Introduction
Rheumatoid arthritis (RA) is primarily characterized
by inflammatory synovitis, with subsequent destruction of articular
cartilage and bone, joint swelling and space narrowing, and joint
stiffness, deformity and dysfunction (1,2). RA
is an autoimmune disease, and the pathological features mostly
affect multiple small symmetrical joints of the hands and feet
(1). The current therapeutic
strategies available for RA include nonsteroidal anti-inflammatory
drugs (NSAIDs), disease modifying anti-rheumatic drugs,
glucocorticoids and surgery (3).
Sinomenine is a bioactive alkaloid, which is extracted from the
stems and roots of the Chinese medicine Sinomenium acutum.
Sinomenine exerts various pharmacological activities, including
anti-arthritic, immunosuppressive, neuroprotective,
anti-inflammatory and anticancer effects (4–6).
Sinomenine is widely used as a traditional Chinese medicine in the
treatment of RA, as it exerts a biological effect on
immunoregulation, anti-inflammation and bone protection (7,8). In
addition, sinomenine is effective in ameliorating morning joint
stiffness and painful joints, and exhibits fewer side effects on
the digestive system compared with NSAIDs (9). Since sinomenine exerts
immunosuppressive and anti-inflammatory effects, it is widely used
to treat RA in Chinese clinical practice (10).
The proteome is an entire set of proteins encoded by
the full genome produced by an organism or system. In addition, the
proteome is the subject to sorting N-terminal peptides, which may
be identified and quantified by mass spectrometrometry (MS)
(11–13). In recent years, proteome analysis
has been employed to enhance understanding of malignant tumors and
malaria (14,15). However, the association between RA
and the serum proteome requires further analysis. The mechanisms
underlying the development of RA are complex and are not fully
understood. Few studies have focused on the association between RA
and the serum proteome (16–18);
therefore, the present study performed proteomic analysis to detect
the anti-RA mechanisms of sinomenine.
The pathological features of collagen-induced
arthritis (CIA) in rats are consistent with typical pathological
alterations in patients with RA; therefore, CIA is the most widely
studied CIA model in preclinical studies (19). The present study demonstrated that
sinomenine exerted anti-inflammatory effects, and alleviated the
hyperplasia of fibrous tissue to exert an anti-arthritic effect. In
addition, the present study investigated serum proteome profiles in
the blank control group, model group, test group and positive
control group, in order to generate a differentially expressed
protein network map and evaluate the effectiveness of sinomenine in
RA via multitarget methods.
Materials and methods
Animals
A total of 60 Sprague-Dawley female rats (aged 6–8
weeks old; 190–200 g) were purchased from the Laboratory Animal
Research Center of Nanjing University of Chinese Medicine (Nanjing,
China). All rats were housed at 26°C under pathogen-free conditions
with a 12 h light/dark cycle and a 60% humidity, and fed with
standard rat chow and water ad libitum. All experiments were
conducted in compliance with the guidelines for the Care and Use of
Laboratory Animals (20), and the
present study was approved by the Institutional Animal Care and Use
Committee of Nanjing University of Chinese Medicine. Rats were
divided into six groups (10 rats per group), five of which
underwent CIA.
Assessment of CIA in rats
The CIA model was established according to a
previously described protocol (19). Briefly, 8 mg type II collagen (CII)
(Chondrex, Redmond, WA, USA) was dissolved in 0.1 mol/l acetic acid
and vortexed at 4°C; the concentration of CII reached 2 mg/ml. A
total of 100 µg CII was emulsified thoroughly with the same volume
of complete Freund's adjuvant (Chondrex) in an ice bath; the final
concentration of CII reached 1 mg/ml. A total of 50 rats were
injected subcutaneously at the tail base with 200 µl CII emulsion
for the first immunization. After 7 days, 100 µg CII was dissolved
and emulsified at the same concentration using incomplete Freund's
adjuvant (Chondrex), and 100 µl emulsion was subcutaneously
administered into the tail as a booster injection. Clinical
arthritis was measured and the Arthritis Index (AI) was analyzed,
as presented in Table I. The AI
for each rat was expressed as the sum of the scores for all four
limbs; therefore, the maximum AI was 16.
 | Table I.Scoring system for the evaluation of
arthritis severity. |
Table I.
