Introduction
Angiotensin II (Ang II), an important effector
molecule of the renin-angiotensin system, regulates the expression
of various cytokines and maintains homeostasis of the
cardiovascular system under physiological conditions (1). Under pathological conditions, Ang II
is involved in the occurrence and development of cardiovascular
diseases, including intimal hyperplasia, cardiac hypertrophy and
cardiac remodeling, by activating multiple signaling pathways
(2,3). Previous studies have shown that Ang
II is involved in the development of various cardiovascular
diseases by disrupting microvessel permeability (4). However, the exact mechanism by which
Ang II affects microvascular permeability remains to be
elucidated.
The integrity of the vascular endothelial cell
barrier is the basis for maintaining normal vascular permeability.
Multiple tight junctions, which are often found around the cells,
form a continuous band to link adjacent vascular endothelial cells
together, thus filling the cell gap and forming a natural physical
barrier. The connected endothelial cells can maintain homeostasis
and selective permeability (5).
Zonula occludens-1 (ZO-1) is a 220-kDa protein of the
membrane-associated guanylate kinase homologs gene family, which
interacts directly with the transmembrane protein occludin, ZO-2,
and AF-6, the target of the oncogene, ras (6). As a key component of junctional
complexes that regulate tight junction formation, the expression
and distribution of ZO-1 in endothelial cells is decisive in the
process of formation of tight junctions between endothelial cells
(7). Therefore, the present study
investigated the effect of Ang II on the expression and
distribution of ZO-1 in endothelial cells.
The dynamics of vascular endothelial (VE)-cadherin
at the plasma membrane are considered to be essential in modulating
endothelial adhesion strength and junction plasticity between
endothelial cells (8). Previous
studies have shown that downregulation of the VE-cadherin
extracellular domain leads to reduced cell-cell adhesion strength
(9). In addition, studies have
found that VE-cadherin is involved in the formation of tight
junctions between endothelial cells, not only as a major component
of junctional complexes but also as a key regulator of the
expression and distribution of other components (10,11).
However, whether VE-cadherin is involved in regulating the
expression of ZO-1 remains to be elucidated.
The present study investigated the effect of Ang II
on the expression and distribution of ZO-1 in endothelial cells and
aimed to elucidate the role of VE-cadherin in endothelial cells in
order to investigate the possible mechanism underlying the effect
of Ang II.
Materials and methods
Animals
Male specific pathogen-free Sprague-Dawley (SD) rats
(50 days old, body weight 150–180 g) were obtained from the Animal
Center Laboratory of Zhejiang Province Institute of Medicine
(Zhejiang, China). Mice were housed in a sterile environment at
25°C with a 12 h light/dark cycle, 40% humidity and food and water
ad libitum.
Reagents
M199 medium, PBS, 0.25% trypsase-EDTA, penicillin,
and streptomycin were purchased from Jinuo Biotech Company
(Hangzhou, China). Ang II was purchased from Sigma; EMD Millipore
(Billerica, MA, USA), dimethyl sulfoxide was from MP Biomedicals;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA), fetal bovine
serum (FBS) was from Gibco; Thermo Fisher Scientific, Inc., and
DAPI was from Roche Diagnostics (Indianapolis, IN, USA). Antibodies
against ZO-1 (cat. no. ab150266), VE-cadherin (cat. no. ab33168)
and β-actin (cat. no. ab8226) were purchased from Abcam (Cambridge,
MA, USA), and horseradish peroxidase-conjugated goat anti-mouse and
goat anti-rabbit IgG antibodies were purchased from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). The other
reagents for the immunoblot assay were purchased from Beyotime
Institute of Biotechnology (Jiangsu, China). The Rneasy mini-kit
was purchased from Qiagen GmbH (Hilden, Germany). All primer
sequences were synthesized by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The human VE-cadherin gene was constructed into
the pcDNA3.1+HA vector by GeneChem Co., Ltd. (Shanghai, China). All
other chemicals were commercially available and of reagent
grade.
