Introduction
Type 2 diabetes mellitus (T2D) is a major metabolic
disease and is a risk to human health worldwide (1,2). T2D
is characterized by reduced sensitivity of insulin receptors in
target organs and absolutely or relatively insufficient secretion
of insulin (3,4). These characteristics result in
persistent hyperglycemia, which affects the heart, blood vessels,
eyes, kidney and nerves, in addition to the wound-healing process,
in diabetic patients (5–10). Although drugs or exogenous insulin
administration can ameliorate hyperglycemia, the amelioration of
peripheral insulin resistance in target tissues is not very
effective.
Currently, cell-based therapy is being explored as a
potential treatment strategy for diabetes (11,12).
Mesenchymal stem cells (MSCs) possess various properties, including
differentiation potential, local microenvironment modulatory and
immunoregulatory effects, and the capacity to secrete various
factors (13). These properties of
MSCs make them excellent candidates for diabetes management. To
date, numerous studies on diabetic animal models have demonstrated
that infusion of MSCs ameliorates hyperglycemia (14–17).
In addition, the majority of registered clinical trials on
MSC-treated type 1 and/or type 2 diabetes in phase I/II have
indicated that MSC administration exerts promising therapeutic
effects in diabetic volunteers (18). Furthermore, our previous study
demonstrated that MSCs alleviated hyperglycemia and insulin
resistance by reversing the reduced expression of glucose
transporter type 4 (Glut4) and insulin receptor substrate-1
(IRS-1), as well as AKT phosphorylation, in peripheral insulin
target tissues of T2D rats, including the skeletal muscle, adipose
and liver (19). These results
concerning the beneficial effect of MSC infusion in alleviating
insulin resistance have been widely verified by different research
institutions (14,20–22).
However, the precise underlying mechanisms require further
investigation.
Insulin resistance is a key pathogenic factor that
presents in several metabolic disorders, including obesity and T2D.
In the peripheral insulin target tissues, skeletal muscle accounts
for 70–90% of insulin-stimulated glucose metabolism (23). The insulin resistance of skeletal
muscle has been the focus of numerous studies worldwide. Recent
studies have demonstrated that the muscle-specific TRIM family
protein mitsugumin 53 (MG53; also termed TRIM72) is implicated in
insulin resistance (24–27). In addition to acting as a key
component of plasma membrane repair during normal cellular
physiology (28,29), MG53 is also an E3 ligase that
interacts with IRS-1 (24,25,27,29).
Certain studies have demonstrated that MG53 expression is elevated
in the skeletal muscle of rodents and humans with insulin
resistance or metabolic disorders (24,27).
In addition, it has been indicated that elevated MG53 in skeletal
muscle may interact with and ubiquitinated IRS-1, thereby
disrupting insulin signaling and inducing insulin resistance or
metabolic disorders (24,27). Notably, the role of MG53 in
metabolic disorders is controversial, as other studies have
indicated that muscle samples derived from human diabetic patients
and mice with insulin resistance exhibit normal expression of MG53
(25,26). Nevertheless, we recently reported
that MG53 was elevated in the cardiac muscle of rats with diabetic
cardiomyopathy (30). Therefore,
the expression of MG53 in skeletal muscle during T2D should be
investigated in greater detail. In addition, the aforementioned
studies (19,24,27)
identified that MSC infusion and MG53 may exert their effects via
certain common molecules in the insulin signaling pathway,
including IRS-1, AKT phosphorylation and Glut4, and our previous
results indicated that MSC infusion inhibited MG53 elevation in the
cardiac muscle of T2D rats (30).
Therefore, it was hypothesized that MG53 in skeletal muscle may be
a promising novel therapeutic target protein for MSC-mediated
amelioration of insulin resistance in T2D.
To test this hypothesis and investigate the specific
therapeutic mechanisms or targets involved in the beneficial
effects of MSC infusion, a T2D rat model was generated and MSC
infusion performed. The effects of MSC infusion on hyperglycemia,
insulin resistance and MG53 expression in skeletal muscle, in
addition to the expression of proteins associated with insulin
signaling, were investigated. The results demonstrated that MSC
infusion ameliorated hyperglycemia through improving insulin
resistance. The underlying mechanisms may include the inhibition of
MG53 elevation and reduced degradation of IRS-1 and
phosphorylated-AKT (p-AKT) in skeletal muscle.
Materials and methods
Animals
A total of 40 adult (aged 8 weeks; weight, 210±12 g)
and 20 immature (aged 4 weeks; weight, 80–100 g) male
Sprague-Dawley rats were supplied by the Experimental Animal Center
of the Chinese PLA General Hospital (Beijing, China). All animal
experiments were conducted in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (Beijing, China) and were approved by the Animal Care and
Use Committee of the Chinese PLA General Hospital. Animals were
maintained in a room with filtered air and a 40–70% relative
humidity, with 12 h light/dark cycle and an ambient temperature of
22–25°C. Unless required to fast, animals had free access to food
and water. At the end of experiments, rats were anesthetized with
pentobarbital sodium intraperitoneally (60 mg/kg) and sacrificed by
cervical dislocation.
Adipose-derived MSC isolation, culture
and identification
Adipose-derived MSCs were isolated and purified from
immature rats as described previously (17,19).
Briefly, rats were anesthetized with pentobarbital sodium
intraperitoneally (60 mg/kg) and sacrificed by cervical
dislocation; adipose tissue isolated from the groin was digested
using 0.05% trypsin and 0.1% collagen I. Following filtration and
centrifugation at 600 × g for 10 min at room temperature, cells
were cultured in low-glucose Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), penicillin (80 U/ml) and streptomycin (0.2
mg/ml) at 37°C in an atmosphere of 5% CO2 and a relative
humidity of ~100%. MSCs were identified as previously described
(17,19). In order to perform surface
immunophenotype analysis, third passage (P3) MSCs were used for
flow cytometry. Once an ~80% confluence was reached, cells were
collected and counted, then cells were randomly divided into six
groups (one group per antibody), each containing 1×106
cells. Cells were then washed with PBS and incubated for 15 min at
room temperature in the dark with the following antibodies:
Allophycocyanin-conjugated CD90 (1:20; cat. no. 561409; BD
Biosciences, San Jose, CA, USA), R-phycoerythrin-conjugated CD54
(1:20; cat. no. 554970; BD Biosciences), fluorescein isothiocyanate
(FITC)-conjugated CD44 (1:20; cat. no. 550974; BD Biosciences),
FITC-conjugated CD34 (1:20; cat. no. 11-0341-82; eBioscience;
Thermo Fisher Scientific, Inc.), FITC-conjugated CD11b (1:20; cat.
no. 554982; BD Biosciences) and FITC-conjugated CD45 (1:20; cat.
no. 554877; BD Biosciences). Following incubation, cells were
washed with PBS and then subjected to flow cytometry analysis,
which was performed using a BD Accuri C6 software system (version
1.0.264.21; BD Biosciences).
In order to perform differentiation potential
analysis, P3 MSCs were cultured in a six-well plate at a density of
104 cells/well at 37°C in an atmosphere of 5%
CO2 and a relative humidity of ~100%. Once cells had
reached a confluency of ~70 or ~100% for osteogenic or adipogenic
differentiation, respectively; the medium was replaced with SD rat
MSCs adipogenic (cat. no. RASMD-90031) or osteogenic (cat. no.