Scoring system for the evaluation of
arthritis severity.
| Severity score | Degree of
inflammation in the joints |
|---|
| 0 | No evidence of
erythema and swelling |
| 1 | Erythema and mild
swelling confined to the tarsals or ankle joint |
| 2 | Erythema and mild
swelling extending from the ankle to the tarsals |
| 3 | Erythema and moderate
swelling extending from the ankle to metatarsal joints |
| 4 | Erythema and severe
swelling encompass the ankle, foot and digits, or ankylosis of the
limb |
In vivo drug administration
CIA rats were randomly separated into the model
control group, low dose group (sinomenine 30 mg/kg/day), middle
dose group (sinomenine 60 mg/kg/day), high dose group (sinomenine
120 mg/kg/day) and positive control group (methotrexate 0.5
mg/kg/day). Sinomenine (cat no. Z20010174; Hunan Zhengqing
Pharmaceutical, Hunan, China) was dissolved in normal saline at
various concentrations and administered every day. Methotrexate
(cat no. H31020644; Shanghai SINE Pharmaceutical Co., Ltd.,
Shanghai, China) was dissolved and 0.5 mg/kg/day methotrexate was
administered every 3 days by gavage. The model group and blank
control group were administered saline (1 ml/100 g) by gavage. The
drug was administered continuously for 28 days.
Rheumatoid serum biochemical
measurements
After treatment, rats were anesthetized with 5%
isoflurane, and blood samples (3 ml) were obtained from the
abdominal aorta, after which the rats were sacrificed. The blood
samples were centrifuged at 3,000 × g for 10 min at 4°C. Serum was
isolated for measurement of rheumatoid factor (RF) and C-reactive
protein (CRP), according to the manufacturer's protocols (Abcam,
Cambridge, UK; cat nos. ab178653 and ab108827).
Measurement of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST)
AST assay kit (GOT kit; cat no. C010-2) and ALT
assay kit (GPT kit; cat no. C009-2) were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). ALT and AST
activity were measured after 6 weeks according to the
manufacturer's protocols.
Histological scoring
Rats were euthanized using CO2
(displacement rate of the chamber volume/min, 10%), and the paw and
knee joints of rats were fixed in 10% paraformaldehyde for 1 h at
room temperature, decalcified in EDTA, embedded in paraffin and
then sectioned (size, 4 µm). Tissue sections were mounted on slides
for staining with hematoxylin and eosin for 1 h at room
temperature. All sections were randomized and evaluated by two
trained observers who were blinded to the treatment groups and the
arthritis severity of each rat. The data were expressed as mean
inflammation score and all scores were based on a scale of 0–5
(Table II). For each section, the
number of positively stained cells was counted in 20 fields using a
phase contrast microscope (magnification, ×200).
 | Table II.Scoring system for the evaluation of
histology. |
Table II.
Scoring system for the evaluation of
histology.
| Score | Degree of
histological scoring |
|---|
| 0 | No staining |
| 1 | Few of the cells were
positively stained |
| 2 | Some (<50%) of
the cells were stained |
| 3 | ~50% of the cells
were stained |
| 4 | >50% of the
cells were stained |
| 5 | All cells
stained |
Serum sample processing
After treatment, rats were anesthetized with 5%
isoflurane, and blood samples were obtained from the abdominal
aorta and transferred to a 1.5 ml Eppendorf protein tube
(Eppendorf, Hamburg, Germany). The blood samples were briefly
vortexed and incubated at 4°C for 6 h to precipitate serum
proteins. Subsequently, the samples were centrifuged at 1,000 × g
for 10 min at 4°C. The collected supernatants were then centrifuged
at 16,000 × g for 10 min at 4°C to remove lipids and the clearest
serum was collected and centrifuged 4,000 × g at 4°C for 10 min to
remove any remaining cells. The extracted serum was transferred to
a 200 µl Eppendorf tube and stored at −20°C.
