Introduction
Benign prostatic hyperplasia (BPH) has become a
common and frequently-occurring disease among elderly men
worldwide, following the improvements in living standards and the
prolongation of average life expectancies (1). Age, growth and testicular function
have previously been revealed to affect the pathogenesis of BPH
(2); however, its underlying
mechanisms remain unclear. In recent years, the role of upregulated
gene 11 (URG11; also termed von Willebrand factor C and EGF
domain-containing protein) in the pathogenesis of BPH has gained
increasing attention.
URG11 was initially identified as an upregulated
gene in hepatitis B virus X protein (HBx)-positive HepG2 cells by
polymerase chain reaction (PCR)-selected cDNA subtraction, and it
has also been demonstrated that URG11 promotes the growth of
hepatocellular carcinoma and tumorigenesis (3). URG11 encodes for a 70 kDa protein,
which comprises of cysteine-rich and lectin domains (4). A further study revealed that lectin
domains are closely associated with cell adhesion, matrix
interaction, migration and invasion (5). Furthermore, it has previously been
demonstrated that the expression of URG11 is enhanced in liver,
thyroid, gastric and bladder cancer (3,4). In
addition, a previous study revealed that URG11 is involved in the
regulatory processes of cell growth, adhesion, migration and tumor
metastasis (6). Furthermore, it
has been demonstrated that URG11 is involved in
epithelial-mesenchymal transition (EMT) via E-cadherin in the human
proximal tubule cells (7).
Therefore, URG11 may be an important gene in the progress of
tumorigenesis and metastasis. However, the function and mechanism
of URG11 in the pathogenesis and development of BPH have not yet
been determined.
EMT refers to the transformation process whereby
epithelial cells lose their polarity and move freely among the cell
matrix with a mesenchymal cell phenotype (8). A previous study has demonstrated that
within the patients with BPH, epithelial cells lose E-cadherin
protein expression, get vimentin protein expression, which suggests
that the development of BPH may be initiated from the dysregulation
of prostate epithelial cells (9).
Transforming growth factor-β (TGF-β) is a pleiotropic cytokine that
is involved in cell reconstruction processes, such as cell
proliferation, apoptosis, differentiation, migration and invasion
(10). TGF-β is considered to have
an important role in EMT (11).
Despite the TGF-β signaling pathway being relatively simple, its
regulatory mechanism is highly complex. Currently, the TGF-β
signaling pathway and its associations with other signaling
pathways has become an important research field.
The Ras protein family of guanosine triphosphates
(GTPases) is comprised of numerous subfamilies. Ras family proteins
include Rho, Rac1 and Cdc42 (12).
Rho acts as a molecular switch in response to cytokines, G-protein
coupled receptors, adhesion molecules and growth factor cell
surface receptors by cycling between active GTP and inactive
guanine diphosphate (13).
Rho-associated protein kinase (ROCK)1 and ROCK2 protein kinases of
the Rho protein family (14) that
regulate actomyosin contractility via phosphorylation of the myosin
light chain and inactivation of the myosin-binding subunit of
myosin phosphatase (15).
Furthermore, Ras homolog family member A (RhoA) and downstream
target ROCK1 are involved in numerous biological and pathological
processes, including cell adherence, apoptosis, proliferation and
migration (16).
In the present study, the expression levels of
URG11, RhoA and ROCK1 in patients with BPH, the effect of TGF-β on
BPH-1 cells, and the roles of URG11 in BPH-1 cell proliferation,
the cell cycle and EMT were investigated. In addition, the role of
URG11 in BPH was investigated. The results of the present study
suggest that URG11 may be a novel therapeutic target for the
treatment of BPH.
Materials and methods
Clinical samples
A total of 5 ml of peripheral blood from 37 male
patients (aged from 40–75 years old; mean, 52.3±3.6) with prostatic
hyperplasia and the healthy prostate glands were collected from
healthy volunteers (aged from 40–75 years old; mean, 49.9±5.2) at
The First Affiliated Hospital of Xinxiang Medical University
(Xinxiang, China) between July 2015 and June 2016. The participants
who were diagnosed as BPH and willing to participate in the present
study were enrolled. The exclusion criteria were as follows:
Prostate carcinoma, bladder cancer, urethral stricture;
cardiovascular, liver, kidney and hematopoietic disease and mental
illness. Serums were separated by centrifugation (1,200 × g, 10
min, 4°C) 2 h following collection, and the supernatant serum was
then frozen at −80°C until further analysis. The present study was
approved by the Ethics Committee of The First Affiliated Hospital
of Xinxiang Medical University, and written informed consent was
obtained from all participants. No patients had undergone
radiotherapy or chemotherapy prior to surgical resection.
Cell culture
Human BPH-1 cells (BPH epithelium) and normal
prostate gland cells (RWPE-1) were obtained from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and
100 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.)
in a humidified atmosphere at 37°C and 5% CO2. Cell
morphology was then observed under a microscope (magnification,
×40).
TGF-β treatment
BPH-1 cells (5×104 cells/well) were
seeded into 6-well plates with 2 ml serum-free medium and then
incubated at 37°C for 8 h. Following this, cells were treated with
10 ng/ml TGF-β for 24 h.
Small interfering (si)RNA
interference
Negative control siRNA (si-NC) and URG11 siRNA
(si-URG11) were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequence of si-NC was
5′-UUCUCCGAACGUGUCACGU-3′, and the sequence of si-URG11 was
5′-CAGACGGAUUGCUGUACUU-3′. BPH-1 cells (2×104
cells/well) were seeded into 6-well plates and incubated at 37°C
overnight. Following this, cells were transfected with si-NC (50
µM) and si-URG11 (50 µM) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 36 h post-transfection, the cells
were collected to proceed with the subsequent experiments.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the treated BPH-1 cells
using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized from the extracted RNA using a reverse
transcription kit, PrimeScript RT reagent kit (Takara Bio, Inc.,
Otsu, Japan) according to manufacturer's instructions. The reaction
mixture was incubated at 37°C, 15 min, and reaction was inactivated
at 85°C for 5 sec. Following this, qPCR was performed using SYBR
Premix Ex Taq™ (Takara Bio, Inc.). The PCR thermocycling conditions
were as follows: 95°C for 10 min, 35 cycles at 95°C for 15 sec and
60°C for 60 sec. Expression levels were quantified using the
2−ΔΔCq method (17).
