Introduction
Lecithin from egg yolk (LE) is one of the best
sources of phosphatides, of which the major components are
phosphatidylcholine (PC) and sphingomyelin (SM). Its PC content is
approximately 70–80%. Its SM content is approximately 15–20%
(1). Lecithin is a natural
emulgator and a nutritious supplement. The functionalities and
features of lecithin as well as its development and utilization
have been receiving increasing attention from scientists worldwide
(2–5). Actual production process and clinical
applications proved that LE was superior to other emulsifiers for
intravenous injection, such as soya bean lecithin (6). Calcium carbonate (CaCO3)
is one of the minerals that exists naturally in abundance and is
the major component of the shells of shellfish. It has been
extensively applied in scientific and industrial fields (7–11).
Biomimetic synthesis is a method of simulating biomineralization
processes regulated by organic media to synthesize inorganic
materials with complicated structures, highly ordered arrangements
and special functionalities (12).
The research on CaCO3 drug carriers mainly investigates
the methods of producing a biomineral with favorable biological
properties via biomimetic synthesis. Compared with other drug
carriers, CaCO3 as a drug carrier has a series of merits
such as a simple preparation process, sensitivity to pH, and
favorable biocompatibility and degradability in addition to being
environmentally friendly and non-toxic to humans (13). The pH sensitivity of it could be
favorable for the release of drugs because of the acidity of the
lysosomes in cancer cells and the weak acid environment outside of
cancer cells (14). Therefore, the
CaCO3 would be an ideal drug carrier. At present, the
anticancer effects of chemotherapy drugs are good in vitro
but not ideal in vivo, the reasons of which might be the
dosage form, administration method and microenvironment inside the
body. Therefore, a change in the dosage form could increase the
utilization of drugs to some extent. The two ends of phosphatide
molecules are a negatively charged polar acyl group and a
positively charged quaternary ammonium group, i.e., a non-polar
hydrophobic end and a polar hydrophilic end. LE and
CaCO3 both have favorable biocompatibility,
degradability, and non-toxicity, among other features. This work
investigated the regulation of LE-ordered systems on
CaCO3 crystallization to prepare compound microparticles
of CaCO3/LE and to realize the controllable release of
anticancer drugs.
Materials and methods
Ethical statement
All animal experiments were performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(Bethesda, MD, USA). The animal protocols were approved by the
Committee on the Ethics of Animal Experiments of the Hubei
University of Technology (Wuhan, China).
Reagents and animals
Calcium chloride (CaCl2) and sodium
carbonate (Na2CO3; Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) were of analytical grade and used
without further purification. The deionized water used in all
experiments was obtained from a Milli-Q system with a resistivity
greater than 18.2 MΩ cm. Doxorubicin hydrochloride (DOX) was
purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai,
China). All glass was cleaned and sonicated in ethanol for 10 min,
soaked in a H2O-HNO3
(65%)-H2O2 (1:1:1 by volume) solution, rinsed
with deionized water and acetone, and then dried in air. H22
hepatoma cells lines (purchased from the China center for type
culture collection), Male Kunming (KM) mice (age, 6–12 wk, weight,
20±2 g) were purchased from the Hubei provincial academy of
preventive medicine.
Polarized light microscopy (PLM)
investigation of the LE ordered systems
PLM (Leica DM4500P; Leica Microsystems GmbH,
Wetzlar, Germany) was employed to characterize the LE ordered
systems. The sample for PLM investigation was prepared by dropping
one drop of the LE-ordered system suspension onto a slide and
placing a glass cover on top. The LE ordered systems were prepared
by reconstituting LE in deionized water. Then, the sample was put
under PLM for observation. The PLM image was recorded by a digital
camera.
Preparation of CaCO3 in LE
LE with different concentrations of Ca2+
was prepared by reconstituting 0, 20, 100 and 250 mg of LE in 10 ml
of a 0.3 mol/l CaCl2 solution by sonication,
respectively. The precipitation reaction was conducted by mixing 10
ml of a 0.3 mol/l Na2CO3 solution with the
Ca2+/LE ordered systems by stirring. The precipitate
formed immediately and was aged for 2 h before being rinsed with
ethanol by centrifugation several times and dried for SEM and TEM
analysis. Because the aging time of each sample was the same, it
should not have any influence on the differences in the morphology
and polymorph of the CaCO3 produced in the different
reaction suspensions. A control sample of CaCO3 prepared
without LE was produced by mixing 10 ml of a 0.3 mol/l
CaCl2 solution with 10 ml of a 0.3 mol/l
Na2CO3 solution by stirring and the
precipitate was aged for 2 h as well. The concentrations of the
CaCl2 and Na2CO3 solutions used in
synthesizing the CaCO3 precipitation for XRD analysis
were both 0.3 mol/l. The precipitation was also conducted in 5, 10
and 15 mg/ml LE, respectively.