Cell culture, transfection and
treatment groups
The primary culture of vascular endothelial cells
was established by isolating the cells from the thoracic and
abdominal aortas, which were resected from 2–3-week-old male SD
rats as described in our previous study (12). The present study was approved by
the Ethical Committee of Shaoxing People's Hospital (no.
2016C33227). The endothelial cells were cultured in M199 medium
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
1% 100 U/ml penicillin and 1% 100 mg/ml streptomycin sulfates. The
cells were incubated in humidified incubators with 5%
CO2 at 37°C.
The rat VE-cadherin gene was constructed into the
pcDNA3.1+HA vector and the empty vector served as the negative
control. For transfection, following culture of the cells to 70–80%
confluence, the pcDNA3.1+HA-VE-cadherin and pcDNA3.1+HA empty
vectors were transfected using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
In the present study, ~105 cells/well
were plated in a six-well plate at 37°C for 24 h. Ang II (1 µmol/l)
was used to establish the cell model. In the valsartan group, the
cells were treated with Ang II (1 µmol/l) and valsartan (10 µmol/l)
for 24 h at 37°C; in the VE-cadherin group, the cells were
transfected with VE-cadherin overexpression vector and subsequently
treated with Ang II (1 µmol/l) for 24 h at 37°C.
Total mRNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Following treatment, total RNA from the treated
cells was extracted using the Rneasy mini-kit. First-strand
complementary DNA (cDNA) was synthesized using the Thermo Script
RT-PCR kit (Invitrogen; Thermo Fisher Scientific, Inc.) with a
10-µl reaction mixture containing 2 µl PrimeScript RT Enzyme Mix I,
2 µl RT Primer Mix, 4 µl RNase Free distilled H2O and 2
µl total RNA, according to the manufacturer's protocol. The
synthesized cDNA (2 µl) was then used for RT-qPCR analysis. The
forward and reverse primer sequences used were as follows: ZO-1,
forward 5′-CCCTCAAGGAGCCATTC-3′ and reverse
5′-CAGTTTGCTCCAACGAGA-3′; VE-cadherin, forward
5′-AAGCGTGAGTCGCAA-3′ and reverse 5′-CTCTCAGGTTTTCGC-3′; GAPDH,
forward 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse
5′-TTGATTTTGGAGGGATCTCG-3′. The reaction conditions were as
follows: Step 1, 95°C for 30 sec; step 2, 40 cycles of 95°C for 5
sec and 60°C for 34 sec; step 3, 95°C for 15 sec, 60°C for 60 sec,
and 95°C for 15 sec. The final densities of ZO-1 and VE-cadherin
were determined relative to the corresponding density of GAPDH from
the same RNA sample with the 2−ΔΔCq method (13).
Western blot analysis
Following incubation with the corresponding
intervention factors for 24 h, cellular protein was obtained
following lysis using radioimmunoprecipitation assay lysis buffer
for western blot analysis. The protein concentrations of the
supernatant were determined using a bicinchoninic acid protein
assay. Proteins (30 µg/lane) were loaded and separated by SDS-PAGE
(10%) and transferred onto polyvinylidene fluoride membranes.
Subsequently, the membranes were blocked with blocking buffer for
30 min at room temperature and then incubated with rabbit anti-ZO-1
and anti-VE-cadherin 1 monoclonal antibodies (1:1,000 dilution) and
rabbit anti-β-actin monoclonal antibody (1:10,000 dilution)
overnight at 4°C. Following incubation, TBS-T was used to wash the
membranes (three times for 10 min), following which the membranes
were incubated with goat anti-rabbit IgG-HRP (1:10,000 dilution)
for 1 h at room temperature. The standard chemical luminescence
method was used to detect the antigen. The bands were scanned on a
gel imaging and analysis system and analyzed using Quantity One 4.4
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical analysis
Following treatment, the cells were washed with PBS,
fixed in 4% paraformaldehyde, and permeabilized using 0.1% Triton
X-100. Subsequently, the cells were blocked with 10% goat serum
(Beyotime Institute of Biotechnology) in PBST and incubated with
ZO-1 antibodies (1:300) in PBST for 1 h at 37°C. After 1 h, the
cells were washed in PBS with 0.1% Tween-20 and then incubated with
anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary
antibody (1:500) for 1 h at 37°C, followed again by washing.