The primer sequences are presented in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction assay. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction assay.
| Gene | Primers
sequence |
|---|
| URG11 | Forward:
5′-GCCCTTAGTCCAATGTTGTC-3′ |
| PSA | Reverse:
5′-TGCCCTCTGGACAGGAGGCT-3′ |
|
| Forward:
5′-AGGGTACGGTATGGGGTGTA-3′ |
|
| Reverse:
5′-TCATCCTCCCACTTCGAACC-3′ |
| RhoA | Forward:
5′-ACCAGTTCCCAGAGGTTTATGT-3′ |
|
| Reverse:
5′-TTTGGTCTTTGCTGAACACT-3′ |
| ROCK1 | Forward:
5′-ACCTGTAACCCAAGGAGATGTG-3′ |
|
| Reverse:
5′-CACAATTGGCAGGAAAGTGG-3′ |
| Cyclin D1 | Forward:
5′-GAACAAACAGATCATCCGCAA-3′ |
|
| Reverse:
5′-CCCTTCTGGTATCAAAATGC-3′ |
| p27 | Forward:
5′-AACGTGCGAGTGTCTAA |
|
| CGG-3′ |
|
| Reverse:
5′-CCCTCTAGGGGTTTGTGAT' |
|
| TCT-3 |
| E-cadherin | Forward:
5′-CGGACGATGATGTGAAC |
|
| ACC-3′ |
|
| Reverse:
5′-TTGCTGTTGTGCTTAACCCC-3′ |
| N-cadherin | Forward:
5′-CATCCCTCCAATCAACTTGC-3′ |
|
| Reverse:
5′-ATGTGCCCTCAAATGAAACC-3′ |
| Vimentin | Forward:
5′-GAGTCCACTGAGTACCG |
|
| GAG-3′ |
|
| Reverse:
5′-ACGAGCCATTTCCTCCTTCA-3′ |
| GAPDH | Forward:
5′-ATGTCGTGGAGTCTACTGGC-3′ |
|
| Reverse:
5′-TGACCTTGCCCACAGCCTTG-3′ |
Western blotting
Cells were rinsed with PBS (cat. no. 37350; Stemcell
Technologies, Inc., Beijing, China) and directly lysed in ice-cold
radioimmunoprecipitation assay buffer (Beijing Solarbio Science
& Technology, Beijing, China). Protein samples (20 mg) were
then separated by performing 10% SDS-PAGE and then transferred onto
polyvinylidene difluoride membranes. Membranes were then blocked
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany; cat. no. A0281) at room temperature. After 2 h, membranes
were then incubated with the following primary antibodies (all
Abcam, Cambridge, UK) at 4°C overnight: Anti-URG11 [1:600 (3)], anti-cyclin D1 (1:10,000; cat. no.
ab134175), anti-cyclin-dependent kinase inhibitor 1B (p27; 1:5,000;
cat. no. ab32034), anti-E-cadherin (1:50; cat. no. ab1416),
anti-N-cadherin (1:500; cat. no. ab18203), anti-vimentin (1:800;
cat. no. ab8978), anti-RhoA (1:5,000; cat. no. ab187027),
anti-ROCK1 (cat. no. ab45171, 1:200), anti-prostate specific
antigen (PSA; 1:5,000; cat. no. ab182031) and anti-GAPDH (1:5,000;
cat. no. ab8245). Following this, the membranes were incubated with
an appropriate horseradish peroxidase-coupled secondary antibody at
room temperature for 2 h (1:5,000; cat. no. ab6721, Abcam).
Proteins were visualized on membranes using an enhanced
chemiluminescent kit (Pierce; Thermo Fisher Scientific, Inc.) on
the ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln,
NE, USA). The data was then analyzed using Quantity One software
version 4.6. (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein expression levels were normalized to GAPDH.
Cell Counting Kit-8 (CCK-8) assay
Cells (2.5×104/ml) were collected with
complete medium and seeded into 96-well plates in a 200 µl cell
suspension. Following the transfection of cells according to the
aforementioned protocol, CCK-8 reagent (Beyotime Institute of
Biotechnology, Haimen, China; 15 µl/well) was added to the wells
and incubated for 3 h at 37°C with 5% CO2. At days 1, 2,
3, 4 and 5 post-transfection, absorbance was then determined at 450
nm using an Elx800 Reader (Bio-Tek Instruments, Inc., Winooski, VT,
USA).
Cell cycle assay
Treated cells were washed with PBS and then fixed
with 70% ethanol for 30 min on ice. Following this, RNA was
degraded with 20 mg/ml RNase (Sigma-Aldrich; Merck KGaA) for 1 h at
37°C. Cells were then labeled with 20 mg/ml propidium iodide
(Sigma-Aldrich; Merck KGaA). Cell cycle images were obtained and
analyzed using the FACSCalibur (BD Biosciences, Franklin Lakes, NJ,
USA) flow cytometer and FlowJo software version 6.2.1 (FlowJo LLC,
Ashland, OR, USA).