Protein extraction
A total of 60 µl elution buffer [7 M urea, 1% (w/v)
CHAPS] was added to 10 µl serum (n=6/group). Subsequently,
dithiothreitol was added to reach a final concentration of 10 mM.
The samples were boiled at 56°C in water for 1 h, followed by the
addition of PBS to achieve a concentration of 55 mM, and were
incubated for 1 h in the dark at room temperature. The addition of
iced pure acetone resulted in the formation of precipitate,
followed by centrifugation at 4,000 × g at 4°C for 10 min, and
removal of the supernatant. The precipitate was dissolved in 300 µl
physiological saline in a vortex tube for 3 min. The proteins were
quantified using a Bradford assay.
Liquid chromatography and MS
Serum proteome was analyzed using LTQ-Orbitrap-Veces
iFunnel (Thermo Fisher Scientific, Inc., Waltham, MA, USA) equipped
with a reversed-phase capillary column and interfaced with the
nanoflow LC system (1,100; Agilent Technologies, Inc., Santa Clara,
CA, USA). The peptides (500 ml) were enriched on the C18 enrichment
column and separated on a 75 µm ×43 mm analytical/separation column
in the protein chip (Agilent HPLC-Chip: G4240-62001ZORBAX
300SB-C18; Agilent Technologies, Inc.) using a gradient mobile
phase consisting of two different solvents, 0.1% formic acid
solution (solvent A) and 90% acetonitrile (solvent B), at a flow
rate of 200 nl/min. The following gradient method was used for the
separation of peptides on the chip over a period of 60 min: From
0–60%. Nitrogen gas was maintained at 120°C with a 9 l/min flow
rate and a nebulizer pressure of 207 kPa. Positive ions were
generated via electrospray and MRM transitions were assessed using
350–1,750 m/z. The MS/MS data were further analyzed using MaxQuant
(version 1.2.2.5; http://www.maxquant.org). MaxQuant is designed as a
three-tiered application for the analysis of data, application
logic and presentation. The spectra data were determined and
subsequently searched using the UniProt database (http://www.uniprot.org/). Pathway analysis was
performed with Ingenuity® Pathway Analysis (IPA)
software version 1 (Ingenuity Systems; Qiagen, Inc., Valencia, CA,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
of at least three independent experiments. Statistical analysis was
performed using SPSS version 16.0 software (SPSS, Inc., Chicago,
IL, USA). A Student's t-test was used to compare the discrepancy
between two groups. One-way analysis of variance followed by
Duncan's test was used to determine the difference between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sinomenine improves clinical arthritic
conditions in CIA rats
The present study investigated the in vivo
efficacy of sinomenine in CIA rats. The CIA model was elicited in a
genetically susceptible rat strain by immunization with CII
emulsified in complete Freund's adjuvant. The body weights of the
rats were monitored weekly, and the results demonstrated that
beginning from day 21, CIA rats gained less weight compared with in
the blank control group. Treatment with sinomenine (week 5, high
dose) significantly reversed weight loss caused by RA compared with
in the model group (Table
III).
 | Table III.Body weights (g) of rats with
collagen-induced arthritis. |
Table III.
Body weights (g) of rats with
collagen-induced arthritis.
