Introduction
Colorectal cancer (CRC) is the fourth most commonly
diagnosed malignant disease worldwide, after lung, stomach and
liver cancer (1). It is estimated
that CRC ranks as the fourth and fifth most common cause of cancer
death among women and men in China. The prevalence of CRC is
rapidly increasing owing to changes in people's lifestyle, such as
smoking, obesity, and red meat consumption (2). Despite advances in traditional
treatments, such as chemotherapy, radiotherapy, targeted therapy
and surgery, there has been no significant breakthrough in the
overall curative therapy. The five-year survival rate after
diagnosis remains less than 60% (3,4).
Like other cancers, CRC exhibits resistance to cell death, the
ability of replication, angiogenesis, tissue invasion and
metastasis, and immune escape (5).
According to a medical view considering Darwinian evolution, the
tumor may be a ‘new species’ in the body that has an increased
ability to proliferate and metastasize (6). Tumors may have highly evolved and
conserved information transmission systems, so that large numbers
of tumor cells can coordinately proliferate, hibernate and
metastasize. Steven Paget's theory of ‘seed and soil’ was
considered a milestone in tumor research. It proposed that a
suitable microenvironment is the primary requirement for malignant
cells to survive in other non-primary focal areas and to continue
to grow and form metastases (7).
In 1971, Folkman et al proposed the concept of
‘angiogenesis’ in tumors (8). In
2001, Seifert et al proposed a phenomenon similar to
angiogenesis, called neurogenesis (9). Subsequent studies confirmed that
neurogenesis in bladder, breast, colorectal and pancreatic cancer
was closely correlated with tumor progression, as well as prognosis
(10–14). Albo et al revealed that
neurogenesis was a marker for tumor invasion and prognosis, and the
five-year survival rate of patients with neurogenesis was reduced
by 50% compared with those lacking neurogenesis (13). Tumors cells secrete many cytokines,
such as nerve growth factor, insulin-like growth factor and
brain-derived nerve growth factor, which not only promote
neurogenesis within the tumor, but also play an important role in
accelerating tumor development (15,16).
Moreover, nerve and tumor cells may interact in an autocrine,
paracrine or endocrine manner via the neuro-neoplastic synapse
(17).
The autonomic nervous system consists of sympathetic
and parasympathetic nerves. Magnon et al (18) revealed that in prostate cancer,
sympathetic nerves are mainly distributed in normal tissue around
the tumor, and parasympathetic nerves are mainly found between the
tumor cells. Both types of nerves show complementary progressive
effects in tumor occurrence and development. Sympathetic nerves
were observed in the early stages of prostate cancer and were
associated with occurrence of disease. Parasympathetic nerves were
mainly expressed in the late stage, associated with poor
progression and metastasis (18).
Cutting parasympathetic nerves or local injection of neurotoxic
drugs significantly reduced tumor appearance and development, but
the effects were limited to regions of innervations (19). Considering this point,
parasympathetic nerves were more closely associated with the
progression of tumors than the sympathetic nerves. Disrupted
cholinergic signals may inhibit Wnt signaling pathways and stem
cell proliferation. Blocking or knockdown of the choline M3
receptor inhibited gastric cancer (20). However, to date, the clinical
significance of sympathetic and parasympathetic nerves in human CRC
has not been reported.
There are multiple neurotransmitter receptors on
tumor cells that may affect tumor development (21). Parasympathetic neurotransmission
involves acetylcholine (ACh) and two types of cholinergic receptor
(acetylcholine receptor, AChR), the toadstool alkali (M) and
nicotinic (N) type receptors. The M type receptor has five
subtypes: those found in the central nervous system are M1, M3 and
M4, and those in peripheral nerves are M1, M2 and M3. The N type
cholinergic receptor (nAChR) is a pentameric gate control ion
channel composed of different subunits (α2-α10, β2-β4) (22). Because cancer cells express many
kinds of AChR (23–26), cholinergic neurotransmitters or
other agonists can affect tumor progression via their corresponding
receptors. Currently, α7nAChR is widely studied because it has a
role in tumor development, such as pancreatic and lung cancer, by
regulating signaling pathways such as PI3K-AKT, NF-κB and STAT
(27–29). Another cholinergic receptor,
α9nAChR, was overexpressed in malignant tumors, especially in
breast cancer (30,31), and promoted breast cancer
metastasis by activating vimentin and fibronectin (32,33).
The expression and significance of α9nAChR in CRC remains unknown.
In this study, we examined the expression of autonomic nerves and
α9nAChR in CRC by immunohistochemical methods, and then analyzed
their relationship with clinical stages, lymph node metastasis and
prognosis.
Materials and methods
Patients and specimens
During January 2008 to January 2011, tissue samples
were collected (by the same surgeon) from 90 patients with CRC at
the Second Department of Surgery of the Forth Hospital of Hebei
Medical University. All patients were undergoing their first CRC
surgery. Before surgery, no patients received radiation or
chemotherapy, and all CRC was confirmed by pathology for
adenocarcinoma. CRC tissues were stock in the paraffin-embeded form
after fixation in formalin and dehydration with
increasing-concentration alcohol. Collection and use of specimens
were approved with the patients' informed consent and by the ethics
committee of the Forth Hospital of Hebei Medical University. Total
survival time was defined from diagnosis to time of patient's death
or the last follow-up visit. Postoperative TNM stage of CRC
followed the 8th Edition of the American Joint Committee on Cancer
grading system (34). Tumor, lymph
node, metastasis (TNM) system are considered as standardized
classification system for evaluating cancer at a population level
in terms of the extent of disease, determined by cancer biology and
habitual nature as well as predicting cancer outcome and response
to treatment.