Statistical analysis
For statistical analyses between two groups, the
Student's t-test was performed. For multiple comparisons, one-way
analysis of variance followed by Turkey's post hoc test was
performed. All statistical analyses were performed using IBM SPSS
Statistics version 21 (IBM Corp., Armonk, NY, USA) software, and
all data are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
URG11, RhoA and ROCK1 are highly
expressed in patients with BPH
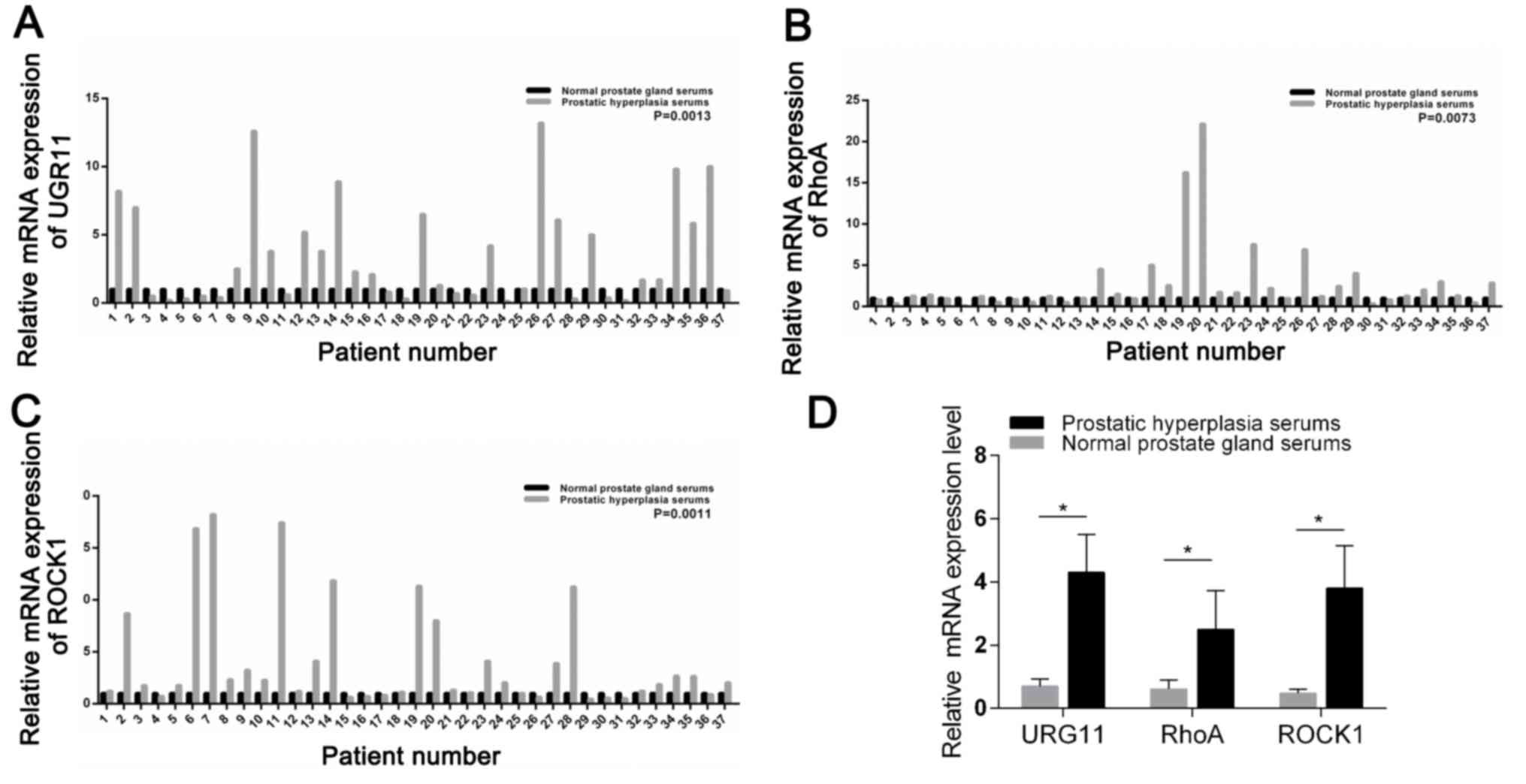
The results of the present study revealed that URG11
expression was markedly higher in serum from patients with
prostatic hyperplasia compared with in the healthy controls (n=37;
P=0.0013; Fig. 1A). The expression
of RhoA was also significantly upregulated in the serum of patients
with BPH compared with the controls (n=37; P=0.0073; Fig. 1B). In addition, ROCK1 expression
was also significantly increased in the serum of patients with
prostatic hyperplasia compared with healthy controls (n=37;
P=0.0011; Fig. 1C). The relative
expression levels of URG11, RhoA and ROCK1 were presented in
Fig. 1D.
URG11 expression is silenced in BPH-1
cells
BPH-1 cells were cultured in RPMI-1640 medium with
10% FBS at 37°C for 48 h and then observed under a light
microscope. Cells grew adhering to the wall of the culture dish,
exhibited rectangular or irregular polygon shapes, had large and
round nuclei, and grew in close contact and irregular arrangements.
These characteristics demonstrated that the cells exhibited typical
epithelial cell morphology (Fig.
2A). To further investigate this, the expression levels of PSA
in cells were determined. The results demonstrated that the
expression level of PSA was significantly enhanced in BPH-1 cells
compared with normal prostate gland cells (Fig. 2B and C). Thus, it was confirmed
that the obtained cells were BPH-1 cells. Furthermore, the
expression level of URG11 in normal prostate gland cells and BPH-1
cells was investigated. The results revealed that the expression
levels of URG11 were significantly enhanced in BHP-1 cells compared
with normal prostate gland cells (Fig.
2B and C). To further investigate the potential roles of URG11
on BPH-1 cell proliferation and migration, BPH-1 cells were either
treated with PBS (control), or transfected with si-NC or si-URG11.
RT-qPCR and western blot assays were used to detect the expression
level of URG11. The results demonstrated that URG11 expression was
significantly suppressed in BPH-1 cells transfected with si-URG11
compared with the si-NC group (Fig. 2D
and E; P<0.001). These results revealed that the efficiency
and specificity of URG11 knockdown were high.