RASMD-90021) differentiation medium (Cyagen Biosciences Inc.,
Guangzhou, China) and following this, the cells were subsequently
cultured at 37°C in an atmosphere of 5% CO2 and a
relative humidity of ~100% for 2 weeks. Following a fixation using
4% paraformaldehyde at room temperature for 30 min, adipogenic
differentiation was identified by staining with 0.5% Oil red O
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room temperature
for 1 h, and osteogenic differentiation was identified via staining
with 0.1% alizarin red S (pH 4.2; Sigma-Aldrich; Merck KGaA) at
room temperature for 30 min. The cells were observed using an
inverted microscope. The freshly harvested, early passage (P4) MSCs
were used in all subsequent experiments.
Induction and treatment of T2D rat
models
A high-fat diet (HFD) combined with streptozotocin
(STZ) injection-induced T2D model was established as previously
described (19). Briefly, adult (8
weeks old) rats were provided with a HFD (40% fat, 41% carbohydrate
and 19% protein) for 8 weeks. Subsequently, a low dose of STZ (20
mg/kg) was administrated intraperitoneally. At 1 week following STZ
administration, the fasting blood-glucose (FBG) and refeeding
blood-glucose levels were measured. In addition, 5 normal chow
diet-induced rats and 15 HFD+STZ-induced rats were randomly chosen
for oral glucose tolerance tests (OGTTs) and insulin tolerance
tests (IPITTs) to verify the T2D model as previously described
(19). For FBG, OGTTs and IPITTs,
rats were fasted overnight prior to measurements or procedures. The
verified T2D model rats were divided into a T2D group and a
MSC-treated group (n=15 each). Each rat in the MSC-treated group
was infused with 2×106 MSCs suspended in 0.3 ml
physiological saline via the tail vein once every 2 weeks, for a
total of four infusions. Rats in the normal group (n=10) were
provided with a normal chow diet and only infused with 0.3 ml
physiological saline.
Determination of the effects of
infused MSCs on hyperglycemia and insulin resistance in T2D
rats
At 24 h (refed) and 48 h (fasted) following each MSC
injection, blood glucose levels were detected with a glucometer
(Accu-Chek Advantage Meter; Roche Diagnostics GmbH, Mannheim,
Germany), and levels were monitored throughout the whole
experiment. At 1 week following the completion of the final MSC
infusion, 5 rats from each group were random chosen for the OGTTs
and IPITTs assessment again. Blood samples were obtained from rats
by squeezing the caudal vein. Following this, serum was isolated
from whole blood samples via centrifugation at 800 × g for 10 min
at 4°C. The serum insulin (FINS) levels were measured using an
ELISA assay kit (cat. no. EZRMI-13K; EMD Millipore, Billerica, MA,
USA) 1 week following the final injection of MSCs. Additionally, at
1 week after the final MSC infusion, 5 rats from each group were
random chosen for the hyperinsulinemic-euglycemic clamp studies to
measure insulin sensitivity, as previously described (19). Briefly, rats were fasted overnight
and 8 mU/kg/min insulin (Novo Nordisk Ltd., Bagsvaerd, Denmark) was
intravenously administered. Blood glucose was monitored at 5 min
intervals and exogenous glucose infusion rates (GIRs) were assessed
until a steady blood glucose level was achieved. In addition, the
homeostatic model assessment (HOMA) was used to assess changes in
insulin resistance (HOMA-IR) and pancreatic β-cell function
(HOMA-β, HBCI), calculated according to the following equations:
HOMA-IR=(FBGⅹFINS)/22.5 and HOMA-β=(20ⅹFINS)/(FBG-3.5) (19).
Immunofluorescence staining
Following the final hyperglycemia/insulin resistance
readings that had been taken 1 week following final MSCs injection,
rats were sacrificed and muscle samples were obtained. The
localization of MG53 protein in rat tibialis anterior muscle was
detected by immunofluorescence staining with 5-µm-thick frozen
sections, which were permeabilized at room temperature for 15 min
using 0.25% TritonX-100 (cat. no. 30632-2I; FARCO Chemical
Supplies, Hong Kong, SAR, China) diluted in PBS. Following this,
sections were incubated with a 1X blocking buffer (cat. no. 12411;
Cell Signaling Technology, Inc., Danvers, MA, USA) at 37°C for 1 h,
and then initially incubated with rabbit polyclonal
anti-MG53/TRIM72 antibody diluted in PBS (8 µg/ml; cat. no.
ab118651; Abcam, Cambridge, UK) overnight at 4°C, followed by
incubation for 1 h at 37°C with anti-rabbit IgG fragment antibody
(1:200; Alexa Fluor 555-conjugated, red; cat. no. 4413; Cell
Signaling Technology, Inc.). Nuclei were stained with DAPI (cat.
no. H-1200; Vector Laboratories, Inc., Burlingame, CA, USA) for 15
min at room temperature. Negative controls were processed
simultaneously by replacing the antibodies with PBS. The sections
were examined by confocal microscopy using a Zeiss 780 system
(Zeiss AG, Oberkochen, Germany).
Western blot analysis
Following the final hyperglycemia/insulin resistance
readings that had been taken 1 week following final MSCs injection,
rats were sacrificed and muscle samples were obtained. Whole cell
lysate from the skeletal muscle was extracted using
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) containing 1% protease
inhibitor cocktail. The protein concentration of lysate was then
determined using bicinchoninic acid assay (Applygen Technologies
Inc., Beijing China). For electrophoresis, a total of 30 µg protein
was loaded onto an 8 or 10% SDS-PAGE gel. Following transfer to
polyvinylidene difluoride membranes, the membranes were blocked in
10% non-fat milk in TBS/Tween-20 (0.2%) for 1 h at 37°C and then
incubated with rabbit antibodies against MG53/TRIM72 (1:500; cat.
no. ab118651; Abcam), Glut4 (1:500; cat. no. sc-7938; Santa Cruz
Biotechnology, Inc.), Na+K+ATPase (1:100,000;
cat. no. ab76020; Abcam), insulin receptor (1:2,000; cat. no.
ab131238; Abcam), IRS-1 (1:500; cat. no. ab131487; Abcam), p-AKT
(1:500; cat. no. 9271; Cell Signaling Technology, Inc.), AKT
(1:1,000; cat. no. 9272; Cell Signaling Technology, Inc.) and
β-actin (1:2,000; cat. no. sc-1616-R; Santa Cruz Biotechnology,
Inc.) diluted in TBS/Tween-20 overnight at 4°C. Following three
washes in TBS-Tween-20, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibodies (cat. no. TA130023; OriGene Technologies, Inc., Beijing,
China) at a dilution of 1:2,000 in TBS-Tween-20 for 40 min at 37°C.
