Introduction
Colorectal cancer (CRC) is the third most common
cancer type worldwide, and the fourth in China (1,2).
Chemotherapy has been the primary treatment for CRC in the past few
decades, although multidrug resistance (MDR) frequently leads to
its failure. Rebucci et al (3) reported a number of mechanisms of drug
resistance, including alterations in drug metabolism, failure of
DNA repair and apoptosis. Although these mechanisms have been
demonstrated to be associated with the development of MDR in CRC,
the regulators of chemotherapy resistance remain to be identified.
Therefore, novel targeted approaches are required to clarify the
underlying mechanisms of chemotherapy resistance, and more
efficient therapies are required to counter MDR.
Long non-coding RNA plasmacytoma variant
translocation 1 (lnc-PVT1) is located at 8q24 on the human
chromosome (4), and a number of
studies have reported that high expression of PVT1 reduces
apoptosis in hepatocellular carcinoma (5), ovarian cancer (6) and breast cancer (7). Xu et al (8) identified that the expression of PVT1
is associated with the short and long-term prognosis of patients
with gastric cancer, and that downregulation of PVT1 may be
regarded as a therapeutic approach for gastric cancer. In addition,
Fang et al (9) demonstrated
that PVT1 is highly expressed in pancreatic cancer cells, and that
sensitivity to chemotherapy is regulated by PVT1. However, whether
PVT1 serves a critical function in the MDR of CRC is unclear.
The present study aimed to investigate the role of
PVT1 in 5-fluorouracil (5-FU)-resistant CRC tissues and cell lines,
and to further study the association between PVT1 expression and
MDR-associated proteins, including MDR protein 1 (MRP1),
P-glycoprotein (P-gp), serine/threonine-protein kinase mTOR (mTOR)
and apoptosis regulator Bcl2 (Bcl-2). The results in the present
study may contribute to a novel therapeutic target for MDR in
patients with CRC.
Materials and methods
Patients and specimens
Samples of human tumor tissues were collected from
30 patients with primary CRC between September 2016 and December
2017. The patients with CRC (13 male and 17 female; 35–76 years old
with a median of 57.4 years) received 5-FU-based neoadjuvant
chemotherapy prior to surgical removal of tumors. 5-FU-sensitive
cases were defined by the following: Shrinkage of the primary
tumor; no enlargement of the primary tumor; and no new occurrence
of metastasis within 6–12 months (n=15); otherwise, the cases were
defined as 5-FU resistant (n=15). The present study was approved by
the Ethics Committees of Ningbo First Hospital (Zhejiang, China)
and Guangdong General Hospital (Guangdong, China), and written
informed consent was obtained from each patient.
Cells
CRC HCT-8 and HCT-116 cell lines (American Type
Culture Collection, Manassas, VA, USA) were cultured, and 5-FU
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to
generate drug-resistant CRC cells. HCT-8 and HCT-116 cells were
exposed to 5-FU in vitro at a continual stepwise increasing
dose (5, 7.5, 10 and 20 µM) (10).
All CRC cells were cultured in RPMI-1,640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C with 5%
CO2.
Cell transfection and cytotoxicity
assay
The lentiviral transfection vector of PVT1
overexpression tagged with green fluorescent protein (LV-PVT1-GFP)
and the negative control (NC) lentiviral vector were purchased from
the Shanghai Tumor Research Institute (Shanghai, China).
Transfection of PVT1 small interfering (si)RNA (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) was performed with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. PVT1
siRNA sense 5′-CGAACCTCCGUCCTCCCTATT-3′ and antisense
5′-TTGAGGAGTCGGAUGTCT-3′ strands were used. The lentiviral vector
that carried a non-targeting sequence was used as the NC of siPVT1.
HCT-8/5-FU and HCT-116/5-FU cells were seeded into 6-well plates
for 24 h, and subsequently transfected with PVT1 siRNA
oligonucleotides or a viral supernatant in Opti-minimum essential
medium (Invitrogen; Thermo Fisher Scientific, Inc.); the
transfected cells were grown at 37°C with 5% CO2 for
24–48 h, and stable cells were harvested for further study. A Cell
Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck KGaA) assay was used to
assess cell viability and survival rates, according to the
manufacturer's protocol (11). CRC
cells were incubated in 96-well plates at a density of
5×104 cells/well and were subsequently exposed to 4
different concentrations of 5-FU (0.25, 0.5, 0.75 and 1.0 µg/ml).
Following incubation, 10 µl CCK-8 reagent was added and the cells
were cultured for a further 2 h at 37°C and 5% CO2. The
absorbance was measured at 450 nm using a microplate reader (Xi'an
Guanyu Bio-Tech Co., Ltd., Xi'an, China).