Loading and release of DOX in the
CaCO3 microspheres
First, 20 mg of CaCO3 microspheres was
added into 2 ml of an aqueous DOX solution (0.5 mg/ml). The mixture
was shaken in a thermostatic shaker for 24 h at 25°C. Then, the
products were collected by centrifugation at 6,000 rpm for 10 min,
washed twice with 1 ml of deionized water, and dried at room
temperature for future use. The DOX content of the supernatant was
determined by measuring the absorbance at 481 nm with a Microplate
spectrophotometer (Epoch2, BioTek, VT, USA) against a calibration
curve. The loading content and entrapment efficiency of DOX was
calculated as follows: Drug loading content=(WD-WF)/WCx100%; and
Entrapment efficiency=(WD-WF)/WDx100%.
where WD is the total weight of DOX in the reaction,
WF is the total weight of free DOX remaining in the supernatant,
and WC is the total weight of the CaCO3 microspheres
loaded with DOX.
For the drug release studies, 20 mg of the dried
DOX-loaded CaCO3 microspheres (DCM) after the washing
treatment was added into 2 ml of PBS solutions at different pH
values (4, 6.5 and 7.4) and placed in a thermostatic shaker at
37°C. At predetermined intervals, 1 ml of the supernatant was taken
and analyzed by measuring the absorbance at 481 nm. The same volume
(1 ml) of fresh PBS buffer at different pH values was added into
the release medium. The release behaviors vs. time were evaluated
based on one sample, and the release measurement was performed in
triplicate.
Characterization
The morphologies of the CaCO3 samples
were characterized with scanning electron microscopy (SEM,
JSM6390LV, JEOL, Ltd., Tokyo, Japan; S-4700; Cold Field Emission
SEM, Hitachi, Ltd., Tokyo, Japan). The samples were sputter-coated
with platinum before being measured with SEM. Transmission electron
microscopy (TEM, JEM-2100; JEOL, Ltd.) was used to investigate the
morphology and microstructure of the precipitation. The content of
LE in the microspheres was measured through thermogravimetric
analysis (TGA; Diamond TG/DTA; PerkinElmer, Inc., Waltham, MA, USA)
at 10°C min−1 in nitrogen gas with a flow rate of 40
cm3 min−1. X-ray diffraction (XRD; X'pert
Powder; PANalytical BV, Almelo, The Netherlands) was employed to
determine the polymorphs of CaCO3 precipitation. The
operating conditions for the XRD were as follows: Radiation of
CuKα, tube voltage of 40 kV, tube current of 30 mA, scanning rate
of 0.6°/min and a collecting angle (2θ) ranging from 10° to 60°.
Fluorescence images of the DCM were captured by a fluorescence
inverted microscope (Axio Vert A1; Zeiss AG, Oberkochen,
Germany).
In vivo antitumor effect
Mice were maintained at 25±1°C and 60±5% humidity
under a 12 h light-dark cycle. All experimental animals were housed
under specific-pathogen-free conditions for 1 week to get
accustomed to the surroundings before initiation of the experiment.
The model of the tumor-bearing mice was created by subcutaneous
injection of H22 cells as previously described (15–17).
Briefly, the H22 cells with ascites were harvested, diluted to a
concentration of 5.0×106 /ml with sterilized normal
saline (NS), and inoculated subcutaneously into the right armpit
region of the mice. The mice were divided into 3 group with 10 mice
in each group, on seventh day of inoculated treatments. The mice in
the control group were treated with NS. The animals in the other 2
groups were admisistered DOS group or DCM group, through the tail
vein at a dose of 5 mg/kg DOX for the single dose. After tail vein
injection for 7 days, the mice were killed by pulling and breaking
of the cervical vertebra. The tumors were excised and weighted. The
inhibitory effect of experimental treatment on tumor growth was
evaluated by tumor inhibition rate. The inhibitory rates of tumor
growth were calculated as follows (17,18):
Tumor inhibition rate=(Wc-Ws)/Wcx100%, where Wc and Ws were donated
as the tumor weight in the control and sample groups,
respectively.
Statistical analyses
All experiments were performed at least three times
and expressed as mean ± standard deviation (SD). Data were analyzed
for statistical significance using SPSS 14.0 (SPSS, Inc., Chicago,
IL, USA), and analysis of variance and the Student-Newman-Keuls
post hoc test were performed for multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
The structure of the LE-H2O
system investigated by PLM
As shown in Fig. 1,
there were many Maltese crosses in the LE-H2O structure
under cross-polarized light.