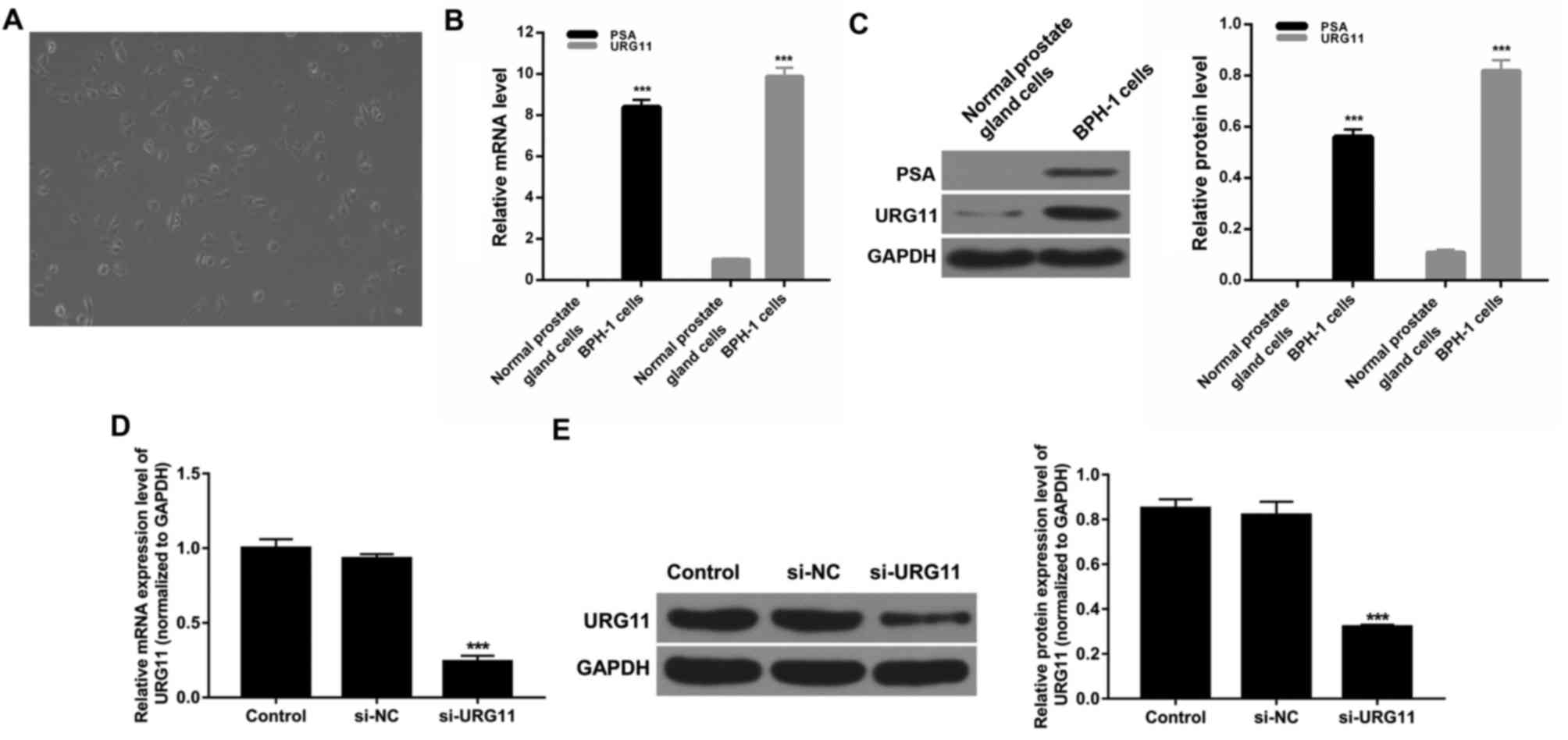 | Figure 2.URG11 expression is suppressed in
BPH-1 cells. (A) The morphology of BPH-1 cells was investigated
using a light microscope. Magnification, ×100; scale bar, 100 µm,
n=3. (B) RT-qPCR was performed to determine the expression level of
URG11 in normal prostate gland cells and BPH-1 cells (n=4). (C)
Western blot assays were performed to determine the expression
levels of PSA and URG11 in normal prostate gland cells and BPH-1
cells (n=3). ***P<0.001 vs. normal prostate gland cells. (D)
BPH-1 cells were transfected with PBS, si-NC and si-URG11. mRNA
expression levels of URG11 were determined using RT-qPCR assays
(n=4). (E) Protein expression level of URG11 was investigated using
western blot assays, and GAPDH was used as control for
normalization (n=3). ***P<0.001 vs. si-NC. PSA, prostate
specific antigen; URG11, upregulated gene 11; BPH, prostatic
hyperplasia; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; si, small interfering RNA; NC, negative
control. |
Decreased expression of URG11
suppresses proliferation and induces cell cycle arrest of BPH-1
cells, regulated by TGF-β
Previous studies have demonstrated that TGF-β
enhances cancer progression by inducing EMT (18). To investigate this further, BPH-1
cells were treated with either PBS (control) or TGF-β (5 ng/ml for
8 h), or transfected with si-NC or si-URG11 and treated with TGF-β.
RT-qPCR analyses revealed that treatment with TGF-β significantly
enhanced URG11 expression, and that transfection with si-URG11
significantly reversed this increase induced by TGF-β (Fig. 3A; P<0.001). The CCK-8 assay
results demonstrated that TGF-β significantly increased the
proliferation of BPH-1 cells, and transfection with si-URG11
significantly suppressed BPH-1 cell proliferation induced by TGF-β
(Fig. 3B; P<0.01 or
P<0.001). In addition, the potential mechanism underlying TGF-β
and URG11-mediated changes in BPH-1 cell proliferation was further
investigated using flow cytometry. The results revealed that the
proportion of cells in the G1 phase was significantly increased in
the TGF-β group compared with the control group, and was
significantly decreased in si-URG11 + TGF-β group compared with the
si-NC + TGF-β group. The proportion of cells in the G2 phase was
decreased in the TGF-β group compared with control group, and was
significantly increased in the si-URG11 + TGF-β group compared with
the si-NC + TGF-β group (Fig. 3C;
P<0.05, P<0.01 or P<0.001), suggesting that the
downregulation of URG11 promoted the cell cycle arrest.
