Introduction
Atopic dermatitis (AD) is a chronic skin
inflammation and one of the most common relapsing allergic
diseases. The incidence of AD has gradually been increasing
worldwide. The etiology of the disease is associated with innate
and adaptive immune responses, which are caused by environmental
and genetic factors; AD mostly occurs in infants and children
(1). The clinical characteristics
of AD are prevalently related to an excessive accumulation of
antigen and imbalance of allergen-specific T helper (Th)1/Th2 cells
and inflammatory cytokines. Those inflammatory cytokines contribute
to increase levels of immunoglobulin (Ig) E and produce other
pro-inflammatory mediators to trigger infiltration of inflammatory
immune cells, such as granulocytes, lymphocytes, macrophages,
eosinophils, and mast cells into skin lesions (2). The repeated exposure of antigen
produces more severe and chronic AD symptoms, including skin
barrier disruption, pruritus, excoriation, and dryness. In most
cases, patients with AD are treated with synthetic steroids.
However, many clinical reports have warned that long-term use of
synthetic steroids may result in side effects such as additional
infections, gastrointestinal ulcers, osteoporosis, and insomnia
(3–5). Therefore, the identification of novel
anti-allergic naturally-derived agents from herbs and medicinal
plants with less side effects are required for AD treatment.
Mast cells play an important role in type I
hypersensitivity reactions via the release of histamine,
chemokines, and various inflammatory cytokines. Secretion and
activation of these strong pro-inflammatory mediators are
stimulated by binding of the cross-linked IgE/antigen complex and
its high affinity receptor FcεRl on surfaces of mast cells
(6). Previous studies have
revealed that IgE/antigen-FcεRl binding activates IκB kinase α and
β (IKKα and IKKβ), leading to the activation of nuclear factor-κB
(NF-κB), which translocates into nucleus to regulate the
inflammatory response. Conversely, IgE/antigen-FcεRl binding
phosphorylates synaptosome-associated protein 23 (SNAP-23) in an
NF-κB-independent manner, which is responsible for late-phase
allergic reactions (7,8). Finally, activated NF-κB increases the
production and secretion of pro-inflammatory cytokines including
tumor necrosis factor α (TNF-α), interleukin (IL)-1β, −4, and −6
(9,10). Accordingly, it is considered that
both the inhibition of pro-inflammatory cytokine production and of
NF-κB dependent signalling molecules are an effective strategies to
alleviate allergic reactions.
Previous studies have revealed that tanshinones,
including tanshinone-I, tanshinone-IIA, and
15,16-dihydrotanshinone-I, reduce allergic reactions in rat mast
cells RBL-2H3 via suppressing their degranulation. More
specifically, 15,16-dihydrotanshinone-I inhibits the activation of
extracellular signal-regulated kinases 1/2 (ERK1/2), spleen
tyrosine kinase (Syk), and phospholipase Cγ2 (PLCγ2) which are
signalling molecules that induce mast cell degranulation (11). Furthermore, components of Salvia
miltiorrhiza Bunge have anti-allergic, anti-inflammatory, and
anticancer activities, (12,13)
and are used to treat cardiovascular disorders (14,15)
Cryptotanshinone (CRT), one of the major natural compounds
extracted from the medicinal herb Salvia miltiorrhiza Bunge,
also belongs to the tanshinone group. In addition, CRT is known as
an inhibitor of signal transducer and activator of transcription 3
(STAT3). Because STAT3 is a transcription factor that increases the
transcription of pro-inflammatory cytokines, CRT is also able to
inhibit the production of these cytokines. Specifically, CRT
strongly inhibits phosphorylation at the Tyr705 residue at STAT3
with a small effect at the Ser727 residue, but has no activity
against STAT1 or STAT5 (16). To
date, little has been reported regarding the precise molecular
target by which CRT inhibits mast cell degranulation.
Materials and methods
Reagents
CRT, Dulbecco's modified Eagle's medium (DMEM),
foetal bovine serum (FBS), phosphate-buffered saline (PBS),
dinitrophenyl-bovine serum albumin (DNP-BSA), anti-dinitrophenyl
IgE isotype (DNP-IgE), 4-nitrophenyl-N-acetyl-D-glucosamine,
citrate buffer, sodium bicarbonate, 1-chloro-2,4-dinitrobenzene
(DNCB), lipopolysaccharide (LPS), and 10% neutral-buffered formalin
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Dimethyl sulfoxide (DMSO) was purchased from Takara Bio Inc.
(Shiga, Japan). Dexamethasone and primary antibody against β-actin
were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX,
USA). Primary antibodies against phospho-IκBα, IkBα, phospho-NF-κB
p65, NF-κB p65, phospho-Lyn, Lyn, phospho-Syk, Syk, phospho-PLCγ1,
PLCγ1, phospho-protein kinase C (PKC), PKC, phospho-ERK1/2, and
ERK1/2 were purchased from Cell Signaling Technology, Inc.,
Danvers, MA, USA.
Cell culture
The rat basophilic leukaemia (RBL) cell line RBL-2H3
was a kind gift from professor Jean-Pierre Kinet (Harvard
University, Cambridge, MA, USA). The RBL-2H3 cell line shares
characteristics with human mucosal mast cells (17–19),
which makes it is an appropriate cell line to use within the
present study. RBL-2H3 cells were cultured in DMEM supplemented
with 10% FBS at 37°C in an incubator under 5% CO2
conditions.
Mast cell degranulation assay
RBL-2H3 cells were plated in 6-well plate
(2×106 cells/well) or 96-well plate (5×104
cells/well and were sensitized with anti-DNP-IgE (0.1 µg/ml) for 16
h. After washing two times with PBS, the cells were pre-treated
with CRT at indicated concentrations for indicated times then
sensitized with DNP-BSA (100 ng/ml) for an additional 1 h. For the
measurement of β-hexosaminidase release (a biomarker of
degranulation) from RBL-2H3 cells, 50 µl of cell supernatant was
incubated with a same volume of solution I [substrate solution: 1.3
mg/ml of 4-nitrophenyl-N-acetyl-D-glucosamine in 0.1 M sodium
carbonate] at 37°C for 1 h and the reaction was terminated by
adding stop solution II [50 mM sodium carbonate] for 15 min at room
temperature. The measurement of β-hexosaminidase release was
determined using a microplate reader at an absorbance of 405 nm
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Luciferase assay
293T cells were transfected with 200 ng of
pGL3-4×NF-kB luciferase reporter plasmid using polyethylenimine
solution (Sigma-Aldrich; Merck KGaA), then incubated overnight.
pEGFP plasmid was used as control. 5, 10, 20 µM CRT was pre-treated
to transfected cells for 1 h. Then, cells were stimulated with LPS.
After 1 h of incubation, the cells were lysed and luciferase
activity was determined using Luciferase Reporter Assay System
(Promega Corp., Madison, WI, USA) according to the manufacturer's
instructions.
Animals
Male 6-week old Balb/c mice (20–25 g; Koatech,
Gyunggi-do, Korea) were housed under 12-h light/12-h dark
conditions and were allowed free access to food and water. The
bedding was changed once a week, and the temperature (22–23°C) and
humidity (40–55%) were controlled. All procedures were conducted at
the animal facilities and this animal experiments were approved by
the Institutional Animal Care and Use Committee of Sookmyung
Women's University, Seoul, Korea (SMWU-IACUC-1611-035).