Effect of LE on the morphology of
CaCO3 particles
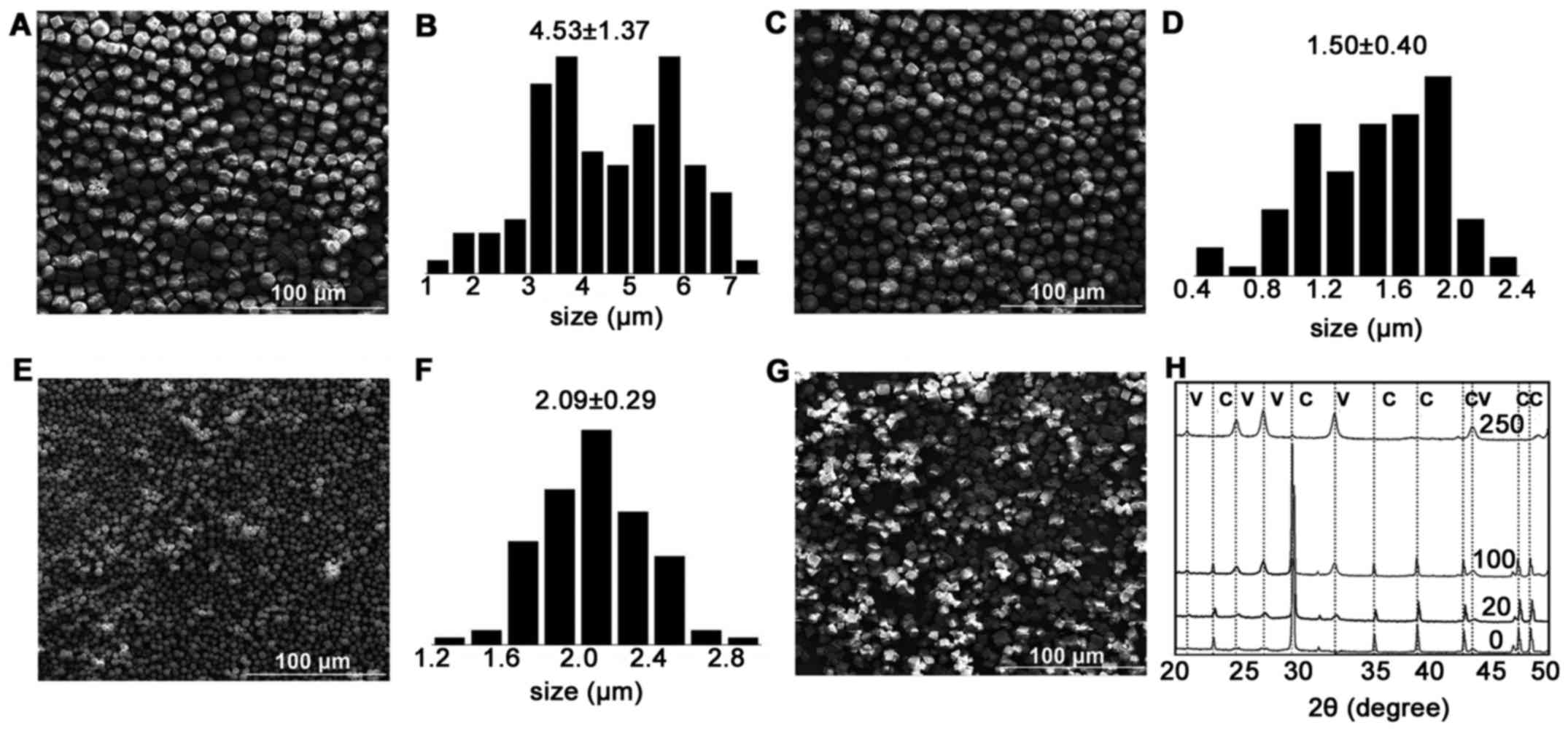
The SEM images shown in Fig. 2 represent the morphology of the
CaCO3 precipitation from the reconstitution of 0, 20,
100 and 250 mg of LE. The precipitation of the control sample, 0 mg
of LE, showed an irregular cubic shape of the particles, which is
quite common for CaCO3. In the precipitation of 20 mg of
LE, most of the particles were uniform cubic crystals with lamellar
structure; the lecithin concentration was less than the critical
micelle concentration (CMC=1.6 mmol/l). In 100 mg LE, the particles
were uniform cubic crystals and, with the exception of some
spherical particles, the amount of rough spherical particles
increased. In 250 mg LE, spherical particles dominated the
precipitation; the spherical particles exhibited fine homogeneity
and a porous structure.
Stability of the CaCO3
microspheres
The formation and stabilization of CaCO3
particles remain fundamental parameters to their unique properties
(19,20). Therefore, the LE-regulated
microspheres were incubated in a flowing water solution to assess
their stability. All typical peaks remained, and no calcite peak
appeared by XRD after the water flow process (Fig. 3), confirming the stability of the
LE-regulated microspheres.
