard
deviation. The control, OGD and OGD + IPSC-MSC groups were tested
for normality within all the time points using repeated measures
analysis of variance followed by the Tukey post hoc test. The null
hypothesis was rejected at the significance level α<0.05. Data
were analyzed statistically using SPSS 22.0 software (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment OGD model and CCK-8
assay
Co-cultured Raw 264.7 and IPSC-MSCs are presented in
Fig. 1. Following establishment of
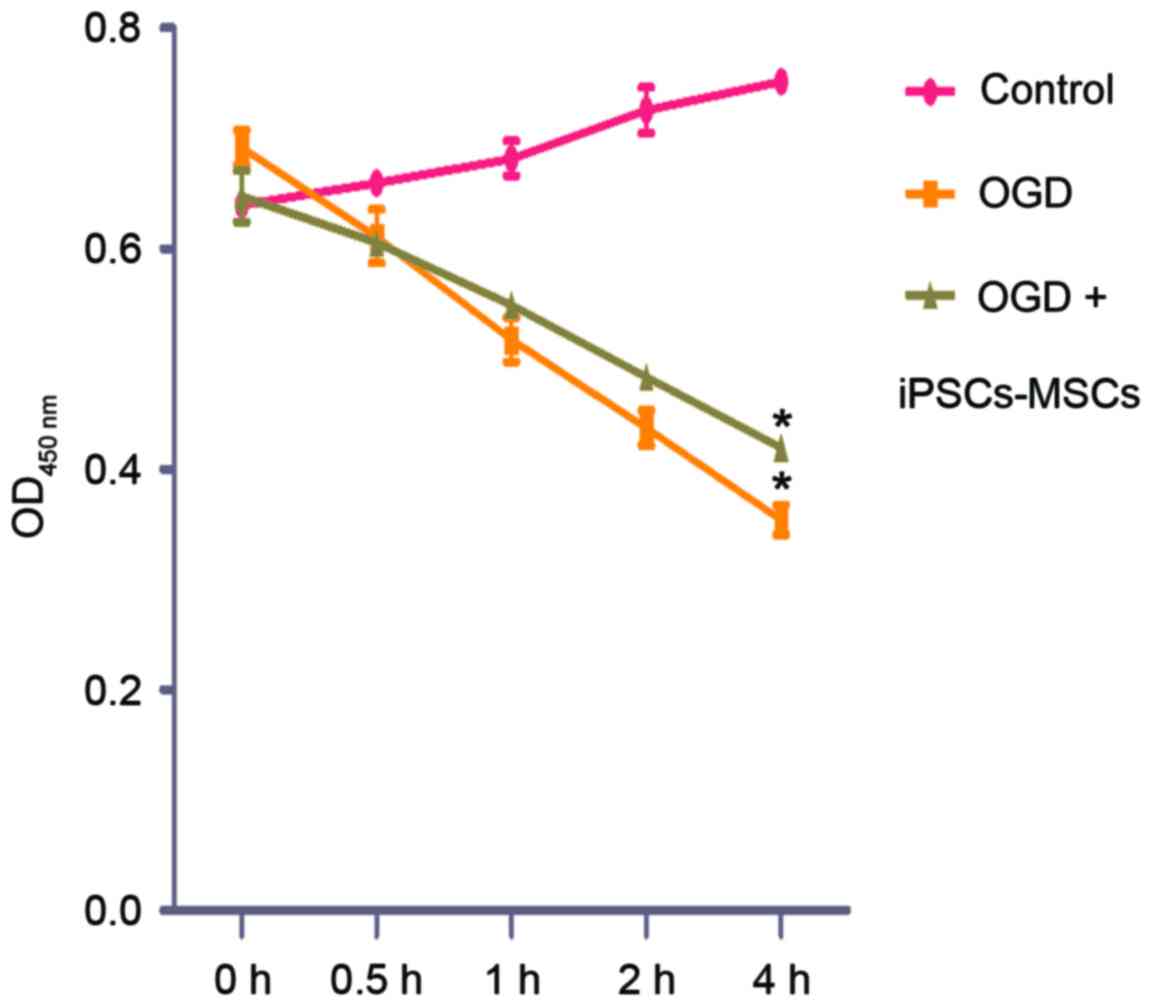
the OGD model, cell viability was determined by assessing the
integrity of mitochondrial function using the CCK-8 assay. The
CCK-8 assay demonstrated that the cell viability of the OGD group
and the OGD + IPSC-MSC group decreased during 0–4 h. The OGD
(0.59+0.02) and OGD + IPSC-MSC groups (0.61+0.01) exhibited no
apparent alterations in cell viability at 0.5 h compared with the
control group (P>0.05); however, cell viability decreased
following 1–2 h of intervention. In addition, OGD cells and OGD +
IPSC-MSC cell viability decreased more significantly following
intervention for 4 h. The cell viability of the OGD group
(0.38±0.13) and the OGD + IPSC-MSC group (0.42±0.10) exhibited a
statistically significant decrease compared with the control group
(0.78±0.05) (P=0.032, P<0.05; Fig.