The immunoreactive bands were visualized using a western blotting
luminol reagent (cat. no. TA100016; OriGene Technologies, Inc.) and
captured on X-ray film. Densitometric analysis was performed using
a Gel-Pro Analyzer 3.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
SPSS software version 19.0 (IBM Corp., Armonk, NY,
USA) was used to process the data. All experiments were performed
at least five times independently. Data are presented as the mean ±
standard deviation. Student's t-test or one-way analysis of
variance with Tukey's post-hoc analysis was used when data passed
the test for normality and equal variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
Development and validation of the T2D
rat model
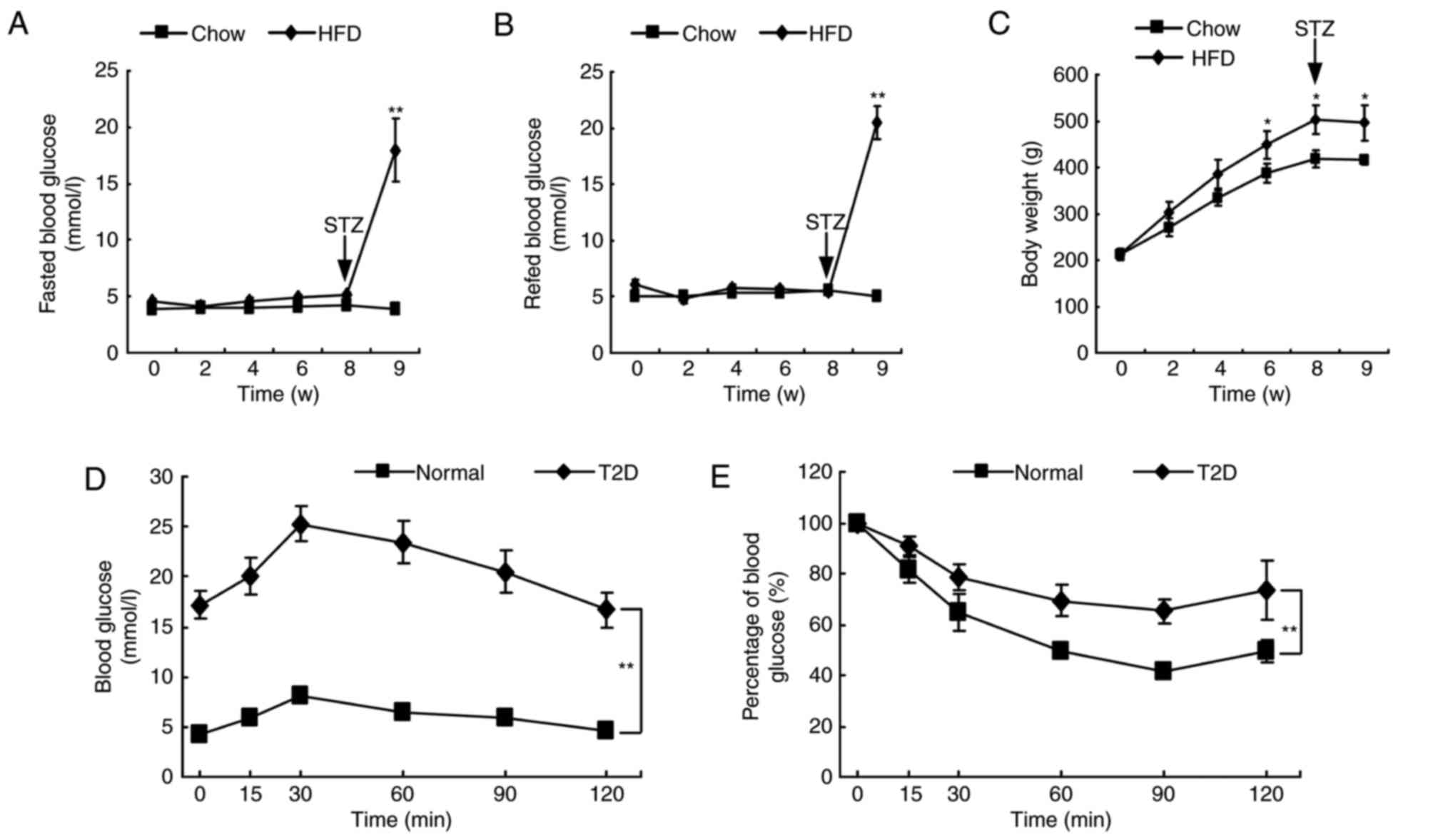
Rats were fed a HFD or normal chow diet for 8 weeks.
No significant differences were observed in the blood glucose level
between the two groups during this 8-week period (Fig. 1A and B). However, HFD induced a
higher body weight compared with the normal chow diet (Fig. 1C). At 1 week following STZ
administration in HFD rats, blood glucose levels significantly
increased by 4–5 fold compared with normal control rats (Fig. 1A and B). In addition, the results
of OGTT and IPITT experiments indicated a notable decrease in
glucose tolerance and insulin sensitivity compared with normal
control rats (Fig. 1D and E).
These results confirmed that the T2D rat model had been established
successfully.
MSC infusion alleviates hyperglycemia
in T2D rats
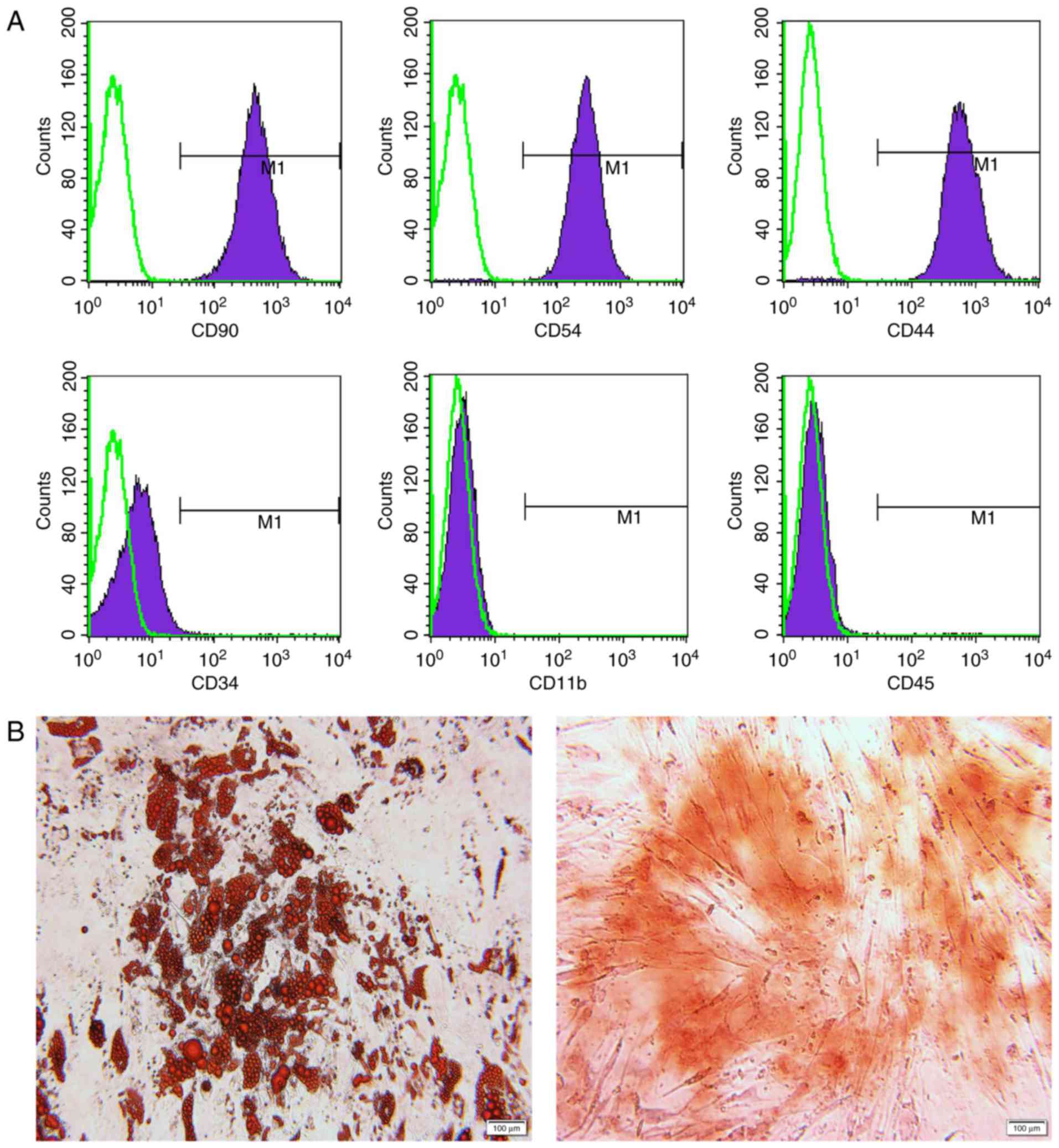
Infused MSCs were identified in advance by their
phenotypes and the potential for differentiating into adipocytes
and osteoblasts (Fig. 2). For
immunological phenotypes, the cells were positive for CD90, CD54
and CD44, and negative for CD34, CD11b and CD45 (Fig. 2A). Successful adipogenic and
osteogenic differentiation was confirmed by oil red O and alizarin
red staining, respectively (Fig.