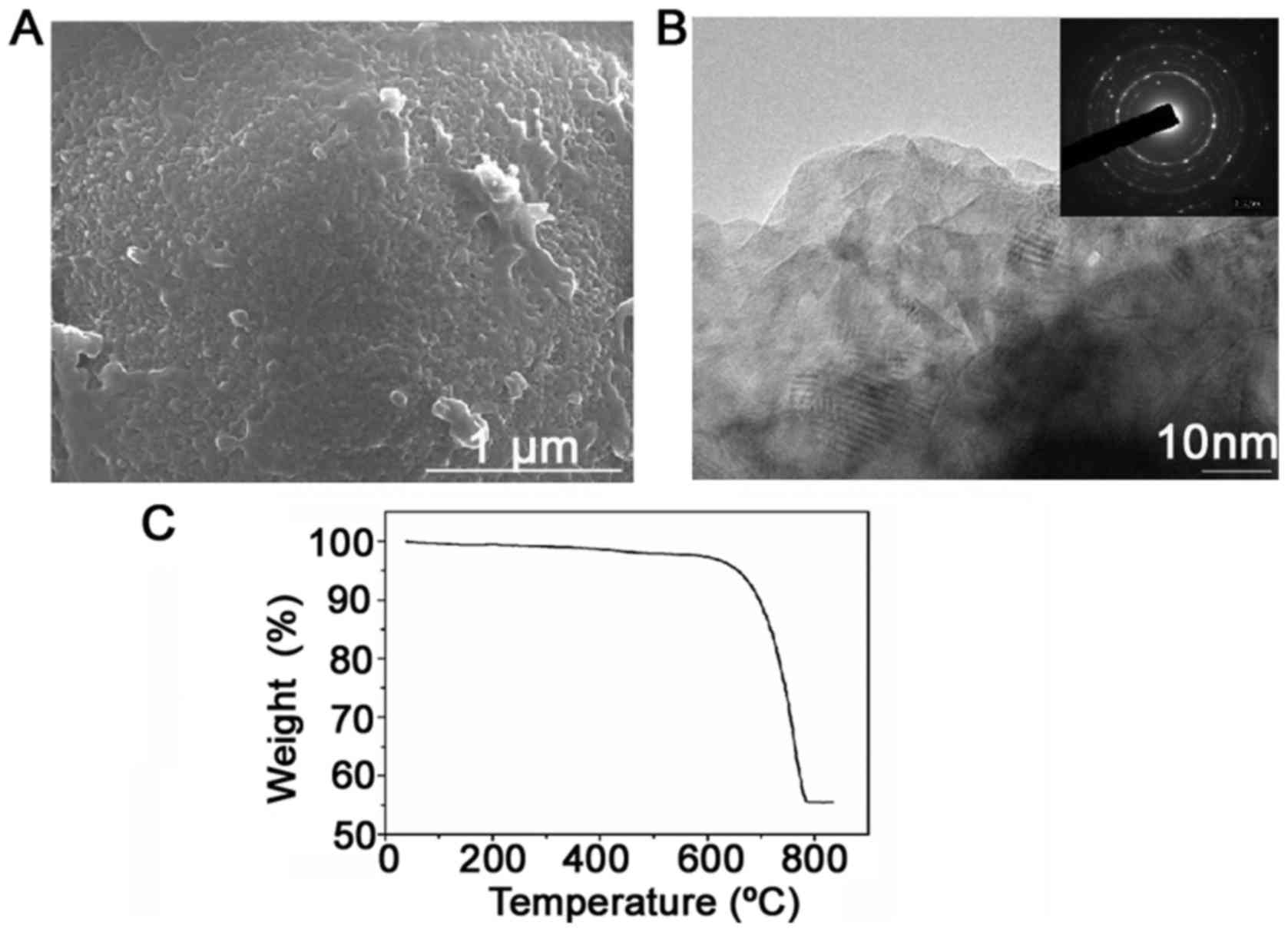
CaCO3 microspheres
formation process
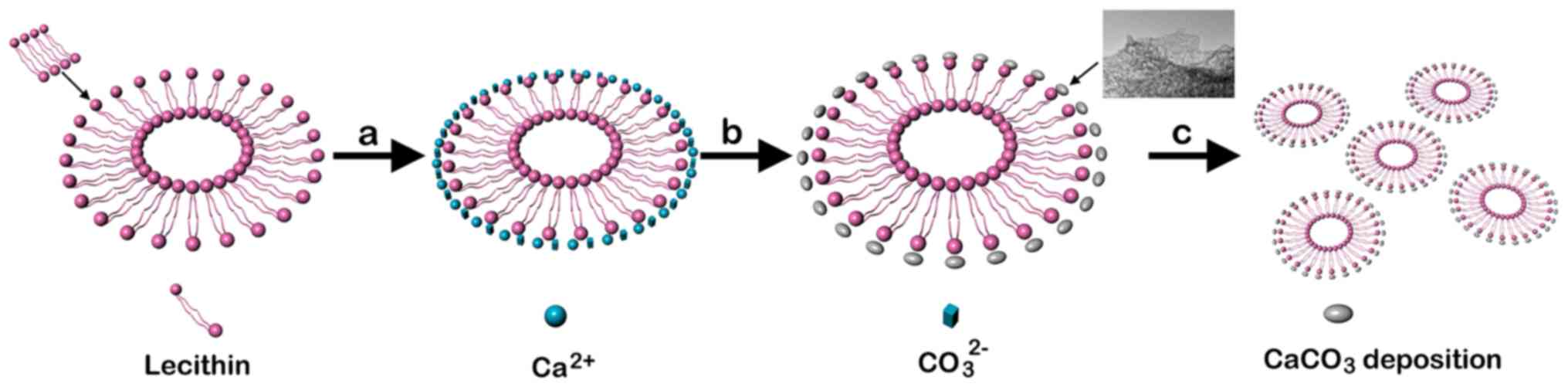
It was confirmed that the LE used as the organic
template for CaCO3 precipitation consisted of vesicles
with multilamellar structure. This indicated that the
microenvironment in the LE suspension increasingly favored the
formation of aggregated balls. Fig.