2).
Effect of OGD and IPSC-MSC
intervention on Raw 264.7 macrophage polarization
To determine whether OGD and IPSC-MSC intervention
affected macrophage polarization, FCM, WB and RT-qPCR analyses were
performed. FCM analysis was used to objectively analyze the
proportion of M1 and M2 macrophages. Following quantitative
analysis, it was observed that the proportion of M1 and M2
macrophages following intervention for 4 h was statistically
significant among the control group (M1, 27.59±1.3%; M2,
52.59±11.0%), OGD + IPSC-MSC group (M1, 50.02±2.4%; M2,
37.08±10.4%), and OGD group (M1, 56.97±12.8%; M2, 33.96±9.7%;
P<0.05). The proportion of M1 and M2 macrophages between the OGD
group (M1, 83.55±7.3%; M2, 11.41±3.2%) and control group (M1,
29.34±4.1%; M2, 52.34±5.4%) was also statistically significant at
6, 12, 24 and 48 h (P=0.026; P<0.05), and over time the trend
was more apparent. The proportion of M1 and M2 macrophages between
the OGD + IPSC-MSC group (M1, 33.48±5.6%; M2, 50.84±6.9%) and OGD
group (M1, 83.55±7.3%; M2, 11.41±3.2%) was statistically
significant at 4, 6, 12, 24 and 48 h (P=0.037, P<0.05), and over
time the trend was more apparent (Fig.
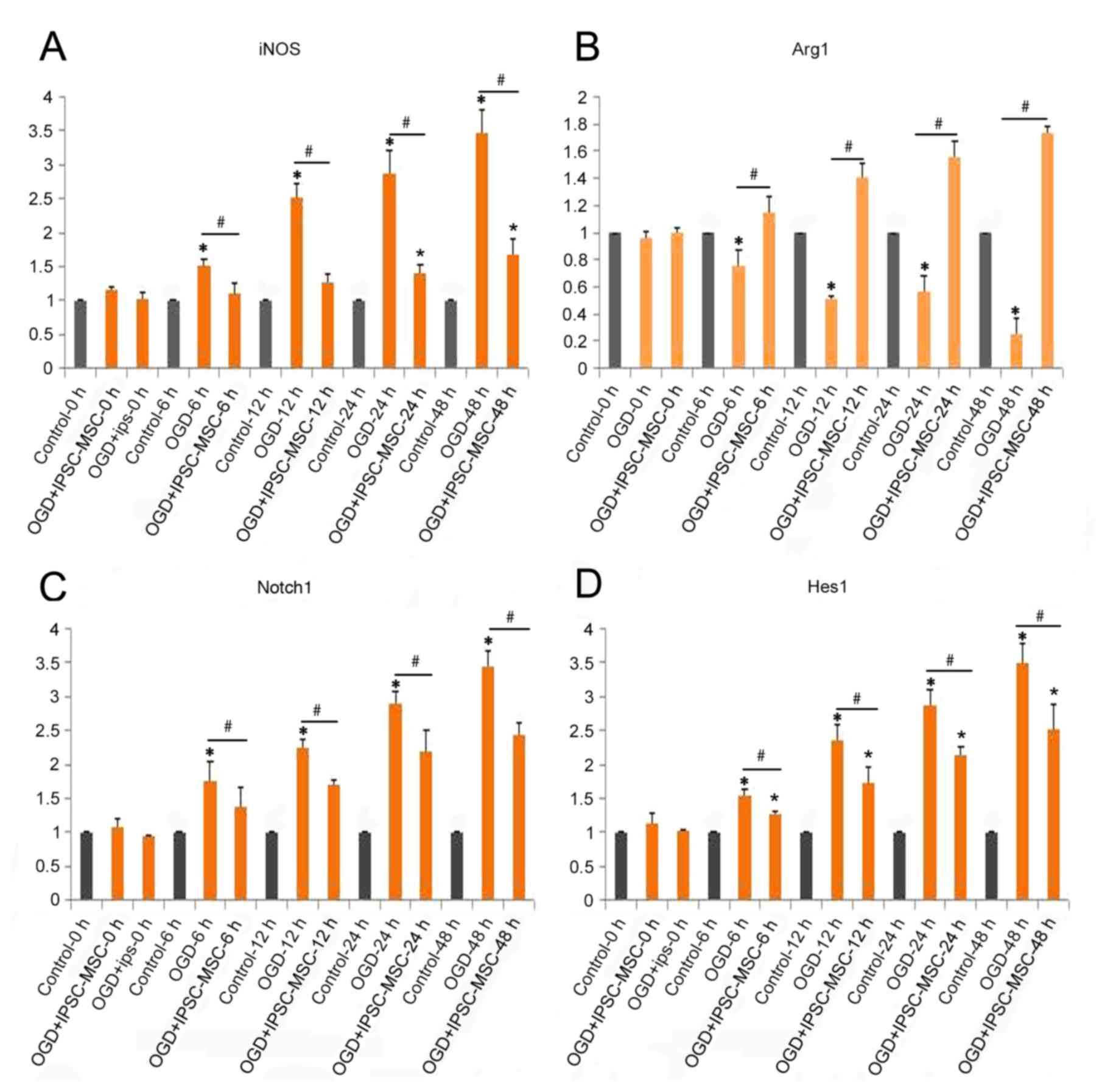
3). To confirm the FCM results, WB and RT-qPCR analyses were
performed. WB and RT-qPCR analysis indicated a decrease in Arg1 and
IL-10 in the OGD group compared with the control group at 6, 12, 24
and 48 h (P=0.041, P<0.05; Figs.