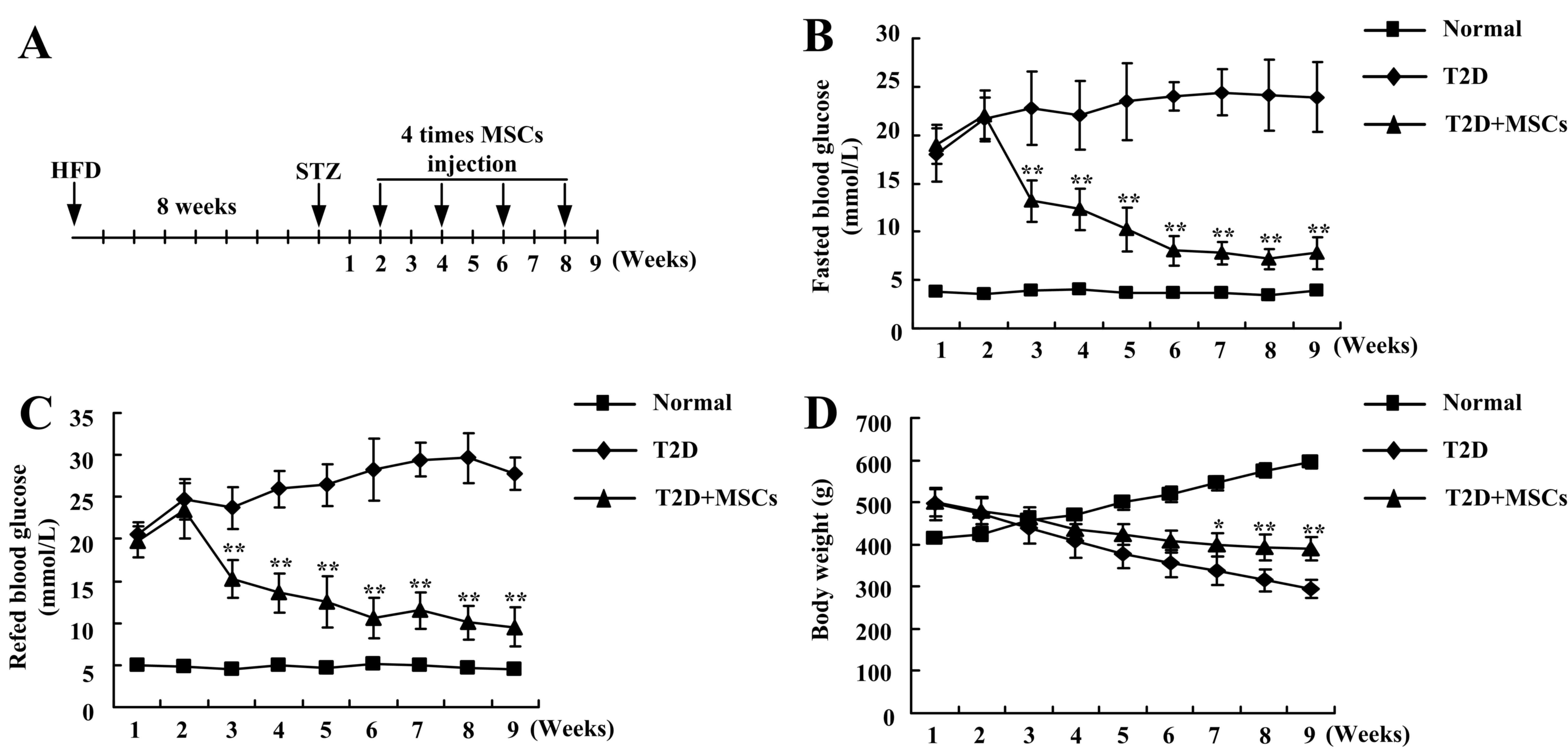
2B). Each rat in the MSC-treated group was infused with MSCs
four times (Fig. 3A). In different
stage phases, untreated T2D rats demonstrated notable hyperglycemia
and decreased body weight (Fig.
3). However, following the four continuous infusions of MSCs,
the blood glucose demonstrated a marked decrease, almost returning
to normal levels (Fig. 3B and C),
and the body weight was significantly increased (Fig. 3D), compared with T2D rats without
treatment. These results indicated that continuous infusion of MSCs
improved the long-term regulation of blood glucose levels in T2D
rats.
MSC infusion improves glucose
metabolism and insulin sensitivity in T2D rats
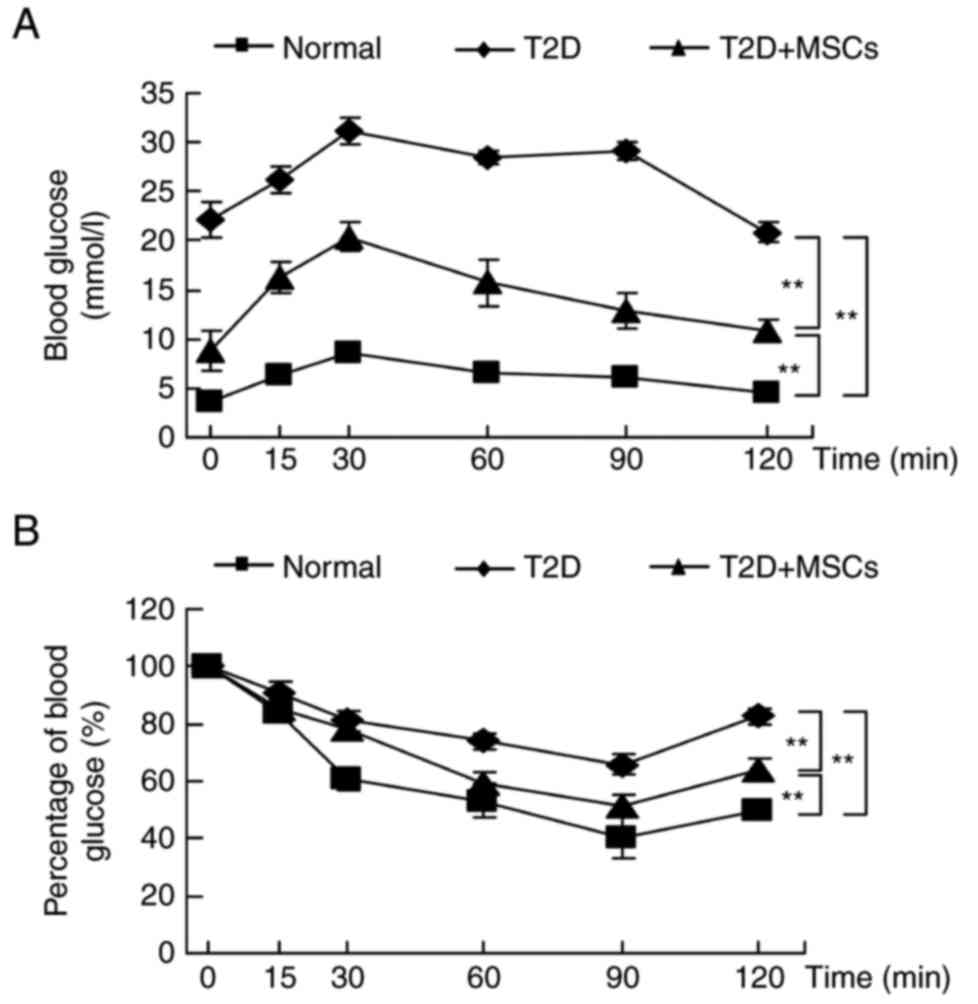
To investigate the effects of MSC infusion on
hyperglycemia alleviation in T2D rats, glucose metabolism and
insulin sensitivity were compared, and hyperinsulinemic-euglycemic
clamp studies performed. As demonstrated in Fig. 4, the results of OGTTs (Fig. 4A) and IPTTs (Fig. 4B) revealed a significant
deterioration in glucose metabolism and insulin sensitivity in T2D
rats, compared with normal control rats. In addition, the GIR was
also significantly decreased in T2D rats compared with normal
control rats (Fig. 5A). Consistent
with hyperglycemia alleviation, MSC-treated rats demonstrated a
significant improvement in glucose metabolism and were more
sensitive to insulin compared with untreated T2D rats (Figs. 4 and 5A). The serum insulin levels were
significantly increased in the T2D and MSC-treated T2D groups
compared with the normal control group; however, no significant
difference was observed between the T2D and MSC-treated T2D groups
(Fig. 5B). Nevertheless, the HOMA
results (IR and HBCI) indicated that insulin resistance and
pancreatic β-cell function were notably improved in MSC-treated T2D
rats (Fig. 5C and D). These
results demonstrated that MSC-mediated hyperglycemia alleviation
may be associated with improvements in target tissue insulin
sensitivity.