DNCB-induced AD animal model
Balb/c mice were randomly divided into three groups
(n=5 per group). The day after shaving the dorsal skin of all mice,
the control, and experimental groups were sensitized by the
application of DNCB solution by painting (dissolved in a 3:1
mixture of acetone and olive oil). After 5 days, 20 µl of 0.2% DNCB
solution was applied on both the left and right ears, and 100 µl
was applied on the shaved dorsal skin every other day; the vehicle
group received applications of DMSO only. In the experimental
group, the same volume of 100 µM CRT was applied by painting on
both ears and on the dorsal skin 1 h before every DNCB challenge.
The thickness of right and left ears of all mice were measured
every other day with a dial caliper (Ozaki Factory, Tokyo, Japan).
All mice were sacrificed on day 31 of the experiment by
CO2 euthanasia and tissues were collected.
Histological analysis
The inflamed ear specimens of each mice were
collected and fixed with 10% neutral-buffered formalin. All fixed
tissues were embedded using a frozen section compound and were cut
into 20 µm-thick sections using a rotary microtome (both Leica
Microsystems, Inc., Buffalo Grove, IL, USA). To compare the
swelling of the epidermis and inflammatory cell accumulation, each
section was stained with haematoxylin and eosin (H&E;
Sigma-Aldrich; Merck KGaA). For immunofluorescence, tissues were
treated with PBS-based 0.1% Triton-X-100 for 10 min to permeabilize
the tissue. After washing with PBS, slides were blocked by
PBS-based 1% BSA for 30 min at room temperature. Then slides were
incubated with phycoerythrin (PE) conjugated-anti-mouse-cluster of
differentiation molecule 11b (CD11b; CA, USA), a marker of
inflammatory granulocytes for 1 h at room temperature in the dark.
After washing with PBS twice, slides were cover-slipped. Confocal
images were obtained with a Zeiss confocal microscope (Carl Zeiss
Microscopy GmbH, Jena, Germany).
Measurement of IgE levels by enzyme
linked immunosorbent assay (ELISA)
Blood was collected by cardiac puncture from
isoflurane-anesthetized mice on the last day of experiments.
Clotted blood samples were centrifuged (3,500 rpm for 20 min) and
serum was collected. Ear tissues from mice were homogenized with
RIPA buffer [20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1%
NP-40, 1% SDS, Complete Protease Inhibitor Cocktail Tablets (Roche,
Basel, Switzerland)] and centrifuged at 12,000 g for 15 min to
obtain tissue lysates. The level of IgE from mice serum and tissue
lysates were determined by a commercial mouse IgE ELISA assay kit
(Shibayagi, Shibukawa, Japan) according to the manufacturer's
instructions.
Quantitative RT-PCR (qRT-PCR) Frozen tissue from
mice or RBL-2H3 cells were lysed with RNAiso plus reagent (Takara
Bio Inc.) and total RNA was extracted according to the
manufacturer's instructions. The isolated total RNA was reverse
transcribed (RT) using M-MuLV RTase (Promega Corp.) at 42°C for 1
h. qRT-PCR was performed using SYBR®-Green master mix
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Applied
Biosystems QuantStudio 3 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). 18s rRNA was used as loading control Fold
changes of indicated mRNA expression were calculated by the
2−ΔΔCt method, where ΔΔCt=(Ct target
gene-Ct18S rRNA). Experimental group-(Ct Target
gene-Ct18S rRNA) Control group. The following primer pairs
were used: IL-1β forward, 5′-AGCCCATCCTCTGTGACTCATG-3′ and reverse,
5′-GCTGATGTACCAGTTGGGGAAC-3′; IL-6 forward,
5′-CCGGAGAGGAGACTTCACAG-3′ and reverse, 5′-TCCACGATTTCCCAGAGAAC-3′;
TNF-α forward, 5′-CCTGTAGCCCACGTCGTAGC-3′ and reverse,
5′-TTGACCTCAGCGCTGAGTTG-3′; monocyte chemoattractant protein 1
(MCP-1) forward, 5′-ATCCCAATGAGTAGGCTGGA-3′ and reverse,
5′-CAGAAGTGCTTGAGGTGGTT-3′; 18s rRNA forward,
5′-AGCTATCAATCTGTCAATCCTGTC-3′, and reverse,
5′-GCTTAATTGACTCAACACGGGA-3′.
Western blot analysis
Cells and tissue from mice were lysed with GST-IP
buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40,
Complete Protease Inhibitor Cocktail Tablets (Roche)] or RIPA
buffer, respectively. For analysing phosphorylated proteins,
PhosSTOP EASYpack (Roche) was added to the lysis buffer. Protein
lysates were obtained by further centrifuge (13,000 rpm for 15
min). Then, obtained lysates were mixed with 5× sodium dodecyl
sulphate (SDS) sample buffer and heated at 95°C for 5 min. Prepared
sample were separated by 12% SDS-Polyacrylamide gel and transferred
to a nitrocellulose membrane (GE Healthcare, Little Chalfont, UK).
After blocking with TBST-based 3% BSA for 30 min at room
temperature, the membranes were incubated with indicated primary
antibodies at 4°C overnight. Then, membranes were further incubated
with horseradish peroxidase (HRP)-conjugated anti-mouse or
anti-rabbit IgG (Fab) secondary antibodies (Enzo Life Sciences
Inc., Farmingdale, NY, USA) for 2 h at room temperature. The target
proteins were analysed by PowerOpti-ECL western blotting reagent
(Thermo Fisher Scientific, Inc.) and evaluated using a luminescent
image analyser Fusion Solo (Vilber Lourmat, Eberhardzell, Germany).