Coverslips were then processed for immunofluorescence
microscopy.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA,
USA). One-way analysis of variance, followed by Tukey's post hoc
analysis was performed to compare multiple experimental groups.
Student's t-test was used for comparisons between two different
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Ang II inhibits the expression of ZO-1
in endothelial cells
Western blot and RT-qPCR analyses were performed to
assess the effect of Ang II on the expression of ZO-1 in
endothelial cells. As shown in Fig. 1A
and B, Ang II significantly inhibited the protein and mRNA
expression of ZO-1 in endothelial cells (P<0.05). In addition,
the immunohistochemical analysis revealed that Ang II also
disturbed the distribution of ZO-1 protein around the cells
(Fig. 1C). Valsartan reversed this
effect of Ang II (P<0.05).
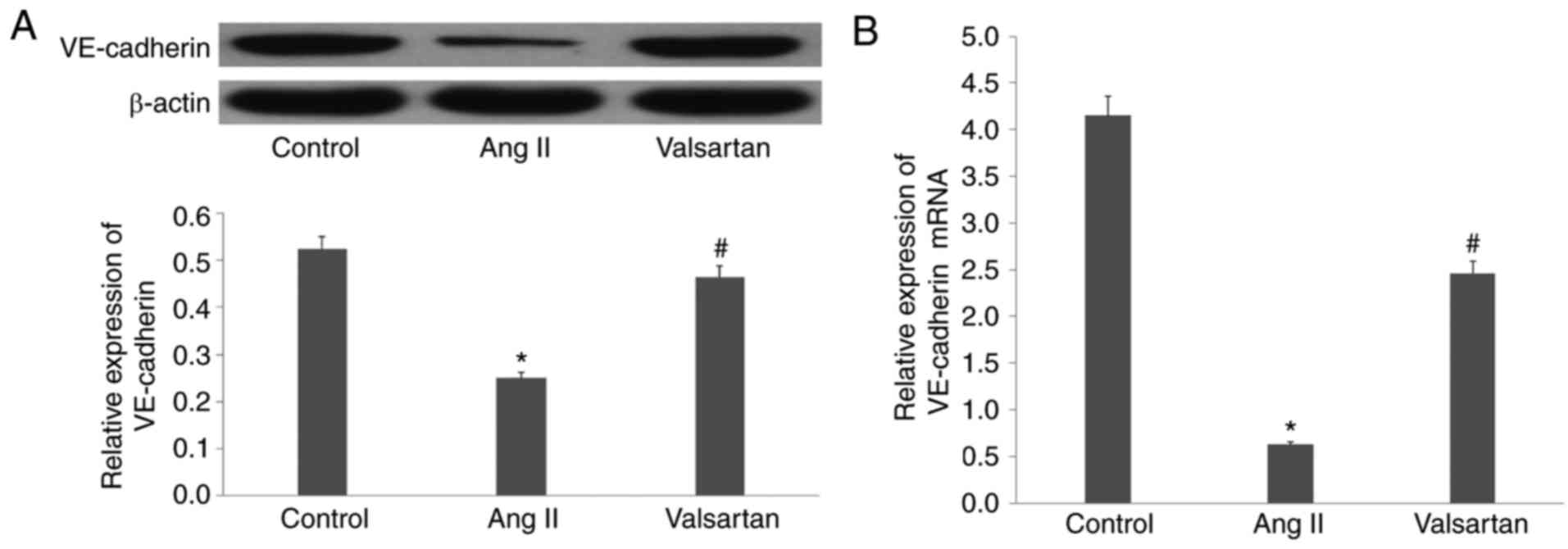
Ang II decreases the expression of
VE-cadherin in endothelial cells
Following incubation of the endothelial cells with
Ang II for 24 h, western blot and RT-qPCR analyses showed that the
expression of VE-cadherin was significantly decreased by Ang II
(P<0.01; Fig. 2A and B).