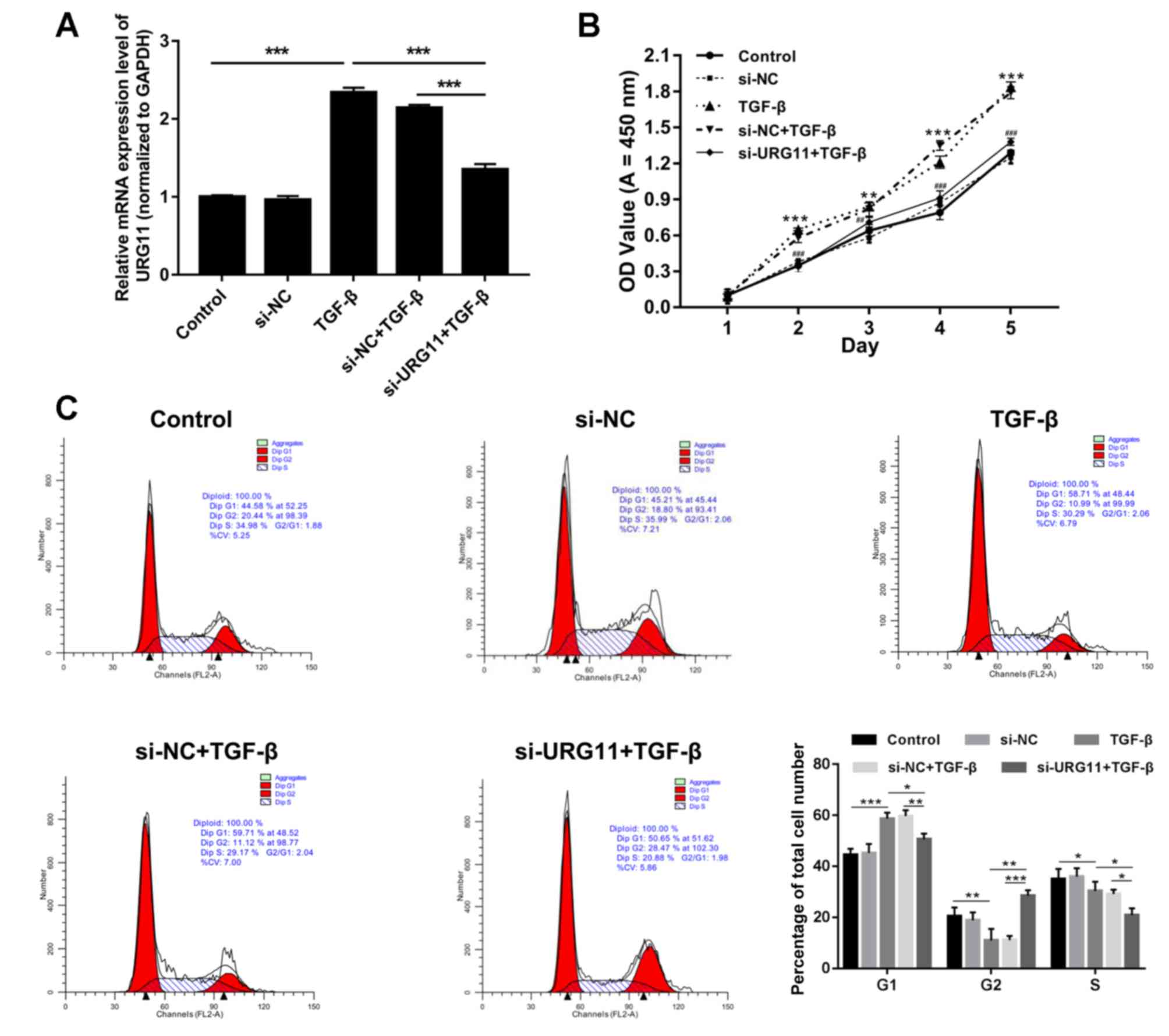 | Figure 3.Silencing of URG11 suppresses
proliferation and induced cell cycle arrest of BPH-1 cells mediated
by TGF-β. BPH-1 cells were transfected with PBS (control), si-NC,
TGF-β, si-NC plus TGF-β, and si-URG11 plus TGF-β, respectively. (A)
Reverse transcription-quantitative polymerase chain reaction assays
were performed to investigate URG11 expression (***P<0.001,
n=4). (B) Cell Counting Kit-8 assays were performed to investigate
BPH-1 cell proliferation. (**P<0.01, ***P<0.001 vs. control
group; ##P<0.01 and ###P<0.001,
si-URG11 + TGF-β vs. si-NC + TGF-β, n=5). (C) Flow cytometry was
performed to investigate cell-cycle distribution. *P<0.05,
**P<0.01, ***P<0.001 (n=4). URG11, upregulated gene 11; si,
small interfering RNA; NC, negative control; TGF-β, transforming
growth factor-β; OD, optical density. All the experiments were
independently repeated at least for 3 times. |
Silencing of URG11 downregulates the
TGF-β-induced expression levels of cyclin D1, N-cadherin and
vimentin, and upregulates the expression levels of p27 and
E-cadherin
According to the aforementioned results, silencing
of URG11 induced cell cycle arrest. The effect of URG11 on cyclin
D1 and p27 expression was investigated further, as these proteins
are associated with the cell cycle, and it was revealed that TGF-β
enhanced cyclin D1 expression and suppressed p27 expression;
however, transfection with si-URG11 reversed these effects in BPH-1
cells (Fig. 4; P<0.01 or