4 shows the morphology of the porous balls formed as aggregates
of the nanoparticles. To investigate the structure of the LE
precipitation, HRTEM and selected area electron diffraction (SAED)
were employed. It revealed the approximate size (50 nm) and
morphology of the nanoparticles. The nanoparticles, which
aggregated and formed the hierarchical structure of the
CaCO3 particles, were more obvious. The CaCO3
spherical particles that appeared were formed and aggregated by
numerous nanosized subparticles. TGA and elemental mapping revealed
the content and distribution of LE in the microspheres (Fig. 4C). During TGA, weight loss from 200
to 600°C was observed, with only a small amount ascribed to the
degradation of the lecithin.
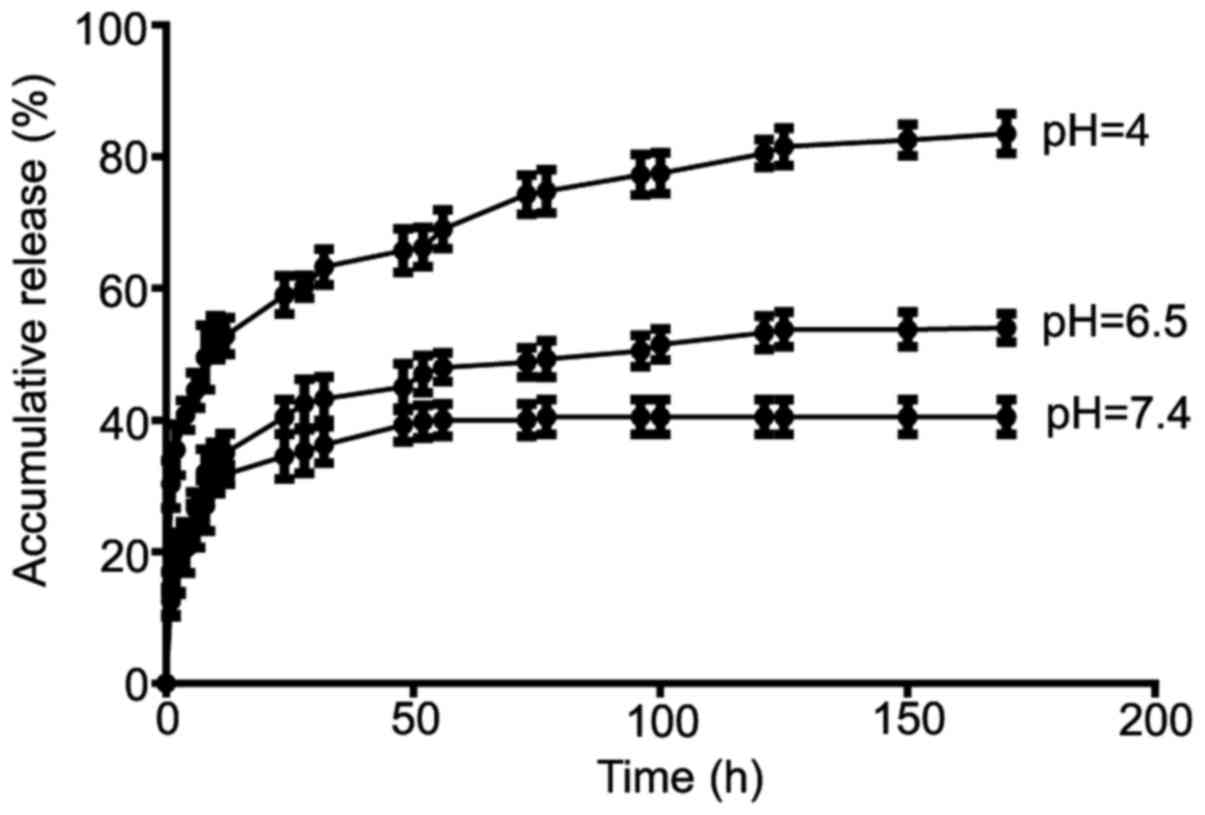
Drug loading and release from
LE-regulated microspheres
The drug loading and release behavior of the
microspheres were assessed with DOX as a model drug. Absorbance
analysis showed that the drug loading content and the entrapment
efficiency of DOX was 5.1±0.1 and 82.5±3.1%, respectively, when the
ratio of the drug to microspheres was 1:20 (w/w). The in
vitro release of DOX from the vaterite microspheres at pH 7.4,
6.5 and 4 is shown in Fig. 5. The
DOX release from the microspheres was also strongly pH
dependenthighest at a pH of 4, then at a pH of 6.5, lowest at a pH
of 7.4. After 8 days, the amount of DOX released at pH 7.4 was 40%
of the total drug load but reached 80% at a pH of 4. At pH 6.5, the
release of DOX was triggered through the initial 38 h only about
40–50% DOX release, and continued at a slower but steady pace.