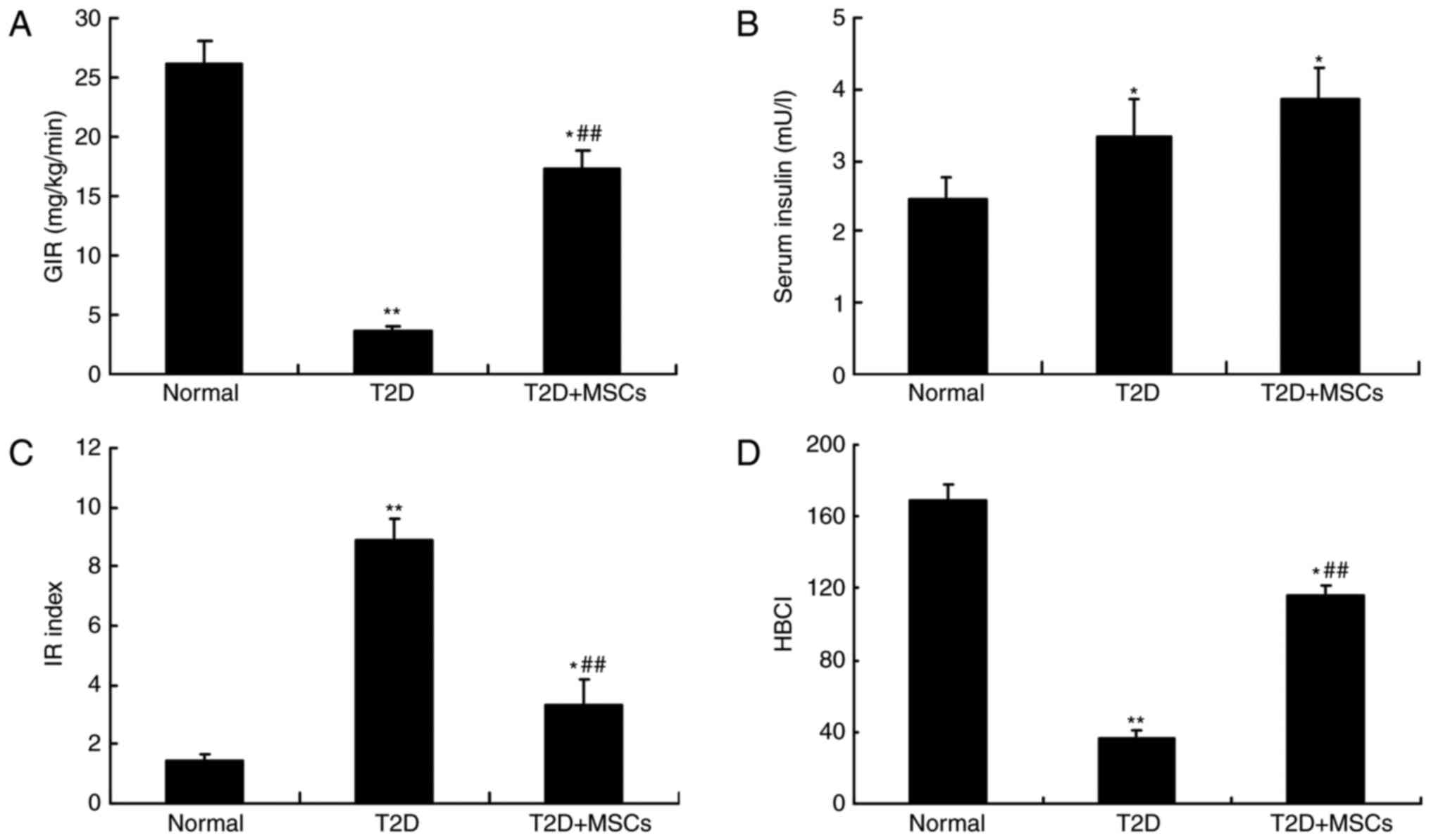 | Figure 5.MSC infusion improved insulin
sensitivity in T2D rats. At 1 week after the completion of the
final MSC infusion, hyperinsulinemic-euglycemic clamp studies were
used to measure the insulin sensitivity of each group. (A)
Exogenous GIRs were assessed by infusion with 8 mU/kg/min insulin
during the hyperinsulinemic clamp, n=5 rats per group. (B) Serum
insulin levels were assessed by ELISA assay kits. The HOMA was used
to assess alterations in (C) IR index and (D) pancreatic β-cell
function, which were calculated according to the following
equations: HOMA-IR=(fasting blood glucoseⅹserum insulin)/22.5 and
HOMA-β=(20ⅹserum insulin)/(fasting blood glucose-3.5). n=10 in
normal group, n=15 in T2D group and n=15 in T2D + MSCs group.