The size of protein in each blot was expected by relative migration
ratio to prestained protein size marker (Thermo Fisher Scientific,
Inc.) and β-actin in each blot was detected to normalize protein
amount. Images were quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All the data were expressed as the mean ± standard
deviation (SD). All multiple comparisons within groups were made
with either one-way or two-way factorial ANOVA. Significances were
determined with Tukey's honest significant difference post hoc
test. Individual group mean differences were determined with
Student's t-test. A maximum level of significance of P<0.05 was
used for all statistical comparisons. All statistical analyses were
performed using GraphPad Prism version 5.0 for Windows (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
CRT suppressed IgE-mediated mast cell
degranulation
In order to identify effective candidates having
anti-allergic activity, we screened 133 natural compounds with an
IgE-mediated degranulation assay using RBL cell line RBL-2H3, which
is widely accepted and applied as a proper model for human mucosal
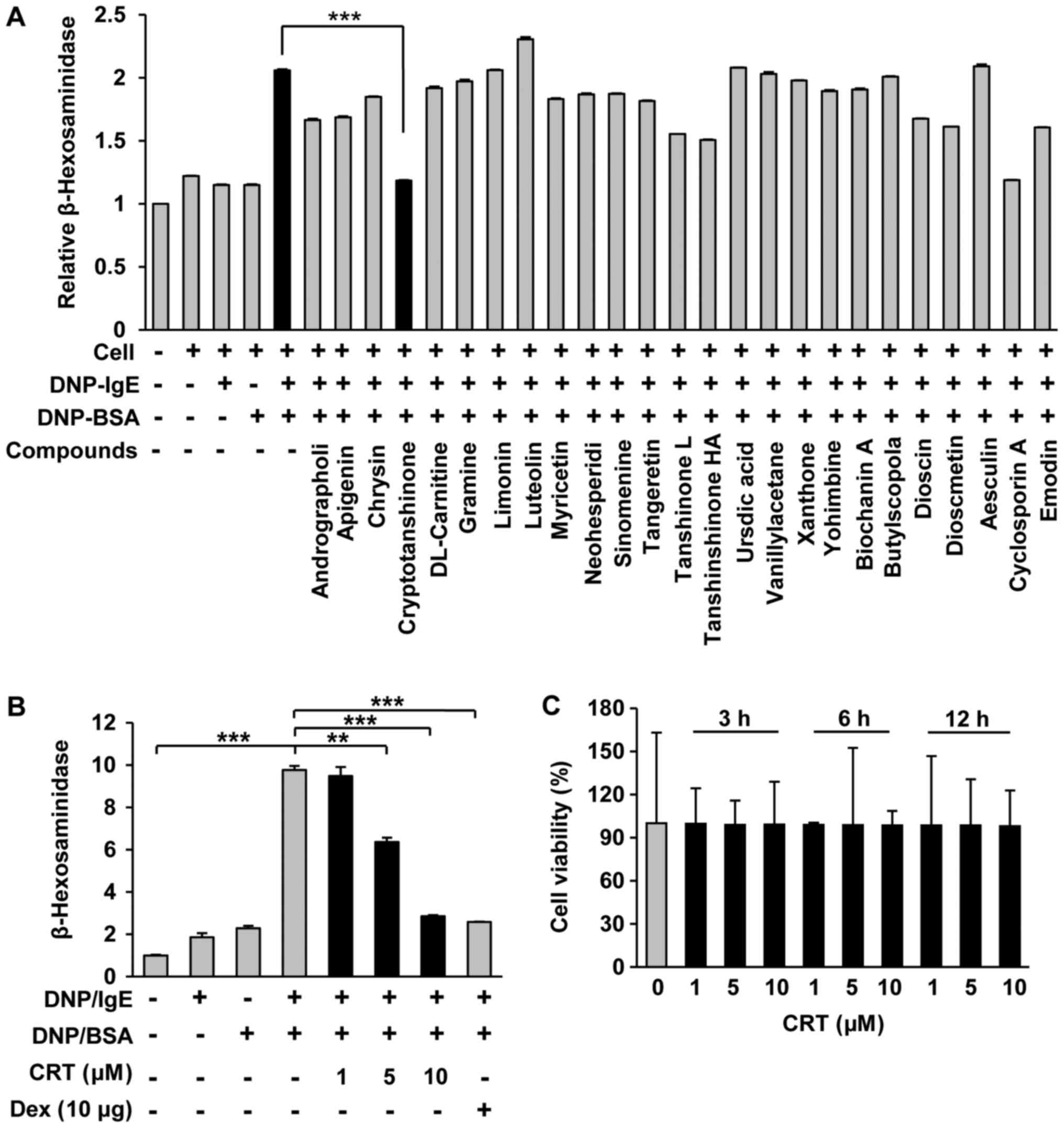
mast cells. Compared to other compounds, CRT showed the greatest
anti-allergy effects as it significantly decreased IgE/DNP
crosslink-mediated degranulation in RBL-2H3 cells (Fig. 1A). Furthermore, CRT suppressed
IgE/DNP-mediated mast cell degranulation dose-dependently and its
effects at the maximum dose was similar to that of dexamethasone at
10 µg/ml (Fig. 1B). No cytotoxic
effects of CRT were observed (Fig.
1C), indicating that the suppressive effect of CRT on mast cell
degranulation was not due to cytotoxicity.
CRT alleviated DNCB-induced AD
symptoms in the mouse model
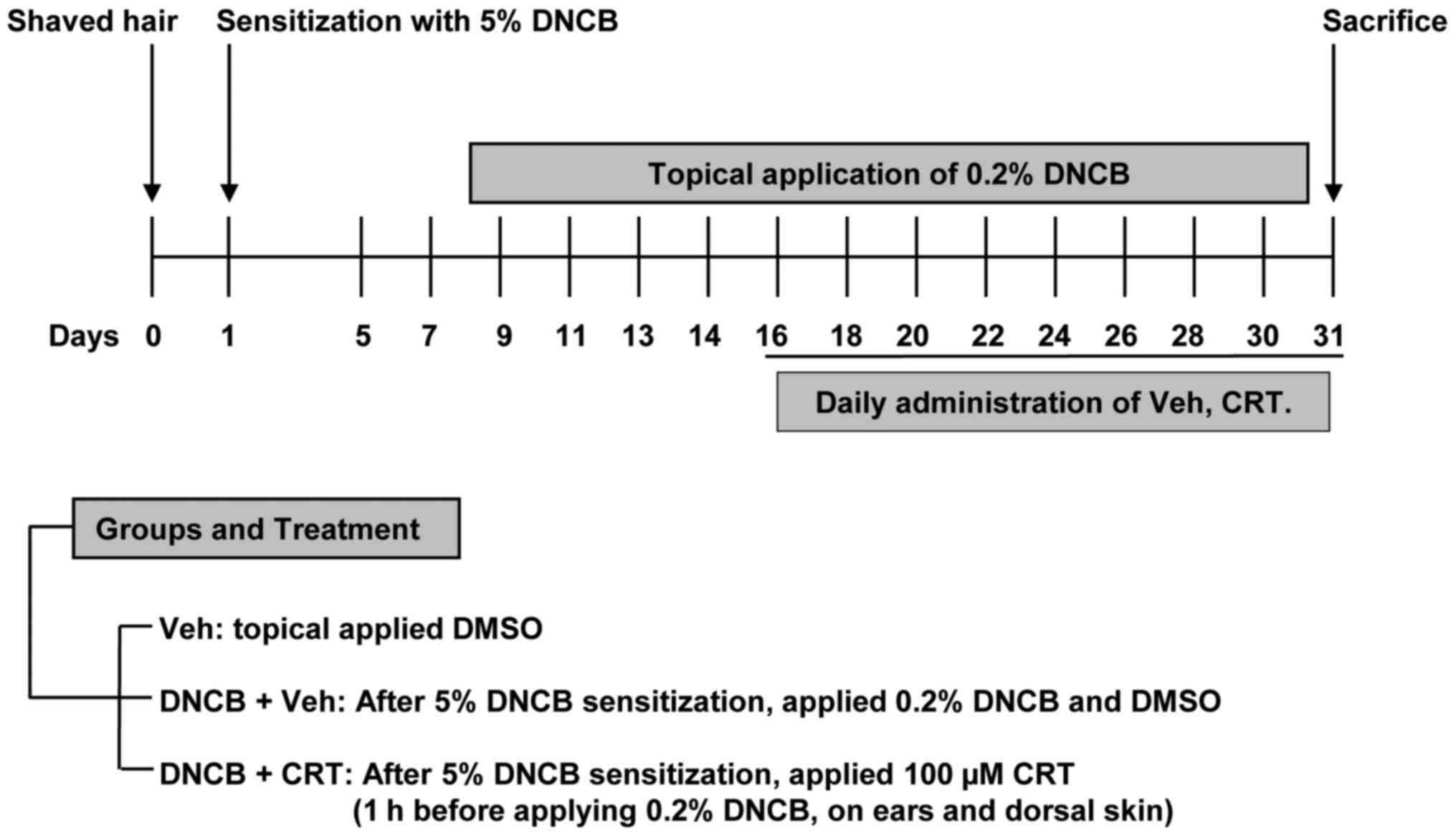
To determine whether CRT has curable effects on
AD-like skin lesions, a DNCB-induced AD animal model was generated
using Balb/c mice and was then subjected to CRT treatment as shown
in the experimental design (Fig.