Measurement of apoptosis
The cells were trypsinized and fixed in 75% ethanol
at 4°C for 25–30 min. The cell pellets were subsequently harvested
and incubated using an Annexin V-fluorescein
isothiocyanate/propidium iodide apoptosis detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.). Cellular apoptosis
was evaluated via flow cytometry (Phoenix Flow Systems, San Diego,
CA, USA) within 5 min and analyzed using a flow cytometer (Coulter
EPICS XL-MCL FACScan, BD Biosciences, Franklin Lakes, NJ, USA). The
data was analyzed using the MultiCycle Software for Windows version
5.0 (Phoenix Flow Systems, San Diego, CA, USA).
Western blotting
The cellular proteins were extracted and separated
electrophoretically via SDS-PAGE, and subsequently probed with
antibodies against MRP1 (cat. no. ab3368, 1:1,500; Abcam,
Cambridge, UK), P-gp (cat. no. ab129450, 1:3,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mTOR (cat. no. ab2732,
1:1,500, Abcam), Bcl-2 (cat. no. ab59348, 1:1,000; Santa Cruz
Biotechnology, Inc.), and anti-GAPDH (cat. no. ab9485, 1:1,000;
Santa Cruz Biotechnology, Inc.) as a control. Quantification of the
expression levels of these proteins was performed using
Quantity-One-Software 29.0 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from fresh frozen CRC
tissues or cells using TRIzol® (Life Technologies;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. cDNA was synthesized using the Hi-Fi Script Reverse
Transcriptase kit (CWBio, Beijing, China). Amplification and
detection of mRNA was performed using SYBRGreen (Applied
Biosystems; Thermo Fisher Scientific, Inc.), with GAPDH as the
internal control. The primer sequences used were as follows: PVT1,
forward 5′-CAGCACTCTGGACGGAC-3′; reverse 5′-CAACAGGAGAAGCAAACA-3′;
MRP1, forward 5′-GCACGTGCACTACCATGTCA-3′; reverse
5′-CTGGTCTCTGCACTCATCTTGCGC-3′; P-gp, forward
5′-GCACGCATCGCCTTAG-3′, reverse 5′-AGTTGTCCATCATTATCCC-3′; mTOR,