4 and 5A-C). In addition, a
significant decrease in iNOS and TNF-α levels in the OGD + IPSC-MSC
group was observed at 6, 12, 24 and 48 h compared with the OGD
group (P=0.018, P<0.05), and significantly increased at 24 and
48 h compared with the control group (P=0.026, P<0.05; Figs. 4 and 5A, D and E).
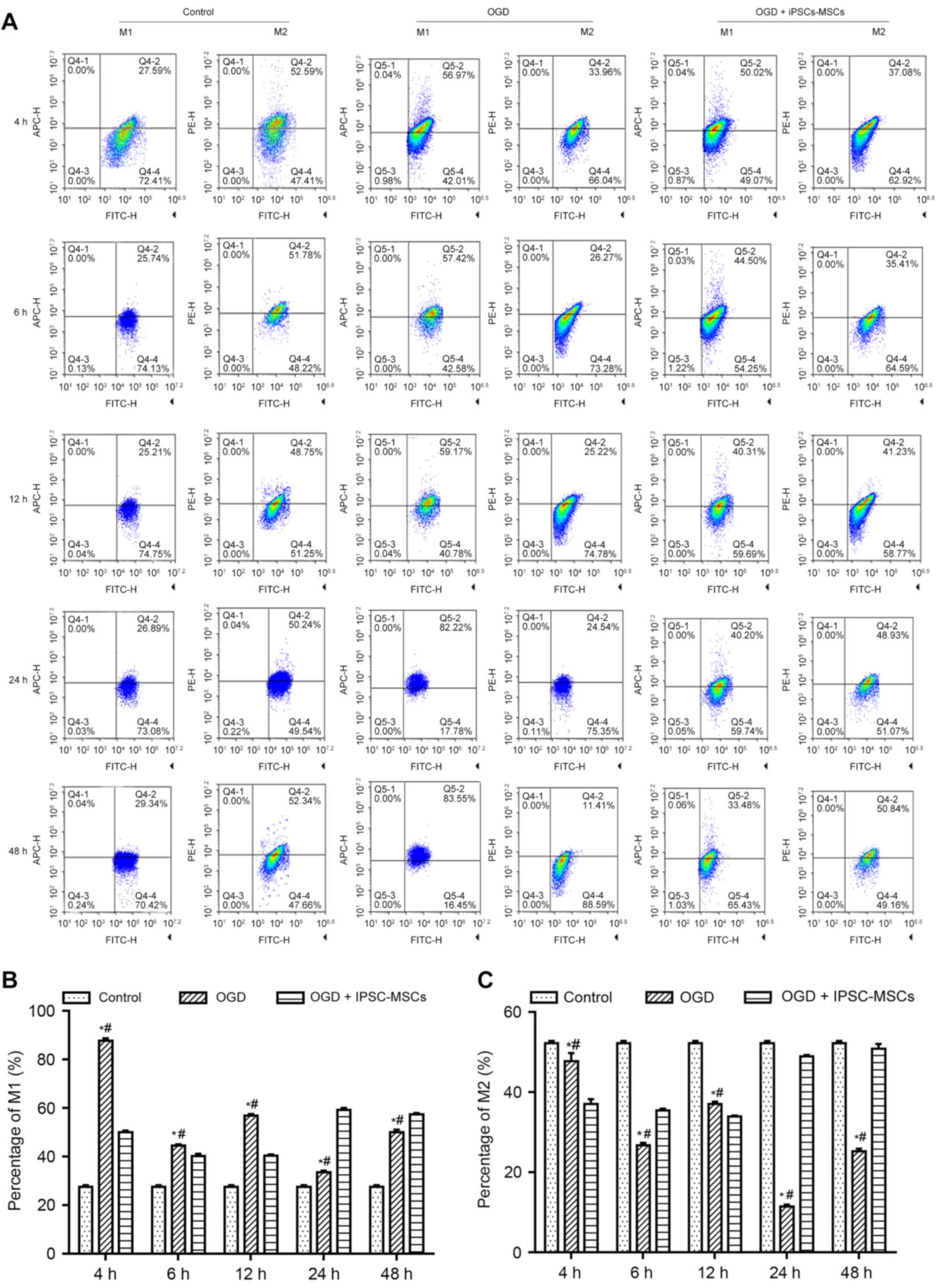 | Figure 3.(A) Flow cytometry analysis of the
control, OGD and OGD + IPSC-MSC groups at the 4, 6, 12, 24 and 48 h
time points (n=3). The bar graphs illustrate the (B) M1 and (C) M2
macrophage proportions in the control, OGD and OGD + IPSC-MSC
groups at the 4, 6, 12, 24 and 48 h time intervals (n=3).