2). The repeated DNCB challenge successfully induced AD-like
symptoms, as mice showed markedly increased ear swelling as
compared to the vehicle group. When CRT was pre-treated 1 h before
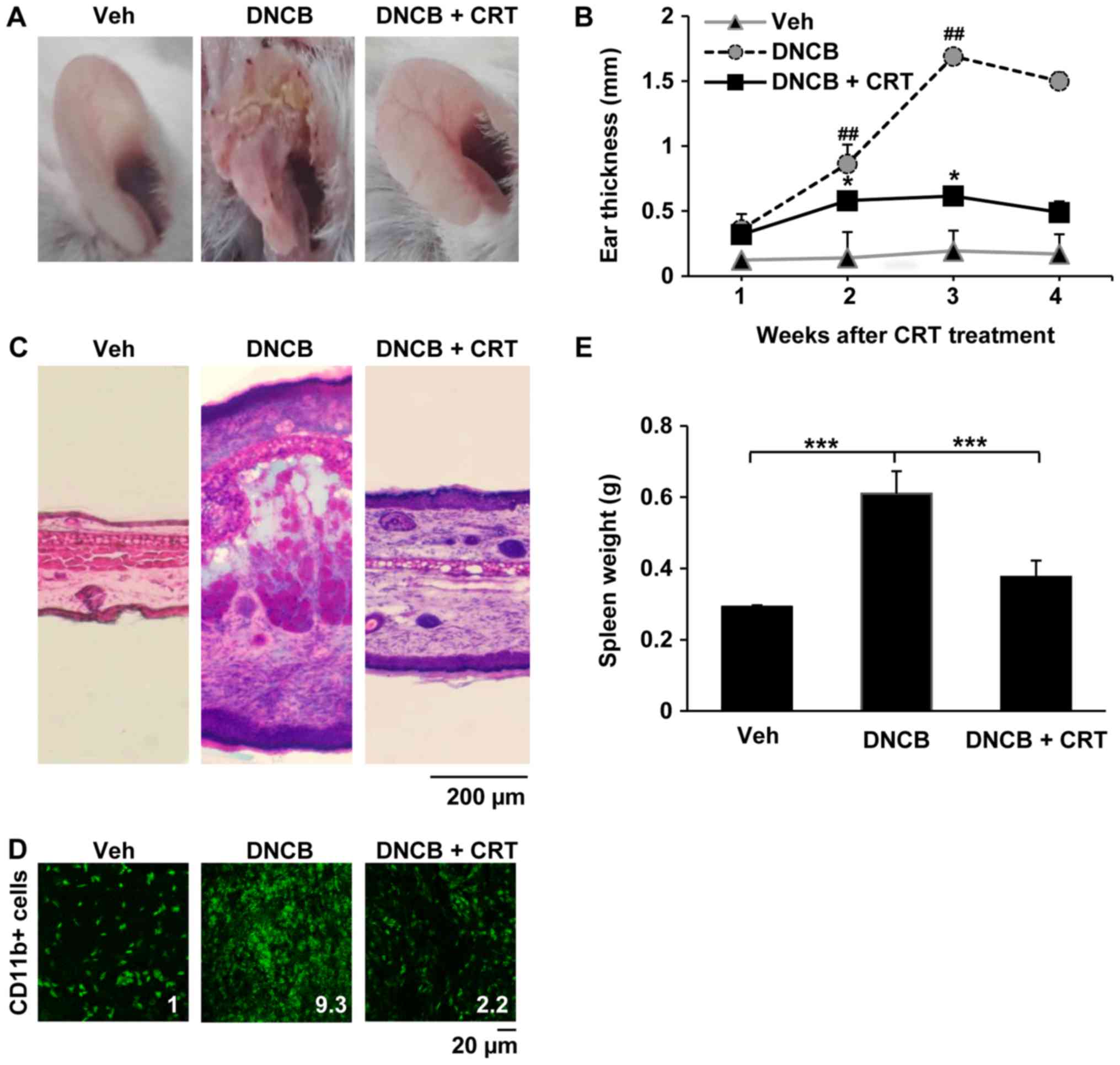
every DNCB challenge, ear swelling was significantly reduced after
2 weeks of CRT treatment (Fig. 3A and
B). Histological analyses also confirmed that CRT treatment
attenuated AD-like inflammation and skin tissue damage induced by
DNCB (Fig. 3C). Next,
immunofluorescence analysis was performed to investigate whether
the effects of mitigation of ear swelling induced by CRT could be
associated with a reduction in immune cell recruitment to the
inflammatory skin lesion. Interestingly, CRT treatment also
restored DNCB-induced excessive accumulation of CD11b-positive
immune cells in the ear skin lesion (Fig. 3D), indicating that CRT alleviates
DNCB-induced AD-like skin symptoms. In addition, CRT greatly
suppressed increased spleen weights by DNCB (Fig. 3E). Given that the spleen weight is
an indicator evaluating the degree of inflammation, those
observations suggest that CRT exerts the anti-AD effect through its
anti-inflammatory effect.
CRT decreased transcription levels of
pro-inflammatory cytokines and inhibited the NF-κB signalling
pathway in the AD-like skin lesion in the DNCB-induced animal
model
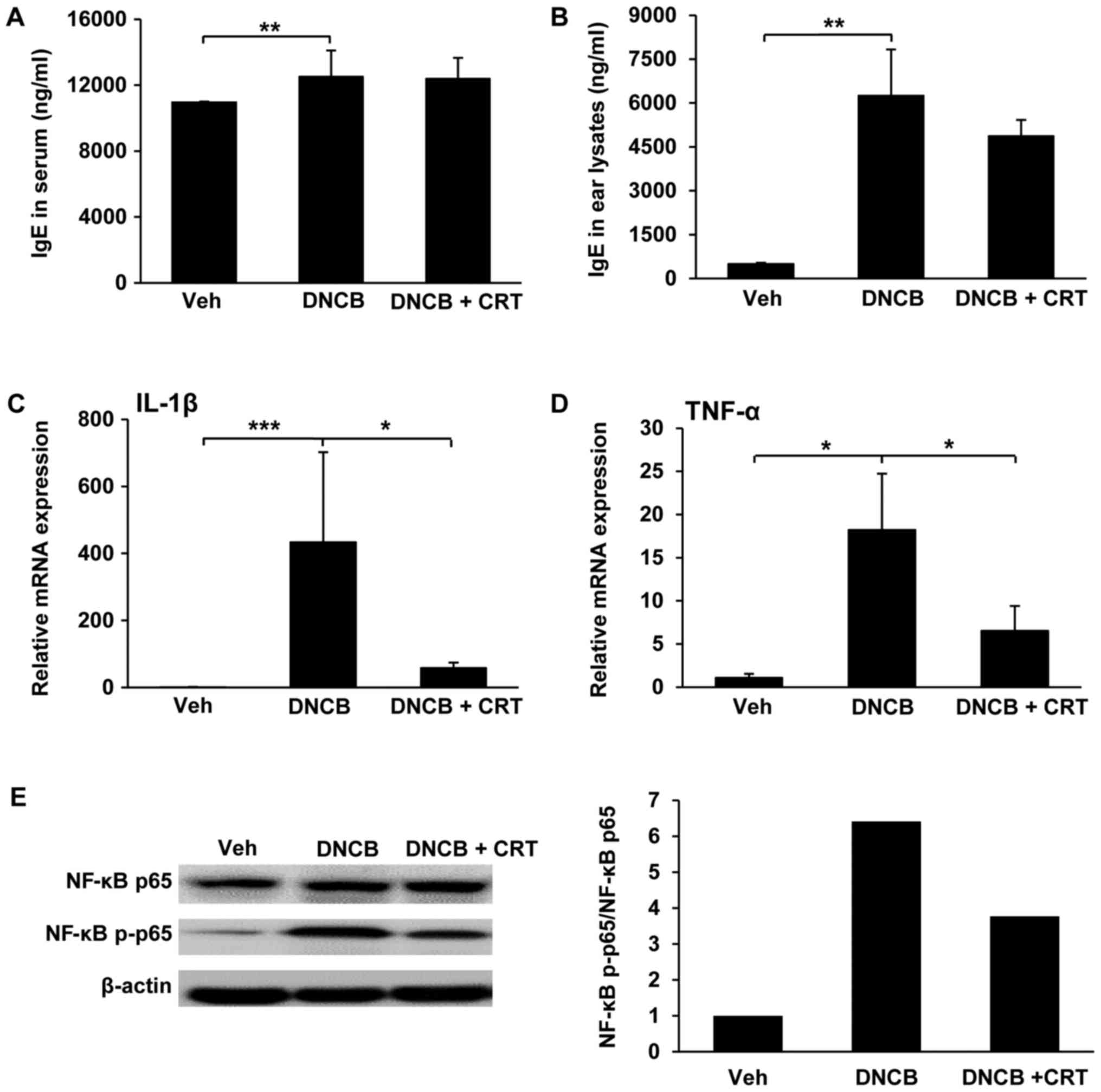
Since the allergen-specific IgE involves in the
initial phase of the allergic response, the increased levels of
allergen-specific IgE is a hallmark of AD. Thus, we isolated serum
and ear tissue lysates and then measured IgE levels by ELISA.