forward 5′-GCCCAGACTGCGATGCCAGTAGG-3′, reverse
5′-GAGCACTGACGACAGTACCAGGCC-3′; Bcl-2, forward
5′-AGCAGCAAGTAGGTGTCCCAG-3′, reverse 5′-CTCCACGCCATCTTGCTTCT-3′;
GAPDH, forward 5′-TCCAGAGTGCAAGGCTTCAG-3′, reverse
5′-ACAGCACGCAGTAGCAGTA-3′. The PCR conditions were as follows: 94°C
for 2 min, followed by 94°C for 30 sec, 60°C for 30 sec and 72°C
for 1 min for 30 cycles, and 72°C for 10 min. The relative
expression of mRNA levels was calculated using the
2−ΔΔCq method (12).
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analysis and graph construction was
conducted using GraphPad version 7.5 software (GraphPad Software,
Inc., La Jolla, CA, USA). All experiments were repeated three
times, and differences between the groups were analyzed using
one-way analysis of variance with Fisher's least significance
difference test as the post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
lncRNA PVT1 is associated with 5-FU
resistance in human CRC tissues and cells
MDR is a key factor leading to failure of
chemotherapy. A previous study indicated that overexpression of
PVT1 in human colonic mucosal cells is associated with increased
morbidity from CRC (13). However,
whether PVT1 is associated with MDR remains to be elucidated. To
determine the association between PVT1 expression and 5-FU
resistance, 5-FU-sensitive (n=15) and 5-FU-resistant (n=15) tissues
were collected from patients with CRC to examine the expression of
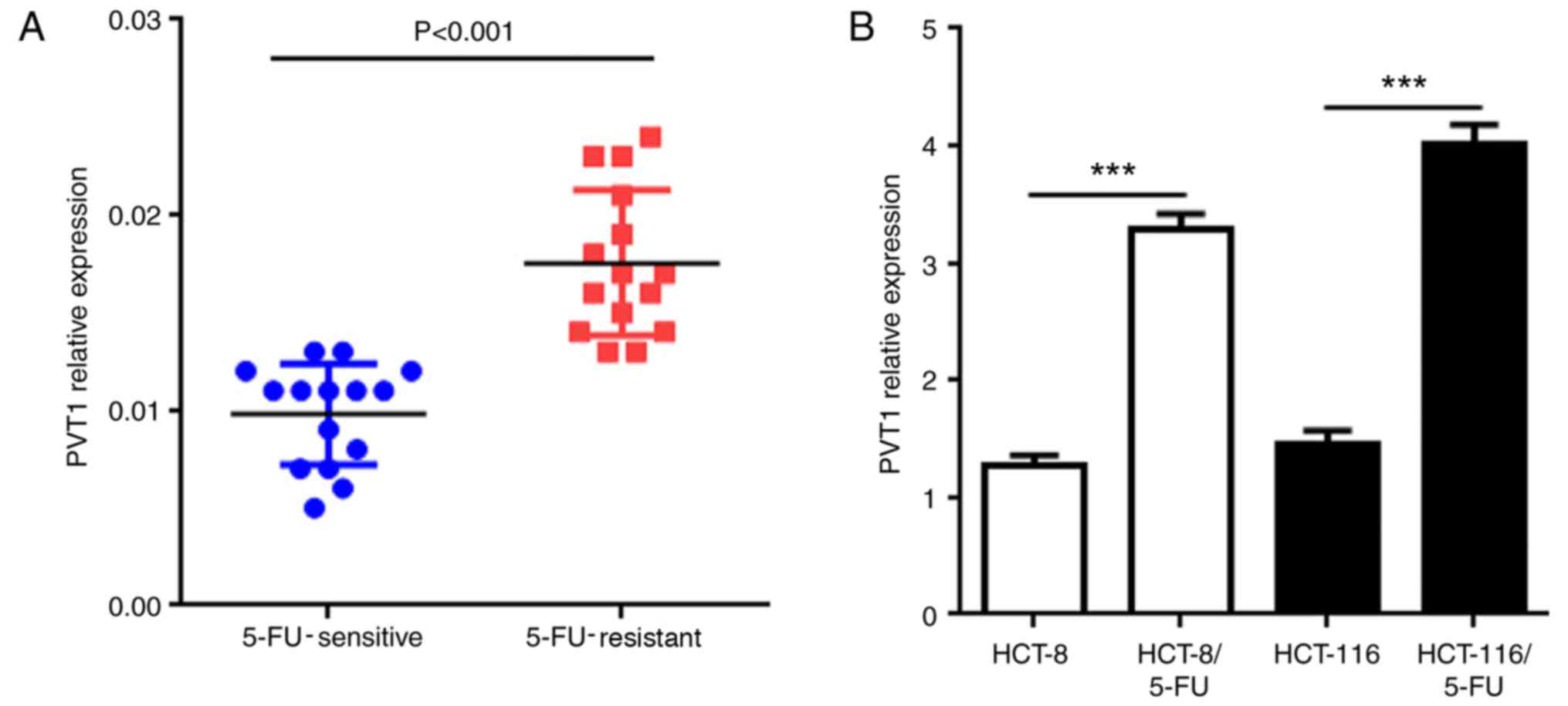
PVT1 mRNA. The results of the present study demonstrated that the
PVT1 mRNA expression was significantly upregulated in
5-FU-resistant CRC tissues compared with that in the 5-FU-sensitive
CRC tissues (Fig. 1A; P<0.001).
To further investigate these different expression levels of PVT1
mRNA, two 5-FU resistant human CRC cell lines (HCT-8/5-FU and
HCT-116/5-FU) were established by exposing the cells to 5-FU. The