*P<0.05 and **P<0.01 vs. normal group; ##P<0.01
vs. T2D group. MSCs, mesenchymal stem cells; T2D, type 2 diabetes
mellitus; GIRs, glucose infusion rates; HOMA, homeostatic model
assessment; IR, insulin resistance; HOMA-β/HBCI, pancreatic β-cell
index. |
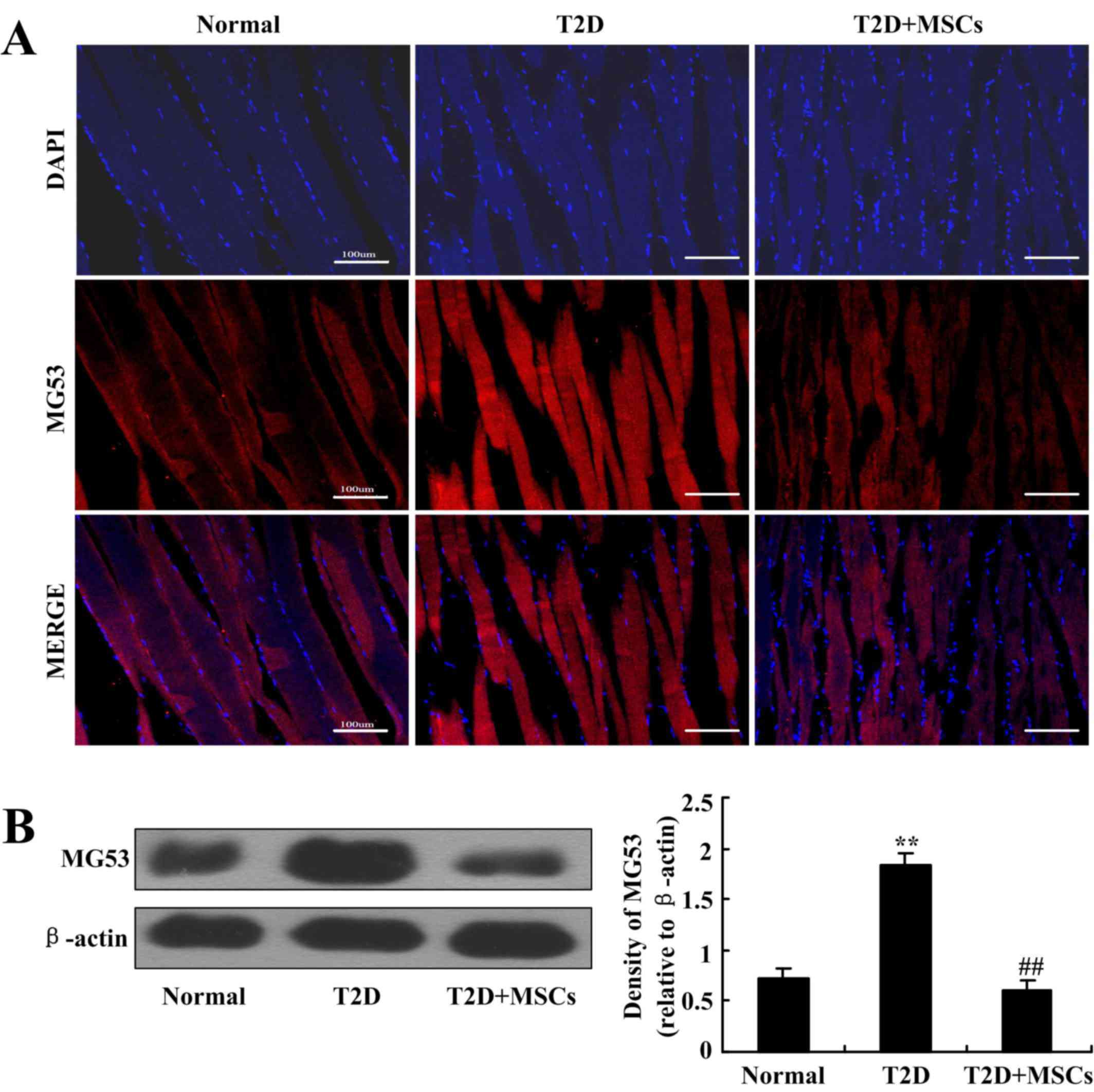
MSC infusion inhibits MG53 elevation
in the skeletal muscle of T2D rats
To further investigate the potential mechanisms of
MSC-induced alleviation of insulin resistance and the potential
association with MG53, MG53 protein expression in the skeletal
muscle of T2D rats with or without MSC administration was assessed
by immunofluorescence staining and western blotting.
Immunofluorescence results demonstrated that MG53 expression was
markedly elevated in the skeletal muscle of T2D rats compared with
normal control rats, but MSC infusion markedly inhibited this MG53
elevation in T2D rats (Fig. 6A).
The results were further confirmed by western blot analysis
(Fig. 6B). These results were
consistent with the amelioration of hyperglycemia and insulin
sensitivity by MSC infusion, which indicates that MG53 in skeletal
muscle may be a potential therapeutic target in the treatment of
T2D with MSCs.
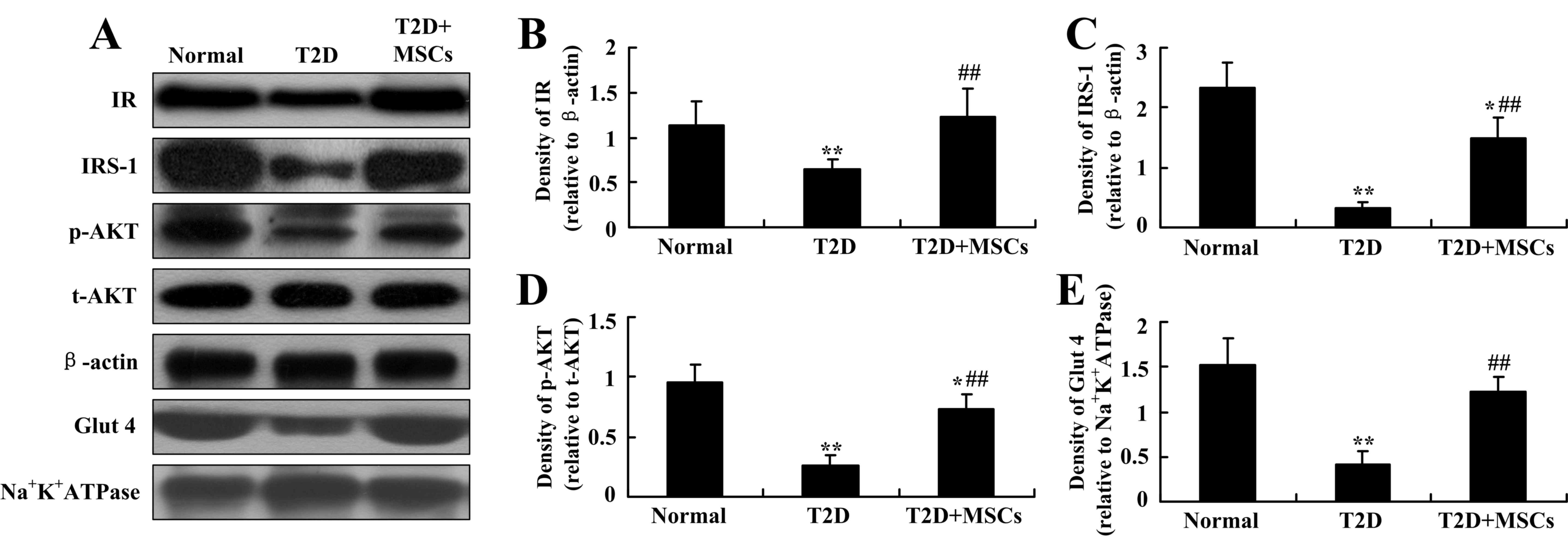
Insulin signaling elements in skeletal
muscle are restored by MSC infusion in T2D rats
The IRS family of proteins serves a central role in
insulin signal transduction. Studies have demonstrated that
elevated MG53 in skeletal muscle may ubiquitinate IRS-1 and
subsequently decrease AKT phosphorylation, which is considered to
be one important mechanism of insulin resistance in T2D or
metabolic disorders (24,27). Thus, in the present study, the
protein expression levels of insulin receptor, IRS-1 and p-AKT in
skeletal muscle were analyzed to further confirm whether skeletal
muscle MG53 may be a potential therapeutic target during the
treatment of T2D by MSC infusion. As demonstrated in Fig. 7A-D, consistent with the alleviation
of insulin resistance and inhibition of MG53 elevation, reductions
in feeding-induced expression of insulin receptor, IRS-1 and p-AKT
in skeletal muscle were markedly restored in T2D rats by MSC
infusion. In addition, the decrease in the expression of Glut4 in
the skeletal muscle of T2D rats was also restored following MSC
infusion. Therefore, these results indicate that MSC infusion in
T2D may inhibit MG53 elevation, subsequently inhibiting insulin
signaling element degradation and alleviating insulin
resistance.