Histological analysis
Immunohistochemistry detected the expression of
autonomic nerves and α9nAChR in a tumor microenvironment. Tyrosine
hydroxylase (TH) and vesicular acetylcholine transporter (VAChT)
were used as specific markers for sympathetic and parasympathetic
nerves, respectively. Tissue samples were fixed in 4% buffered
formaldehyde, decalcified, paraffin-embedded and sectioned to 3–5
µm. Paraffin sections were dewaxed, rehydrated and treated for
standard antigen retrieval, and incubated with anti-TH antibody
(rabbit anti-human monoclonal antibody, ab6211, Massachusetts, USA,
1:500) and anti-VAChT antibody (rabbit anti-human monoclonal
antibody, ab68984, Massachusetts, USA, 1:100). An antibody for
a9nAChR (rabbit anti-human monoclonal antibody, ab177119,
Massachusetts, USA, 1:100) was used to detect expression of
α9nAChR. Goat anti-rabbit IgG-HRP antibody (Aorui Dongyuan
Biotechnology, Wuxi, China) was used as secondary antibody and
specific binding detected using DAB (Zhongshan Jinqiao
Biotechnology, Beijing, China). An OLYMPUS BX61 universal
microscope was used to analyze slide images, with nerve fibers
stained brown or yellow designed as positive. For α9nAChR
detection, the presence of brown-yellow or brown particles in the
cell membrane or in the plasma was defined as a positive image.
According to the proportion of positive cells and the staining
strength of positive cells, the experimental results of α9nAChR
were determined. A: The proportion of positive cells <1/3 was
scored as 1 point; the proportion of positive cells 1/3 ~ 2/3 was
scored as 2 point; the proportion of positive cells >2/3 was
scored as 3 point. B: According to the staining of the cells, the
non-positive cells was scored as 0 point; the light yellow was
scored as 1 point; the claybank was scored as 2 point; and the tan
was scored as 3 point. The integral is equal to A × B. A × B=0 was
classified as (−); A × B=1 ~ 2 was classified as (+); A × B=3 ~ 4
was classified as (+ +); A × B=6 ~ 9 was classified as (+ + +).
Statistical analysis
Relationships between the presence of autonomic
nerves or α9nAChR and the clinical pathology were assessed using
chi-square and correlation tests. Cox proportional risk regression
analysis was used for single variable and multivariate analysis to
examine the underlying prognostic factors of overall survival. Cox
regression analyses were used to analyze survival status. Survival
curve differences were analyzed using the log-rank test.
Statistical analysis was done using SPSS22.0 statistical analysis
software. Two-side tests were used to compare statistical
differences, and P<0.05 was regarded as significant.
Results
Clinicopathological features and
prognosis of patients with CRC
The clinicopathologic features and prognosis of
