Introduction
Mandibular defects are common and can be cause by
various traumas. Mandibular regeneration and reconstruction are
continuously difficult problems in clinical treatment. At present,
the most common bone repair procedures in the clinic are
transplantation with autogenous or allogeneic bone, and filling
with artificial biomaterials. However, there are numerous of
disadvantages of autogenous or allogeneic bone transplantation,
including difficult obtainment of suitable bone, insufficient
supply, dissatisfactory matching, susceptibility to infection,
rejection reaction and increased pain (1,2).
Therefore, bone transplantation creates great difficulties in
clinical treatments (3,4). In order to improve bone tissue
repair, bone tissue engineering has become a popular method. Stent
composites provide a novel strategy for bone repair in the wake of
the continuous research and development of bone tissue engineering
(5). Currently, it has been
reported that artificial biomaterials have significant effects on
bone repair, however there are also numerous deficiencies (6). Therefore, it is important to increase
the effectiveness of the filling material for the repair of
defective bone.
As special engineering plastic,
polyether-ether-ketone (PEEK) is a type of hypocrystalline organic
polymer with preferable and stable biocompatibility,
biodegradability, thermoplasticity, high temperature resistance,
corrosion resistance and wear resistance (7–9).
Additionally, it also has the advantages of radiation transparency
and nuclear magnetic resonance scanning without the generation of
artifacts (10), thus, it is used
as a substitute for spinal implants. This allows the extensive
application of PEEK in the medical field and it has become an
important orthopedic implant (11). PEEK had been widely accepted and
employed as a substitute for prosthesis and metal implants,
including lumbar fusion cage and cardiac valves, and has produced
satisfactory results (12,13). In addition, a variety of novel PEEK
composites have been successfully developed and applied to treat
oral bone defects. Bioceramic material has been extensively used in
oral clinics due to its superior biological characteristics
(14). Composites produced from
the combination of PEEK and biphasic bioceramic material are more
similar to endogenous biological structures, which will enhance the
repair function of filling materials on bone defects. However,
currently, the molecular mechanisms involved in the repair function
of PEEK-biphasic bioceramic (BBC) composites on bone defect require
further investigation.
Bone morphogenetic protein-2 (BMP-2) is a member of
the transforming growth factor-β superfamily. Numerous studies have
confirmed that BMP-2 can induce undifferentiated mesenchymal cells
to differentiate into bone cells, improving the activity and
proliferation of bone cells at bone defect sites, and sequentially
promoting the bone reconstruction and regeneration (15). BMP-2 is one of the strongest
factors with osteogenic capability in the BMP family (16); BMP-2 may promote bone repair
directly or indirectly.
Therefore, the present study aimed to reveal whether
BMP-2 is involved in the bone repair process of PEEK-BBC composites
by investigating the alterations in BMP-2 expression in the repair
effects of PEEK-BBC composites on mandibular defects in a rabbit
model.
Materials and methods
Materials
Bovine serum albumin (BSA) and Goldner trichrome
staining kit were obtained from Beijing Solarbio Science &
Technology Co., Ltd. (Beijing, China). A hematoxylin and eosin
(H&E) staining kit, rabbit anti-β-actin antibody (cat. no.
TA-09) and horseradish peroxidase (HRP)-labeled goat anti-rabbit
immunoglobulin G (IgG; H+L; cat. no. ZB-2301) were purchased from
OriGene Technologies, Inc. (Beijing, China). Rabbit anti-BMP-2
antibody was obtained from Abcam (Cambridge, MA, USA; cat. no.
ab6285). HiFiScript cDNA synthesis kit was from Jiangsu Kang for
the Century Biological Technology Co., Ltd. (Beijing, China).
Radioimmunoprecipitation assay (RIPA) lysis buffer was from
Applygen Technologies, Inc. (Beijing, China). The RNA extraction
kit was purchased from Tiangen Biotech Co., Ltd. (Beijing,
China).
Preparation of PEEK-BBC
composites
Following the obtainment of ethical approval from
the Institutional Ethical Committee of Hubei University of Medicine
(Shiyan, China) and written informed consent from ten patients,
twenty human tooth tissues were collected and pretreated to remove
the peripheral tissues. Subsequently, the tooth tissues were
immersed in 5% sodium hypochlorite solution for 24 h. Ultrasonic
washing was performed three times for 1 h each time. Following
dehydration in absolute ethanol for 1 h, first stage calcination
was initiated by calcining for 1 h at 800°C. The teeth were
immersed in 0.1 mol/l (NH4)2HPO4
for 24 h following natural cooling, and were then dried and
calcined for 1 h at 800°C again. The temperature was elevated to
1,150°C to calcine for another 2 h followed by natural cooling. The
teeth were ground and sieved to prepare bioceramic powder.
Organic foam materials were immersed in 10% NaOH and
heated to 60°C for 15 min, washed with pure water and dried.
Polyvinyl butyral (15 ml) and tooth powder (50 g) were dissolved in
300 ml of absolute ethanol by continuous stirring for 2 h.
Subsequently, the sponge was immersed in the mixture and baked at
80°C for 1 day. The composites were finally calcined at 1,250°C for
3 h. Following natural cooling, the composites were characterized
under a Schottky field emission scanning electron microscope
(Olympus Corporation, Tokyo, Japan).
The elemental components of the composites were
determined by inductively coupled plasma-atomic emission
spectrometry (ICP-AES) based on elemental analysis.
Animals
A total of 60 New Zealand white rabbits (2–3 months
old, 2–2.5 kg) were obtained from experimental animal center of
Medical College, Nanchang University (Nanchang, China). The animals
were fed in a room with 22–25°C, 40–60% humidity, and natural
light/dark cycle; prior to fasting food intake is free food and
water are changes once a day. The animals had free access to food
and water and were fasted 12 h prior to experiments. The study
protocol was approved by the Institutional Animal Care and Use
Committee of Hubei University of Medicine and was in accordance
with the guidelines established by the Chinese Council of Animal
Care (17).