Discussion
Numerous studies and clinical trials have
demonstrated that MSC infusion is able to alleviate hyperglycemia
in diabetes mellitus and that MSCs are potential candidates for the
treatment of T2D (11,14,18,19).
Despite extensive research in this field, the specific underlying
mechanisms remain poorly understood. Our previous study
demonstrated that infusion of MSCs contributed to ameliorating
hyperglycemia by improving peripheral insulin sensitivity in rats
with T2D (19). These findings
have been confirmed a number of times by different institutes
(14,20–22).
However, the precise mechanisms remain unclear.
The mechanism of insulin resistance is complex. In
previous publications, it has been emphasized that defects in the
glucose signaling pathway are a major obstacle, which typically
manifest as decreased expression of Glut4 and defective Glut4
traffic to the surface membrane, and disruption of
phosphatidylinositol 3-kinase (PI3K)-AKT phosphorylation (31,32).
Our previous study demonstrated that infusion of MSCs contributed
to improving peripheral insulin sensitivity in rats with T2D by
reversing the reduced expression of Glut4 and IRS-1, as well as AKT
phosphorylation, in peripheral insulin target tissues, including
skeletal muscle, adipose and liver tissues (19). A recent study indicated a novel
mechanism by which MSCs alleviated insulin resistance, which
involved the regulation of M2 macrophage polarization and promotion
of interleukin-6 production in adipose tissue (33). Another study indicated that MSCs
improved hyperglycemia by regulating hepatic glucose metabolism in
an AMP-activated protein kinase signaling pathway-dependent manner
(17). However, in the peripheral
insulin target tissues, skeletal muscle accounts for ~70–90% of
insulin-stimulated glucose disposal, which serves an important role
in the modulation of insulin resistance (23). Previous studies have demonstrated
that muscle-specific MG53 may be implicated in insulin resistance
(24–27). Other studies have demonstrated that
in addition to acting as a key component of plasma membrane repair
during normal cellular physiology (28,29),
MG53 is elevated in the skeletal muscle of insulin resistance or
metabolic disorder models, and that elevated MG53 may interact with
and ubiquitinate IRS-1, thereby disrupting insulin signaling
(24,27). By contrast, certain studies have
indicated that muscle samples originating from human patients with
diabetes and mice with insulin resistance did not exhibit an
abnormal expression of MG53 (25,26).
The key point of contention is whether MG53 expression is elevated
in skeletal muscle in rodent or human metabolic disorders. Thus,
further investigation is required to investigate MG53 expression
and its effects on insulin resistance. We recently reported that
MG53 was elevated in cardiac muscle in diabetic cardiomyopathy in
rats (30). The present study
indicated that MG53 was also elevated in the skeletal muscle of T2D
rats. Therefore, it is hypothesized that MSC-mediated MG53
reduction in skeletal muscle may be a novel mechanism for
alleviating insulin resistance. Until now, the association between
MSCs and skeletal muscle MG53 expression, and the associated
effects on insulin signaling, were unknown.
The results of the current study indicated that MSC
infusion inhibited MG53 elevation in the skeletal muscle of T2D
rats. These results are consistent with the hyperglycemia and
insulin resistance alleviation effects of MSCs also observed in the
present study. Thus, MG53 in skeletal muscle may be a promising
novel therapeutic target protein of MSCs during their alleviation
of insulin resistance in T2D. To further verify this hypothesis,
the protein levels of insulin receptor, IRS-1 and p-AKT, components
of the insulin signaling pathway, were analyzed by western
blotting. Notably, consistent with the alleviation of insulin
resistance and inhibition of MG53 elevation, the decreased
expression of insulin receptor, IRS-1 and p-AKT in the skeletal
muscle of T2D rats was markedly restored by MSC infusion. The IRS
family of proteins (IRS-1-4) are important components in insulin
signal transduction. Studies of single-gene knockout mice have
demonstrated that the roles of IRS-1 and IRS-2 may be more
distinctive and partially overlapping, while IRS-3 and IRS-4 do not
appear to be as important in terms of the effects of insulin on
glucose homeostasis (34,35). However, double knockout of IRS-1
and IRS-3 has been reported to induce severe phenotypes of
diabetes, indicating a strong compensatory role of IRS-1 and IRS-3;
and Laustsen et al (34)
also demonstrated that the major factor in the development of this
diabetic phenotype was the deficiency of IRS-1 and IRS-3 in adipose
tissue and the associated decreased level of adipose-derived
leptin. As IRS-3 is reported to be most abundant in adipocytes,
with its mRNA also detected in the liver, heart, lungs and kidneys
(34), the IRS-1 expression in
skeletal muscle may be more important in regulating insulin
resistance. MG53 is a muscle-specific E3-ligase that has been
reported to ubiquitinate IRS-1 and subsequently inactivate the
downstream PI3K-AKT signaling pathway to impair glucose homeostasis
in skeletal muscle (25,27). Thus, MSC infusion may inhibit MG53
elevation and subsequently restore insulin receptor, IRS-1 and
feeding-induced p-AKT levels in the skeletal muscle of T2D rats.
This may explain why MSC infusion has been demonstrated to
alleviate insulin resistance.
Furthermore, in the present study, the decreased
expression of Glut4 in the skeletal muscle of T2D rats was also
restored following MSC infusion. Previous studies have demonstrated
that decreased levels of Glut4 are implicated in insulin
resistance, acting as an obstacle for glucose disposal (31,32).
Therefore, the results of the present study indicate that MSC
infusion in T2D may inhibit MG53 elevation in skeletal muscle,
subsequently inhibiting insulin signaling element degradation and
alleviating insulin resistance. However, certain limitations remain
that should be addressed in future studies, including the specific
mechanisms involved in the negative regulation of MG53 expression
by MSCs.
In conclusion, the results of the present study
demonstrated that MSC infusion may ameliorate hyperglycemia by
alleviating insulin resistance. The specific mechanisms involved
may include inhibiting the elevation of skeletal muscle MG53 and
the subsequent degradation of IRS-1 and p-AKT in skeletal muscle.
These findings indicate that MG53 may be a potential therapeutic
target in the treatment of T2D with MSCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81471052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZD, HX, WH and GC designed and conducted
experiments, performed data analyses and contributed to the writing
of the manuscript. JZ, CY, LJ, JL and HS conducted the experiments
and performed data analyses. YS designed and conducted experiments,
performed data analyses and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals and were approved by the Animal Care and Use
Committee of the Chinese PLA General Hospital (Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen L, Magliano DJ and Zimmet PZ: The
world wide epidemiology of type 2 diabetes mellitus-present and
future perspectives. Nat Rew Endocrinol. 8:228–236. 2011.