*P<0.05 vs. control group; #P<0.05 vs. OGD +
IPSC-MSC group. OGD, oxygen and glucose deprivation; IPSC-MSCs,
induced pluripotent stem cell-derived mesenchymal stem cells. |
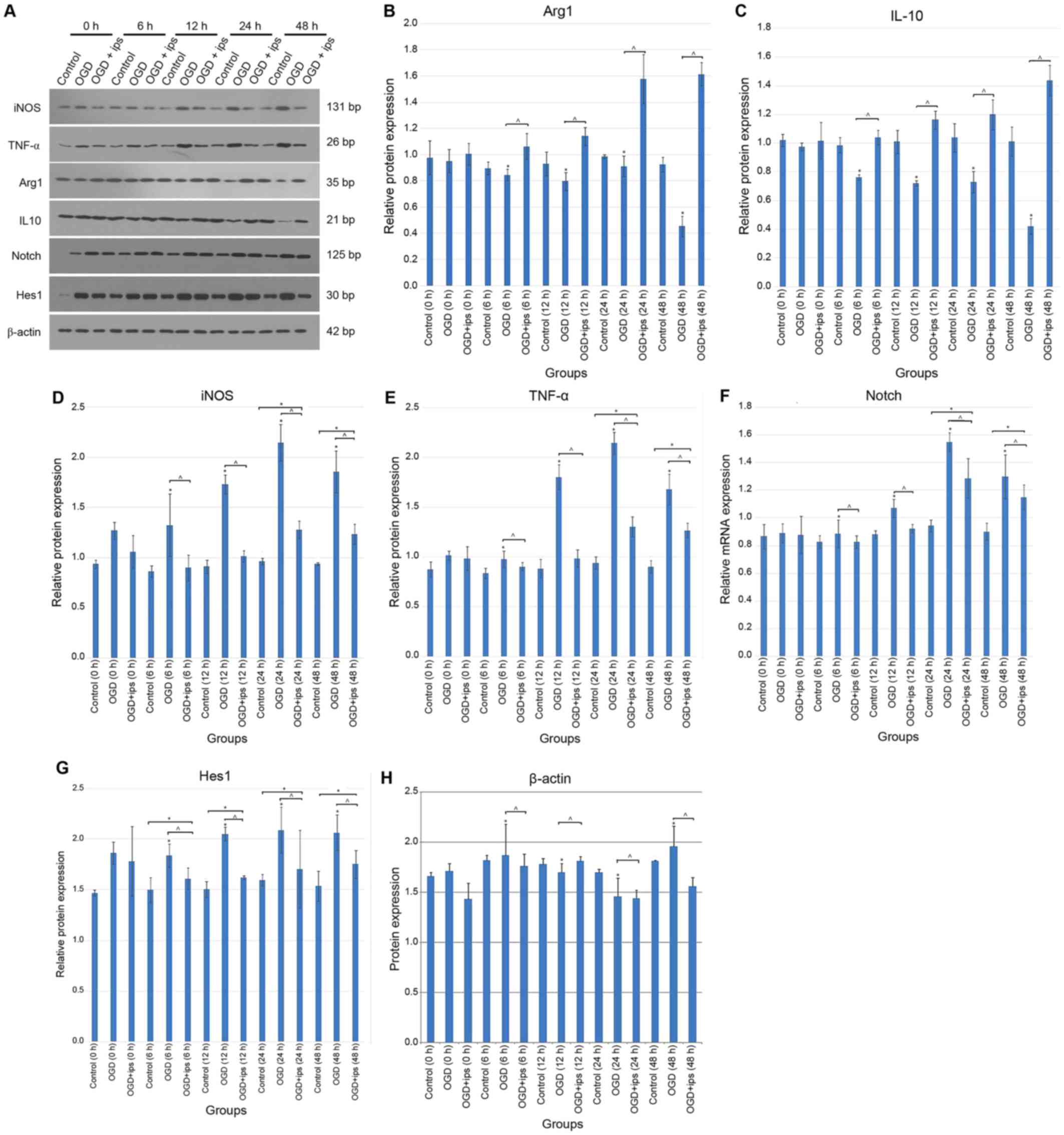 | Figure 5.(A) Representative western blot
analysis of the control, OGD and OGD + IPSC-MSC groups at the 0, 6,
12, 24 and 48 h time points. Quantification of the expression of
(B) Arg1, (C) IL-10, (D) iNOS, (E) TNF-α (F) Notch-1 (G) Hes1 and
(H) β-actin at 6, 12, 24 and 48 h time intervals for the
corresponding control, OGD and OGD + IPSC-MSC groups. Each bar
represents the mean ± standard error of the mean (n=3). *P<0.05
vs. control; ^P<0.05 vs. OGD + IPSC-MSC group. IL-10,
interleukin 10; Notch-1, neurogenic locus notch homolog protein-1;
Hes1, transcription factor HES-1; iNOS, inducible nitric oxide
synthase; TNF-α, tumor necrosis factor-α; Arg1, arginase 1; OGD,
oxygen and glucose deprivation; IPSC-MSCs, induced pluripotent stem
cell-derived mesenchymal stem cells; OGD + ips, OGD + IPSC-MSC
group. |
Effects of IPSC-MSC intervention on
the polarization of Raw 264.7 macrophages through the Notch-1
pathway
The Notch gene encodes a highly conserved cell
surface receptor that regulates the development of a variety of
biological cells, ranging from sea urchins to humans. Therefore,
the present study aimed to examine the expression of Notch-1 in the
OGD + IPSC-MSC group. Notch-1 protein and mRNA levels were
determined in each group. WB quantitative analysis demonstrated
that Notch-1 expression in the OGD + IPSC-MSC group [0.60±0.05
Absorbance Unit (A.U.)] was significantly decreased at 6, 12, 24
and 48 h compared with the OGD group (1.12±0.11 A.U.), and
significantly increased at 24 and 48 time intervals compared with
the control group (0.86±0.07 A.U.) (P=0.034, P<0.05; Fig. 5A and F). Additionally, RT-qPCR
analysis confirmed the effect of IPSC-MSC intervention on the
polarization of Raw 264.7 macrophages through the Notch-1 pathway.