Establishment of mandibular defect
model
A total of 60 rabbits were divided into four groups
(n=15): Control, sham, surgery and PEEK. Each rabbit was fixed on
an operating table and 30 mg/kg pentobarbital sodium (1 ml/kg) was
injected into the ear vein. The skin was incised to expose the
groove part of molars. The bone was ground with a dental grinder to
produce a square hole with 12×10×2 mm (length × width × depth). All
the groups were modeled on the same side. In the PEEK group, the
holes were filled with the PEEK-BBC composite stents. In the
surgery group, the holes were produced but not filled with the
composite stents. In the sham group, only the molar grooves were
exposed and defect treatment was not performed. Animals without any
treatment served as the control. Following the operation, 800,000
units of penicillin were injected for 3 consecutive days to prevent
infection. Rabbits in all groups were euthanized at 4, 8 and 16
weeks postoperation (n=5 per group) and the samples in the molding
sites were collected.
H&E staining
Mandibular samples were fixed in 10% neutral
formaldehyde buffer at room temperature for 1 h and decalcified in
5% hydrochloric acid for 3–4 days. Following paraffin embedding,
the samples were cut into slices (4-µm). The slices were incubated
in hematoxylin alcoholic solution (6%) for 2 min at room
temperature. Then, they were immersed in acid water and ammonia
water for color separation for several sec, respectively. Following
washing with running water for 1 h, the slices were immersed in
distilled water for a moment. The sections were then dehydrated in
70 and 90% ethanol for 10 min each. Subsequently, the slices were
incubated in eosin solution (0.5%) for 2–3 min at room temperature
and dehydrated in absolute ethanol; sections were transparentized
with 100% xylene for 5 min at room temperature and mounted with
Canada balsam. Finally, the sections were observed under a light
microscope (CKX41; Olympus Corporation).
Goldner trichrome staining
Following xylene deparaffination (100%) for 5 min at
room temperature, the paraffin sections were washed with ethanol
(80, 95, and 100%; each 5 min) at room temperature and immersed in
water. Then the sections were incubated in Weigert iron hematoxylin
solution for 25 min at room temperature. Prior to immersion in acid
alcohol solution (1%) for 3 sec at room temperature for
differentiation, the sections were washed with running water for 1
min. After washing with running water and distilled water, sections
were stained in acid Ponceau solution (0.1%) for 5 min at room
temperature. In the aforementioned process, a weak acid working
solution was prepared according to the 4:1 ratio of distilled water
to weak acid solution and the wash was conducted with the weak acid
working solution. Sections were stained in Orange G solution, till
Ponceau solution (0.1%) for 3–5 min at room temperature followed by
a wash with the weak acid working solution (pH 6.5) for 10 sec at
room temperature. Immediately, the sections were stained in
brilliant green solution for 5 min at room temperature and washed
three times with the weak acid working solution (pH 6.5) for 15 sec
at room temperature. Following washing with distilled water, the
slices were blotted up or dried in the air and then rapidly
dehydrated in absolute ethanol, and mounted with neutral balsam.
Finally, the sections were observed under a light microscope
(CKX41; Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from the samples and reverse
transcribed into cDNA according to the protocols of the HiFiScript
cDNA synthesis kit. The product was stored at −80°C. The RT system
(20 µl) comprised of: dNTP mix (4 µl), primer mix (2 µl), RNA
template (7 µl), 5X RT buffer (4 µl), dithiothreitol (DTT; 2 µl),
and HiFiScript (1 µl). Total RNA in diethylpyrocarbonate water was
mixed by vortexing and then transiently centrifuged at 4°C and
10,000 × g for 15 min to collect the solution on the tube wall into
the bottom of the tube; dNTP mix, primer mix, and RNA template were
then added. Following incubation at 70°C for 10 min, the mixture
was rapidly placed in ice bath for 2 min. Subsequently 5X RT
buffer, DTT and HiFiScript were added and the final mixture was
incubated at 50°C for 15 min and at 85°C for 5 min. The qPCR
reaction system (25 µl) constituted: RNase free double distilled
water (9.5 µl), cDNA/DNA (1 µl), forward primer (1 µl), reverse
primer (1 µl), and 2X UltraSYBR Mixture (12.5 µl, CW0957; ComWinBIO
Co., Ltd., Beijing, China). The sequences of primers are presented
in Table I. The qPCR parameters
were set as follows: Predegeneration for 3 min at 95°C,
denaturation for 10 sec at 95°C, annealing for 30 sec at 50–55°C,
elongation for 30 sec at 72°C, 40 cycles, and final elongation for
10 min at 72°C. The amplification products were separated via
agarose gel electrophoresis. β-actin served as internal control.
Relative expression levels of genes were calculated by using the
2−ΔΔCq method (18).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer (5′-3′) |
|---|
| Bone | Forward:
CGTGAGGATTAGCAGGTCTT |
| morphogenetic
protein-2 | Reverse:
GCTGGATTTGAGGCGTTT |
| β-actin | Forward:
ATCGTCCACCGTAAATGC |
|
| Reverse:
TGAAGTGGTAGTCGGGTG |
Western blotting
The samples were removed from liquid nitrogen
storage. The areas required were sawed and ground into fine powder.
The powder was added to RIPA buffer containing
phenylmethanesulfonyl fluoride. The lysate were centrifuged at
10,000 × g at 4°C for 10 min. The supernatant was collected and 5X
loading buffer was added. The mixture was boiled with boiling water
for 5 min and then centrifuged again at 10,000 × g at 4°C for 3 min
and the supernatant was collected. Protein concentration was
determined by bicinchoninic acid kit. SDS-PAGE gels were prepared
(15% separation gel for BMP-2, 15% separation gel for β-actin).