P<0.001). In addition, the effect of URG11 on N-cadherin,
vimentin and E-cadherin expression levels was investigated, as
these proteins are associated with EMT, and it was revealed that
treatment with TGF-β enhanced the expression levels of N-cadherin
and vimentin, and decreased the expression level of E-cadherin
(Fig. 4; P<0.01 or P<0.001).
Suppression of URG11 expression via transfection with si-URG11
significantly reversed these effects on BPH-1 cells (Fig. 4; P<0.01 or P<0.001).
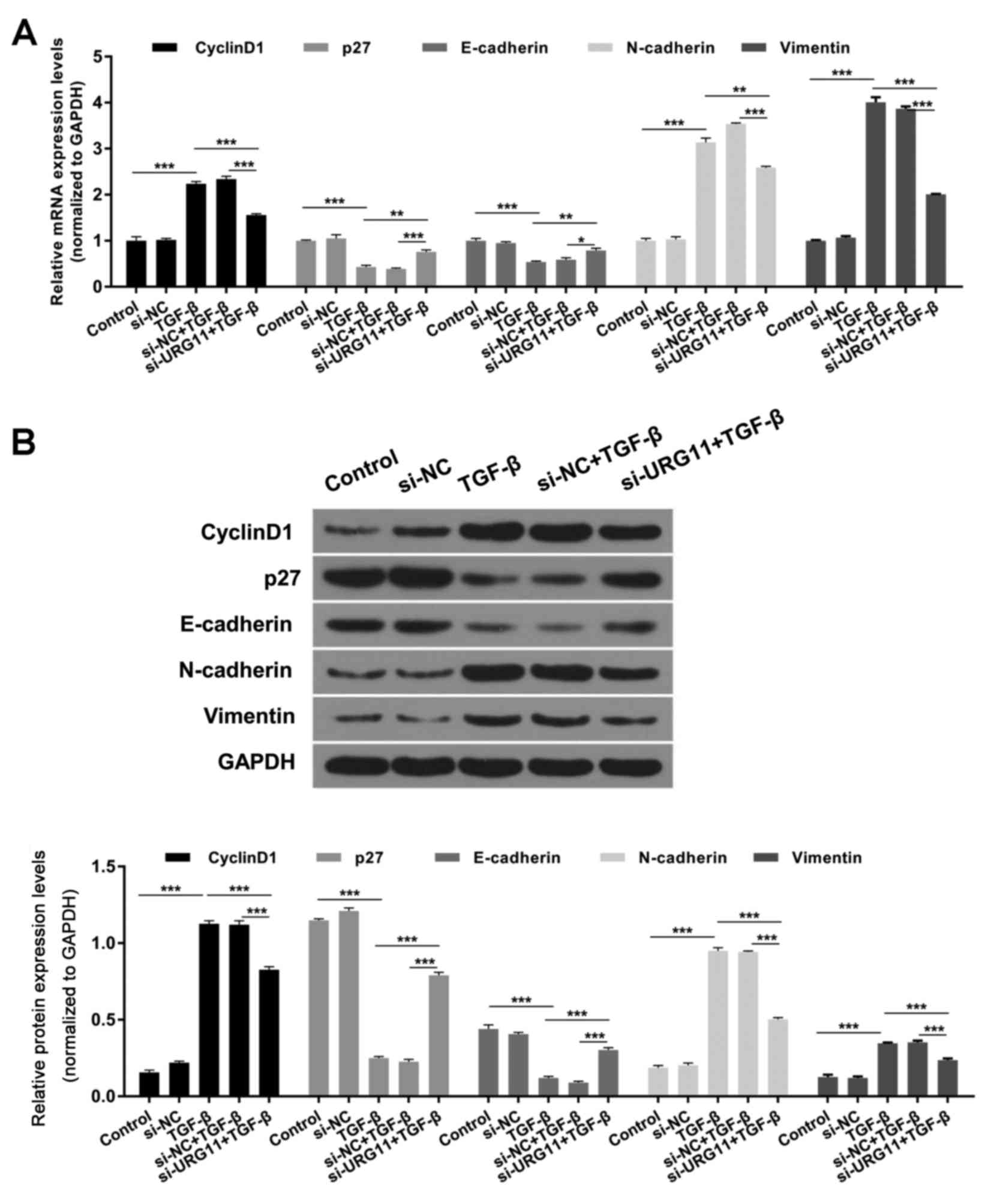 | Figure 4.Silencing of URG11 expression
decreases cyclin D1, N-cadherin and vimentin expression levels, and
enhances p27 and E-cadherin expression levels, regulated by TGF-β.
(A) mRNA expression levels of cyclin D1, p27, E-cadherin,
N-cadherin and vimentin were investigated by reverse
transcription-quantitative polymerase chain reaction. (B) Protein
expression levels of cyclin D1, p27, E-cadherin, N-cadherin and
vimentin were determined by western blot assays. *P<0.05,
**P<0.01 and ***P<0.001. p27, cyclin-dependent kinase
inhibitor 1B; si, small interfering RNA; NC, negative control;
TGF-β, transforming growth factor-β; URG11, upregulated gene
11. |
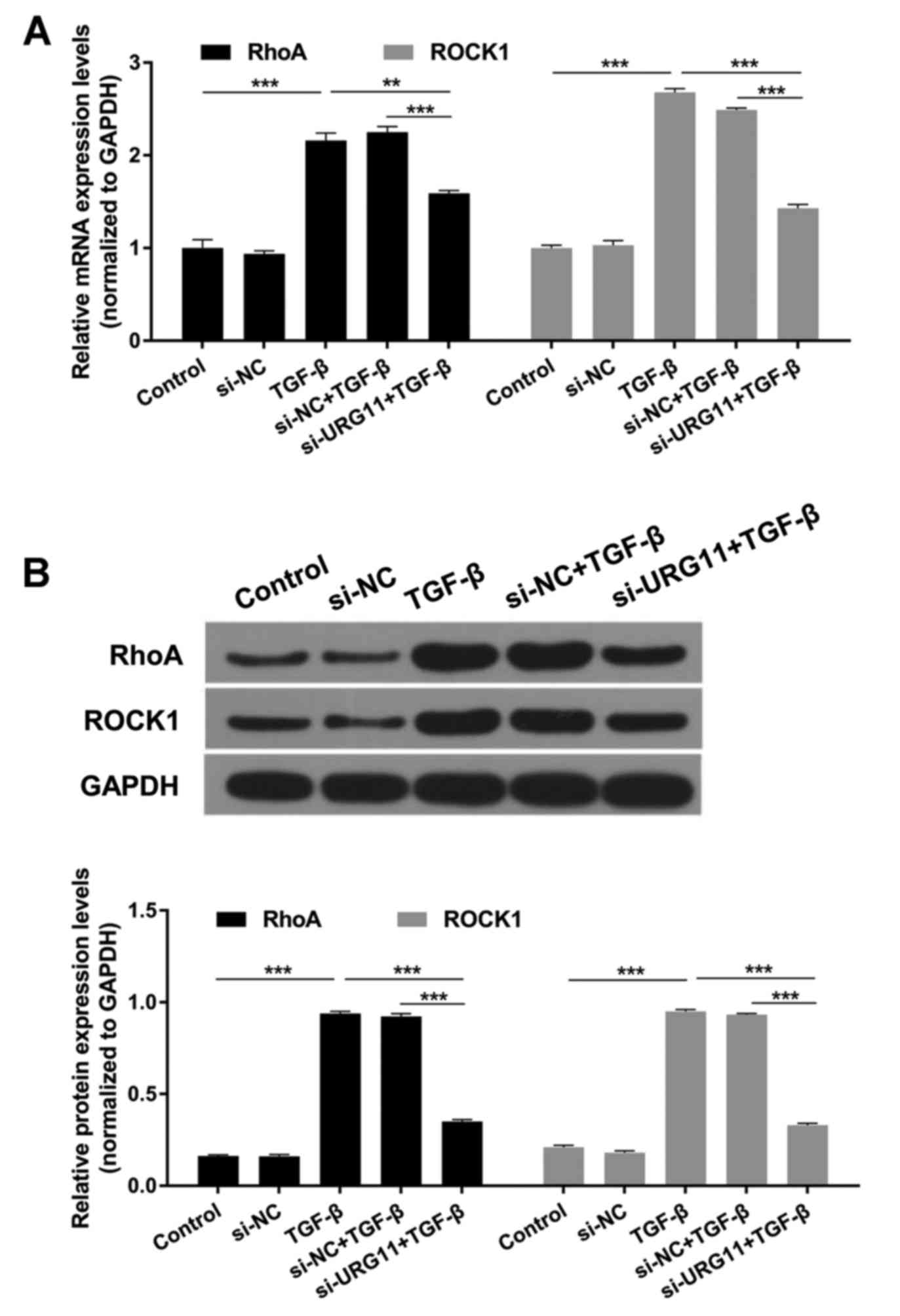
Suppression of URG11 expression
decreases TGF-β-induced RhoA and ROCK1 expression levels
Studies revealed that RhoA and ROCK1 are downstream
targets of TGF-β signaling and are involved in cell proliferation,
migration and apoptosis processes (19–22).