results demonstrated that PVT1 mRNA exhibited significantly higher
expression in HCT-8/5-FU and HCT-116/5-FU cells compared with the
untreated cells of the same cell type (Fig. 1B; P<0.001). These results
suggested that the expression of PVT1 may be associated with the
development of 5-FU resistance in human CRC.
Knockdown of lncRNA PVT1 reverses drug
resistance in 5-FU-resistant CRC cells
To further investigate the potential associations
between the expression levels of PVT1 and 5-FU resistance in CRC,
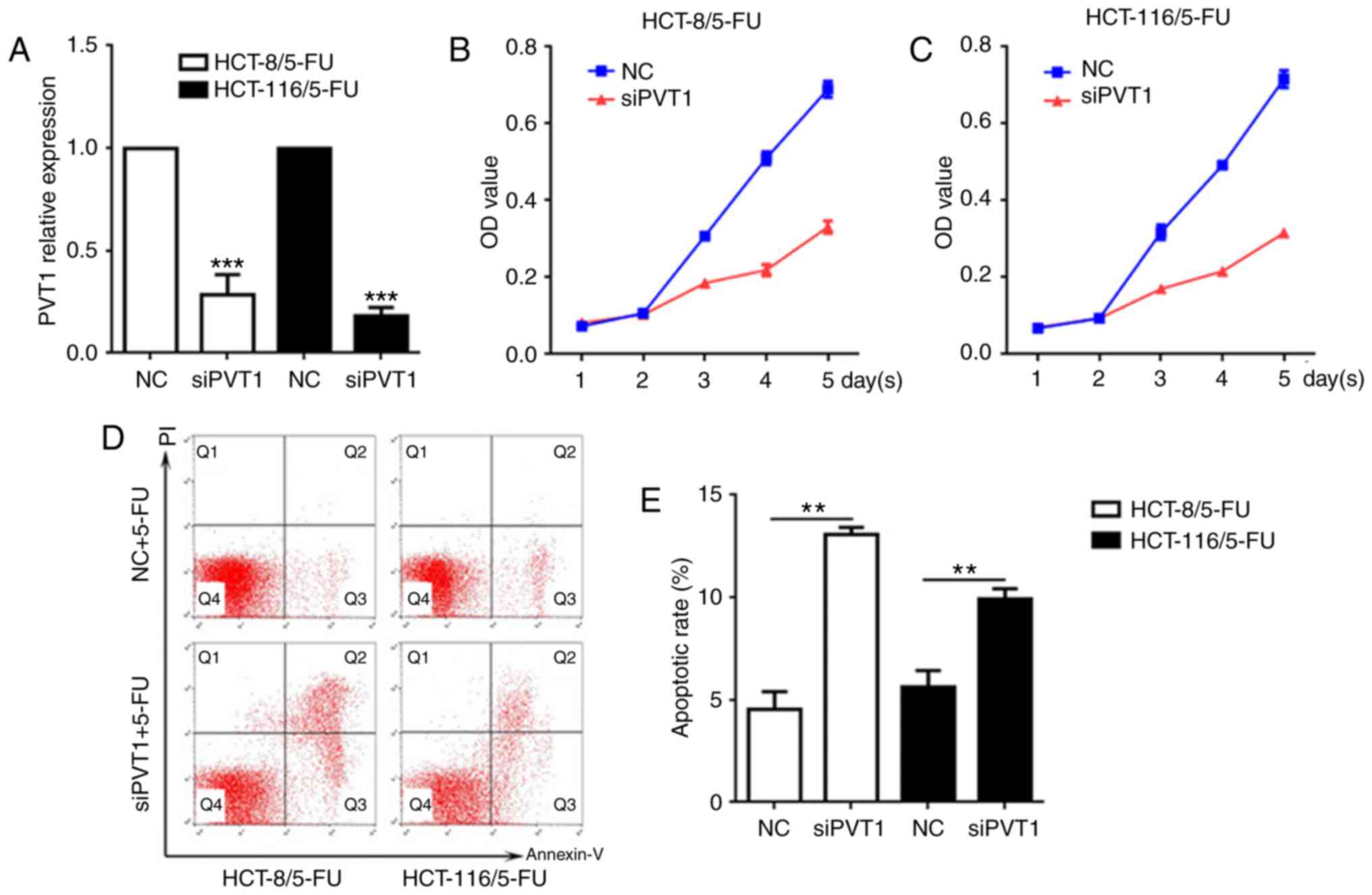
PVT1 was knocked down in HCT-8/5-FU and HCT-116/5-FU cells, and
cellular cytotoxicity and apoptosis were examined. The expression
of PVT1 mRNA was significantly decreased in HCT-8/5-FU and
HCT-116/5-FU cells exposed to siPVT1 compared with the NC of each
respective cell line (Fig. 2A;
P<0.001). Following transfection with siPVT1, the two drug
resistant cell lines were treated with 5-FU for 5 days (The median
effective dose value was the smallest at 0.5 µg/ml 5-FU, thus 0.5
µg/ml 5-FU was used in the experiment). The CCK-8 assay
demonstrated that the cells transfected with siPVT1 had decreased
viability and survival rates compared with the NC groups of the
same cell line at the same time points following treatment with
5-FU (Fig. 2B and C). To confirm
whether the decreased viability and survival rates of these cells
were induced by apoptosis, the apoptosis rate was determined
following transfection with siPVT1 and treatment with 5-FU (0.5
µg/ml). The results of the present study demonstrated that the
proportion of apoptotic cells was significantly increased in the
siPVT1 groups of the two cell lines compared with the NC groups
(Fig. 2D and E; P<0.01),
suggesting that the knockdown of PVT1 may reverse the drug
resistance of 5-FU-resistant CRC cell lines by inducing
apoptosis.
Overexpression of lncRNA PVT1