patients with CRC in this trial are shown in Table I. A return visit for 90 patients
was completed via telephone. Seventy-three cases provided survival
data, and 19 cases died before the return visit, all of whom died
of postoperative tumor distant metastasis (pulmonary, bone and
multiple metastases in 11, 4 and 5 cases, respectively). The
overall survival rate was 73.97%. The prognosis was not
significantly associated with gender, location and stage of cancer
(P>0.05). The prognosis of lymph node metastatic negative
patients was greater than that of lymph node metastasis positive
patients (P<0.05). Considering the few cases of CRC in stage I,
we combined the cases in stages I and II, and compared them with
stage III cases. Patients with stages I+II CRC had a better
prognosis than those with stage III CRC (P<0.05).
 | Table I.Clinicopathological features and
prognosis of 73 patients with colorectal cancer. |
Table I.
Clinicopathological features and
prognosis of 73 patients with colorectal cancer.
|
| 5-year survival
rate | OS |
|---|
|
|
|
|
|---|
| Characteristic | Total no. | Sur no. | Sur R (%) | HR | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
Male | 43 | 34 | −79.10 | 1 | – | – |
|
Female | 47 | 37 | −78.70 | 0.968 | 0.393–2.384 | 0.944 |
| Location |
|
|
|
|
|
|
|
Rectum | 55 | 43 | −78.20 | 1 | – | – |
|
Colon | 35 | 28 | −80.00 | 0.904 | 0.356–2.298 | 0.833 |
| T stage |
|
|
|
|
|
|
| T1 | 2 | 1 | −50.00 | 1 | – | – |
| T2 | 20 | 18 | −90.00 | 0.235 | 0.021–2.598 | 0.238 |
| T3 | 29 | 22 | −75.90 | 0.641 | 0.057–4.995 | 0.648 |
| T4 | 39 | 30 | −76.90 | 0.534 | 0.068–4.218 | 0.552 |
| LN |
|
|
|
|
|
|
|
(+) | 42 | 29 | −69.00 | 1 | – | – |
|
(−) | 48 | 42 | −87.50 | 0.357 | 0.135–0.939 | 0.037a |
| TNM |
|
|
|
|
|
|
|
I+II | 50 | 45 | −90.00 | 1 | – | – |
|
III | 40 | 26 | −65.00 | 4.273 | 1.536–11.888 | 0.005a |
| PN |
|
|
|
|
|
|
|
(+) | 45 | 29 | −64.40 | 1 | – | – |
|
(−) | 45 | 42 | −93.30 | 0.161 | 0.047–0.554 | 0.005a |
| SN |
|
|
|
|
|
|
|
(+) | 48 | 42 | −87.50 | 1 | – | – |
|
(−) | 42 | 29 | −69.00 | 2.822 | 1.070–7.444 | 0.036a |
| α9 |
|
|
|
|
|
|
|
(+) | 40 | 26 | −56.00 | 1 | – | – |
|
(−) | 50 | 45 | −90.00 | 0.228 | 0.082–0.634 | 0.005a |
Expression of autonomic nerves and
α9nAChR in CRC
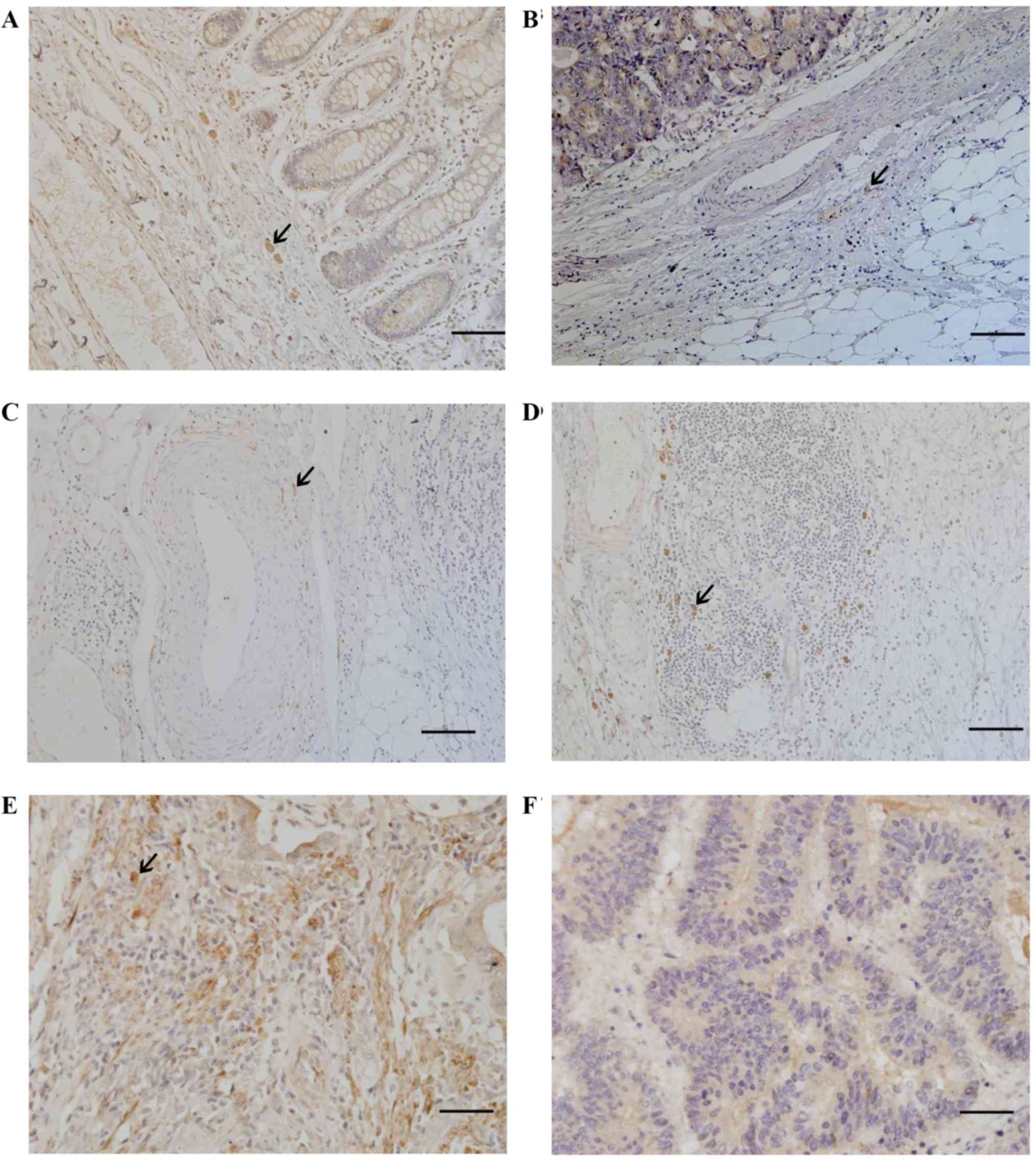
Expression of autonomic nerves and α9nAChR in CRC is
shown in Fig. 1. Most of the
sympathetic fibers were seen in the stroma adjacent to cancer cells
(Fig. 1A). Most of the
parasympathetic fibers were seen in the stroma away from cancer
cells (Fig. 1B). Some
parasympathetic fibers surrounded the blood vessels (Fig. 1C) and were sporadically located in
tertiary lymphoid tissue (Fig.
1D). The α9nAChR was expressed in CRC tissues (Fig. 1E), and was not detected in normal
rectal tissues (Fig. 1F).