In the present study, it was revealed that RhoA and ROCK1
expression levels were significantly increased by TGF-β compared
with the control group, and significantly decreased in the si-URG11
+ TGF-β group compared with the si-NC + TGF-β group (Fig. 5; P<0.01 or P<0.001).
Discussion
BPH is one of the most prevalent diseases of the
urinary system that affects elderly men. The major clinical
manifestations of BPH include frequent urination, urgent urination
and dysuria (23). Currently,
population aging is a major public health problem, particularly in
China. With the aging population, the incidence of BPH is expected
to increase further and thus seriously affect the quality of life
of older men, and may even result in the emergence of serious
complications (24,25).
URG11 is an HBx-upregulated gene and regulates
HBx-mediated hepatocarcinoma development (26). A previous study demonstrated that
silencing of URG11 expression suppresses gastric cancer cell
proliferation, adhesion, invasion and metastasis abilities, and
prevents cell transition from G1 phase to S phase in vitro
(6). Furthermore, previous studies
have revealed that knockdown of URG11 suppresses lung cancer
proliferation and invasion via β-catenin (27) and induces EMT via E-cadherin and
β-catenin; and overexpression of URG11 enhances hepatocellular
carcinoma growth (4). These
results suggest that URG11 may regulate tumor progression via
regulation of EMT and the β-catenin signaling pathway. However, the
function and underlying mechanisms of URG11 in BPH have not yet
been determined. In the present study, it was revealed that the
expression of URG11 was significantly enhanced in patients with BPH
compared with matched controls, and that decreased URG11 expression
suppressed BPH-1 cell proliferation and induced cycle arrest via
regulation of cyclin D1 and p27 expression levels.
EMT is an essential early step and a critical
process in tumor metastasis (28).
During EMT, tumor cells lose their epithelial characteristics,
including cell-cell adhesion and polarity, and obtain
mesenchymal-like phenotypes (29).
Furthermore, phenotypic markers commonly change during EMT, for
example, the epithelial cell phenotypic marker E-cadherin is
downregulated and mesenchymal cell phenotypic markers, N-cadherin
and vimentin, are upregulated post-EMT (30). Previous studies have demonstrated
that URG11 is associated with E-cadherin expression (31,32).
E-cadherin, an epithelial cell adhesion protein, is important for
the formation and maintenance of embryonic epithelial cells
(33). In addition, E-cadherin is
important for the maintenance of structural integrity and cell
polarity (34). In the present
study, it was demonstrated that suppression of URG11 expression
significantly enhances E-cadherin expression, and significantly
suppresses the expression of N-cadherin and vimentin. TGF-β is a
multifunctional protein of the TGF-β protein superfamily and
regulates the growth, differentiation, apoptosis and immunity of
numerous cell types (35).
Previous studies have demonstrated that TGF-β functions as an
inducer of EMT and is closely associated with cancer progression,
angiogenesis, pathological staging and prognosis (18,36).
Thus, the results suggested that silencing of URG11 suppresses EMT
progress in BPH.
Rho proteins have GTPase activity and are involved
in cell division and proliferation (37). RhoA, an important member of the Rho
family, regulates numerous downstream genes, alters actin structure
and morphology and regulates cell proliferation, apoptosis,
metastasis and other biological processes (38). A recent study demonstrated that
ROCK, an important RhoA regulator, is associated with cell
apoptosis, EMT, migration and metastasis (39). In the present study, it was
revealed that knockdown of URG11 suppresses TGF-β-induced RhoA and
ROCK1 expression levels, indicating that URG11 regulates BPH
progression via the RhoA/ROCK1 signaling pathway.
In conclusion, the results of the present study
revealed that URG11, RhoA and ROCK1 expression levels were enhanced
in patients with PBH. Additionally, suppression of URG11 expression
inhibited TGF-β-regulated BPH-1 cell proliferation and induced cell
cycle arrest via the regulation of cell cycle-associated genes
(cyclin D1 and p27). Furthermore, URG11 knockdown suppressed
TGF-β-mediated EMT of BPH-1 cells. In addition, silencing of URG11
expression significantly decreased RhoA and ROCK1 expression levels
regulated by TGF-β. Therefore, URG11 may represent a novel
prognostic marker and candidate drug target for the treatment of
BPH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GZ wrote the main manuscript. FZ, GH and ZL
performed the experiments. GZ and JL designed the study. QY and ZHL
performed data analysis. GZ, FZ and JL contributed to manuscript
revisions and all authors reviewed the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xinxiang Medical
University (Xinxiang, China), and written informed consent was
obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mobley D, Feibus A and Baum N: Benign
prostatic hyperplasia and urinary symptoms: Evaluation and
treatment. Postgrad Med. 127:301–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corona G, Vignozzi L, Rastrelli G, Lotti
F, Cipriani S and Maggi M: Benign prostatic hyperplasia: A new
metabolic disease of the aging male and its correlation with sexual
dysfunctions. Int J Endocrinol. 2014:3294562014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lian Z, Liu J, Li L, Li X, Clayton M, Wu
MC, Wang HY, Arbuthnot P, Kew M, Fan D and Feitelson MA: Enhanced
cell survival of Hep3B cells by the hepatitis B × antigen effector,
URG11, is associated with upregulation of beta-catenin. Hepatology.
43:415–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie H and Liu J: Increased expression
URG11 in hepatocellular carcinoma tissues promotes the growth of
hepatocellular carcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 31:1523–1527. 2015.(In Chinese). PubMed/NCBI
|
|
5
|
Zou X, Li X, Liu J, Lian Z, Fan R, Du R,
Xie H, Song J and Fan D: Preparation and characterization of a
specific monoclonal antibody against a new gene product: URG11.