suppresses the apoptosis of CRC cells
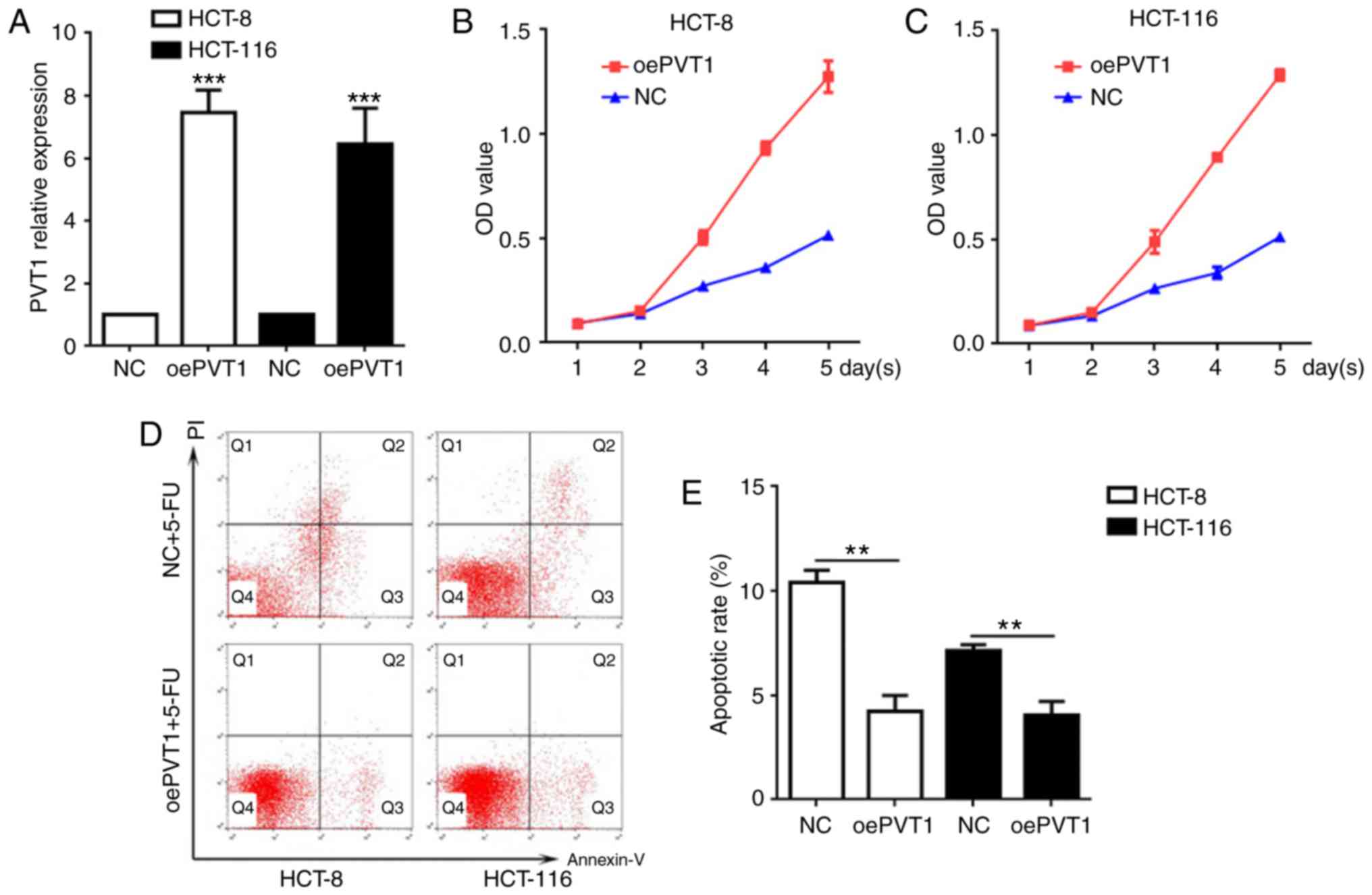
Following transfection with LV-PVT1-GFP and
treatment with 5-FU, the association between PVT1 overexpression
and cellular apoptosis was further evaluated. The PVT1 mRNA levels
in LV-PVT1-GFP-transfected HCT-8 and HCT-116 cells were 7.6- and
7.2-fold higher, respectively, compared with cells in the NC groups
(Fig. 3A). These results indicated
that LV-PVT1-GFP-transfected HCT-8 and HCT-116 cells contributed to
the upregulation of PVT1 mRNA levels. Furthermore, the CCK-8 assay
demonstrated that LV-PVT1-GFP transfected HCT-8 and HCT-116 cells
had higher viability compared with the respective NC groups
(Fig. 3B and C), and the flow
cytometric analysis indicated that LV-PVT1-GFP transfected HCT-8
and HCT-116 cells had a significantly decreased apoptosis rate
compared with cells in the NC groups (Fig. 3D and E; P<0.01). These results
indicated that PVT1 overexpression suppressed 5-FU induced
apoptosis in HCT-8 and HCT-116 cells.
Association between lncRNA PVT1 and
MDR-associated proteins
High expression of MDR-related proteins is
considered to be one of the primary MDR mechanisms in tumor cells
(14). To study the effect of PVT1
overexpression in 5-FU resistant CRC cell lines, the mRNA of
MDR-associated proteins was detected by RT-qPCR, including MRP1,
P-gp and mTOR and an inhibitor of apoptosis, Bcl-2. The mRNA
expression levels of MRP1, P-gp, mTOR and Bcl-2 in
LV-PVT1-GFP-transfected HCT-8 and HCT-116 cells were significantly
increased compared with the cells of the NC groups (Fig. 4A and B; P<0.001). In addition,
the protein expression levels of MRP1, P-gp, mTOR and Bcl-2 were
examined via western blot analysis. The protein expression levels
of MRP1, P-gp, mTOR and Bcl-2 were significantly increased in the
LV-PVT1-GFP-transfected HCT-8 and HCT-116 cells, compared with the
cells of the NC groups (Fig.
4C-F).