Relationship between autonomic nerves
as well as α9nAChR and the survival rate of patients with CRC
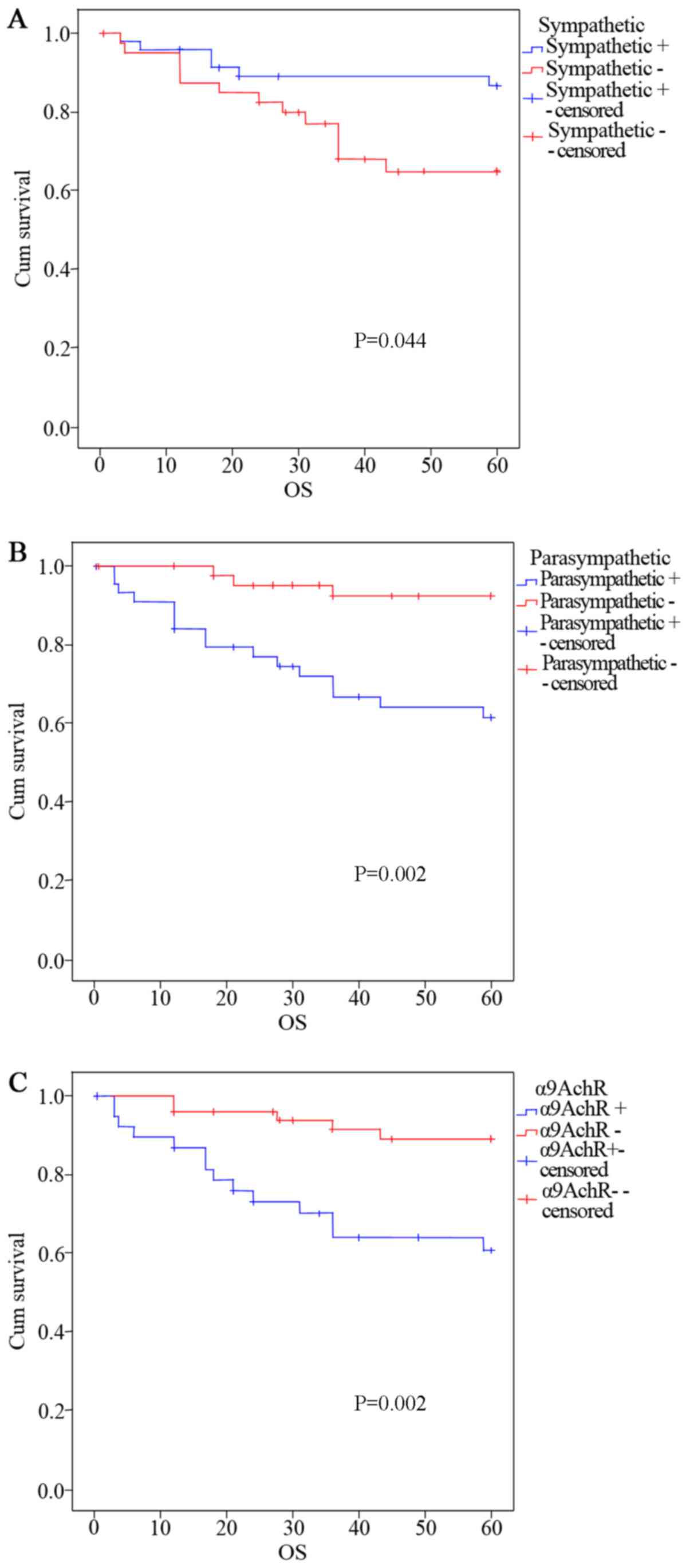
Relationships between autonomic nerves as well as
α9nAChR and the survival rate of patients with CRC are shown in
Fig. 2. Comparison of survival
rates of patients with CRC showed that patients with sympathetic
nerve positive CRC tissue had a better prognosis compared with
those having sympathetic nerve negative tissue. There was a
significant difference (P<0.05) between the survival rates of
these two groups (Fig. 2A).
Patients with parasympathetic nerve positive tissue had a worse
prognosis compared with patients that had parasympathetic nerve
negative tissue, shown by the significant difference (P<0.05)
between the survival rates of these two groups (Fig. 2B). Patients with α9nAChR positive
CRC tissue had a worse prognosis compared with patients that had
α9nAChR negative tissue, shown by the significant difference (P
< 0.05) between the survival rates of these two groups (Fig. 2C).
Relationship between autonomic nerves
as well as α9nAChR and the clinical pathology of CRC
The relationships between sympathetic nerves and
clinical pathological features are shown in Table II. The presence of sympathetic
nerves had no significant correlation with age, tumor location,
gender, and cancer stages (T and TNM criteria). The lymph node
invasion rate of patients with sympathetic nerve positive tissue
was significantly lower than that of patients with sympathetic
nerve negative tissue (41.7 vs. 66.7%, P=0.018), suggesting that
sympathetic nerves are inversely related to the lymph node
invasion.
 | Table II.Association between sympathetic
nerves and clinical pathological features. |
Table II.
Association between sympathetic
nerves and clinical pathological features.
|
| Sympathetic
nerve |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristic/group | − | + | N | Expression rate
(%) | P-value |
|---|
| Gender |
|
|
|
| 0.382 |
|
Male | 18 | 25 | 43 | 58.1 |
|
|
Female | 24 | 23 | 47 | 48.9 |
|
| Age |
|
|
|
| 0.571 |
|
≤60 | 22 | 28 | 50 | 56.0 |
|
|
>60 | 20 | 20 | 40 | 50.0 |
|
| Location |
|
|
|
| 0.58 |
|
Rectum | 21 | 14 | 35 | 40.0 |
|
|
Colon | 27 | 28 | 55 | 50.9 |
|
| T |
|
|
|
| 0.555 |
| T1 | 0 | 2 | 2 | 100.0 |
|
| T2 | 9 | 11 | 20 | 55.0 |
|
| T3 | 15 | 14 | 29 | 48.3 |
|
| T4 | 19 | 20 | 39 | 51.3 |
|
| N |
|
|
|
| 0.018a |
| N0 | 14 | 28 | 42 | 66.7 |
|
| N+ | 28 | 20 | 48 | 41.7 |
|
The relationships between parasympathetic nerves and
clinical pathological characteristics are shown in Table III. The presence of
parasympathetic nerves was not significantly associated with tumor
location and gender (P>0.05). Patients >60 years of age had a
higher incidence of parasympathetic nerve expression compared with
those ≤60 years of age (62.5 vs. 40.0%, P=0.034). Patients with
lymph node invasion had a higher incidence of parasympathetic nerve
expression than those without lymph nodes invasion (62.5 vs. 35.7%,
P=0.011). As the cancer (T stage) advanced, parasympathetic nerve
positive rates gradually increased (T1 50.0 T2 25.0 T3 48.3 and T4
64.1%, P=0.043), suggesting that the presence of parasympathetic
nerves was positively correlated with age, lymph node invasion and
cancer T stage.
 | Table III.Association between parasympathetic
nerves and clinical pathological features. |
Table III.