Tumor inhibition rates
After mice were killed, the solid tumors were
removed from tumor-bearing mice and the tumor inhibition rate was
shown in Table I. Although the two
DOX formulations both inhibited the growth of tumor compared with
NS, the treatment of DCM showed a significantly better efficacy
than that of free DOX. The average tumor weight in the DCM group
was just half of that in the free DOX group (P<0.01). The tumor
inhibition rates in DCM group was significantly higher than in the
DOX group (P<0.01).
 | Table I.Inhibitory effect of DCM on the
growth of H22 tumors transplanted in mice. |
Table I.
Inhibitory effect of DCM on the
growth of H22 tumors transplanted in mice.
| Group | Tumor weight
(g) | Inhibition rate
(%) |
|---|
| NS | 2.04±0.67 | – |
| DOS |
1.28±0.28a | 37.30 |
| DCM |
0.73±0.19b,c |
64.23c |
Discussion
The Maltese cross corresponded to a multilamellar
vesicle structure. The PLM results proved that in the
LE-H2O system suspension, LE molecules self-assembled
and packed into multilamellar liposome vesicles. Thus, it was
confirmed that the LE liposomes used as the organic template for
CaCO3 precipitation were vesicles with multilamellar
structure.
The SEM results showed that as the concentration of
LE increased, the amount of aggregated spherical particles in the
precipitation increased. From the results and discussion above, it
can be summarized that as the concentration of LE increased, the
number of aggregated spherical particles in the precipitation
increased accordingly. This indicates that the microenvironment in
LE-H2O increasingly favored the formation of aggregated
balls. The interaction of LE and calcium ions was likely
responsible for this result. LE induced a localized supersaturation
zone of Ca2+, which remarkably increased the number of
nuclei.
In the stability study, the CaCO3
microspheres were incubated in water after 10 days and retained
their initial spherical morphology and size, suggesting the
stability of those features. Here, in this research, it was
suggested that in the LE suspension, the negatively charged
phosphatidyl group can selectively bind Ca2+. When the
LE formed in Ca2+ solution, the calcium ions were
trapped and enriched by the binding sites on the negatively charged
surface of the outermost bilayer of vesicles. Therefore, the outer
surface of the LE vesicles acted as the template to modify the
nucleation and growth of CaCO3. LE and CaCO3
must be closely combined to prevent the dissolution and
recrystallization of the CaCO3.
The CaCO3 microsphere formation process
results suggest that the lecithin must be closely packed with
CaCO3 precipitation to provide sufficient stabilization
and prevent transformation. As shown in Fig. 6, the nucleation and formation of
the nanoparticles in the CaCO3 precipitate was
illustrated. The external additives adsorbed on these nanoparticles
cause the nanoparticles to be attracted to each other, driving the
directed self-assembly process. Eventually, the nanoparticles
integrated with each other to form mesocrystals, and the additives
existed between the particles. Therefore, the nanoparticle
aggregation pathway is that which minimizes the surface energy and
interfacial crystal energy, and this pathway is the directed
accretion process. More significantly, the amphiphilic molecules
attached to the microcrystal planes prevent the transformation.
The release of DOX from CaCO3 microsphere
showed distinct pH dependence. The higher amount of drug release at
pH 4.5 could be caused by special structure of the CaCO3
microsphere based on ultra-pH-sensitivity, which was beneficial for
rapid killing tumor cells. The amount of drug release at pH 6.5 was
little high, but in vivo drug release might be less because
of the increased acidity distribution from outer layer to the core
of tumor tissue (21–23). Vaterite is a useful candidate for
drug release due to its large surface area, biocompatibility,
biodegradability, lower toxicity, low production costs, and
pH-dependent dissolution (24,25).