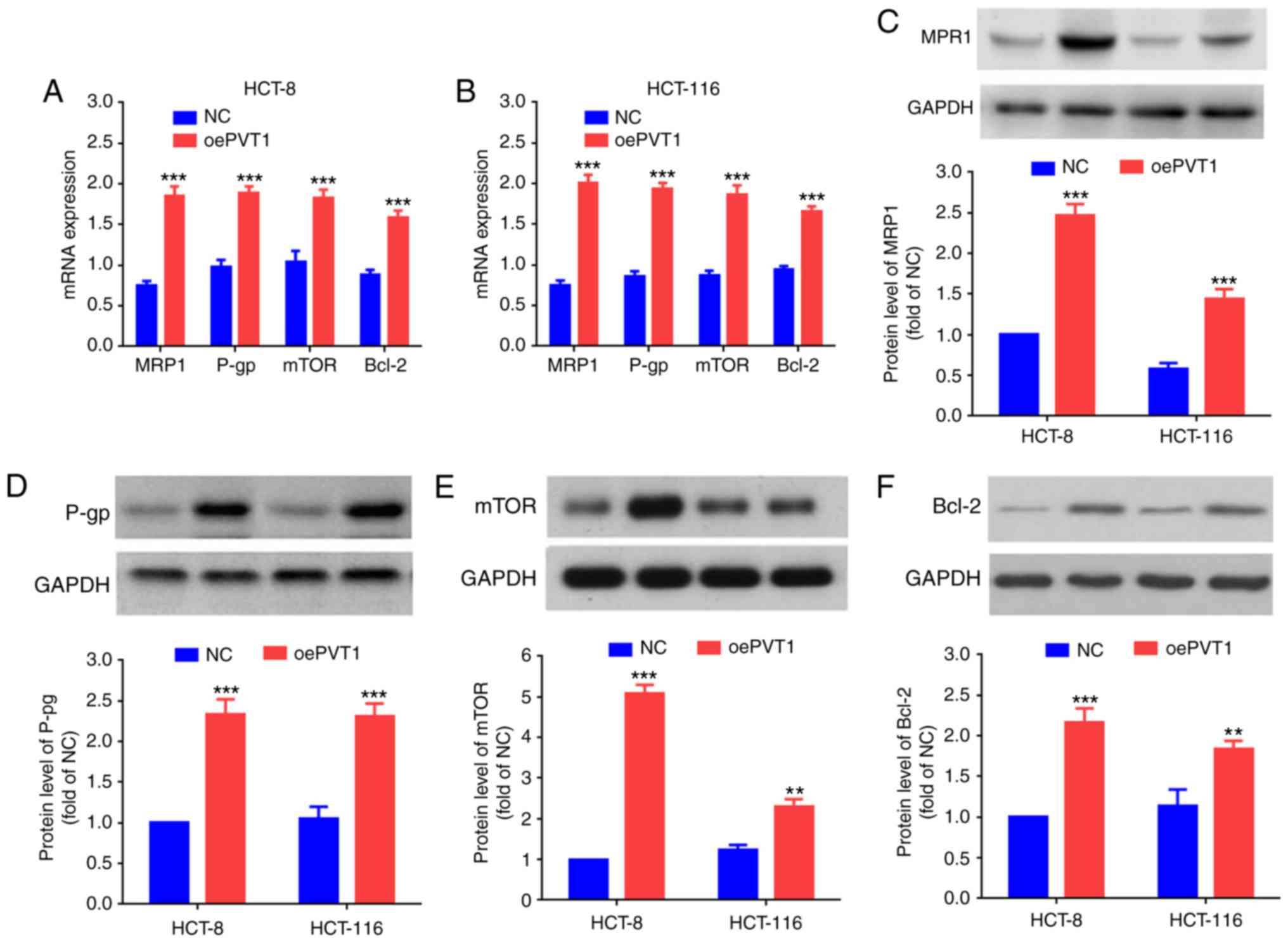 | Figure 4.PVT1 influences the expression of
genes and proteins associated with MDR. mRNA expression levels of
MRP1, P-gp, mTOR and Bcl-2 in (A) the HCT-8 cells transfected with
LV-PVT1-GFP and (B) the HCT-116 cells transfected with LV-PVT1-GFP.
Protein expression levels of (C) MRP1, (D) P-gp, (E) mTOR and (F)
Bcl-2 in the HCT-8/5-FU cells and HCT-116/5-FU cells transfected
with LV-PVT1-GFP. GAPDH was used as a loading control. All values
are presented as the mean ± standard deviation. **P<0.01 and
***P<0.001 vs. respective NC. PVT1, plasmacytoma variant
translocation 1; 5-FU, 5-fluorouracil; LV-PVT1-GFP, lentiviral
vector-PVT1-green fluorescent protein; MDR, multidrug resistance;
NC, negative control; MRP1, multidrug resistance protein 1; P-gp,
P-glycoprotein; mTOR, serine/threonine-protein kinase mTOR; Bcl-2,
apoptosis regulator Bcl2; oePVT1, overexpression of PVT1. |
Discussion
Mortality from CRC has been reduced due to
commitment to CRC screening, the reduction of risk factors, and
improvements in therapeutic measures, although the morbidity in a
number of countries continues to increase (15). 5-FU has been widely used as an
important chemotherapeutic agent in CRC, as it is able to rapidly
shrink tumor mass; however, MDR may be a cause of failure to
eliminate tumor cells thoroughly. Therefore, it is necessary to
clarify the exact mechanisms of MDR.