View Article : Google Scholar
|
|
2
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fonseca VA: Defining and characterizing
the progression of type 2 diabetes. Diabetes Care. 32 Suppl
2:S151–S156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stumvoll M, Goldstein BJ and van Haeften
TW: Type 2 diabetes: Principles of pathogenesis and therapy.
Lancet. 365:1333–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Russell ND and Cooper ME: 50 years
forward: Mechanisms of hyperglycemia driven diabetic complications.
Diabetologia. 58:1708–1714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZV and Hill JA: Diabetic
cardiomyopathy: Catabolism driving metabolism. Circulation.
131:771–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y and Vanhoutte PM: Macro- and
microvascular endothelial dysfunction in diabetes. J Diaberes.
9:434–449. 2017. View Article : Google Scholar
|
|
8
|
Shih KC, Lam KS and Tong L: A systematic
review on the impact of diabetes mellitus on the ocular surface.
Nutr Diabetes. 7:e2512017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim AKh: Diabetic
nephropathy-complications and treatment. Int J Nephrol Renovasc
Dis. 7:361–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Volmer-Thole M and Lobmann R: Neuropathy
and diabetic foot syndrome. Int J Mol Sci. 17:pii: E917. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lilly MA, Davis MF, Fabie JE, Terhune EB
and Gallicano GI: Current stem cell based therapies in diabetes. Am
J Stem Cells. 5:87–98. 2016.PubMed/NCBI
|
|
12
|
Liu X, Wang Y, Li Y and Pei X: Research
status and prospect of stem cells in the treatment of diabetes
mellitus. Sci China Life Sci. 56:306–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orbay H, Tobita M and Mizuno H:
Mesenchymal stem cells isolated from adipose and other tissues:
Basic biological properties and clinical applications. Stem Cells
Int. 2012:4617182012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao M, Pan Q, Dong H, Yuan X, Li Y, Sun Z,
Dong X and Wang H: Adipose-derived mesenchymal stem cells improve
glucose homeostasis in hight-fat diet-induced obese mice. Stem Cell
Res The. 6:2082015. View Article : Google Scholar
|
|
15
|
Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou
Q, Tong C, Ti D, Dong L, Cheng Y, et al: Multiple intravenous
infusion of bone marrow mesenchymal stem cells reverse
hyperglycemia in experimental type 2 diabetes rats. Biochem Biophys
Res Commun. 436:418–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aali E, Mirzamohammadi S, Ghaznavi H,
Madjd Z, Larijani B, Rayegan S and Sharifi AM: A comparative study
of mesenchymal stem cell transplantation with its paracrine effect
on control of hyperglycemia in type 1 diabetic rats. J Diabetes
Metab Disord. 13:762014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie M, Hao HJ, Cheng Y, Xie ZY, Yin YQ,
Zhang Q, Gao JQ, Liu HY, Mu YM and Han WD: Adipose-derived
mesenchymal cells ameliorate hyperglycemia through regulating
hepatic glucose metabolism in type 2 diabetic rats. Biochem Biophys
Res Commun. 483:435–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng SK, Park EY, Pehar A, Rooney AC and
Gallicano GI: Current progress of human trials using stem cell
therapy as a treatment for diabetes mellitus. Am J Stem Cells.
5:74–86. 2016.PubMed/NCBI
|
|
19
|
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y,
Shen J, Cheng Y, Fu X and Han W: Infusion of mesenchymal stem cells
ameliorates hyperglycemia in type 2 diabetes rats: Identification
of a novel role in improving insulin sensitivity. Diabetes.
61:1616–1625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pde Bueno G, Yochite JN, Derigge-Pisani
GF, de Farias Malmegrim KC, de Avó LR, Voltarelli JC and Leal ÂM:
Metabolic and pancreatic effects of bone marrow mesenchymal stem
cells transplantation in mice fed high-fat diet. PLoS One.
10:e01243692015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hughey CC, Ma L, James FD, Bracy DP, Wang
Z, Wasserman DH, Rottman JN, Hittel DS and Shearer J: Mesenchymal
stem cell transplantation for the infracted heart: Therapeutic
potential for insulin resistance beyond the heart. Cardiovasc
Diabetol. 12:1282013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shree N and Bhonde RR: Conditioned media
from adipose tissue derived mesenchymal stem cells reverse insulin
resistance in cellular models. J Cell Biochem. 118:2037–2043. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shulman GI, Rothman DL, Jue T, Stein P,
DeFronzo RA and Shulman RG: Quantitation of muscle glycogen
synthesis in normal subjects and subjects with
non-insulin-dependent diabetes by 13C nuclear magnetic resonance
spectroscopy. N Engl J Med. 322:223–228. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song R, Peng W, Zhang Y, Lv F, Wu HK, Guo
J, Cao Y, Pi Y, Zhang X, Jin L, et al: Central role of E3 ubiquitin
ligase MG53 in insulin resistance and metabolic disorders. Nature.
494:375–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi JS, Park JS, Ham YM, Nguyen N, Lee NR,
Hong J, Kim BW, Lee H, Lee CS, Jeong BC, et al: MG53-induced IRS-1
ubiquitination negatively regulates skeletal myogenesis and insulin
signaling. Nat Commun. 4:23542013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma H, Liu J, Bian Z, Cui Y, Zhou X, Zhou
X, Zhang B, Adesanya TM, Yi F, Park KH, et al: Effects of metabolic
syndrome on mitsugumin 53 expression and function. PLoS One.
10:e01241282015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee H, Park JJ, Nguyen N, Park JS, Hong J,
Kim SH, Song WY, Kim HJ, Choi K, Cho S, et al: MG53-IRS-1
(Mitsugumin 53-insulin receptor substrate-1) interaction disruptor
sensitizes insulin signaling in skeletal muscle. J Biol Chem.
291:26627–26635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai C, Masumiya H, Weisleder N, Matsuda N,
Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, et al: MG53
nucleates assembly of cell membrane repair machinery. Nat Cell
Biol. 11:56–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan T, Ko YG and Ma J: Dual function of
MG53 in membrane repair and insulin signaling. BMB Rep. 49:414–423.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang C, Deng ZH, Chen S, Chen S, Zhang JY,
Jin LY, Si YL and Chen GH: Adipose-derived mesenchymal stem cells
alleviating heart dysfunction through suppressing MG53 protein in
rat model of diabetic cardiomyopathy. Int J Clin Exp Pathol.
10:4009–4022. 2017.
|
|
31
|
Rea S and James DE: Moving GLUT4: The
biogenesis and trafficking of GLUT4 storage vesicles. Diabetes.
46:1667–1677. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sylow L, Kleinert M, Pehmøller C, Prats C,
Chiu TT, Klip A, Richter EA and Jensen TE: Akt and Rac1 signaling
are jointly required for insulin-stimulated glucose uptake in
skeletal muscle and downregulated in insulin resistance. Cell
Signal. 26:323–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang
Y, Si Y, Guo Y, Zang L, Mu Y and Han W: Human umbilical
cord-derived mesenchymal stem cells elicit macrophages into an
anti-inflammatory phenotype to alleviate insulin resistance in type
2 diabetic rats. Stem Cells. 34:627–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laustsen PG, Michael MD, Crute BE, Cohen
SE, Ueki K, Kulkarni RN, Keller SR, Lienhard GE and Kahn CR:
Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice.
Genes Dev. 16:3213–3222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bunner AE, Chandrasekera PC and Barnard
ND: Knockout mouse models of insulin signaling: Relevance past and
future. Word J Diabetes. 5:146–159. 2014. View Article : Google Scholar
|