| Group | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|
| Blank control | 244.0±15.4 | 285.3±16.3 | 324.3±22.9 | 358.5±30.2 | 380.5±30.7 |
| Model | 244.8±23.6 | 263.4±30.2 |
286.7±31.9a |
312.0±31.7a |
300.3±29.5b |
| Positive
control | 246.0±24.2 | 276.4±39.7 | 299.8±41.2 | 318.2±44.0 |
313.2±41.9b |
| High dose | 240.4±27.6 | 267.7±32.7 | 293.8±30.8 |
331.9±36.7a,d |
319.6±33.2b,c |
| Middle dose | 234.1±26.2 |
251.0±34.0a |
278.8±36.5a |
309.1±42.5a |
308.6±40.8b |
| Low dose | 246.8±27.2 | 260.1±39.8 |
281.8±36.8a |
310.4±34.4a |
313.8±32.1b |
Clinical score was used to measure the progression
of arthritis development. The model group developed severe
swelling, erythema and joint rigidity of the hind paws after 6
weeks (data not shown). Conversely, the experimental and positive
control groups exhibited showed a lower AI (P<0.05) compared
with in the model group. In addition, in the high dose sinomenine
group, the AI was significantly attenuated compared with in the
model group (P<0.01; Table
IV). These results indicated that sinomenine and methotrexate
may improve clinical arthritic conditions in CIA rats, and high
dose sinomenine treatment exerted the optimal efficacy in CIA
rats.
 | Table IV.Arthritis index of rats with
collagen-induced arthritis at week 6. |
Table IV.
Arthritis index of rats with
collagen-induced arthritis at week 6.
| Group | Arthritis
index |
|---|
| Model | 13.5±0.9 |
| Positive
control |
8.1±2.5a |
| High dose |
6.4±2.3b |
| Middle dose |
10.5±2.0a |
| Low dose |
10.5±1.8a |
Sinomenine improves histological
parameters in CIA rats
The histology of tissues from CIA rats was analyzed,
in order to determine whether sinomenine prevented articular
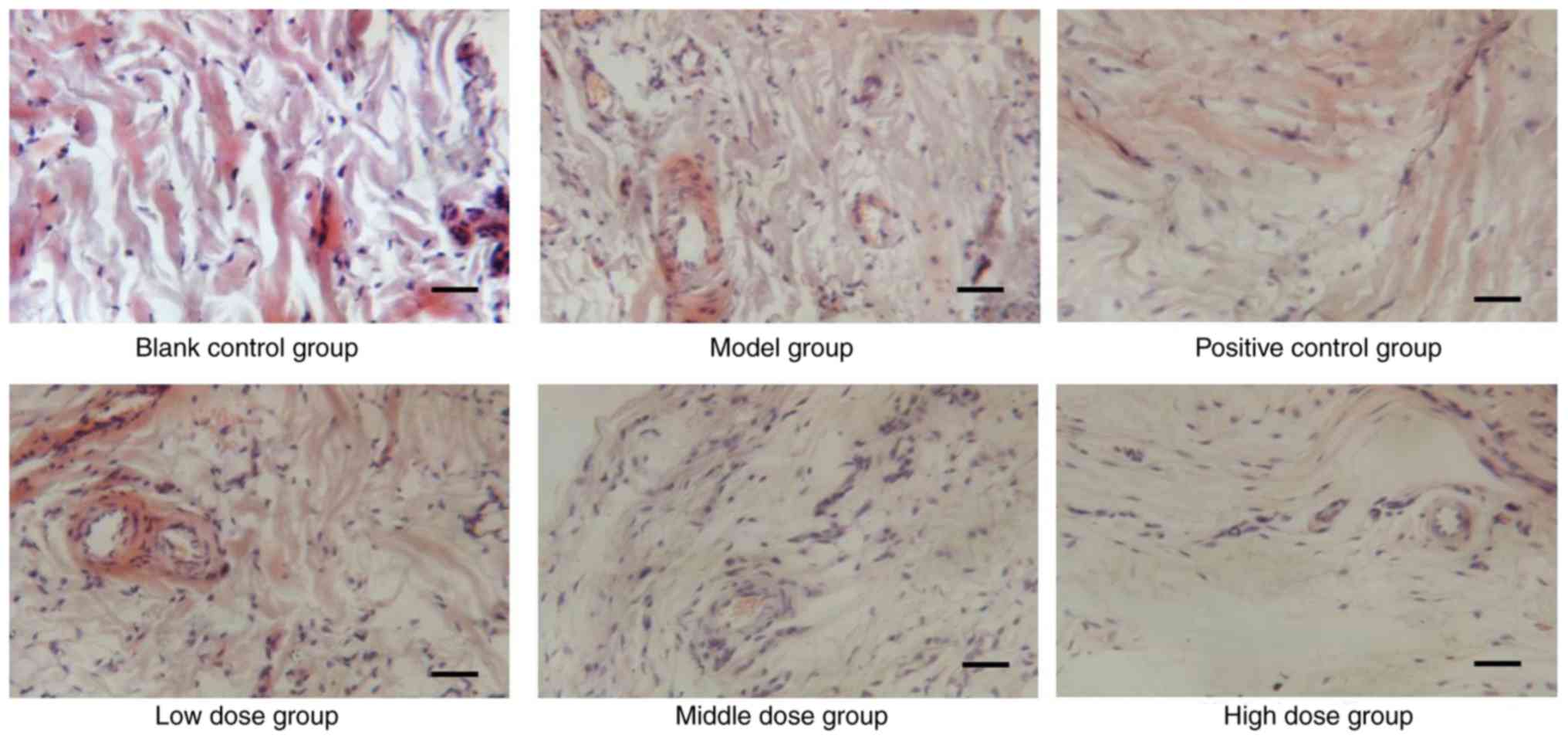
destruction of talocrural joints. As shown in Fig. 1, infiltration of inflammatory
cells, pannus invasion, cartilage damage and subchondral bone
erosion were detected in CIA rats (Fig. 1). Histological scores of the
individual groups are shown in Table
V. The histological score of the model group was significantly