A significant increase in Notch-1 in the OGD group was observed
compared with the control group at 6, 12, 24 and 48 h (OGD,
3.44±0.22 A.U.; P=0.014, P<0.05; Fig. 4).
Effects of IPSC-MSC intervention on
Hes1 and the polarization of Raw 264.7 macrophages post-OGD
In order to further characterize the effects of
Notch-1 in the OGD model, Hes1, a downstream effector of Notch-1,
was examined by WB and RT-qPCR analyses. Densitometric analysis of
Hes1 WB bands demonstrated a significant decrease in Hes1
expression in the OGD + IPSC-MSC group (1.69±0.17 A.U.) at 6, 12,
24 and 48 h compared with the OGD group (2.01±0.07 A.U.); however,
Hes1 expression in the OGD + IPSC-MSC group was significantly
increased compared with control groups at 6, 12, 24 and 48 h time
intervals (1.58±0.21 A.U.; P=0.036, P<0.05; Fig. 5A and G). Quantitative analysis of
the PCR results demonstrated a significant decrease in Hes1
expression in the OGD + IPSC-MSC group (2.5±0.37 A.U.) compared
with the OGD group (3.50±0.27 A.U), and a significant increase in
Hes1 expression in the OGD + IPSC-MSC group compared with the
control group (1.00 A.U.; P=0.031, P<0.05; Fig. 4).
Discussion
Cerebral hypoxic ischemia injury is a primary cause
of mortality and disability in emergency medicine (15). Although improvements in CPR
performance and the increasing success rate in achieving recovery
of spontaneous respiration and circulation in recent years, the
survival and discharge rate of patients post-sudden cardiac arrest
remain poor (16). However, in
Denmark and the USA, greater survival and favorable neurological
status were associated with hospital based post-resuscitative care
guidelines; therefore, study of the associated mechanisms is
required (17,18). Macrophages, a particular type of
immune cell, serve a role in innate immunity, including killing
pathogens. A previous study demonstrated the role of macrophage
polarization direction in anti-tumor immunity, infection, immune
responses, atherosclerosis, cardiovascular disease and diabetes
(19). In addition, glucose
tolerance abnormalities and other diseases have served an important
role (19). A study demonstrated
that axonal regeneration may be the primary target of and key
concern with nerve injury repair (20). Previous studies aiming to promote
axonal regeneration have demonstrated that the primary reason for
the failure of early regeneration in the adult mammalian central
nervous system (CNS) is the existence of myelin inhibitors
(21,22). Studies have confirmed that
macrophages/glial cells in the CNS exhibit chemotaxis, phagocytosis
of myelin fragments, and that it is possible to improve the
prognosis of neurological function (23,24).
However, previous studies have demonstrated that macrophages
mediate inflammatory responses in the process of neuronal repair,
resulting in secondary neuronal damage (25,26).
Therefore, in-depth examination of the regulation of macrophages in
the CNS may improve the prognosis of neurological function
following cardiac arrest. In the present study, the results
demonstrated that intervention with IPSC-MSCs affected the
polarization of Raw 264.7 macrophages via the Notch-1/Hes1
signaling pathway. However, additional experiments are required to
support the present results.
In previous studies, IPSC-MSCs have been induced by
an alteration in macrophage polarization direction to achieve a
protective effect in inflammatory diseases (27–29).