Considering the importance of VE-cadherin in the formation of tight
junctions between endothelial cells, these results led to a focus
on VE-cadherin in the subsequent experiments.
Overexpression of VE-cadherin reverses
the effect of Ang II on the expression of ZO-1 in endothelial
cells
To further verify the role of VE-cadherin in the Ang
II-induced inhibited expression of ZO-1 in endothelial cells,
pcDNA3.1+HA-VE-cadherin was transfected into the endothelial cells
to induce the overexpression of VE-cadherin. Fluorescence analysis
showed that the plasmid had been successfully transfected into the
endothelial cells (Fig. 3A). The
results of the RT-qPCR analysis revealed that the mRNA levels of
VE-cadherin were significantly higher in the cells transfected with
pcDNA3.1+HA-VE-cadherin, compared with those in the cells
transfected with the empty vector (P<0.01; Fig. 3B).
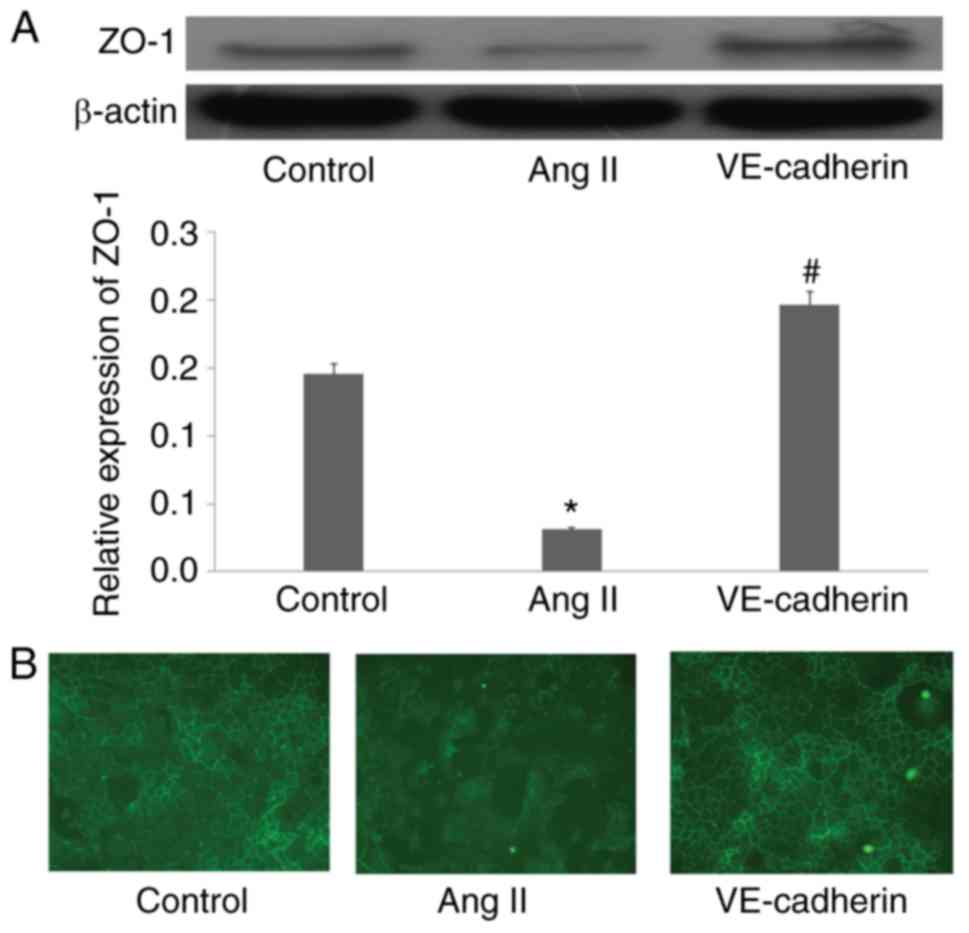
Following successful transfection of the
pcDNA3.1+HA-VE-cadherin or empty plasmid, the cells were incubated
with Ang II. In the group overexpressing VE-cadherin, VE-cadherin
not only suppressed the Ang II-induced inhibition of ZO-1
(P<0.01; Fig. 4A) but also
reversed the Ang II-induced disordered distribution of ZO-1 around
the cells (P<0.01; Fig. 4B).