Association between parasympathetic
nerves and clinical pathological features.
|
| Sympathetic
nerve |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristic/group | − | + | N | Expression rate
(%) | P-value |
|---|
| Gender |
|
|
|
| 0.291 |
|
Male | 19 | 24 | 43 | 55.80 |
|
|
Female | 26 | 21 | 47 | 44.70 |
|
| Age |
|
|
|
| 0.034a |
|
≤60 | 30 | 20 | 50 | 40.00 |
|
|
>60 | 15 | 25 | 40 | 62.50 |
|
| Location |
|
|
|
| 0.052 |
|
Rectum | 22 | 13 | 35 | 37.10 |
|
|
Colon | 23 | 32 | 55 | 58.20 |
|
| T |
|
|
|
| 0.043a |
| T1 | 1 | 1 | 2 | 50.00 |
|
| T2 | 15 | 5 | 20 | 25.00 |
|
| T3 | 15 | 14 | 29 | 48.30 |
|
| T4 | 14 | 25 | 39 | 64.10 |
|
| N |
|
|
|
| 0.011a |
| N0 | 27 | 15 | 42 | 35.70 |
|
| N+ | 18 | 30 | 48 | 62.50 |
|
The relationships between α9nAChR and clinical
pathological features are shown in Table IV. The expression of α9nAChR was
not associated with gender or tumor location of CRC patients (P
> 0.05). The expression of α9nAChR in patients >60 years of
age was higher than that of patients ≤60 years of age (57.5 vs.
34.0%, P=0.026). The expression of α9nAChR in patients with lymph
node invasion was significantly higher than that of patients
without lymph nodes invasion (66.7 vs. 42.9%, P=0.023). As the
cancer T stage advanced, the expression of α9nAChR gradually
increased (0 T1, 20.0 T2, 44.8 T3, 59.0% T4, P=0.021), suggesting
that α9nAChR expression was positively correlated with age, cancer
T stage and lymph node invasion.
 | Table IV.Association between α9nAChR and
clinical pathological features. |
Table IV.
Association between α9nAChR and
clinical pathological features.
|
| Α9nachr |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristic/group | − | + | N | Expression rate
(%) | P-value |
|---|
| Gender |
|
|
|
| 0.962 |
|
Male | 24 | 19 | 43 | 44.20 |
|
|
Female | 26 | 21 | 47 | 44.70 |
|
| Age |
|
|
|
| 0.026a |
|
≤60 | 33 | 17 | 50 | 34.00 |
|
|
>60 | 17 | 23 | 40 | 57.50 |
|
| Location |
|
|
|
| 0.134 |
|
Rectum | 19 | 16 | 35 | 45.70 |
|
|
Colon | 21 | 34 | 55 | 61.80 |
|
| T |
|
|
|
| 0.021a |
| T1 | 2 | 0 | 2 | 0 |
|
| T2 | 16 | 4 | 20 | 20.00 |
|
| T3 | 16 | 13 | 29 | 44.80 |
|
| T4 | 16 | 23 | 39 | 59.00 |
|
| N |
|
|
|
| 0.023a |
| N0 | 24 | 18 | 42 | 42.90 |
|
| N+ | 16 | 32 | 48 | 66.70 |
|
Discussion
In the present study, we revealed that there are
sympathetic and parasympathetic nerves in the human CRC
microenvironment. Sympathetic fibers were mainly found in the
stroma adjacent to cancer cells. Patients with sympathetic nerves
detected in CRC tissue have less lymph node invasion compared with
patients exhibiting tissue with no detectable sympathetic nerves.
The presence of sympathetic nerves in CRC had no significant
correlation with age, tumor location, gender, and cancer (T and
TNM) stage. The prognosis of patients with sympathetic nerve
positive CRC was better than patients with sympathetic negative
tissue. Parasympathetic fibers were mainly detected in the stroma
away from cancer cells, and some parasympathetic fibers were
observed around the blood vessels. The expression of α9nAChR was
mainly located in cellular membrane and cytoplasm of CRC tissues.
The detection of parasympathetic nerves and α9nAChR was positively
related to cancer T stage, lymph node invasion and age; as
expression of parasympathetic nerves and α9nAChR increased in CRC
tissue, the prognosis of patients became poor, suggesting that
parasympathetic nerves may participate in the late development of
tumors via α9nAChR.
Tumors are not an isolated structure, and are
associated with the microenvironment of the host tissues (35). Recently, researchers reported that
neurogenesis was present in colorectal tumors (36). Our study also revealed that there
were sympathetic and parasympathetic nerves in CRC. Parasympathetic
nerves were closely associated with the development of tumors.
Acetylcholine is a neurotransmitter in parasympathetic nerves,
which function via the AChR (37,38).
The AChR is also expressed in some non-neuronal cells, such as lung
cancer and CRC cells (39).
Nicotine, as well as its structural analogues nitrosamines
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and
N'-nitrosonornicotine, can activate a variety of nAChR subtypes
found in the parasympathetic nervous system, resulting in a various
biological responses (40).
Nicotine can induce the proliferation of a variety of cancer cells,
such as small cell lung cancer, non-small cell lung cancer,
pancreatic and colon cancer cells, in receptor-dependent manner
(41,42). In human bronchial epithelial cells
and lung cancer cells, the α7nAChR inhibitors mecamylamine reduced
cell proliferation mediated by nicotine (43). Moreover, the nAChR antagonist
d-tubocurarine reduced the proliferation of MSTO-211 mesothelioma
cells in vitro (44).