Protein samples (10 µg) and a marker were loaded, and
electrophoresis was initiated. The voltages were set as 60 and 80 V
to compress and separate proteins, respectively. Transfer buffer
was prepared and precooled. A polyvinylidene fluoride membrane was
tailored in accordance with the position of strips and then
activated in methanol for 15 sec. Sizeable gel containing target
bands was cut to prepare a ‘sandwich’ (sponge-filter
paper-gel-membrane-filter paper-sponge). According to the voltage
and molecular weight, duration of transfer was controlled to ~1.5
h. The membrane was immersed in 5% BSA overnight at 4°C for
blocking. Following washing, the membrane was incubated in primary
antibody buffer (rabbit anti-β-actin antibody, 1:2,000; rabbit
anti-BMP-2 antibody, 1:1,000) for 3 h at room temperature.
Following three washes for 10 min each time, the membrane was
incubated in secondary antibody buffer [HRP-labeled goat
anti-rabbit IgG (H+L), 1:2,000] for 2 h at room temperature, and
washed three times for 10 min. A luminescent solution
(SuperSignal® west pico chemiluminescent substrate,
RJ239676; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
applied onto the membrane. Finally, the membrane and the gray
values of the bands were analyzed by using a gel imaging system
(ChemiDoc™ XRS; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
a Quantity One software (v4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
A total of three repeats were performed and data was
presented as the mean ± standard deviation. Statistical analysis
was performed with one way analysis of variance followed by Tukey's
post hoc test using SPSS software (version 17.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
significantly significant difference.
Results
Preparation and characterization of
PEEK-BBC composites
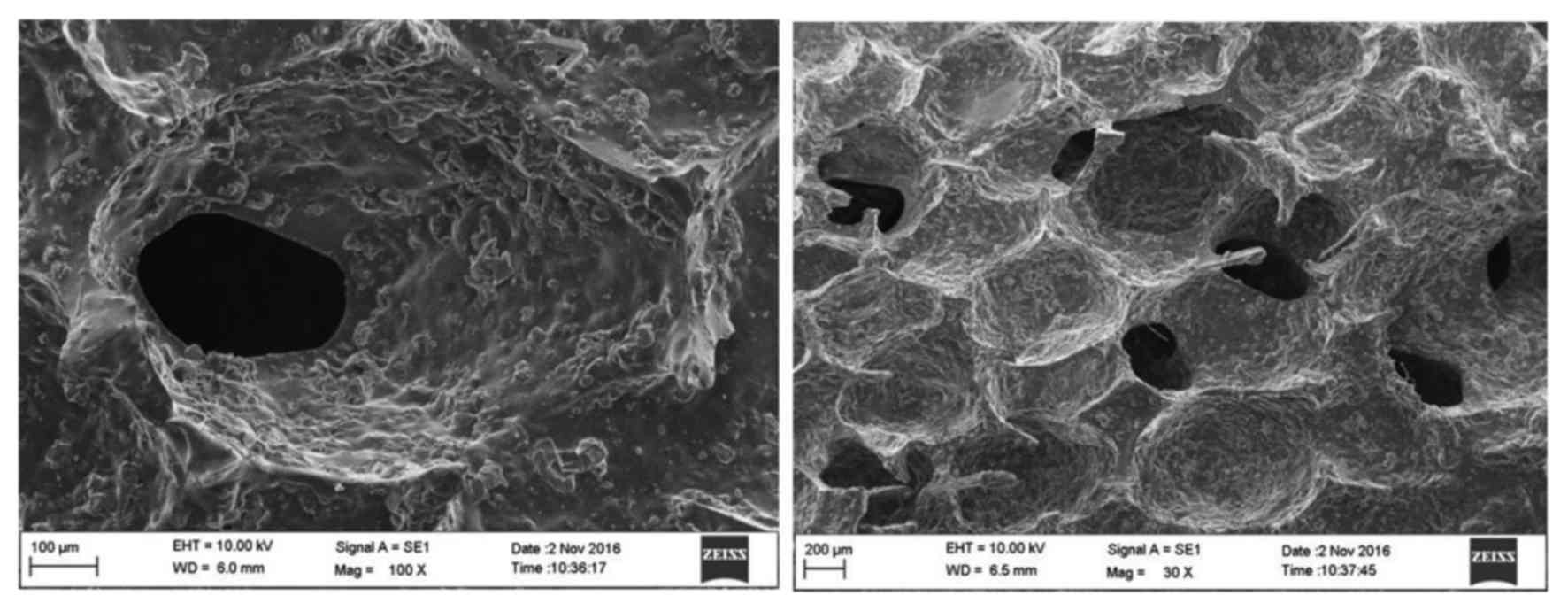
The microstructure of the PEEK-BBC composites
evaluated by scanning electron microscope is presented in Fig. 1. There were abundant rounded or
sub-rounded pores interconnected within the PEEK-BBC composites. It
was suggested that the PEEK-BBC composites are porous matrixes,
which may be favorable for the adhesion and growth of cells.
The elemental components of PEEK-BBC composites
determined by ICP-AES are presented in Table II. The analysis showed that the
composites were 40.5% calcium and 19.8% phosphorus. The atomic
ratio of calcium to phosphorus was 1.73. In addition, the presence
of magnesium, zinc, and potassium was detected. The elemental
analysis was performed to detect major elements and the remaining
elements may of other microelements that have not been determined
yet.
 | Table II.Elemental analysis of
polyether-ether-ketone biphasic bioceramic composites. |
Table II.