These results suggested that Ang II suppressed the expression of
ZO-1 in the endothelial cells by downregulating VE-cadherin.
Discussion
The effect of Ang II on the function of vascular
endothelial cells has attracted increasing attention from
cardiologists. Previous studies have shown that Ang II can promote
cellular functions by stimulating reactive oxygen species
production, promoting thrombosis (14), inhibiting nitric oxide production,
and promoting vascular endothelial cell apoptosis (15). Our previous studies also showed
that Ang II can affect the normal function of endothelial cells by
inhibiting nitric oxide synthase or inducing inflammatory reactions
in cells (12,16). Studies have also revealed another
mechanism by which Ang II is involved in the development of various
cardiovascular diseases, wherein it acts by disrupting capillary
permeability and the tight junctions between endothelial cells
(4,17). However, few reports have been
published on this topic.
Tight junctions form a continuous apicolateral
paracellular barrier between epithelial cells, and this barrier
enables the selective and regulated movement of solutes between
apical and basolateral compartments. Its disturbance can eventually
result in several cardiovascular diseases (18,19).
To date, numerous proteins involved in the formation of tight
junctions, including the transmembrane claudin family of proteins,
occludin, and the ZO scaffolding family of proteins, have been
identified (20,21). ZO proteins consist of three
isoforms, ZO-1, ZO-2 and ZO-3, all of which form a heteromeric
complex. All the ZO proteins contain the fusion protein (PDZ), SRC
homology 3 domain and glucose kinase domains, through which the
occludin, claudin and actin are linked (22). Therefore, these structures connect
the tight junction with the cytoskeleton to finally form a stable
ligation system between cells. ZO-1 is an important marker of tight
junction integrity, which is disrupted in several intestinal
diseases and invasive cancer types (23); ZO-1 has also been shown to be
downregulated in poorly differentiated invasive breast cancer cell
lines (24). Studies have also
shown that absence of ZO-1 protein prevents the formation of tight
junctions between cells (25). In
addition, Su et al (7)
demonstrated that the downregulation of ZO-1 protein reduced the
number of tight junctions, eventually resulting in dysfunction of
the blood-testosterone barrier. Furthermore, Guo et al
(26) reported that via the
miR-181d-5p-mediated downregulation of ZO-1, the long non-coding
RNA, nuclear paraspeckle assembly transcript 1, regulated the
permeability of the blood-tumor barrier. Wei et al (27) also found that it was possible for
the function of the intestinal barrier to be regulated by
modulating the expression of ZO-1 through the protein kinase
Cε-dependent pathway. In the present study, it was found that Ang
II reduced the protein expression of ZO-1 in endothelial cells and
also caused a disturbance in the distribution of ZO-1 protein,
which is typically distributed around the cell membrane. This
result suggested that Ang II inhibited the formation of tight
junctions between endothelial cells, which may be the mechanism by
which Ang II decreases capillary permeability, eventually resulting
in the development of cardiovascular diseases.
The cadherin family is a group of calcium-dependent
type I transmembrane glycoproteins that mediate islet cell
adhesion, and are involved in the formation and maintenance of
normal cell-cell connections and polarity, differentiation of stem
cells, and invasion and metastasis of tumor cells (28). VE-cadherin, which is specific to
endothelial cells, belongs to the class of classical cadherins, is
mainly distributed in endothelial cells and mediates endothelial
cell-cell connections (29).