Another type of AChR was found in mammalian ear vestibular hair
cells type II, called α9nAChR (45). The α9nAChR was closely associated
with the development of breast cancer (46,47).
To date, there have been no reports regarding the role of α9nAChR
in CRC. In the current study, α9nAChR was mainly expressed in the
cellular cytoplasm and membranes of CRC tissue, and was not
detected in normal colorectal tissues and tumor stroma, which
suggested that α9nAChR may not be essential for normal
neurotransmitter signaling. We propose that α9nAChR may be induced
by some factors found in the tumor, and then plays a role in
promoting tumor development by downstream signaling events.
In addition, we found that parasympathetic nerve
fibers also exist in tumor tertiary lymphoid tissue, which
suggested that parasympathetic nerves are closely associated with
immune cells. Immune cells, such as T and B cells, and monocytes,
express all five kinds of toadstool alkali AChR (mAChR M1-M5) and
different types of nAChR, such as α3, α5, α7, α9 and α10, which
provide the structural basis for parasympathetic nerves to regulate
the immune system (48).
Borovikova et al (49)
proposed the concept of the ‘cholinergic anti-inflammatory
pathway’, in which ACh released by parasympathetic nerves acted
upon α7nAChR in macrophages, which inhibited immune function by
suppressing a variety of inflammatory factors (such as TNF-α,
IL-1β, IL-6 and high mobility group protein (49,50).
The electrical stimulation of parasympathetic nerves or
α7nAChR-specific agonists both reduced local or systemic
inflammatory responses (51).
These findings have been applied to the study of Alzheimer's
disease (52), rheumatoid
arthritis (53), and endotoxin
blood disease (54). Furthermore,
nicotine increased the expression of phosphorylated STAT5 by
activating α7nAChR on regulatory T cells, ultimately resulting in
elevated activity of regulatory T cells, which restrained T cell
immunosuppression (55).
Therefore, parasympathetic nerves may directly act on tumor cells,
as well as inhibit immune cells in the tumor microenvironment, with
combined AChR receptor responses promoting tumor progression. The
current study may ultimately provide a new avenue for the treatment
of CRC.
We found that the expression of parasympathetic
nerves in CRC was positively related to the age of the patient. It
is well known that the prevalence of both cancer and Alzheimer's
disease increases gradually with age (56). Musicco et al reported that
cancer rates in patients with Alzheimer's disease were reduced by
50% compared with normal people of the same age. However, the rates
of Alzheimer's disease in cancer patients were reduced by 35%
compared with healthy people of the same age (57). Another epidemiological study showed
a reduction of malignant tumor risk was associated with Alzheimer's
disease, and the risk of malignant tumors was further reduced using
stringent criteria for the diagnosis of dementia (58), which excluded the point that the
incidence of malignant tumors affected cognitive impairment.
Elderly cognitive impairment may involve chronic inflammation,
together with reduced excitability of parasympathetic nerves or the
reduction of ACh synthesis and release. The tumor is usually
associated with abnormal activation of parasympathetic nerves,
which may inhibit immune and inflammatory responses and promote
tumor progression. Hence, parasympathetic nerves may provide a
‘bridge’ connecting cognitive disorders with tumors. Ongoing
investigations into the use of parasympathetic treatments for these
two diseases will have to consider the complications that may arise
from multiple AChR actions. Regulating the excitability of
parasympathetic nerves to maintain an ‘appropriate’ state is likely
to be an important subject of geriatric medicine in the future.
In conclusion, sympathetic and parasympathetic
nerves were found in human CRC. Sympathetic nerves were mostly
detected in early phases of cancer associated with good prognosis,
whereas parasympathetic nerves and α9nAChR were mostly observed in
late phases of cancer associated with bad prognosis. The expression
of parasympathetic nerves and α9nAChR were positively correlated.
These results suggest that parasympathetic nerves may promote the
progression of CRC through α9nAChR. The exact role and mechanism of
autonomic nervous system actions in CRC deserve further study.
Acknowledgements
The present study was supported by The Program of
Clinical Excellent Talents of Hebei Province.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
α9nAChR
|
α9 nicotinic acetylcholine
receptor
|
|
Ach
|
acetylcholine
|
|
AChR
|
acetylcholine receptor
|
|
nAChR
|
N type cholinergic receptor
|
|
TNM
|
tumor, lymph node, metastasis
|
|
TH
|
Tyrosine hydroxylase
|
|
VAChT
|
vesicular acetylcholine
transporter
|
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng B, Sun J, Ling TL, Lu AG, Wang ML,
Chen XY, Ma JJ, Li JW, Zang L, Han DP and Zheng MH: Laparoscopic
complete mesocolic excision (CME) with medial access for right-hemi
colon cancer: Feasibility and technical strategies. Surg Endosc.
26:3669–3675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J1, Jiang T, Qiu Z, Cen G, Cao J,
Huang K, Pu Y, Liang H, Huang R and Chen S: Short-term and
medium-term clinical outcomes of laparoscopic-assisted and open
surgery for colorectal cancer: A single center retrospective
case-control study. BMC Gastroenterol. 11:852011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pienta KJ, McGregor N, Axelrod R and
Axelrod DE: ecological therapy for cancer: Defining tumors using an
ecosystem paradigm suggests new opportunities for novel cancer
treatments. Transl Oncol. 1:158–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q: Circulating tumor cells: Determining
its number and what it means. Cytometry A. 77:211–212.