The vaterite particles with smaller and homogeneous sizes are
preferred for drug delivery because of their improved efficient and
homogeneous distribution of drugs as well as their improved
cellular uptake (26,27). Since the submicrometer
CaCO3 carriers have larger specific surface areas, the
increased drug entrapment efficiency of the microspheres in the
present work may be due to the lecithin embedded in the particles,
where the hydrophilic group could form strong interactions with
DOX. Because of the stronger interactions of DOX and LE, an initial
burst release, as in previous CaCO3-based release
systems (28–34), was significantly reduced, resulting
in sustained release for more than 1 week. The DOX release from the
microspheres was also strongly pH sensitive. Compared with previous
other microspheres (30), the
slower release from vaterite microspheres suggested stronger
interactions between DOX and microspheres.
In vivo tumor inhibition examination, the
tumor treated with NS could not controlled and grew up rapidly. The
tumor treated with free DOX and DCM was grew steadily. DCM was
successful to a certain extent in controlling the tumor growth.
Importantly, mice treated with DCM significantly reduced the tumor
progression and displayed a remarkable tumor inhibition effect on
tumor, which was possibly due to its enhanced drug accumulation and
cell killing ability.
In conclusion, the results of the present study
demonstrated that CaCO3 microspheres with smaller sizes
can be prepared with LE as a modifier. The polymorphology and
nanostructure of these microspheres were regulated by altering
experimental parameters such as the LE concentration. The
microspheres were stabilized by LE to prevent transformation into
calcite. Higher DOX encapsulation and improved interactions
supported sustained drug release and pH-sensitive release behavior,
suggesting the utility of the microspheres as drug carriers. These
results provide new insight into the relationship between LE and
CaCO3 biomineralization as well as a facile, efficient
way to rapidly synthesize well-controlled CaCO3
particles. In addition, this DCM were found good anti-tumor effect
in H22 tumor-bearing mice in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JS designed experiments, and JS, RW and ZL carried
out experiments. JS and HZ analyzed the experimental results, and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal protocols were approved by the Committee
on the Ethics of Animal Experiments of the Hubei University of
Technology (Wuhan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chi Y and Lin S: Research advances in the
extraction and application of egg-yolk lecithin. Food Ferment Ind.
28:50–53. 2002.
|
|
2
|
Li Y, Liu JB and Lin SY: Study on
extraction of high pure egg yolk lecithin. Food Sci. 27:851–853.
2006.
|
|
3
|
Palacios LE and Wang T: Extraction of
egg-yolk lecithin. J Am Oil Chem Soc. 82:565–569. 2005. View Article : Google Scholar
|
|
4
|
Chang H, Wang EL, Gong XT and Liu JB: Over
view on study of yolk lecithin. Sci Tech Food Ind. 5:414–420.
2010.
|
|
5
|
Ali AH, Zou X, Lu J, Abed SM, Yao Y, Tao
G, Jin Q and Wang X: Identification of phospholipids classes and
molecular species in different types of egg yolk by using
UPLC-Q-TOF-MS. Food Chem. 221:58–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossi J and Leroux JC: Principles in the
development of intravenous lipid emulsionsWasan KM and Wiley: John
Wiley & Sons, Inc.; New York: pp. 88–123. 2006, PubMed/NCBI
|
|
7
|
Foran E, Weiner S and Fine M: Biogenic
fish-gut calcium carbonate is a stable amorphous phase in the
gilt-head seabream, sparus aurata. Sci Rep. 3:17002013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vlieg E: Materials science. Complexity
from simplicity. Science. 340:822–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim S and Park CB: Bio-inspired synthesis
of minerals for energy, environment, and medicinal applications.
Adv Funct Mater. 23:10–25. 2013. View Article : Google Scholar
|
|
10
|
Zhao Y, Luo Z, Li M, Qu Q, Ma X, Yu SH and
Zhao Y: A preloaded amorphous calcium carbonate/doxorubicin@silica
nanoreactor for pH-responsive delivery of an anticancer drug. Angew
Chem Int Ed Engl. 54:919–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Chen SQ, Yu Q, Hu FQ and Yuan H:
Taking advantage of the disadvantage: Employing the high aqueous
instability of amorphous calcium carbonate to realize burst drug
release within cancer cells. J Mater Chem B. 5:2068–2073. 2017.
View Article : Google Scholar
|
|
12
|
Zan G and Wu Q: Biomimetic and bioinspired
synthesis of nanomaterials/nanostructures. Adv Mater. 28:2099–2147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han MR, Kwon MC, Lee HY, Kim JC, Kim JD,
Yoo SK, SIN IS and Kim SM: pH-dependent release property of
alginate beads containing calcium carbonate particles. J
Microencapsul. 24:787–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei W, Ma GH, Hu G, Yu D, Mcleish T, Su ZG
and Shen ZY: Preparation of hierarchical hollow CaCO3
Particles and the application as anticancer drug carrier. J Am Chem
Soc. 130:15808–15810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiaoguang C, Hongyan L, Xiaohong L, Zhaodi
F, Yan L, Lihua T and Rui H: Cancer chemopreventive and therapeutic
activities of red ginseng. J Ethnopharmacol. 60:71–78. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao L, Chen L, Fei XH, Qiu HY, Zhou H and
Wang JM: STI571 combined with vincristine greatly suppressed the
tumor formation of multidrug-resistant K562 cells in a human-nude
mice xenograft model. Chin Med J (Engl). 119:911–918.