Several studies have shown that VE-cadherin not only mediates
intercellular junctions, but also transduces signals between cells
(30–32). Taddei et al (33) reported that the
VE-cadherin-mediated upregulation of claudin-5 increased the
formation of tight junctions. In addition, studies have shown that
VE-cadherin and vascular endothelial growth factor receptor 2
eventually affected endothelial cell plasticity in the course of
angiogenesis (34,35). VE-cadherin can also stabilize
cell-cell contacts and organize the endothelial barrier through an
original outside-in signaling mechanism involving calcium signaling
and microtubule dynamics (36). In
the present study, it was found that Ang II inhibited the
expression of VE-cadherin in endothelial cells and downregulated
the expression of ZO-1. Therefore, considering the
signal-transducing role of VE-cadherin, it was hypothesized that
Ang II inhibited the expression of ZO-1 by downregulating
VE-cadherin. To further validate this hypothesis, a
VE-cadherin-overexpression plasmid was constructed, and it was
found that Ang II did not inhibit the protein expression of ZO-1 in
endothelial cells transfected with the VE-cadherin-overexpression
plasmid. This result suggested that VE-cadherin was involved in the
Ang II-induced downregulation of ZO-1.
The present study had a number of limitations. The
effect of the overexpression of VE-cadherin was examined, however,
no knockdown of VE-cadherin was performed to confirm the role of
VE-cadherin in the Ang II-reduced expression of ZO-1. In addition,
the experiments were performed in cells only, with no experiments
performed in animals. These experiments are to be included in
future investigations.
In conclusion, the present study found that Ang II
reduced the expression of ZO-1 and caused a disturbance in the
distribution of ZO-1 in endothelial cells. It was then showed that
Ang II decreased the expression of VE-cadherin and that the
overexpression of VE-cadherin reversed the inhibitory effect of Ang
II on ZO-1. Taken together, these results suggested that Ang II
inhibited the protein expression of ZO-1 in vascular endothelial
cells by downregulating the expression of VE-cadherin. This may be
the molecular mechanism by which Ang II decreases the formation of
tight junctions between cells, eventually resulting in the
development of cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by the Plan Project of
Zhejiang Province Department of Health (grant no. 2016RCA027).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL, PZ, HL, JC and FP performed the cell
experiments. LM performed the statistical analysis. HG designed the
study.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Shaoxing People's Hospital (grant no. 2016RCA027).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bois Du P, Tortola Pablo C, Lodka D, Kny
M, Schmidt F, Song K, Schmidt S, Bassel-Duby R, Olson EN and
Fielitz J: Angiotensin II induces skeletal muscle atrophy by
activating TFEB-mediated MuRF1 expression. Circ Res. 117:424–436.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwai M, Chen R, Li Z, Shiuchi T, Suzuki J,
Ide A, Tsuda M, Okumura M, Min LJ, Mogi M and Horiuchi M: Deletion
of angiotensin II type 2 receptor exaggerated atherosclerosis in
apolipoprotein E-null mice. Circulation. 112:1636–1643. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones A, Deb R, Torsney E, Howe F, Dunkley
M, Gnaneswaran Y, Gaze D, Nasr H, Loftus IM, Thompson MM and
Cockerill GW: Rosiglitazone reduces the development and rupture of
experimental aortic aneurysms. Circulation. 119:3125–3146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi Z, Li Z, Hao D, Wang T, Xia Y, Sun T,
Wang J, Zhuang F and Wang X: Association between angiopoietin-2 and
enterovirus 71 induced pulmonary edema. Indian J Pediatr.
83:391–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Z, Liu H, Ren W, Dai F, Chang J and Li
B: VE-cadherin involved in the pulmonary microvascular endothelial
cell barrier injury induced by angiotensin II through modulating
the cellular apoptosis and skeletal rearrangement. Am J Transl Res.
8:4310–4319. 2016.PubMed/NCBI
|
|
6
|
Yamamoto T, Harada N, Kano K, Taya S,
Canaani E, Matsuura Y, Mizoguchi A, Ide C and Kaibuchi K: The Ras
target AF-6 interacts with ZO-1 and serves as a peripheral
component of tight junctions in epithelial cells. J Cell Biol.
139:785–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su L, Mruk DD, Lui WY, Lee WM and Cheng
CY: P-glycoprotein regulates blood-testis barrier dynamics via its
effects on the occludin/zonula occludens 1 (ZO-1) protein complex
mediated by focal adhesion kinase (FAK). Proc Natl Acad Sci USA.