Elemental analysis of
polyether-ether-ketone biphasic bioceramic composites.
|
| Elements |
|---|
|
|
|
|---|
| Sample number | P (%) | Ca (%) | Mg (%) | Zn (%) | K (%) |
|---|
| 1 | 17.4 | 42.5 | 7.2 | 5.9 | 3.0 |
| 2 | 19.4 | 38.6 | 5.7 | 6.1 | 2.9 |
| 3 | 20.5 | 39.8 | 5.9 | 5.4 | 2.8 |
| 4 | 19.9 | 41.3 | 6.9 | 5.2 | 3.8 |
| 5 | 18.4 | 40.6 | 6.5 | 5.8 | 3.7 |
| Mean | 19.8 | 40.5 | 6.4 | 5.7 | 3.2 |
Establishment of the mandibular defect
model
By using a dental grinder, relatively large and deep
defect holes were produced. The success rate of the model
establishment was 100% with no mortalities. All rabbits lived well
with appropriate wound healing and did not exhibit any
complications, such as inflammatory reaction.
Pathological characterization by
H&E staining
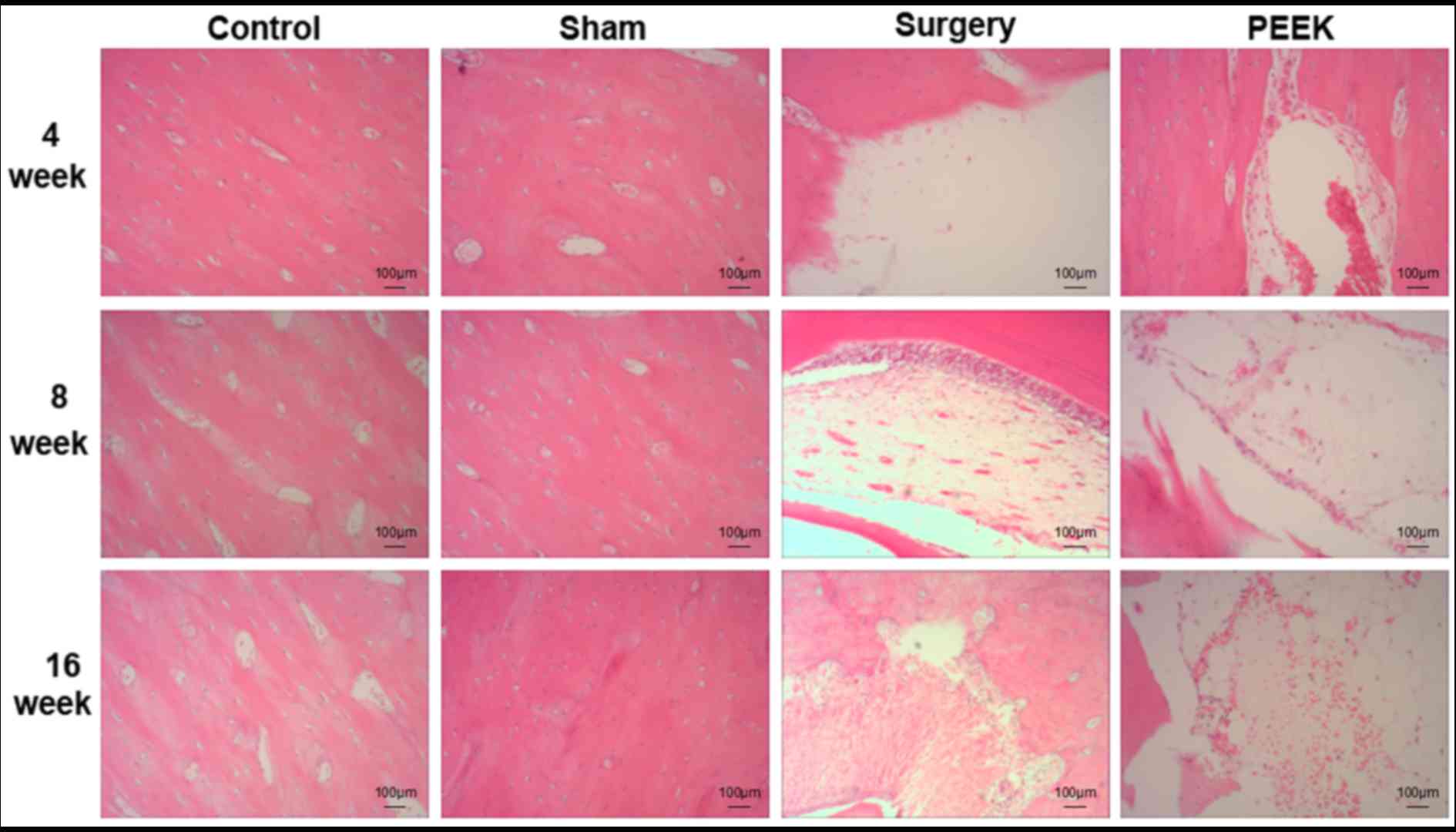
Pathological results of the mandibular tissues among
the four groups assessed by H&E staining were revealed in
Fig. 2. At all time intervals,
bone structures were intact in the control and sham groups. In the
bone defect positions in the surgery group, few markedly positive
alterations were observed, such as growth of very few cells at 4
weeks; however, the number of osteocytes was did not markedly
increase at 16 weeks. In the PEEK group, numerous osteocytes formed
within the pores of the PEEK-BBC composites at 4 weeks, and were
widely distributed in the composites at 8 and 16 weeks.