Numerous studies have elucidated the effectiveness
of lncRNA PVT1 in human cancer, including breast (7), cervical (16), thyroid (17), gastric (18) and ovarian (19) cancer. In these previous studies,
PVT1 has been demonstrated to exert different effects. Liu et
al (6) demonstrated that the
overexpression of PVT1 facilitates cellular apoptosis in ovarian
cancer and inhibits tumor growth, suggesting that PVT1 has
antitumor properties. By contrast, Wan et al (20) identified that PVT1 overexpression
is associated with increased lung cancer lymph node metastasis and
poor overall survival, while PVT1 knockdown may inhibit cell
proliferation and induce apoptosis, indicating that PVT1 has
potential oncogenic activity. In addition, certain studies have
reported that PVT1 facilitates the development of MDR in certain
cancer cases (5,21). Zhang et al (21) suggested that PVT1 overexpression
promotes the development of cisplatin-resistance in gastric
carcinoma and that PVT1 may be a potential target for reversing
MDR.
The results of the present study demonstrated that
PVT1 was highly expressed in the CRC tissues of 5-FU resistant
patients, and in HCT-8/5-FU and HCT-116/5-FU cells, and that PVT1
overexpression was associated with the development of 5-FU
resistance. Previous studies have demonstrated that cellular
apoptosis serves an important function in MDR, and the majority of
chemotherapeutic drugs induce tumor cellular apoptosis to exert
their anticancer activity (22–24).
Therefore, inhibiting tumor cellular apoptosis is one of the
principal mechanisms of MDR. Takahashi et al (25) reported that PVT1 had anti-apoptotic
activity in CRC. However, no current study, to the best of the
authors' knowledge, has demonstrated the exact role of PVT1 in the
induction of apoptosis in CRC. In the present study, knockdown of
PVT1 significantly inhibited viability and increased the apoptosis
rate following transfection with siPVT1 and treatment with 5-FU. By
contrast, PVT1 overexpression had the property of inhibiting
apoptosis, and suppressed the apoptosis of HCT-8 and HCT-116 cells
treated with 5-FU. These results suggested that siPVT1 may reverse
the 5-FU resistance of 5-FU resistant cells.
MRP1 is a member of the adenosine
triphosphate-binding cassette superfamily of transmembrane
transporters, and, via overexpression of P-gp, causes MDR (26). P-gp is regarded as a transmembrane
efflux pump, which prevents the accumulation of chemotherapeutic
drugs (27). The expression level
of MRP1/P-gp is regulated by various signaling pathways and may
affect the therapeutic efficacy of chemotherapeutic agents
(28). MRP1, P-gp and mTOR are
regarded as important drug resistance molecular targets in cancer
therapy. Yan et al (29)
reported that MRP1 and mTOR contributed to the upregulation of
P-gp, leading to inhibition of accumulation of chemotherapeutic
drugs in cells. These proteins were investigated in the present
study to clarify the critical role of PVT1 in MDR. The results
indicated that PVT1 upregulated the expression of MRP1, P-gp, mTOR
and Bcl-2, supporting the hypothesis that PVT1 may promote the
development of MDR in CRC.
The present study demonstrated that PVT1 knockdown
reverses drug resistance in 5-FU resistant CRC cell lines, and that
PVT1 overexpression promotes the development of MDR in CRC
primarily by inhibiting apoptosis and upregulating the expression
of MRP1, P-gp, mTOR and Bcl-2. The results of the present study
indicated that PVT1 is a potential therapeutic target for the
treatment of MDR in CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Medical and Health Science Fund (grant no.
2018KY667).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HF and JHZ performed the experiments and drafted the
manuscript. XQY designed the experiments and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ningbo First Hospital (Zhejiang, China) and Guangdong
General Hospital (Guangdong, China), and written informed consent
was obtained from each patient.
Consent for publication
All patients provided written informed consent for
the publication of this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu M, Chen YM, Huang J, Fang YJ, Huang WQ,
Yan B, Lu MS, Pan ZZ and Zhang CX: Flavonoid intake from vegetables
and fruits is inversely associated with colorectal cancer risk: A
case-control study in China. Br J Nutr. 116:1275–1287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rebucci M and Michiels C: Molecular
aspects of cancer cell resistance to chemotherapy. Biochem
Pharmacol. 85:1219–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beck-Engeser GB, Lum AM, Huppi K, Caplen
NJ, Wang BB and Wabl M: Pvt1-encoded microRNAs in oncogenesis.
Retrovirology. 5:42008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Xie X, Xiao YF, Xie R, Hu CJ, Tang
B, Li BS and Yang SM: The emergence of long non-coding RNAs in the
tumorigenesis of hepatocellular carcinoma. Cancer Lett.
360:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu E, Liu Z and Zhou Y:
Carboplatin-docetaxel-induced activity against ovarian cancer is
dependent on up-regulated lncRNA PVT1. Int J Clin Exp Pathol.