2010.PubMed/NCBI
|
|
8
|
Folkman J, Merler E, Abernathy C and
Williams G: Isolation of a tumor factor responsible for
angiogenesis. J Exp Med. 133:275–288. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seifert P and Spitznas M: Tumours may be
innervated. Virchows Arch. 438:228–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchell BS, Schumacher U and Kaiserling
E: Are tumours innervated? Immunohistological investigations using
antibodies against the neuronal marker protein gene product 9.5
(PGP 9.5) in benign, malignant and experimental tumours. Tumour
Biol. 15:269–274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seifert P, Benedic M and Effert P: Nerve
fibers in tumors of the human urinary bladder. Virchows Arch.
440:291–297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kayahara M, Nakagawara H, Kitagawa H and
Ohta T: The nature of neural invasion by pancreatic cancer.
Pancreas. 35:218–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albo D, Akay CL, Marshall CL, Wilks JA,
Verstovsek G, Liu H, Agarwal N, Berger DH and Ayala GE:
Neurogenesis in colorectal cancer is a marker of aggressive tumor
behavior and poor outcomes. Cancer. 117:4834–4845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lolas G, Bianchi A and Syrigos KN:
Tumour-induced neoneurogenesis and perineural tumour growth: A
mathematical approach. Sci Rep. 6:206842016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giger RJ, Hollis ER IId and Tuszynski MH:
Guidance molecules in axon regeneration. Cold Spring Harb Perspect
Biol. 2:a0018672010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mancino M, Ametller E, Gascón P and
Almendro V: The neuronal influence on tumor progression. Biochim
Biophys Acta. 1816:105–118. 2011.PubMed/NCBI
|
|
17
|
Zänker KS: The neuro-neoplastic synapse:
Does it exist? Prog Exp Tumor Res. 39:154–161. 2007.PubMed/NCBI
|
|
18
|
Magnon C, Hall SJ, Lin J, Xue X, Gerber L,
Freedland SJ and Frenette PS: Autonomic nerve development
contributes to prostate cancer progression. Science.
341:12363612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang J, Li Z, Lu L and Cho CH:
β-Adrenergic system, a backstage manipulator regulating tumour
progression and drug target in cancer therapy. Semin Cancer Biol.
23:533–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani
S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman
RA, Renz BW, et al: Denervation suppresses gastric tumorigenesis.
Sci Transl Med. 6:250ra1152014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marjolaine PR, Michèle L, Benjamin D,
Bruno B and Muriel JS: Role of cholinergic receptors in colorectal
cancer: Potential therapeutic implications of vagus nerve
stimulation? J Cancer Ther. 4:1116–1131. 2013. View Article : Google Scholar
|
|
22
|
Hulme EC, Birdsall NJ and Buckley NJ:
Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol.
30:633–673. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia Y, Jia Y, Zu S, Zhang Y, Xiao D, Wang
D and Ma X: Hypoxia-induced overexpression of alpha5 nicotinic
acetylcholine receptor of human lung cancer cell lines. 2014 IEEE
Workshop on Electronics, Computer and Applications. IEEE. 969–971.
2014.
|
|
24
|
Zhao Q, Yue J, Zhang C, Gu X, Chen H and
Xu L: Inactivation of M2 AChR/NF-κB signaling axis reverses
epithelial-mesenchymal transition (EMT) and suppresses migration
and invasion in non-small cell lung cancer (NSCLC). Oncotarget.
6:29335–29346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grando SA: Connections of nicotine to
cancer. Nat Rev Cancer. 14:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Zhou W, Xue L, Zhang W and Zhan Q:
Nicotine activates YAP1 through nAChRs mediated signaling in
esophageal squamous cell cancer (ESCC). PLoS One. 9:e908362014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Wadei MH, Al-Wadei HA and Schuller HM:
Pancreatic cancer cells and normal pancreatic duct epithelial cells
express an autocrine catecholamine loop that is activated by
nicotinic acetylcholine receptors alpha3, α5, and α7. Mol Cancer
Res. 10:239–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang N, Meng X and Song H: Nicotinic
acetylcholine receptors and cancer. Biomed Rep. 4:515–518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yue Y, Liu R, Cheng W, Hu Y, Li J, Pan X,
Peng J and Zhang P: GTS-21 attenuates lipopolysaccharide-induced
inflammatory cytokine production in vitro by modulating the Akt and
NF-κB signaling pathway through the α7 nicotinic acetylcholine
receptor. Int Immunopharmacol. 29:504–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu C, Hu Z, Yu D, Huang L, Jin G, Liang J,
Guo H, Tan W, Zhang M, Qian J, et al: Genetic variants on
chromosome 15q25 associated with lung cancer risk in Chinese
populations. Cancer Res. 69:5065–5072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Changeux JP: Nicotine addiction and
nicotinic receptors: Lessons from genetically modified mice. Nat
Rev Neurosci. 11:389–401. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ,
Chang YJ, Tam KW, Wei PL, Cheng TC, Chu JS, et al: Overexpression
and activation of the alpha9-nicotinic receptor during
tumorigenesis in human breast epithelial cells. J Natl Cancer Inst.
102:1322–1335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tu SH, Lin YC, Huang CC, Yang PS, Chang
HW, Chang CH, Wu CH, Chen LC and Ho YS: Protein phosphatase
Mg2+/Mn2+ dependent 1F promotes smoking-induced breast cancer by
inactivating phosphorylated-p53-induced signals. Oncotarget.
7:77516–77531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. Ca Carcer J Clin.
67:93–99. 2017. View Article : Google Scholar
|
|
35
|
Ho YJ, Wang TC, Fan CH and Yeh CK: Current
progress in antivascular tumor therapy. Drug Discov Today.