Pathological characterization by
Goldner trichrome staining
Fig. 3 demonstrates
the pathology of the mandibular tissues among the four groups
following Goldner trichrome staining. The mineralized bone, osteoid
and cartilage were stained green, orange-red and purple,
respectively. Additionally, the results of Goldner trichrome
staining verified those of H&E staining. Intact bone structures
in both control and sham groups were observed at all time
intervals. Bone defect status in the surgery group did not markedly
alter at 4 weeks due to the formation of a minute quantity of
cells. At 16 weeks, some osteocytes were observed; however, the
cell number remained low. By contrast, in the PEEK group,
osteocytes were evidently present in the PEEK-BBC composites at 4
weeks. At the subsequent 8 and 16 weeks, there were large numbers
of osteocytes in the pores of the composites.
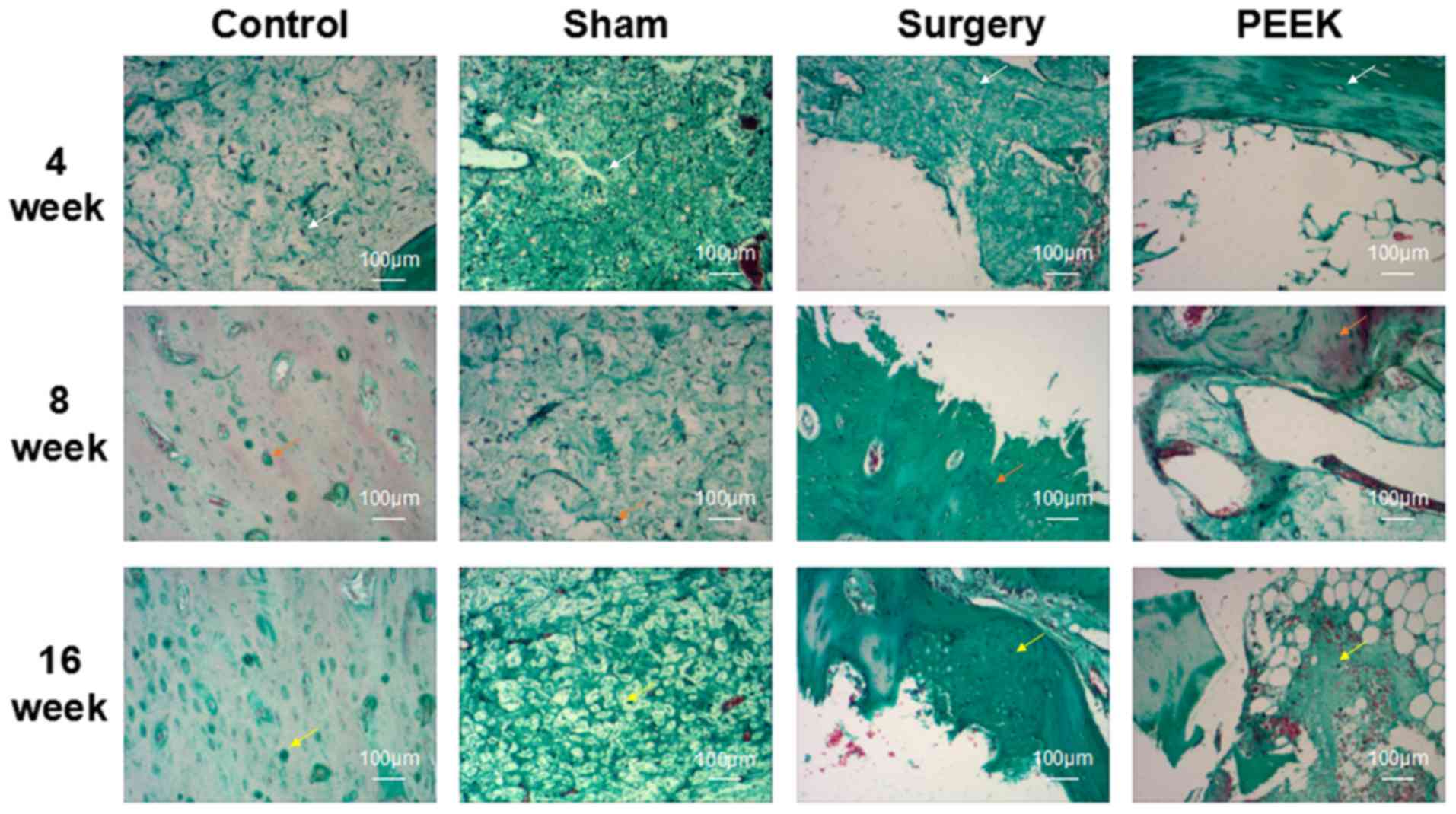 | Figure 3.Pathology of the mandibular tissues
among the control, sham, surgery and PEEK groups at 4, 8 and 16
weeks postoperation demonstrated by Goldner trichrome staining
(magnification, ×100). The mineralized bone, osteoid, and cartilage
were stained as white, orange and yellow, respectively. Arrows
indicate mineralized bone, osteoid and cartilage. PEEK,
polyether-ether-ketone. |
BMP-2 mRNA expression levels