higher than in the blank control group rats (P<0.01), and was
reduced in the experimental groups, which indicated that sinomenine
improved joint histological conditions, synovial swelling,
congestion and hyperplasia in CIA rats (Table V). The joints of CIA rats treated
with a high dose of sinomenine demonstrated less inflammatory cell
infiltration and synovial hyperplasia than the positive control
group. These results suggested that sinomenine improved the
histological parameters in CIA rats.
 | Table V.Histological score of joints in
rats. |
Table V.
Histological score of joints in
rats.
| Group | Number | Score |
|---|
| Blank control | 8 | 1.00±0.00 |
| Model | 8 |
5.50±1.41a |
| Positive
control | 8 |
4.00±1.07b |
| Low dose | 8 | 4.25±1.04 |
| Middle dose | 8 |
4.00±1.07b |
| High dose | 8 |
3.75±1.04b |
Sinomenine attenuates the inflammatory
response in the joints of CIA rats
The present study next investigated the mechanisms
underlying the decreased occurrence and severity of CIA following
sinomenine treatment. The results of RF detection detected an
apparent discrepancy between sinomenine-treated groups and the
model group (P<0.05). Notably, high dose sinomenine exhibited an
improved anti-arthritic effect compared with low dose sinometine
(Table VI).
 | Table VI.Expression levels of rheumatoid
factor in serum at week 6. |
Table VI.
Expression levels of rheumatoid
factor in serum at week 6.
| Group | Rheumatoid factor
(IU/ml) |
|---|
| Blank control |
5,007.8±3,168.2 |
| Model |
9,176.5±3,757.2a |
| Positive
control |
5,048.2±3,132.2b |
| High dose |
4,578.0±3,489.5b |
| Middle dose |
4,750.8±4,764.3b |
| Low dose |
5,756.3±4,963.4c |
The expression levels of CRP in the positive control
group were significantly lower than in the model group (Table VII). Sinomenine treatment
attenuated the secretion of CRP in CIA rats compared with in the
model or positive control groups. These findings indicated that
sinomenine exhibited an improved anti-arthritic effect compared
with methotrexate.
 | Table VII.Expression levels of C-reactive
protein in serum at week 6. |
Table VII.