DNCB-challenged mice exhibited a significant increase in serum IgE
levels, but no significant changes were observed with CRT treatment
compared to DNCB-challenged mice (Fig.
4A). However, IgE levels in lysates from ear tissues were
decreased by CRT treatment (Fig.
4B), implying that CRT decreases the local IgE level at nearby
inflamed region. Next, we evaluated whether the CRT has inhibitory
effects on pro-inflammatory cytokines including IL-1β and TNF-α,
which are known to be upregulated and play important roles
associated with the NF-κB signalling pathway under
inflammation-challenged conditions. Therefore, the transcription
levels of pro-inflammatory cytokines in ear tissues were determined
by qRT-PCR. As expected, CRT treatment significantly reduced
DNCB-challenged upregulation of IL-1β (Fig. 4C) and TNF-α (Fig. 4D). Next, we examined the inhibitory
effect of CRT on the activation of the NF-κB pathway in inflamed
ear tissues isolated from a DNCB-induced AD animal model. CRT
showed a suppressive effect on DNCB-induced NF-κB p65
phosphorylation (Fig. 4E).
CRT inhibited the transcriptions of
inflammatory cytokines by suppression of IgE-mediated ERK 1/2 and
NF-κB activation in RBL-2H3 cells
Cross-linking of the IgE/antigen complex stimulates
mast cells to produce pro-inflammatory cytokines through activation
of the MAPK ERK 1/2 and NF-κB signalling pathways. Thus, the effect
of CRT on ERK 1/2 and NF-κB signalling pathway activation was
examined using RBL-2H3 cells. IgE-cross-linking by DNP/BSA
treatment activated EKR 1/2 by increasing phosphorylation, but
pre-treatment with CRT dose-dependently suppressed ERK 1/2
activation in RBL-2H3 cells. Phosphorylation of NF-κB p65 and IκBα
were increased by IgE cross-linking; however, CRT treatment
inhibited their phosphorylations in a dose-dependent manner
(Fig. 5A).
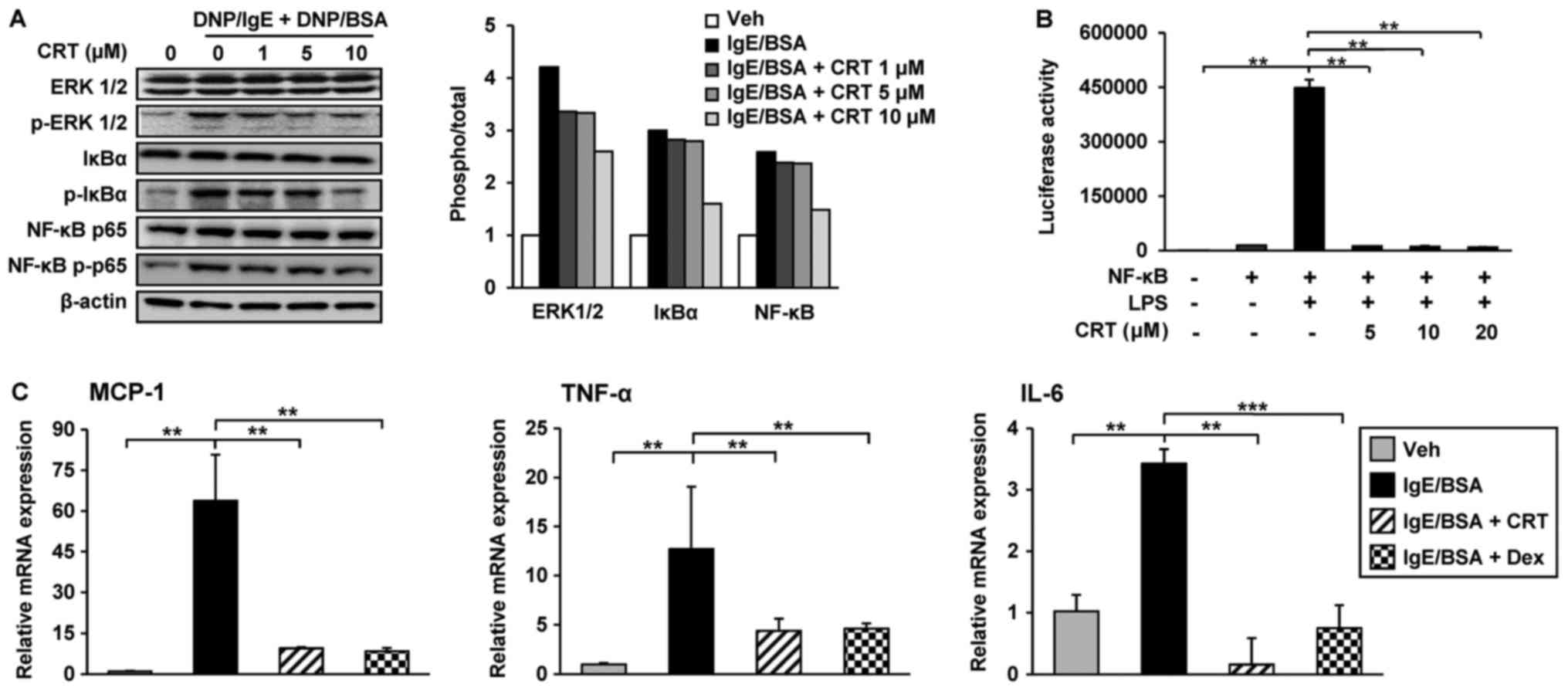 | Figure 5.CRT downregulates pro-inflammatory
cytokines by inhibiting the IgE/antigen complex-induced
extracellular signal regulated kinase 1/2 and NF-κB signalling
pathways. (A) RBL-2H3 cells were sensitised with anti-DNP/IgE for
16 h followed by treatment with various concentrations of CRT 30
min prior to DNP/BSA stimulation. Levels of p-ERK1/2, p-NF-κB p65
and p-IκBα were determined by western blot analysis 1 h post
DNP/BSA stimulation and relative ratios to total protein levels
were quantified. (B) 293T cells were transfected with NF-κB
reporter plasmid. LPS was treated to activate NF-κB pathway and
then its activity was evaluated using luciferase assay kit. (C)
RBL-2H3 cells were sensitised with anti-DNP-IgE for 16 h followed
by treatment with 10 µM CRT 30 min before DNP/BSA stimulation.