108:19623–19628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brinkmann BF, Steinbacher T, Hartmann C,
Kummer D, Pajonczyk D, Mirzapourshafiyi F, Nakayama M, Weide T,
Gerke V and Ebnet K: VE-cadherin interacts with cell polarity
protein Pals1 to regulate vascular lumen formation. Mol Biol Cell.
27:2811–2821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iden S, Rehder D, August B, Suzuki A,
Wolburg-Buchholz K, Wolburg H, Ohno S, Behrens J, Vestweber D and
Ebnet K: A distinct PAR complex associates physically with
VE-cadherin in vertebrate endothelial cells. EMBO Rep. 7:1239–1246.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Xi Y, Mu X, Zhao R, Chen H, Zhang
L, Wu Y and Li Q: Antitumor immunity induced by VE-cadherin
modified DC vaccine. Oncotarget. 8:67369–67379. 2017.PubMed/NCBI
|
|
11
|
Li S, Ai N, Shen M, Dang Y, Chong CM, Pan
P, Kwan YW, Chan SW, Leung GPH, Hoi MPM, et al: Discovery of a ROCK
inhibitor, FPND, which prevents cerebral hemorrhage through
maintaining vascular integrity by interference with VE-cadherin.
Cell Death Discov. 3:170512017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji Z, Zhao F, Meng LP, Zhou CZ, Tang WL,
Xu FK, Liu LB, Lv HT, Chi JF, Peng F and Guo HY: Chinese yellow
wine inhibits production of matrixmetalloproteinase-2 induced by
homocysteine in rat vascular endothelial cells. Int J Clin Exp Med.
9:838–852. 2016.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mogielnicki A, Chabielska E, Pawlak R,
Szemraj J and Buczko W: Angiotensin II enhances thrombosis
development in renovascular hypertensive rats. Thromb Haemost.
93:1069–1076. 2005.PubMed/NCBI
|
|
15
|
Du J, Leng J, Zhang L, Bai G, Yang D, Lin
H and Qin J: Angiotensin II-induced apoptosis of human umbilical
vein endothelial cells was inhibited by blueberry anthocyanin
through bax- and Caspase 3-dependent pathways. Med Sci Monit.
22:3223–3228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao F, Ji Z, Chi J, Tang W, Zhai X, Meng
L and Guo H: Effects of Chinese yellow wine on nitric oxide
synthase and intercellular adhesion molecule-1 expressions in rat
vascular endothelial cells. Acta Cardiol. 71:27–34. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gurnik S, Devraj K, Macas J, Yamaji M,
Starke J, Scholz A, Sommer K, Di Tacchio M, Vutukuri R, Beck H, et
al: Angiopoietin-2-induced blood-brain barrier compromise and
increased stroke size are rescued by VE-PTP-dependent restoration
of Tie2 signaling. Acta Neuropathol. 131:753–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu N, Wang Z, Chen Y, Yang J, Lu X, Guo Y,
Chen Z and Xu Z: The ameliorative effect of bloodletting puncture
at hand twelve Jing-well points on cerebral edema induced by
permanent middle cerebral ischemia via protecting the tight
junctions of the blood-brain barrier. BMC Complement Altern Med.
17:4702017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang C, Liu H, Wei C, Chang L, Liang J,
Bei H, Li H, Liu S and Wu Y: Tongxinluo regulates expression of
tight junction proteins and alleviates endothelial cell monolayer
hyperpermeability via ERK-1/2 signaling pathway in oxidized
low-density lipoprotein-induced human umbilical vein endothelial
cells. Evid Based Complement Altern Med. 2017:41984862017.