M2 macrophages serve an important role in combatting excessive
inflammatory injury and tissue repair during pathogen infection
(30). Following an injection of
IPSC-MSCs, the expression of CD206+ (M2) macrophages was
observed to increase in acute kidney-injured mice (31). IPSC-MSCs are able to reduce renal
tubular injury, reduce interstitial fibrosis, and increase the
CD206+/CD206− proportion of macrophages in
patients with unilateral fallopian tube obstruction caused by
aseptic nephritis (32). IPSC-MSC
intervention in a myocardial infarction model was confirmed to
alter the direction of M2 macrophage polarization (33). In addition, IPSC-MSCs exert a
protective effect on the phenotype and function of macrophages
during severe infections. Krasnodembskaya et al (34) demonstrated that an injection of
IPSC-MSCs may increase the proportion of CD206+
macrophages in the spleens of mice. A previous study demonstrated
that mice lacking the Notch-recombining binding protein suppressor
of hairless pathway in macrophages produce lower levels of specific
types of M1 macrophage and, therefore, exhibit a low-inflammation
phenotype (35). These results
indicated that IPSC-MSCs may regulate the surface molecules of
macrophages to increase the levels of CD206+
macrophages, accompanied by the role of tissue repair and other
protective effects (36–39). Therefore, it was hypothesized that
IPSC-MSCs may alter the polarization of macrophages, which may be a
central link in the immune regulatory network of brain
resuscitation and improve outcomes following cardiac arrest.
Arg1, IL-10, iNOS and TNF-α expression levels
confirmed that IPSC-MSCs may regulate the balance between
inflammation and anti-inflammation, reduce the inflammatory
responses of the body, inhibit inflammatory reactions, and induce
phenotypic alterations and functions of macrophages (40,41).
However, the mechanisms of how IPSC-MSCs alter the polarization of
macrophages remain unclear.
Previous studies have determined that IPSC-MSC
signaling pathways are involved in the differentiation of
macrophages, including the c-Jun N-terminal kinase,
phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein
kinase, Notch and tyrosine-protein kinase JAK/signal transducer and
activator of transcription signaling pathways (42,43).
The Notch gene encodes a highly conserved cell
surface receptor that regulates the development of a variety of
biological cells, ranging from sea urchins to humans. The Notch
signaling pathway consists of the Notch receptor,
delta-serrate-LAG-2 protein (Notch ligand), CSL protein, DNA
binding proteins and other regulatory molecules. The Notch-1
signaling pathway is directly associated with inflammation and
anti-inflammatory reactions. Upregulation of the Notch-1 signaling
pathway enhances the ability of macrophages to kill pathogens
(44). During the activation of
the Notch-1 signaling pathway, TNF-a and IL-6 expression levels
increase, inhibiting IL-6, which causes Notch-1 levels to decrease
and leads to a reduced inflammatory response. Therefore, Notch-1
may be an important signaling pathway in the regulation of
macrophage polarization and functional status (45). IPSC-MSC intervention may decrease
the apoptosis of macrophages in the OGD model (46,47).
The use of a Notch-1 receptor blocking agent or the disruption of a
downstream signaling pathway may lead to a decrease in the
expression of pro-inflammatory factors and an increase in the
expression of anti-inflammatory factors, which cause M2 macrophages
to bypass activation. The strength of the present study is that it
demonstrated that intervention with IPSC-MSCs affected the
polarization of Raw 264.7 macrophages via the Notch-1/Hes-1
pathway. However, there were limitations to the present study. A
Notch inhibitor was not used and only macrophage polarization was
observed. A grouping experiment for the dose of IPSCs-MSCs was not
performed. Other channel-associated factors were not detected and,
in future, in vivo experiments are required for functional
verification.
In the present study, WB and RT-qPCR analyses
confirmed that IPSC-MSC intervention affected the polarization of
Raw 264.7 macrophages via the Notch-1 pathway. RT-qPCR analysis
demonstrated a significant decrease in Hes1 expression in the OGD +
IPSC-MSCs group compared with the OGD group; and demonstrated a
significant increase compared with the control group. Therefore,
the results of the present study demonstrated that intervention
with IPSC-MSCs may influence the polarization of Raw 264.7
macrophages via the Notch-1/Hes1 signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
NSFC-2013-81372022), Science and Technology Planning Project of
Guangdong Province, China (grant no. 20140221), the Department of
Cardiology, Heart Center, The First Affiliated Hospital, Sun
Yat-Sen University, and the Key Laboratory on Assisted Circulation,
Ministry of Health (Guangzhou, China).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ made substantial contributions to the conception
and design of the present study. YY and DW interpreted the data. HL
and YL gave final approval to the manuscript to be published and
co-cultured the IPSC-MSCs and Raw 264.7 cells. ZX and JW performed
RT-qPCR, western blot analysis, FCM and the CCK-8 assay.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American heart association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X,
Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, et al: Functional
mesenchymal stem cells derived from human induced pluripotent stem
cells attenuate limb ischemia in mice. Circulation. 121:1113–1123.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng Y, Cai W, Zhou S, Xu L and Jiang C:
Protective effect of bone marrow derived mesenchymal stem cells in
lipopolysaccharide-induced acute lung injury mediated by claudin-4
in a rat model. Am J Translat Res. 8:3769–3779. 2016.
|
|
5
|
Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L,
Lu W and Han X: Mesenchymal stromal cell treatment prevents H9N2
avian influenza virus-induced acute lung injury in mice. Stem Cell
Res Ther. 7:1592016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang ZX, Sun JP, Wang P, Tian Q, Yang Z
and Chen LA: Bone marrow-derived mesenchymal stem cells protect
rats from endotoxin-induced acute lung injury. Chin Med J (Engl).