Total RNA was isolated 1 h after DNP/BSA stimulation and mRNA
levels of MCP-1, TNF-α and IL-6 were determined by reverse
transcription-quantitative polymerase chain reaction. Dex was used
as the positive control. Experiments were performed at triplicates
three times and representative data are presented as the mean ±
standard deviation. **P<0.01; ***P<0.001. CRT,
cryptotanshinone; IL, interleukin; TNF, tumor necrosis factor; NF,
nuclear factor; p-, phosphorylated; IgE, immunoglobulin E; MCP,
monocyte chemoattractant protein 1; DNP/IgE, anti-dinitrophenyl IgE
isotype; BSA, bovine serum albumin; IκBα, NF-κB inhibitor; ERK,
extracellular signal-regulated kinases; LPS,
lipopolysaccharide. |
Next, we asked whether CRT directly regulates the
activity of NF-κB. To address this, 293T cells were transfected
with NF-κB luciferase reporter gene, because 293T cells show higher
transient transfection efficiency than RBL-2H3 cells. The
transfected cells were treated with LPS treatment to activate
NF-κB. Following co-treatment with CRT and LPS, CRT significantly
suppressed LPS-activated NF-κB luciferase activity (Fig. 5B). These results indicated that CRT
significantly mitigates the IgE-mediated NF-κB activation in
RBL-2H3 cells. Moreover, CRT treatment also decreased the
transcription levels of TNF-α and IL-6 increased by IgE
cross-linking in RBL-2H3 cells (Fig.
5C). In addition to these inflammatory cytokines, the
expression of MCP-1, a key chemokine involved in the stimulation of
infiltration and migration of leukocytes towards the inflammatory
lesion, was also examined. Interestingly, the IgE
cross-linking-induced upregulation of MCP-1 was completely restored
to normal levels by CRT treatment (Fig. 5C), suggesting that the decreased
accumulation of CD11b positive cells in AD-like skin lesions from
CRT-treated mice might be caused by a reduced expression of
chemoattractant MCP-1.
CRT inhibited IgE-mediated mast cell
activation through suppression of Lyn/Syk phosphorylation and its
downstream signalling pathway
Next, to reveal exact molecular targets of CRT, we
examined effect of CRT on phosphorylation of Lyn and Syk kinases,
which are the most upstream kinases responsible for mast cell
activation. CRT treatment suppressed phosphorylation levels of Lyn
and Syk in activated RBL-2H3 cells (Fig. 6A). We also examined phosphorylation
levels of PLCγ, PKCδ, and IKKβ, which are downstream target
molecules of p-Lyn and p-Syk. CRT also greatly suppressed
phosphorylation of PLCγ, PKC, and IKKβ in a dose-dependent manner
(Fig. 6B). CRT effects on p-PLCγ,
p-PKC, and p-IKKβ showed its best efficiency with 10 µM of
concentration. These results suggest that CRT suppresses
IgE-mediated mast cell degranulation by inhibiting the activation
of Lyn and Syk kinases (Fig.
6C).
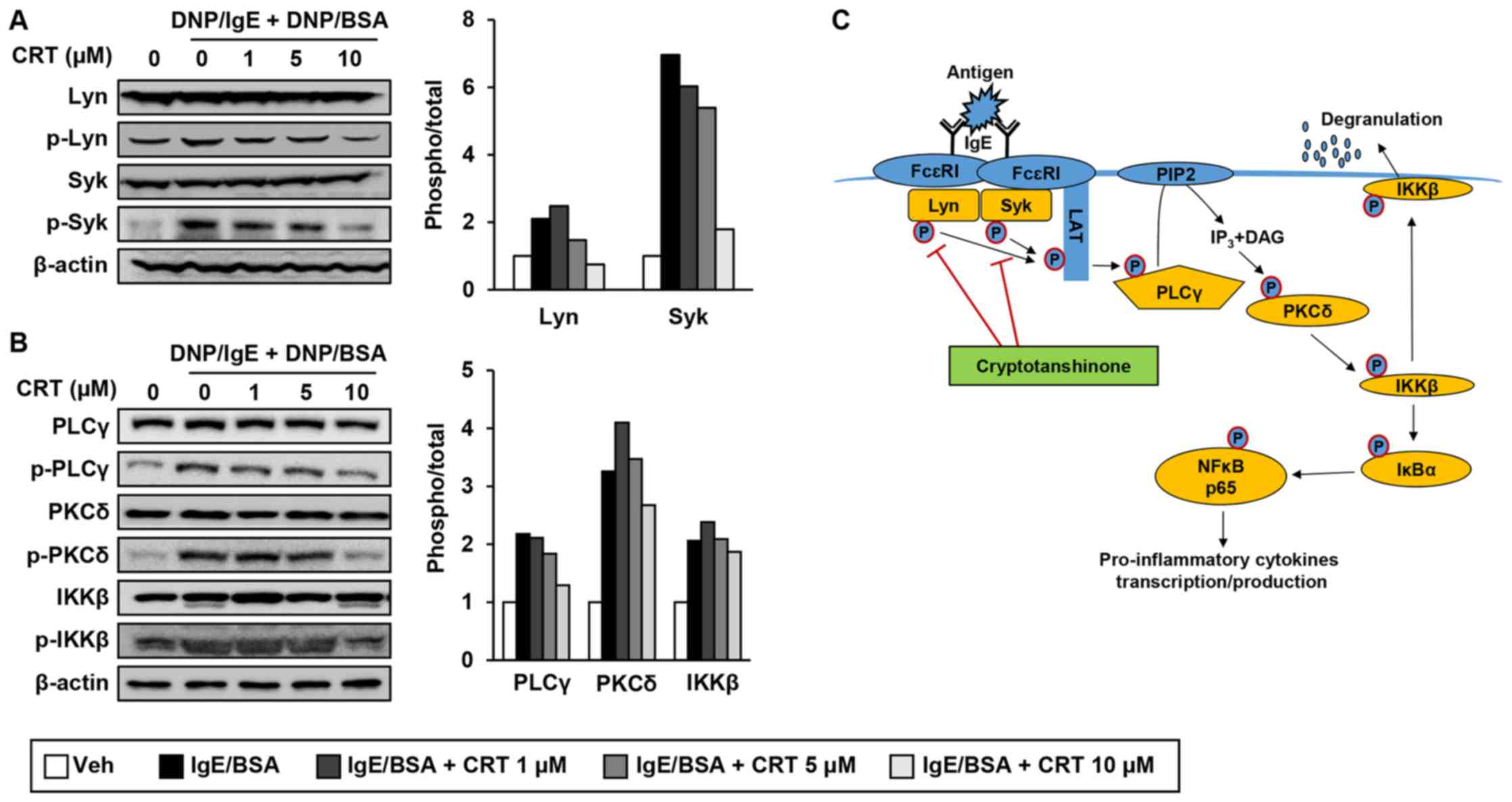 | Figure 6.CRT inhibits the signalling pathways
of Lyn and Syk (A and B) RBL-2H3 cells were sensitised with
anti-DNP-IgE for 16 h followed by treatment with various
concentrations of CRT 30 min before DNP/BSA stimulation. Levels of
phosphorylated (A) Lyn and Syk, and (B) PLCγ, PKCδ and IKKβ were
determined by western blot analysis 1 h after DNP/BSA stimulation.