22:1503–1515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Yang C, Liu X, Yuan L, Zhang F, Wang
M, Miao D, Gu X, Jiang S, Cui B, et al: EphA3 promotes malignant
transformation of colorectal epithelial cells by upregulating
oncogenic pathways. Cancer Lett. 383:195–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu X and Tüzün E: Are linear AChR epitopes
the real culprit in ocular myasthenia gravis. Med Hypotheses.
99:26–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wong HP, Yu L, Lam EK, Tai EK, Wu WK and
Cho CH: Nicotine promotes colon tumor growth and angiogenesis
through beta-adrenergic activation. Toxicol Sci. 97:279–287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu CH, Lee CH and Ho YS: Nicotinic
acetylcholine receptor-based blockade: Applications of molecular
targets for cancer therapy. Clin Cancer Res. 17:3533–3541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee CH, Chang YC, Chen CS, Tu SH, Wang YJ,
Chen LC, Chang YJ, Wei PL, Chang HW, Chang CH, et al: Crosstalk
between nicotine and estrogen-induced estrogen receptor activation
induces α9-nicotinic acetylcholine receptor expression in human
breast cancer cells. Breast Cancer Res Treat. 129:331–345. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guertin KA, Gu F, Wacholder S, Freedman
ND, Panagiotou OA, Reyes-Guzman C and Caporaso NE: Time to first
morning cigarette and risk of chronic obstructive pulmonary
disease: Smokers in the PLCO cancer screening trial. PLoS One.
10:e01259732015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luberto CM, Hyland KA, Streck JM, Temel B
and Park ER: Stigmatic and sympathetic attitudes toward cancer
patients who smoke: A qualitative analysis of an online discussion
board forum. Nicotine Tob Res. 18:2194–2201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nuutinen S, Panula P and Salminen O:
Different hypothalamic nicotinic α7 receptor expression and
response to low nicotine dose in alcohol-preferring and
alcohol-avoiding rats. Alcohol Clin Exp Res. 40:329–334. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cesario A, Russo P, Nastrucci C and
Granone P: Is α7-nAChR a possible target for lung cancer and
malignant pleural mesothelioma treatment. Curr Drug Targets.
13:688–694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holt JC, Lioudyno M, Athas G, Garcia MM,
Perin P and Guth PS: The effect of proteolytic enzymes on the
alpha9-nicotinic receptor-mediated response in isolated frog
vestibular hair cells. Hear Res. 152:25–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guha P, Bandyopadhyaya G, Polumuri SK,
Chumsri S, Gade P, Kalvakolanu DV and Ahmed H: Nicotine promotes
apoptosis resistance of breast cancer cells and enrichment of side
population cells with cancer stem cell-like properties via a
signaling cascade involving galectin-3, α9 nicotinic acetylcholine
receptor and STAT3. Breast Cancer Res Treat. 145:5–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou T, Wang Y, Guo CK, Zhang WJ, Yu H,
Zhang K and Kong WJ: Two distinct channels mediated by m2mAChR and
α9nAChR co-exist in type II vestibular hair cells of guinea pig.
Int J Mol Sci. 14:8818–8831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kawashima K, Fujii T, Moriwaki Y and
Misawa H: Critical roles of acetylcholine and the muscarinic and
nicotinic acetylcholine receptors in the regulation of immune
function. Life Sci. 91:1027–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bencherif M, Lippiello PM, Lucas R and
Marrero MB: Alpha7 nicotinic receptors as novel therapeutic targets
for inflammation-based diseases. Cell Mol Life Sci. 68:931–949.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang R, Wugeti N, Sun J, Yan H, Guo Y,
Zhang L, Ma M, Guo X, Jiao C, Xu W, et al: Effects of vagus nerve
stimulation via cholinergic anti-inflammatory pathway activation on
myocardial ischemia/reperfusion injury in canine. Int J Clin Exp
Med. 7:2615–2623. 2014.PubMed/NCBI
|
|
52
|
Vicens P, Ribes D, Heredia L, Torrente M
and Domingo JL: Motor and anxiety effects of PNU-282987, an alpha7
nicotinic receptor agonist, and stress in an animal model of
Alzheimer's disease. Curr Alzheimer Res. 10:516–523. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koopman FA, Schuurman PR, Vervoordeldonk
MJ and Tak PP: Vagus nerve stimulation: A new bioelectronics
approach to treat rheumatoid arthritis? Best Pract Res Clin
Rheumatol. 28:625–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mazloom R, Eftekhari G, Rahimi-Balaei M,
Khori V, Hajizadeh S, Dehpour AR and Mani AR: Correction: The role
of α7 nicotinic acetylcholine receptor in modulation of heart rate
dynamics in endotoxemic rats. PLoS One. 10:e01278262015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Marrero MB and Bencherif M: Convergence of
alpha 7 nicotinic acetylcholine receptor-activated pathways for
anti-apoptosis and anti-inflammation: Central role for JAK2
activation of STAT3 and NF-kappaB. Brain Res. 1256:1–7. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Daianu M, Jahanshad N, Nir TM, Toga AW,
Jack CR Jr, Weiner MW and Thompson PM: Alzheimer's
DiseaseNeuroimaging Initiative: Breakdown of brain connectivity
between normal aging and Alzheimer's disease: A structural k-core
network analysis. Brain Connect. 3:407–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Musicco M, Adorni F, Di Santo S, Prinelli
F, Pettenati C, Caltagirone C, Palmer K and Russo A: Inverse
occurrence of cancer and Alzheimer disease: A population-based
incidence study. Neurology. 81:322–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Roe CM, Fitzpatrick AL, Xiong C, Sieh W,
Kuller L, Miller JP, Williams MM, Kopan R, Behrens MI and Morris
JC: Cancer linked to Alzheimer disease but not vascular dementia.
Neurology. 74:106–112. 2010. View Article : Google Scholar : PubMed/NCBI
|