124:2715–2722. 2011.PubMed/NCBI
|
|
7
|
Lai TS, Wang ZH and Cai SX: Mesenchymal
stem cell attenuates neutrophil-predominant inflammation and acute
lung injury in an in vivo rat model of ventilator-induced lung
injury. Chin Med J (Engl). 128:361–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Zhao T, Liu B, Halaweish I,
Mazitschek R, Duan X and Alam HB: Inhibition of histone deacetylase
6 improves long-term survival in a lethal septic model. J Trauma
Acute Care Surg. 78:378–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kikuchi S, Nishihara T, Kawasaki S, Abe N,
Kuwabara J, Choudhury ME, Takahashi H, Yano H, Nagaro T, Watanabe
Y, et al: The ameliorative effects of a hypnotic bromvalerylurea in
sepsis. Biochem Biophys Res Commun. 459:319–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Sun G, Yu Y and Coy DH: Is Notch
signaling a specific target in hepatocellular carcinoma? Anticancer
Agents Med Chem. 15:809–815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tachikawa Y, Matsushima T, Abe Y, Sakano
S, Yamamoto M, Nishimura J, Nawata H, Takayanagi R and Muta K:
Pivotal role of Notch signaling in regulation of erythroid
maturation and proliferation. Eur J Haematol. 77:273–281. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kritis A, Pourzitaki C, Klagas I,
Chourdakis M and Albani M: Proteases inhibition assessment on PC12
and NGF treated cells after oxygen and glucose deprivation reveals
a distinct role for aspartyl proteases. PLoS One. 6:e259502011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai JCY, Rocha-Ferreira E, Ek CJ, Wang X,
Hagberg H and Mallard C: Immune responses in perinatal brain
injury. Brain Behav Immun. 63:210–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chokengarmwong N, Ortiz LA, Raja A,
Goldstein JN, Huang F and Yeh DD: Outcome of patients receiving CPR
in the ED of an urban academic hospital. Am J Emerg Med.
34:1595–1599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stub D, Schmicker RH, Anderson ML,
Callaway CW, Daya MR, Sayre MR, Elmer J, Grunau BE, Aufderheide TP,
Lin S, et al: Association between hospital post-resuscitative
performance and clinical outcomes after out-of-hospital cardiac
arrest. Resuscitation. 92:45–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wissenberg M, Lippert FK, Folke F, Weeke
P, Hansen CM, Christensen EF, Jans H, Hansen PA, Lang-Jensen T,
Olesen JB, et al: Association of national initiatives to improve
cardiac arrest management with rates of bystander intervention and
patient survival after out-of-hospital cardiac arrest. JAMA.
310:1377–1384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zanganeh S, Hutter G, Spitler R, Lenkov O,
Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M,
et al: Iron oxide nanoparticles inhibit tumour growth by inducing
pro-inflammatory macrophage polarization in tumour tissues. Nat
Nanotechnol. 11:986–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woolf CJ and Bloechlinger S: Neuroscience.
It takes more than two to Nogo. Science. 297:1132–1134. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ntranos A and Casaccia P: Bromodomains:
Translating the words of lysine acetylation into myelin injury and
repair. Neurosci Lett. 625:4–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Kelamangalath L, Kim H, Han SB,
Tang X, Zhai J, Hong JW, Lin S, Son YJ and Smith GM: NT-3 promotes
proprioceptive axon regeneration when combined with activation of
the mTor intrinsic growth pathway but not with reduction of myelin
extrinsic inhibitors. Exp Neurol. 283:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang K, Zheng J, Bian G, Liu L, Xue Q,
Liu F, Yu C, Zhang H, Song B, Chung SK, et al: Polarized
macrophages have distinct roles in the differentiation and
migration of embryonic spinal-cord-derived neural stem cells after
Grafting to injured sites of spinal cord. Mol Ther. 23:1077–1091.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gruber RC, Ray AK, Johndrow CT, Guzik H,
Burek D, de Frutos PG and Shafit-Zagardo B: Targeted GAS6 delivery
to the CNS protects axons from damage during experimental
autoimmune encephalomyelitis. J Neurosci. 34:16320–16335. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong XY, Liu L and Yang QW: Functions and
mechanisms of microglia/macrophages in neuroinflammation and
neurogenesis after stroke. Prog Neurobiol. 142:23–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rawji KS, Mishra MK, Michaels NJ, Rivest
S, Stys PK and Yong VW: Immunosenescence of microglia and
macrophages: Impact on the ageing central nervous system. Brain.
139:653–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song X, Xie S, Lu K and Wang C:
Mesenchymal stem cells alleviate experimental asthma by inducing
polarization of alveolar macrophages. Inflammation. 38:485–492.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao S, Mao F, Zhang B, Zhang L, Zhang X,
Wang M, Yan Y, Yang T, Zhang J, Zhu W, et al: Mouse bone
marrow-derived mesenchymal stem cells induce macrophage M2
polarization through the nuclear factor-κB and signal transducer
and activator of transcription 3 pathways. Exp Biol Med (Maywood).
239:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang QZ, Su WR, Shi SH, Wilder-Smith P,
Xiang AP, Wong A, Nguyen AL, Kwon CW and Le AD: Human
gingiva-derived mesenchymal stem cells elicit polarization of m2
macrophages and enhance cutaneous wound healing. Stem Cells.
28:1856–1868. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashley JW, Hancock WD, Nelson AJ, Bone RN,
Tse HM, Wohltmann M, Turk J and Ramanadham S: Polarization of
Macrophages toward M2 Phenotype Is Favored by Reduction in
iPLA2beta (Group VIA Phospholipase A2). J Biol Chem.
291:23268–23281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng Y, Zhang L, Fu B, Zhang J, Hong Q, Hu
J, Li D, Luo C, Cui S, Zhu F and Chen X: Mesenchymal stem cells
ameliorate rhabdomyolysis-induced acute kidney injury via the
activation of M2 macrophages. Stem Cell Res Ther. 5:802014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duffy MM, McNicholas BA, Monaghan DA,
Hanley SA, McMahon JM, Pindjakova J, Alagesan S, Fearnhead HO and
Griffin MD: Mesenchymal stem cells and a vitamin D receptor agonist
additively suppress T helper 17 cells and the related inflammatory
response in the kidney. Am J Physiol Renal Physiol.
307:F1412–F1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dayan V, Yannarelli G, Billia F, Filomeno
P, Wang XH, Davies JE and Keating A: Mesenchymal stromal cells
mediate a switch to alternatively activated monocytes/macrophages
after acute myocardial infarction. Basic Res Cardiol.
106:1299–1310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krasnodembskaya A, Samarani G, Song Y,
Zhuo H, Su X, Lee JW, Gupta N, Petrini M and Matthay MA: Human
mesenchymal stem cells reduce mortality and bacteremia in
gram-negative sepsis in mice in part by enhancing the phagocytic
activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol.
302:L1003–L1013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu H, Zhu J, Smith S, Foldi J, Zhao B,
Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, et al: Notch-RBP-J
signaling regulates the transcription factor IRF8 to promote
inflammatory macrophage polarization. Nat Immunol. 13:642–650.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tran TH, Mattheolabakis G, Aldawsari H and
Amiji M: Exosomes as nanocarriers for immunotherapy of cancer and
inflammatory diseases. Clin Immunol. 160:46–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun W, Pang Y, Liu Z, Sun L, Liu B, Xu M,
Dong Y, Feng J, Jiang C, Kong W and Wang X: Macrophage inflammasome
mediates hyperhomocysteinemia-aggravated abdominal aortic aneurysm.
J Mol Cell Cardiol. 81:96–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim OS, Seo CS, Kim Y, Shin HK and Ha H:
Extracts of Scutellariae Radix inhibit low-density lipoprotein
oxidation and the lipopolysaccharide-induced macrophage
inflammatory response. Mol Med Rep. 12:1335–1341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sánchez-Quesada C, López-Biedma A and
Gaforio JJ: Maslinic Acid enhances signals for the recruitment of
macrophages and their differentiation to m1 state. Evid Based
Complement Alternat Med. 2015:6547212015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herbert DR, Hölscher C, Mohrs M, Arendse
B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann
H, et al: Alternative macrophage activation is essential for
survival during schistosomiasis and downmodulates T helper 1
responses and immunopathology. Immunity. 20:623–635. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou D, Huang C, Lin Z, Zhan S, Kong L,
Fang C and Li J: Macrophage polarization and function with emphasis
on the evolving roles of coordinated regulation of cellular
signaling pathways. Cell Signal. 26:192–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeng KW, Song FJ, Wang YH, Li N, Yu Q,
Liao LX, Jiang Y and Tu PF: Induction of hepatoma carcinoma cell
apoptosis through activation of the JNK-nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase-ROS self-driven death signal
circuit. Cancer Lett. 353:220–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Singla RD, Wang J and Singla DK:
Regulation of Notch 1 signaling in THP-1 cells enhances M2
macrophage differentiation. Am J Physiol Heart Circ Physiol.
307:H1634–H1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fleming BD and Mosser DM: Regulatory
macrophages: Setting the threshold for therapy. Eur J Immunol.
41:2498–2502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu J, Chi F, Guo T, Punj V, Lee WN, French
SW and Tsukamoto H: NOTCH reprograms mitochondrial metabolism for
proinflammatory macrophage activation. J Clin Invest.
125:1579–1590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li B, Zhang H, Zeng M, He W, Li M, Huang
X, Deng DY and Wu J: Bone marrow mesenchymal stem cells protect
alveolar macrophages from lipopolysaccharide-induced apoptosis
partially by inhibiting the Wnt/beta-catenin pathway. Cell Biol
Int. 39:192–200. 2015. View Article : Google Scholar : PubMed/NCBI
|