The relative ratio of phosphorylated protein to total protein
levels were quantified. (C) Schematic diagram indicates how CRT
suppresses mast cell degranulation. CRT, cryptotanshinone; DNP/IgE,
anti-dinitrophenyl IgE isotype; DNA/BSA, dinitrophenyl-bovine serum
albumin; PLCγ, phospholipase Cγ; PKC, phospho-protein kinase C;
IKK, IκB kinase; IgE, immunoglobulin E; Veh, vehicle; Syk, spleen
tyrosine kinase. |
Discussion
Chronic AD patients have a higher risk of developing
allergic rhinitis and asthma, which are triggered by
pro-inflammatory mediators released from activated and infiltrated
immune cells including mast cells, neutrophils, and macrophages
into skin lesions (20,21). To prevent complications and relieve
AD symptoms, powerful immunosuppressive steroids are used for
treatment, but the beneficial effects are short-lived; thus,
patients have to take these steroid drugs chronically. Furthermore,
taking steroid drugs continuously for prolonged periods of time
leads to severe side effects such as blood disorders, irregular
heartbeat, and psychological interference (22). Accordingly, identifying
biologically active natural compounds from medicinal plants and
developing alternative anti-AD drugs with fewer side effects are in
demand to alleviate AD.
In this study, we evaluated the anti-AD effects of
CRT using a DNCB-induced AD mouse model, which is typically used
for studying the pathogenesis of AD (4). Topical application of CRT attenuated
ear swelling induced by DNCB. In addition, the excessive
accumulation of CD11b-positive immune cells in skin lesions
triggered by repeated exposure to DNCB on Balb/c mice was
dramatically decreased by CRT treatment. In addition, DNCB is known
to significantly increase TNF-α and IL-1β levels in mice and these
cytokines enhance the expression of adhesion molecules and increase
vascular permeability and facilitating the recruitment of
inflammatory cells to the skin lesion (23–26).
In our study, CRT strongly suppressed mRNA levels of DNCB-induced
TNF-α and IL-1β, likely resulting in the observed blocked
recruitment of immune cells to the skin lesion. However, further
studies are required to identify immune cell types decreased by
CRT, because monocytes, neutrophils, basophils, mast cells, and
eosinophils all express CD11b on their cell surfaces. Conversely,
it has previously been reported that extracts from Salvia
miltiorrhiza Bunge show immunomodulatory effects by increasing
the population of host immune cells, including macrophages, natural
killer (NK) cells, and peripheral lymphocytes, and by decrease in
serum levels of IgE and the pro-inflammatory cytokine IL-1β against
Listeria monocytogenes infection in Balb/c mice (27). Thus, CRT could be the major
component in Salvia miltiorrhiza Bunge extracts which exerts
the potent anti-AD effects.
We showed that CRT exerts anti-AD effect through
inhibition of the mast cell degranulation in mast cells. Upon
IgE/antigen stimulation, the immunoreceptor tyrosine-based
activation motif (ITAM) region of FcεRI receptor which is on the
mast cell surface is phosphorylated and the initial signalling
protein kinases Lyn and Syk are recruited to the ITAM. Then, Lyn
and Syk are activated through autophosphorylation, which leads to
phosphorylation of the transmembrane adaptor linker for activation
of T cells (LAT). Phosphorylated LAT which is a scaffold for
multimolecular signalling complexes and activates PLCγ through
phosphorylation. The activated PLCγ hydrolyses phosphatidylinositol
biphosphate (PIP2) to generate second signalling
molecules IP3 and DAG, which activate PKCδ through
phosphorylation. Then, activated PKCδ phosphorylates IKKβ so IKKβ
moves to the plasma membrane, resulting in the induction of mast
cell degranulation (6,11,28,29).
In this study, novel function of CRT on phosphorylations of Lyn/Syk
kinases in mast cells is elucidated for the first time.
Furthermore, it is likely that this inhibitory effect of CRT on
Lyn/Syk kinases negatively affected activities of their downstream
signalling molecules including PLCγ, PKCδ, and IKKβ, which leads to
decrease in mast cell degranulation by CRT treatment.
Besides the inhibitory effect of CRT on mast cell
degranulation, here we provide additional evidence that CRT exerts
anti-AD effects through inactivation of MAPK and NF-κB. It has been
reported that CRT regulates the activities of MAPK and NF-κB in
various cell types. In rhabdomyosarcoma, hepatoma, and breast
carcinoma, CRT activates MAPK p38/JNK and suppresses ERK1/2,
followed by caspase-independent apoptosis (10,30,31).
In chronic myeloid leukaemia cells, CRT enhances TNF-α-induced
apoptosis through the activation of MAPK p38 (32). In smooth muscle cells, CRT exerts
anti-migration/invasion effect as it inhibits TNF-α/NF-κB
signalling pathway (33). In this
study, we elucidated the anti-inflammatory role of CRT in mast
cells as CRT suppresses the IgE/antigen-induced phosphorylation of
ERK1/2 and IκBα/NF-κB. Furthermore, the luciferase assay revealed
that CRT directly inhibits the LPS-induced NF-κB activity,
suggesting that CRT decreases the transcriptions of
pro-inflammatory cytokines by downregulation of NF-κB activity.
Given that IKKβ regulates IκBα/NF-κB signalling pathway and that
the activity of IKKβ is controlled by the Lyn/Syk signalling
pathway, the suppressive effect of CRT on NF-κB activity is also
considered to be a downstream effect of CRT-induced inhibition of
the Lyn/Syk signalling pathway as well as degranulation.
Nonetheless, the limitation of this study is that the NF-κB
activity was not measured in mast cells because of low transfection
efficiency.
In conclusion, we provide evidence that CRT could be
developed as an anti-AD drug because it targets Lyn/Syk kinases
which are the most upstream signalling molecules for mast cell
degranulation and the production of inflammatory cytokines
(Fig. 6C). Further studies
examining whether CRT can directly inhibit autophosphorylations of
Lyn/Syk kinases and suppress the recruitment of Lyn/Syk kinases to
ITAM of FcεRI will be more valuable for therapeutic drug
development using CRT.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(grant nos. NRF-2016R1A2B2011683, 2016R1A5A1011974,
NRF-2015M3A9B6027818 and NRF-2016R1A6A3A11931083).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SB and SH designed the study, performed the
experiments, interpreted the data and were major contributors in
writing the manuscript. SL setup the degranulation assay using the
RBL-2H3 cell line. ALJ interpreted the results. HIK, JYP and AB
performed the RT-PCR and western blot analysis. J-SL setup of the
DNCB-induced AD mouse model. M-SL contributed to the preliminary
screening of 133 natural compounds. YY conceived the project and
interpreted the data. All authors read and approved the final
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Sookmyung Women's University
(approval no. SMWU-IACUC-1611-035).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park S, Lee JB and Kang S: Topical
application of Chrysanthemum indicum L. attenuates the development
of atopic dermatitis-like skin lesions by suppressing serum IgE
levels, IFN-γ, and IL-4 in Nc/Nga mice. Evid Based Complement
Alternat Med. 2012:8219672012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SR, Choi HS, Seo HS, Ku JM, Hong SH,
Yoo HH, Shin YC and Ko SG: Oral administration of herbal mixture
extract inhibits 2,4-dinitrochlobenzene-induced atopic dermatitis
in BALB/c mice. Mediators Inflamm. 2014:3194382014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martel BC, Lovato P, Bäumer W and Olivry
T: Translational animal models of atopic dermatitis for preclinical
studies. Yale J Biol Med. 90:389–402. 2017.PubMed/NCBI
|
|
4
|
Lee KS, Jeong ES, Heo SH, Seo JH, Jeong DG
and Choi YK: A novel model for human atopic dermatitis: Application
of repeated DNCB patch in BALB/c mice, in comparison with NC/Nga
mice. Lab Anim Res. 26:95–102. 2010. View Article : Google Scholar
|
|
5
|
Kim SR, Choi HS, Seo HS, Choi YK, Shin YC
and Ko SG: Topical application of herbal mixture extract inhibits
ovalbumin- or 2,4-dinitrochlorobenzene-induced atopic dermatitis.