2006.PubMed/NCBI
|
|
17
|
Jin Y, Li J, Rong LF, Li YH, Guo L and Xu
SY: Anti-hepatocarcinoma effects of 5-fluorouracil encapsulated by
galactosylceramide liposomes in vivo and in vitro. World J
Gastroenterol. 11:2643–2646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Takahashi S, Imamura M, Okutani E,
Zhang ZG, Chayama K and Chen BA: Earthworm fibrinolytic enzyme:
Anti-tumor activity on human hepatoma cells in vitro and in vivo.
Chin Med J (Engl). 120:898–904. 2007.PubMed/NCBI
|
|
19
|
Naka K, Huang SC and Chujo Y: Formation of
stable vaterite with poly(acrylic acid) by the delayed addition
method. Langmuir. 22:7760–7767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naka K, Tanaka Y and Chujo Y: Effect of
anionic starburst dendrimers on the crystallization of
CaCO3 in aqueous solution: Size control of spherical
vaterite particles. Langmuir. 18:3655–3658. 2002. View Article : Google Scholar
|
|
21
|
Helmlinger G, Yuan F, Dellian M, Dellian M
and Jain RK: Interstitial pH and pO2 gradients in solid tumors in
vivo: High-resolution measurements reveal a lack of correlation.
Nat Med. 3:177–182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirschhaeuser F, Menne H, Dittfeld C, West
J, Mueller-Klieser W and Kunz-Schughart LA: Multicellular tumor
spheroids: An underestimated tool is catching up again. J
Biotechnol. 148:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chauhan VP and Jain RK: Strategies for
advancing cancer nanomedicine. Nat Mater. 12:958–962. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parakhonskiy BV, Haase A and Antolini R:
Sub-micrometer vaterite containers: Synthesis, substance loading,
and release. Angew Chem Int Ed Engl. 51:1195–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt S and Volodkin D: Microparticulate
biomolecules by mild CaCO3 templating. J Mater Chem B.
1:1210–1218. 2013. View Article : Google Scholar
|
|
26
|
Qi C, Zhu YJ and Chen F: Microwave
hydrothermal transformation of amorphous calcium carbonate
nanospheres and application in protein adsorption. ACS Appl Mater
Interfaces. 6:4310–4320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Svenskaya Y, Parakhonskiy B, Haase A,
Atkin V, Lukyanets E, Gorin D and Antolini R: Anticancer drug
delivery system based on calcium carbonate particles loaded with a
photosensitizer. Biophys Chem. 182:11–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang YH, Liu CH, Liang YH, Lin FH and Wu
KCW: Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with
enhanced drug loading and PH-responsive release properties for
intracellular drug delivery. J Mater Chem B. 1:2447–2450. 2013.
View Article : Google Scholar
|
|
29
|
Qi C, Zhu YJ and Chen F: Microwave
hydrothermal transformation of amorphous calcium carbonate
nanospheres and application in protein adsorption. ACS Appl Mater
Interfaces. 6:4310–4320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Chen JS, Zong JY, Zhao D, Li F,
Zhou RX and Cheng SX: Calcium carbonate/carboxymethyl chitosan
hybrid microspheres and nanospheres for drug delivery. J Phys Chem
C. 114:18940–18945. 2010. View Article : Google Scholar
|
|
31
|
Ying X, Shan C, Jiang K, Chen Z and Du Y:
Intracellular pH-sensitive delivery CaCO3 nanoparticles
templated by hydrophobic modified starch micelles. RSC Adv.
4:10841–10844. 2014. View Article : Google Scholar
|
|
32
|
Kurapati R and Raichur AM: Composite
cyclodextrin-calcium carbonate porous microparticles and modified
multilayer capsules: Novel carriers for encapsulation of
hydrophobic drugs. J Mater Chem B. 1:3175–3184. 2013. View Article : Google Scholar
|
|
33
|
Peng C, Zhao Q and Gao C: Sustained
delivery of doxorubicin by porous CaCO3 and
chitosan/alginate multilayers-coated CaCO3
microparticles. Colloids Sur A: Physicochem Eng Aspects.
353:132–139. 2010. View Article : Google Scholar
|
|
34
|
Parakhonskiy BV, Foss C, Carletti E, Fedel
M, Haase A, Motta A, Migliaresi C and Antolini R: Tailored
intracellular delivery via a crystal phase transition in 400 nm
vaterite particles. Biomater Sci. 1:1273–1281. 2013. View Article : Google Scholar
|