Hybridoma (Larchmt). 25:378–381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du R, Xia L, Sun S, Lian Z, Zou X, Gao J,
Xie H, Fan R, Song J, Li X, et al: URG11 promotes gastric cancer
growth and invasion by activation of beta-catenin signalling
pathway. J Cell Mol Med. 14:621–635. 2010.PubMed/NCBI
|
|
7
|
Du R, Huang C, Bi Q, Zhai Y, Xia L, Liu J,
Sun S and Fan D: URG11 mediates hypoxia-induced
epithelial-to-mesenchymal transition by modulation of E-cadherin
and beta-catenin. Biochem Biophys Res Commun. 391:135–141. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao R, Shi J, Liu H, Shi X, Du X, Klocker
H, Lee C, Zhu Y and Zhang J: Epithelial-to-mesenchymal transition
and estrogen receptor alpha mediated epithelial dedifferentiation
mark the development of benign prostatic hyperplasia. Prostate.
74:970–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Syed V: TGF-β signaling in cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chew TW, Liu XJ, Liu L, Spitsbergen JM,
Gong Z and Low BC: Crosstalk of ras and rho: Activation of RhoA
abates Kras-induced liver tumorigenesis in transgenic zebrafish
models. Oncogene. 33:2717–2727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bryan BA, Dennstedt E, Mitchell DC, Walshe
TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK and
D'Amore PA: RhoA/ROCK signaling is essential for multiple aspects
of VEGF-mediated angiogenesis. FASEB J. 24:3186–3195. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riou P, Kjær S, Garg R, Purkiss A, George
R, Cain RJ, Bineva G, Reymond N, McColl B, Thompson AJ, et al:
14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif
to inhibit G proteins. Cell. 153:640–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao ZS and Manser E: PAK and other
Rho-associated kinases-effectors with surprisingly diverse
mechanisms of regulation. Biochem J. 386:201–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan CH, Lee SW, Li CF, Wang J, Yang WL,
Wu CY, Wu J, Nakayama KI, Kang HY, Huang HY, et al: Deciphering the
transcriptional complex critical for RhoA gene expression and
cancer metastasis. Nat Cell Biol. 12:457–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wendt MK, Tian M and Schiemann WP:
Deconstructing the mechanisms and consequences of TGF-β-induced EMT
during cancer progression. Cell Tissue Res. 347:85–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riento K and Ridley AJ: Rocks:
Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu B, Xu C, Cao P, Tian Y, Zhang Y, Shi C,
Xu J, Yuan W and Chen H: TGF-β stimulates expression of chondroitin
polymerizing factor in nucleus pulposus cells through the Smad3,
RhoA/ROCK1 and MAPK signaling pathways. J Cell Biochem.
119:566–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu ML, Chen CH, Lin YT, Jheng YJ, Ho YC,
Yang LT, Chen L, Layne MD and Yet SF: Divergent signaling pathways
cooperatively regulate TGFβ induction of cysteine-rich protein 2 in
vascular smooth muscle cells. Cell Commun Signal. 12:222014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohamed JS and Boriek AM: Stretch augments
TGF-beta1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway
smooth muscle cells. Am J Physiol Lung Cell Mol Physiol.
299:L413–L424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bechis SK, Otsetov AG, Ge R and Olumi AF:
Personalized medicine for the management of benign prostatic
hyperplasia. J Urol. 192:16–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong X, Chang ES, Zeng P and Simon MA:
Suicide in the global chinese aging population: A review of risk
and protective factors, consequences, and interventions. Aging Dis.
6:121–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Guo Y, Zhang D, Tian Y and Zhang
X: The prevalence of benign prostatic hyperplasia in mainland
China: Evidence from epidemiological surveys. Sci Rep. 5:135462015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lian Z, Liu J, Li L, Li X, Tufan NL,
Clayton M, Wu MC, Wang HY, Arbuthnot P, Kew M and Feitelson MA:
Upregulated expression of a unique gene by hepatitis B × antigen
promotes hepatocellular growth and tumorigenesis. Neoplasia.
5:229–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu ZL, Wu J, Wang LX, Yang JF, Xiao GM,
Sun HP and Chen YJ: Knockdown of upregulated gene 11 (URG11)
inhibits proliferation, invasion, and β-catenin expression in
Non-small cell lung cancer cells. Oncol Res. 24:197–204. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hyun KA, Koo GB, Han H, Sohn J, Choi W,
Kim SI, Jung HI and Kim YS: Epithelial-to-mesenchymal transition
leads to loss of EpCAM and different physical properties in
circulating tumor cells from metastatic breast cancer. Oncotarget.
7:24677–24687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nijkamp MM, Span PN, Hoogsteen IJ, van der
Kogel AJ, Kaanders JH and Bussink J: Expression of E-cadherin and
vimentin correlates with metastasis formation in head and neck
squamous cell carcinoma patients. Radiother Oncol. 99:344–348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Lian Z, Han S, Waye MM, Wang H, Wu
MC, Wu K, Ding J, Arbuthnot P, Kew M, et al: Downregulation of
E-cadherin by hepatitis B virus X antigen in hepatocellullar
carcinoma. Oncogene. 25:1008–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arzumanyan A, Friedman T, Kotei E, Ng IO,
Lian Z and Feitelson MA: Epigenetic repression of E-cadherin
expression by hepatitis B virus × Antigen in liver cancer.
Oncogene. 31:563–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riethmacher D, Brinkmann V and Birchmeier
C: A targeted mutation in the mouse E-cadherin gene results in
defective preimplantation development. Proc Natl Acad Sci USA.
92:855–859. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Talar B and Czyz M: TGF-β signaling
pathways in cancers. Postepy Hig Med Dosw (Online). 67:1008–1017.
2013.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao J, Zhu Y, Nilsson M and Sundfeldt K:
TGF-β isoforms induce EMT independent migration of ovarian cancer
cells. Cancer Cell Int. 14:722014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Surma M, Wei L and Shi J: Rho kinase as a
therapeutic target in cardiovascular disease. Future Cardiol.
7:657–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marjoram RJ, Lessey EC and Burridge K:
Regulation of RhoA activity by adhesion molecules and
mechanotransduction. Curr Mol Med. 14:199–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lotz-Jenne C, Lüthi U, Ackerknecht S,
Lehembre F, Fink T, Stritt M, Wirth M, Pavan S, Bill R, Regenass U,
et al: A high-content EMT screen identifies multiple receptor
tyrosine kinase inhibitors with activity on TGFβ receptor.
Oncotarget. 7:25983–26002. 2016. View Article : Google Scholar : PubMed/NCBI
|