8:3803–3810. 2015.PubMed/NCBI
|
|
7
|
Paci P, Colombo T and Farina L:
Computational analysis identifies a sponge interaction network
between long non-coding RNAs and messenger RNAs in human breast
cancer. BMC Syst Biol. 8:832014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu MD, Wang Y, Weng W, Wei P, Qi P, Zhang
Q, Tan C, Ni SJ, Dong L, Yang Y, et al: A positive feedback loop of
lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and
invasion. Clin Cancer Res. 23:2071–2080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang XY, Pan HF, Leng RX and Ye DQ: Long
noncoding RNAs: Novel insights into gastric cancer. Cancer Lett.
356:357–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uchibori K, Kasamatsu A, Sunaga M, Yokota
S, Sakurada T, Kobayashi E, Yoshikawa M, Uzawa K, Ueda S, Tanzawa H
and Sato N: Establishment and characterization of two
5-fluorouracil-resistant hepatocellular carcinoma cell lines. Int J
Oncol. 40:1005–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Zhou J, Wang Z, Wang P and Li S:
Upregulation of SOX2 activated LncRNA PVT1 expression promotes
breast cancer cell growth and invasion. Biochem Biophys Res Commun.
493:429–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farhana L, Antaki F, Anees MR,
Nangia-Makker P, Judd S, Hadden T, Levi E, Murshed F, Yu Y, Van
Buren E, et al: Role of cancer stem cells in racial disparity in
colorectal cancer. Cancer Med. 5:1268–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iden M, Fye S, Li K, Chowdhury T,
Ramchandran R and Rader JS: The lncRNA PVT1 contributes to the
cervical cancer phenotype and associates with poor patient
prognosis. PLoS One. 11:e01562742016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Q, Chen J, Feng J and Wang J: Long
noncoding RNA PVT1 modulates thyroid cancer cell proliferation by
recruiting EZH2 and regulating thyroid-stimulating hormone receptor
(TSHR). Tumour Biol. 37:3105–3113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong R, Zhang EB, Yin DD, You LH, Xu TP,
Chen WM, Xia R, Wan L, Sun M, Wang ZX, et al: Long noncoding RNA
PVT1 indicates a poor prognosis of gastric cancer and promotes cell
proliferation through epigenetically regulating p15 and p16. Mol
Cancer. 14:822015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu E, Liu Z, Zhou Y, Mi R and Wang D:
Overexpression of long non-coding RNA PVT1 in ovarian cancer cells
promotes cisplatin resistance by regulating apoptotic pathways. Int
J Clin Exp Med. 8:20565–20572. 2015.PubMed/NCBI
|
|
20
|
Wan L, Sun M, Liu GJ, Wei CC, Zhang EB,
Kong R, Xu TP, Huang MD and Wang ZX: Long non-coding RNA PVT1
promotes non-small cell lung cancer cell proliferation through
epigenetically regulating LATS2 expression. Mol Cancer Ther.
15:1082–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XW, Bu P, Liu L, Zhang XZ and Li J:
Overexpression of long non-coding RNA PVT1 in gastric cancer cells
promotes the development of multidrug resistance. Biochem Biophys
Res Commun. 462:227–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duarte N, Varga A, Cherepnev G, Radics R,
Molnár J and Ferreira MJ: Apoptosis induction and modulation of
P-glycoprotein mediated multidrug resistance by new macrocyclic
lathyrane-type diterpenoids. Bioorg Med Chem. 15:546–554. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wobst I, Ebert L, Birod K, Wegner MS,
Hoffmann M, Thomas D, Angioni C, Parnham MJ, Steinhilber D, Tegeder
I, et al: R-flurbiprofen traps prostaglandins within cells by
inhibition of multidrug resistance-associated protein-4. Int J Mol
Sci. 18:pii: E68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang MZ, Qiu CZ, Yu WS, Guo YT, Wang CX
and Chen ZX: GOLPH3 expression promotes the resistance of HT29
cells to 5-fluorouracil by activating multiple signaling pathways.
Mol Med Rep. 17:542–548. 2018.PubMed/NCBI
|
|
25
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et
al: Amplification of PVT-1 is involved in poor prognosis via
apoptosis inhibition in colorectal cancers. Br J Cancer.
110:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weerasinghe P, Hallock S, Tang SC, Trump B
and Liepins A: Sanguinarine overcomes p-glycoprotein-mediated
multidrug-resistance via induction of apoptosis and oncosis in
CEM-VLB 1,000 cells. Exp Toxicol Pathol. 58:21–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Y, Yu N, Chen Y, Zhang K, Ma HY and Di
Q: HMGB1 regulates P-glycoprotein expression in status epilepticus
rat brains via the RAGE/NF-κB signaling pathway. Mol Med Rep.
16:1691–1700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gottesman MM and Pastan IH: The role of
multidrug resistance efflux pumps in cancer: Revisiting a JNCI
publication exploring expression of the MDR1 (P-glycoprotein) gene.
J Natl Cancer Inst. 107:pii: djv222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan LH, Wei WY, Cao WL, Zhang XS, Xie YB
and Xiao Q: Overexpression of CDX2 in gastric cancer cells promotes
the development of multidrug resistance. Am J Cancer Res.
5:321–332. 2014.PubMed/NCBI
|