determined by RT-qPCR
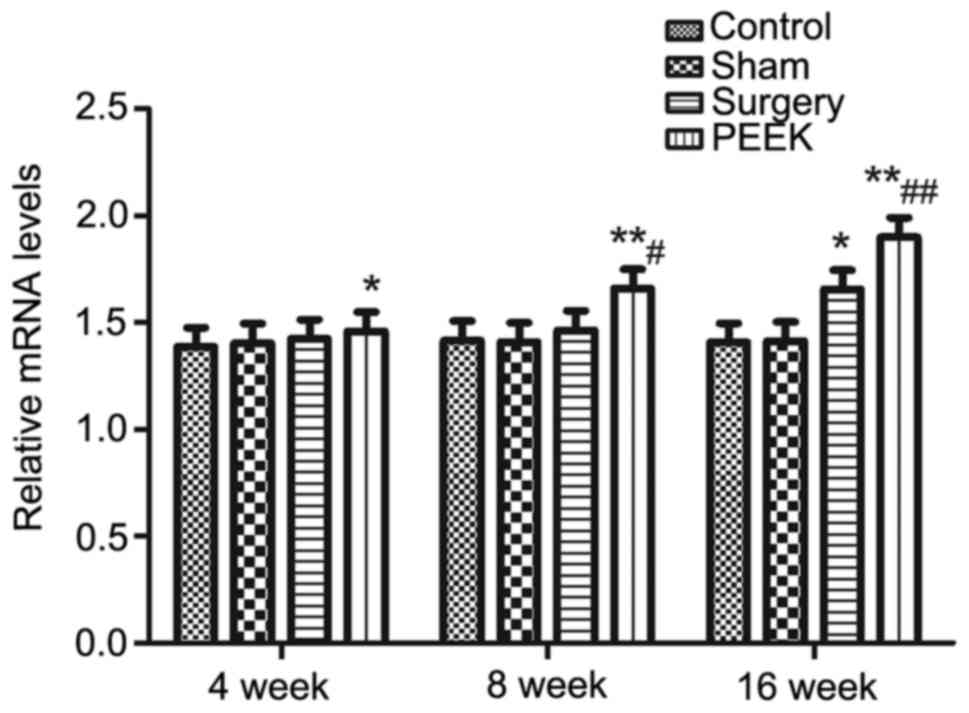
mRNA expression levels of BMP-2 in the mandibular
tissues among the various groups at different time intervals were
determined by RT-qPCR. The results are presented in Fig. 4 and β-actin served as the internal
control. The mRNA expression levels of BMP-2 between the control
and sham groups were similar and they were stable without any
difference among the three time intervals. Until 16 weeks, the mRNA
expression levels of BMP-2 in the surgery group was significantly
elevated compared with in the control group (P<0.05). However,
at 4, 8 and 16 weeks, the mRNA expression levels of BMP-2 in the
PEEK group were significantly increased as compared with in the
control group (P<0.05, P<0.01 and P<0.01, respectively).
As for the difference between the surgery and PEEK groups,
significantly higher levels of BMP-2 were detected in the PEEK
group at 8 and 16 weeks compared with in the surgery group
(P<0.05 and P<0.01, respectively).
BMP-2 protein expression levels
evaluated by western blotting
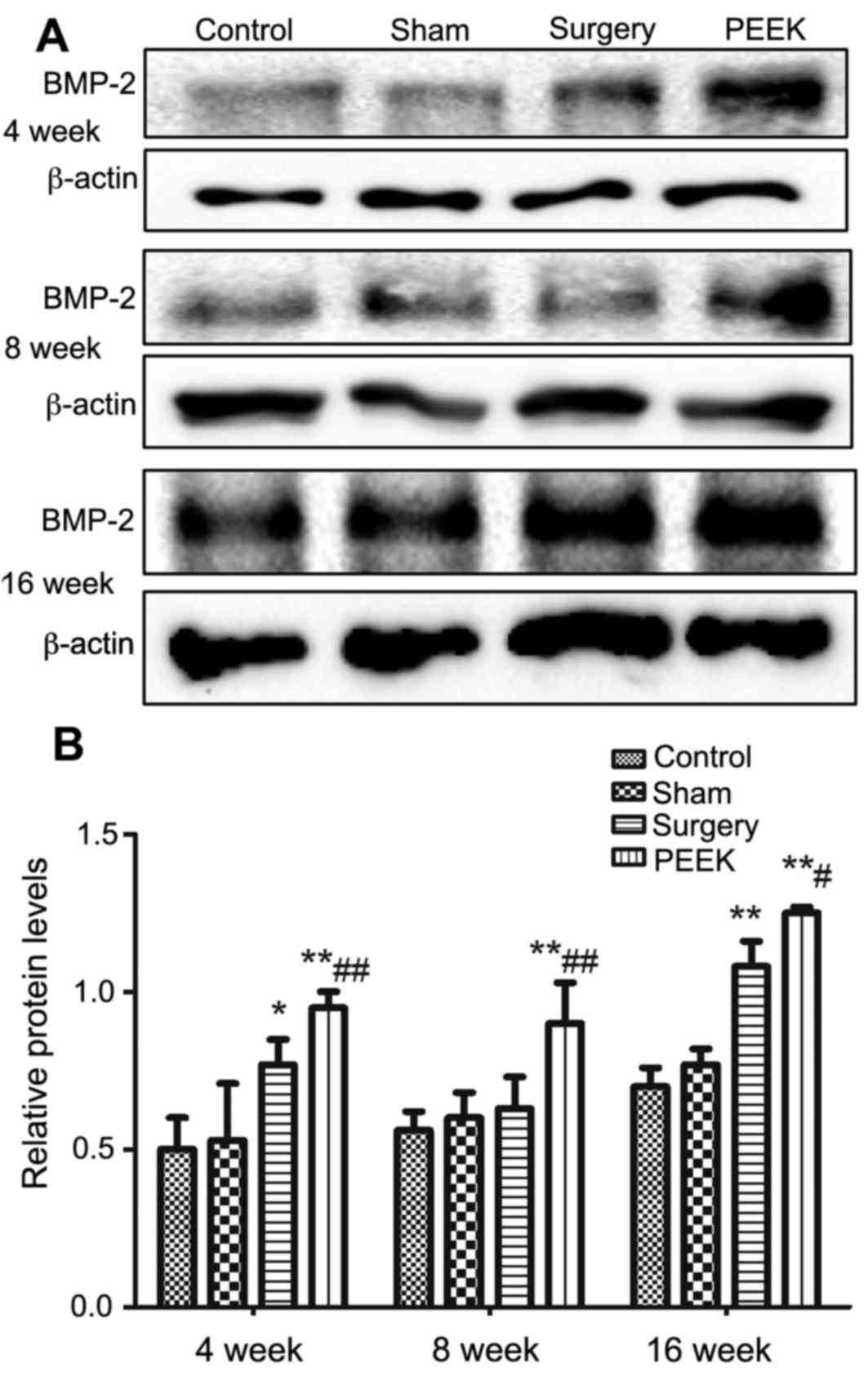
The protein expression levels of BMP-2 in the
mandibular tissues among diverse groups at different time intervals
was evaluated by western blotting. β-actin served as the internal
control for analysis (Fig. 5).