Evid Based Complement Alternat Med. 2012:5454972012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shim J, Kennedy RH, Weatherly LM,
Hutchinson LM, Pelletier JH, Hashmi HN, Blais K, Velez A and Gosse
JA: Arsenic inhibits mast cell degranulation via suppression of
early tyrosine phosphorylation events. J Appl Toxicol.
36:1446–1459. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki K and Verma IM: Phosphorylation of
SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation.
Cell. 134:485–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hepp R, Puri N, Hohenstein AC, Crawford
GL, Whiteheart SW and Roche PA: Phosphorylation of SNAP-23
regulates exocytosis from mast cells. J Biol Chem. 280:6610–6620.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH, Lee B, Kim HK, Kim EY, Kim JH,
Min JH, Kim S, Sohn Y and Jung HS: Peimine inhibits the production
of proinflammatory cytokines through regulation of the
phosphorylation of NF-κB and MAPKs in HMC-1 cells. Pharmacogn Mag.
13 Suppl 2:S359–S364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian Q, Cheng Y, Zhong C and Wang F:
Inhibition of the IgE-mediated activation of RBL-2H3 cells by TIPP,
a novel thymic immunosuppressive pentapeptide. Int J Mol Sci.
16:2252–2268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi HS and Kim KM: Tanshinones inhibit
mast cell degranulation by interfering with IgE receptor-mediated
tyrosine phosphorylation of PLCgamma2 and MAPK. Planta Med.
70:178–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang BQ: Salvia miltiorrhiza: Chemical and
pharmacological review of medicinal plant. J Med Plants Res.
4:pp2813–2820. 2010.
|
|
13
|
Gao H, Sun W, Zhao J, Wu X, Lu JJ, Chen X,
Xu QM, Khan IA and Yang S: Tanshinones and diethyl blechnics with
anti-inflammatory and anti-cancer activities from Salvia
miltiorrhiza Bunge (Danshen). Sci Rep. 6:337202016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Du Y, Cong L, Jia X and Yang G:
Danshen (Salvia miltiorrhiza) compounds improve the biochemical
indices of the patients with coronary heart disease. Evid Based
Complement Alternat Med. 2016:97817152016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W and Chen G: Danshen (Salvia
miltiorrhiza Bunge): A prospective healing sage for cardiovascular
diseases. Curr Pharm Des. 23:5125–5135. 2017.PubMed/NCBI
|
|
16
|
Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ,
Han DC and Kwon BM: Cryptotanshinone inhibits constitutive signal
transducer and activator of transcription 3 function through
blocking the dimerization in DU145 prostate cancer cells. Cancer
Res. 69:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Passante E and Frankish N: The RBL-2H3
cell line: Its provenance and suitability as a model for the mast
cell. Inflamm Res. 58:737–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Metzger H, Alcaraz G, Hohman R, Kinet JP,
Pribluda V and Quarto R: The receptor with high affinity for
immunoglobulin E. Annu Rev Immunol. 4:419–470. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seldin DC, Adelman S, Austen KF, Stevens
RL, Hein A, Caulfield JP and Woodbury RG: Homology of the rat
basophilic leukemia cell and the rat mucosal mast cell. Proc Natl
Acad Sci USA. 82:3871–3875. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bantz SK, Zhu Z and Zheng T: The atopic
march: Progression from atopic dermatitis to allergic rhinitis and
asthma. J Clin Cell Immunol. 5:pii: 202. 2014.PubMed/NCBI
|
|
21
|
Akdis CA, Akdis M, Bieber T,
Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A,
Leung DY, Lipozencic J, et al: Diagnosis and treatment of atopic
dermatitis in children and adults: European Academy of Allergology
and Clinical immunology/American Academy of Allergy, Asthma and
Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol.
118:152–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ku JM, Hong SH, Kim HI, SEo HS, Shin YC
and Ko SG: Effects of Angelicae dahuricae Radix on 2,
4-Dinitrochlorobenzene-induced atopic dermatitis-like skin lesions
in mice model. BMC Complement Altern Med. 17:982017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Junghans V, Gutgesell C, Jung T and
Neumann C: Epidermal cytokines IL-1beta, TNF-alpha, and IL-12 in
patients with atopic dermatitis: Response to application of house
dust mite antigens. J Invest Dermatol. 111:1184–1188. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin G, Gao S, Cheng J, Li Y, Shan L and Hu
Z: 1β-hydroxyalantolactone, a sesquiterpene lactone from Inula
japonica, attenuates atopic dermatitis-like skin lesions induced by
2,4-dinitrochlorobenzene in the mouse. Pharm Biol. 54:516–522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pokharel YR, Lim SC, Kim SC, Heo TH, Choi
HK and Kang HK: Sopungyangjae-tang inhibits development of
dermatitis in Nc/Nga mice. Evid Based Complement Alternat Med.
5:173–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao D, Mendoza A, Lu S and Lawrence DA:
Immunomodulatory effects of Danshen (Salvia miltiorrhiza) in BALB/c
mice. ISRN Inflamm. 2012:9540322012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stone KD, Prussin C and Metcalfe DD: IgE,
mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 125
2 Suppl 2:S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakami Y, Kitaura J, Satterthwaite AB,
Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings
DJ, Witte ON and Kawakami T: Redundant and opposing functions of
two tyrosine kinases, Btk and Lyn, in mast cell activation. J
Immunol. 165:1210–1219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee M, Lee NY, Chung KS, Cheon SY, Lee KT
and An HJ: Roxatidine attenuates mast cell-mediated allergic
inflammation via inhibition of NF-κB and p38 MAPK activation. Sci
Rep. 7:417212017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Lui L, Luo Y, Odaka Y, Awate S,
Zhou H, Shen T, Zheng S, Lu Y and Huang S: Cryptotanshinone
activates p38/JNK and inhibits Erk1/2 leading to
caspase-independent cell death in tumor cells. Cancer Prev Res
(Phila). 5:778–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JH, Jeong SJ, Kwon TR, Yun SM, Jung
JH, Kim M, Lee HJ, Lee MH, Ko SG, Chen CY and Kim SH:
Cryptotanshinone enhances TNF-α-induced apoptosis in chronic
myeloid leukemia KBM-5 cells. Apoptosis. 16:696–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK,
Lee SH, Jeon SJ, Son KH, Chang YC, Lee YC and Kim CH:
Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory
effect on TNF-alpha-induced matrix metalloproteinase-9 production
and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem
Pharmacol. 72:1680–1689. 2006. View Article : Google Scholar : PubMed/NCBI
|