View Article : Google Scholar
|
|
20
|
Furuse M, Fujita K, Hiiragi T, Fujimoto K
and Tsukita S: Claudin-1 and −2: Novel integral membrane proteins
localizing at tight junctions with no sequence similarity to
occludin. J Cell Biol. 141:1539–1550. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stevenson BR, Siliciano JD, Mooseker MS
and Goodenough DA: Identification of ZO-1: A high molecular weight
polypeptide associated with the tight junction (zonula occludens)
in a variety of epithelia. J Cell Biol. 103:755–766. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fredriksson-Lidman K, Van Itallie CM,
Tietgens AJ and Anderson JM: Sorbin and SH3 domain-containing
protein 2 (SORBS2) is a component of the acto-myosin ring at the
apical junctional complex in epithelial cells. PLoS One.
12:e01854482017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruan YC, Wang Y, Da Silva N, Kim B, Diao
RY, Hill E, Brown D, Chan HC and Breton S: CFTR interacts with ZO-1
to regulate tight junction assembly and epithelial differentiation
through the ZONAB pathway. J Cell Sci. 127:4396–4408. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sommers CL, Byers SW, Thompson EW, Torri
JA and Gelmann EP: Differentiation state and invasiveness of human
breast cancer cell lines. Breast Cancer Res Treat. 31:325–335.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li N, Lee WM and Cheng CY: Overexpression
of plastin 3 in Sertoli cells disrupts actin microfilament bundle
homeostasis and perturbs the tight junction barrier.
Spermatogenesis. 6:e12063532016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo J, Cai H, Zheng J, Liu X, Liu Y, Ma J,
Que Z, Gong W, Gao Y, Tao W and Xue Y: Long non-coding RNA NEAT1
regulates permeability of the blood-tumor barrier via
miR-181d-5p-mediated expression changes in ZO-1, occludin, and
claudin-5. Biochim Biophys Acta. 1863:2240–2254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei SC, Yang-Yen HF, Tsao PN, Weng MT,
Tung CC, Yu LCH, Lai LC, Hsiao JH, Chuang EY, Shun CT, et al:
SHANK3 regulates intestinal barrier function through modulating
ZO-1 expression through the PKCε-dependent pathway. Inflamm Bowel
Dis. 23:1730–1740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Priest AV, Shafraz O and Sivasankar S:
Biophysical basis of cadherin mediated cell-cell adhesion. Exp Cell
Research. 358:10–13. 2017. View Article : Google Scholar
|
|
29
|
Delgado-Bellido D, Serrano-Saenz S,
Fernández-Cortés M and Oliver FJ: Vasculogenic mimicry signaling
revisited: Focus on non-vascular VE-cadherin. Mol Cancer.
16:652017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang R and Ge J: Proteinase-activated
receptor-2 modulates Ve-cadherin expression to affect human
vascular endothelial barrier function. J Cell Biochem.
118:4587–4593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sauteur L, Affolter M and Belting HG:
Distinct and redundant functions of Esama and VE-cadherin during
vascular morphogenesis. Development. 144:1554–1565. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu W, Lv C, Zhang B, Zhou Q and Cao Z:
MicroRNA-27b functions as a new inhibitor of ovarian
cancer-mediated vasculogenic mimicry through suppression of
VE-cadherin expression. RNA. 23:1019–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taddei A, Giampietro C, Conti A, Orsenigo
F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S and
Dejana E: Endothelial adherens junctions control tight junctions by
VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol.
10:923–934. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lampugnani MG, Orsenigo F, Gagliani MC,
Tacchetti C and Dejana E: Vascular endothelial cadherin controls
VEGFR-2 internalization and signaling from intracellular
compartments. J Cell Biol. 174:593–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gaengel K, Niaudet C, Hagikura K, Laviña
B, Muhl L, Hofmann JJ, Ebarasi L, Nyström S, Rymo S, Chen LL, et
al: The sphingosine-1-phosphate receptor S1PR1 restricts sprouting
angiogenesis by regulating the interplay between VE-cadherin and
VEGFR2. Dev Cell. 23:587–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Komarova YA, Huang F, Geyer M, Daneshjou
N, Garcia A, Idalino L, Kreutz B, Mehta D and Malik AB: VE-cadherin
signaling induces EB3 phosphorylation to suppress microtubule
growth and assemble adherens junctions. Mol Cell. 48:914–925. 2012.
View Article : Google Scholar : PubMed/NCBI
|