Variation tendency of BMP-2 protein expression levels were similar
to those of corresponding mRNA levels. At 4 and 8 weeks, compared
with the other three groups, markedly darker bands were observed in
the PEEK group. The expression of BMP-2 protein was significantly
upregulated by the PEEK-BBC composites treatment (P<0.01)
compared with the surgery group at 8 weeks. The longest duration
(16 weeks), revealed a slight enhancement of BMP-2 protein
expression of the control and sham groups compared with the earlier
time points. However, the BMP-2 band in the surgery group was
markedly darker, revealing higher expression of BMP-2 protein
compared with in the control group. Additionally, BMP-2 protein
expression levels were further upregulated in the PEEK group
compared with the surgery group (P<0.05). Consistently, the
expression of BMP-2 protein was similar between the control and
sham groups.
Discussion
The use of artificial biomaterials, such as
composites, have been reported to avoid the disadvantages of using
traditional autogenous or allograft bone to repair bone defects.
Additionally, artificial biomaterials are biocompatible; they are
able to promote cell proliferation and differentiation, and
facilitate bone tissue repair. Numerous studies have reported that
tissue engineering of bone using composites may be a novel strategy
for bone repair and reconstruction (3,19,20).
Bioceramic material is one of the most frequently
used materials in treating oral bone defects. It possesses
corresponding biological functions, commendable mechanical
properties and biocompatibility, corrosion resistance and wear
resistance. These characteristics make the potential used of
bioceramic materials very extensive (21–24).
It has been reported that numerous factors including, pore size,
structure distribution and morphology, influence the biological
function of biomaterials (25–27).
In the present study, PEEK/BBC composites were prepared via
calcination. Its major components were β-tricalcium phosphate and
hydroxylapatite, and the major elements were calcium and
phosphorus. Therefore, the chemical composition was similar to that
of human skeleton. Furthermore, scanning electron microscopy
revealed the network structure of these composites with
interconnected pores. This porous structure of composites may
provide an ideal environment for the growth and repair of bone
tissues. The degradation of the calcium component in the composites
may also participate in human metabolism, promoting bone repair and
reconstruction (28).
Mandibular defects in rabbits were produced using a
dental grinder. Following various treatments, such as composite
implanting, pathological evaluations were conducted using H&E
and Goldner trichrome staining, which demonstrated the
osteogenesis-promoting ability of PEEK-BBC composites. It was
demonstrated that PEEK-BBC composites were biocompatible and able
to effectively promote the growth and differentiation of
osteocytes, and consequently repair the defective bone tissues.
Bioactive factors have an important role in the
process of bone reconstruction. BMP has been verified to promote
the formation of bone tissue (29). BMP-2 is reported to promote cell
proliferation and differentiation, improve cell viability and
specifically induce osteoblast formation; BMP-2 may exhibit the
greatest effect on bone repair among >40 types of BMP. Wang
et al (30) revealed that
one of the characteristics of BMP-2 was bone induction; BMP-2 was
able to induce and accelerate the differentiation of bone marrow
stem cells into osteocytes. In the present study, the mRNA and
protein expression levels of BMP-2 were investigated by RT-qPCR and
western blotting, respectively. Within the groups with bone defect,
BMP-2 expression was upregulated. The results of the present study
indicated that that when bone was damaged, the tissues may produce
BMP-2 to induce and promote bone repair. Furthermore, the
upregulation of the BMP-2 expression was further enhanced by
implantation of the PEEK-BBC composites, suggesting that PEEK-BBC
composites efficiently elevated the expression of BMP-2 at the mRNA
and protein levels to promote bone reconstruction.
In conclusion, PEEK-BBC composites with
interconnected porous structure and biocompatibility were prepared
in the present study. These composites promoted the growth of
osteocytes and repaired mandibular defects in rabbits and
upregulating the expression of BMP-2. BMP-2 is reportedly involved
in the bone repair process of PEEK-BBC composites. These findings
may provide a novel strategy for drug development and clinical
treatment of mandibular defects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 51541202 and
81671831).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HY and WL designed study and wrote paper. YC, MM, DL
and JA collected and analyzed data. All authors performed the
study.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Animal Care and Use Committee of Hubei University of Medicine and
was in accordance with the guidelines established by the Chinese
Council of Animal Care (17).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Crane GM, Ishaug SL and Mikos AG: Bone
tissue engineering. Nat Med. 1:1322–1324. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rozen N, Lewinson D, Bick T, Meretyk S and
Soudry M: Role of bone regeneration and turnover modulators in
control of fracture. Crit Rev Eukaryot Gene Expr. 17:197–213. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Li M, Yu B, Cao L, Yang Q and Su
J: Nanocalcium-deficient hydroxyapatite-poly
(e-caprolactone)-polyethylene glycol-poly (e-caprolactone)
composite scaffolds. Int J Nanomedicine. 7:3123–3131.
2012.PubMed/NCBI
|
|
4
|
Levengood SK, Poellmann MJ, Clark SG,
Ingram DA, Yoder MC and Johnson AJ: Human endothelial colony
forming cells undergo vasculogenesis within biphasic calcium
phosphate bone tissue engineering constructs. Acta Biomater.
7:4222–4228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi Y, Yamamoto M and Tabata Y:
Enhanced osteoinduction by controlled release of bone morphogenetic
protein-2 from biodegradable sponge composed of gelatin and
beta-tricalcium phosphate. Biomaterials. 26:4856–4865. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jazayeri HE, Tahriri M, Razavi M, Khoshroo
K, Fahimipour F, Dashtimoghadam E, Almeida L and Tayebi L: A
current overview of materials and strategies for potential use in
maxillofacial tissue regeneration. Mater Sci Eng C Mater Biol Appl.
70:913–929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH, Jang HL, Lee KM, Baek HR, Jin K,
Hong KS, Noh JH and Lee HK: In vitro and in vivo evaluation of the
bioactivity of hydroxyapatite-coated polyetheretherketone
biocomposites created by cold spray technology. Acta Biomater.
9:6177–6187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salerno S, Piscioneri A, Laera S, Morelli
S, Favia P, Bader A, Drioli E and De Bartolo L: Improved functions
of human hepatocytes on NH3 plasma-grafted PEEK-WC-PU membranes.
Biomaterials. 30:4348–4356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dennes TJ and Schwartz J: A nanoscale
adhesion layer to promote cell attachment on PEEK. J Am Chem Soc.
131:3456–3457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ying Y: The study of the MRI artifacts
caused by two alloys of porcelain-fused-to-metal crown. Chin J Dent
Mater Dev. 19:72–74. 2010.(In Chinese).
|
|
11
|
Deng C, Liu D, Liu J and Liu X: Advance in
polyetheretherketone (PEEK) and its composite material for
orthopaedic implants. Biomed Eng Clin Med. 13:473–476. 2009.(In
Chinese).
|
|
12
|
Kurtz SM and Devine JN: PEEK biomaterials
in trauma, orthopedic, and spinal implants. Biomaterials.
28:4845–4869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pace N, Marinelli M and Spurio S:
Technical and histologic analysis of a retrieved carbon
fiber-reinforced poly-ether-ether-ketone composite alumina-bearing
liner 28 months after implantation. J Arthroplasty. 23:151–155.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Converse GL, Conrad TL, Merrill CH and
Roeder RK: Hydroxyapatite whisker-reinforced polyetherketoneketone
bone ingrowth scaffolds. Acta Boimater. 6:856–863. 2010. View Article : Google Scholar
|
|
15
|
Lü K, Zeng D, Zhang Y, Xia L, Xu L, Kaplan
DL, Jiang X and Zhang F: BMP-2 gene modified canine bMSCs promote
ectopic bone formation mediated by a nonviral PEI derivative. Ann
Biomed Eng. 39:1829–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seol YJ, Kim KH, Park YJ, Lee YM, Ku Y,
Rhyu IC, Lee SJ, Han SB and Chung CP: Osteogenic effects of
bone-morphogenetic-protein-2 plasmid gene transfer. Biotechnol Appl
Biochem. 49:85–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
General Administration of Quality
Supervision, Inspection and Quarantine of China: Laboratory
animal-Guideline of welfare ethical review (Draft for approval).
Mar 18–2017.(In Chinese).
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao F, Chen Y, Li Z, Wang Y, Shi B, Gong
Z and Cheng X: A novel bioactive three-dimensional beta-tricalcium
phosphate/chitosan scaffold for periodontal tissue engineering. J
Mater Sci Mater Med. 21:489–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu G and Fan Y: Preparation of
poly(D,L-lactic acid) scaffolds using alginate particles. J
Biomater Sci Polym Ed. 19:87–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G, Li W, Yu X and Sun K: Study of the
cohesion of TTCP/DCPA phosphate cement through evolution of
cohesion time and remaining percentage. J Mater Sci. 44:8282009.
View Article : Google Scholar
|
|
22
|
Sayer M, Stratilatov AD, Reid J, Calderin
L, Stott MJ, Yin X, MacKenzie M, Smith TJ, Hendry JA and Langstaff
SD: Structure and composition of silicon-stabilized tricalcium
phosphate. Biomaterials. 24:369–382. 2013. View Article : Google Scholar
|
|
23
|
Reid JW, Pietak AM, Sayer M, Dunfield D
and Smith TJ: Phase formation and evolution in the silicon
substituted tricalcium phosphate/apatite system. Biomaterials.
26:2887–2897. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang JH, Castano O and Kim HW: Electrospun
materials as potential platforms for bone tissue engineering. Adv
Drug Deliv Rev. 61:1065–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishikawa K: Bone substitute fabrication
based on dissolution-precipitation reactions. Materials.
3:1138–1155. 2010. View Article : Google Scholar :
|
|
26
|
Becker A, Epple M, Müller KM and Schmitz
I: A comparative study of clinically well-characterized human
atherosclerotic plaques with histological, chemical, and
ultrastructural methods. J Inorg Biochem. 98:2032–2038. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dorozhkin SV: Amorphous calcium
(ortho)phosphates. Acta Biomater. 6:4457–4475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonner M, Ward IM, McGregor W, Tanner KE
and Bonfield W: Hydroxyapatite/polypropylene composite: A novel
bone substitute material. J Mater Sci Lett. 20:pp2049–2051. 2001.
View Article : Google Scholar
|
|
29
|
Dean DB, Watson JT, Moed BR and Zhang Z:
Role of bone morphogenetic proteins and their antagonists in
healing of bone fracture. Front Biosci (Landmark Ed). 14:2878–2888.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang EA, Israel DI, Kelly S and Luxenberg
DP: Bone morphogenetic protein-2 causes commitment and
differentiation in C3H10T1/2 and 3T3 cells. Growth Factors.
9:57–71. 1993. View Article : Google Scholar : PubMed/NCBI
|