Expression levels of C-reactive
protein in serum at week 6.
| Group | C-reactive protein
(mg/ml) |
|---|
| Blank control | 261.0±50.2 |
| Model |
636.3±123.4a |
| Positive
control |
578.1±164.2a,b |
| High dose |
340.0±85.4b,c |
| Middle dose |
489.2±107.1d,e |
| Low dose | 679.9±21.2 |
The results revealed that the secretion of ALT and
AST was not significantly different between the sinomenine-treated
groups and the blank control group; whereas ALT was significantly
decreased in the high dose and positive control groups compared
with the model group. Furthermore, a marked decrease of ALT and AST
expression levels were observed in the high dose group compared
with the middle and low dose groups (Table VIII). ALT and AST are the major
markers of hepatic damage in the plasma. Taken together, the
results suggest that a high dose of sinomenine exerted significant
liver function improvement in CIA rats (Table VIII) compared with the model
group. Furthermore, the positive control group demonstrated a
significant decrease in leukocyte, erythrocyte and hemoglobin
levels compared with the blank control group. Bone marrow
suppression represents the decrease in the production of
leukocytes, erythrocytes and/or platelets (21). Therefore, it can be suggested that
methotrexate caused bone marrow suppression in CIA rats (Table IX). However, there were no
significant alterations between the sinomenine-treated groups and
the blank control group, which indicated that sinomenine did not
induce bone marrow suppression and liver damage. In addition, the
number of platelets in the low dose sinomenine group was
significantly increased compared with in the blank control group,
which indicated that low dose sinomenine treatment exerted a marked
increase in platelet production or release. Therefore, sinomenine
may exert an improved anti-arthritic effect, associated with no
liver damage and bone marrow inhibition compared with methotrexate,
which is associated with chronic liver damage (22). These results suggested that
sinomenine may be used in the clinical treatment of RA.
 | Table VIII.Expression levels of AST and ALT in
serum. |
Table VIII.
Expression levels of AST and ALT in
serum.
| Group | ALT (U/l) | AST (U/l) |
|---|
| Blank control | 7.99±3.38 | 10.84±4.08 |
| Model | 10.30±2.11 | 14.39±1.98 |
| Positive
control |
7.63±1.62a | 12.13±2.34 |
| High dose |
7.15±2.33a |
11.77±3.69a |
| Middle dose | 7.96±2.11 | 13.15±1.16 |
| Low dose | 8.51±3.69 | 13.13±5.48 |
 | Table IX.Blood analysis. |
Table IX.
Blood analysis.
| Group | Leukocytes | Erythrocytes | Hemoglobin | Platelets |
|---|
| Blank control | 6.57±1.54 | 7.64±0.71 | 151.38±9.40 | 1,031.8±107.3 |
| Model | 6.58±0.97 | 6.75±1.10 | 130.75±18.30 | 939.4±124.4 |
| Positive
control |
5.60±1.22a,b |
6.74±0.49a |
134.25±8.96a |
1,342.0±138.1a,b |
| High dose | 6.66±1.69 | 7.01±1.53 | 133.88±34.66 | 1,115.3±270.9 |
| Middle dose | 6.95±2.20 | 7.68±0.76 | 151.57±18.44 | 1,063.1±172.8 |
| Low dose | 6.93±1.75 | 7.57±0.53 | 139.29±19.90 |
1,308.1±197.1a |
Identification of differentially
expressed proteins between sinomenine-treated groups and model
group
To investigate the underlying mechanisms involved in
sinomenine-treated CIA rats, proteomic analysis was performed. The
data revealed that 320 differential proteins were expressed in the
sinomenine-treated groups compared with in the model group. There
were 79 differentially expressed proteins identified in the low
dose group, and among them, 36 proteins were upregulated. The top
12 up- and downregulated proteins were presented in Table X. In addition, five highly relevant
biological processes were identified from 16 relevant biological
processes by gene ontology enrichment analysis (13). These biological processes were cell
cycle (P-value 6.66×10−3-8.91×10−6; 14
associated genes); cell morphology (P-value,
6.43×10−3-2.82×10−4; 24 associated genes);
cellular function and maintenance (P-value,
6.43×10−3-4.52×10−4; 22 associated genes);
cellular assembly and organization (P-value,
6.43×10−3-7.78×10−4; 24 associated genes);
and post-translational modification (P-value,
3.22×10−3-7.78×10−4; 6 associated genes)
(Table XI). In addition,
biological processes were associated with statistically relevant
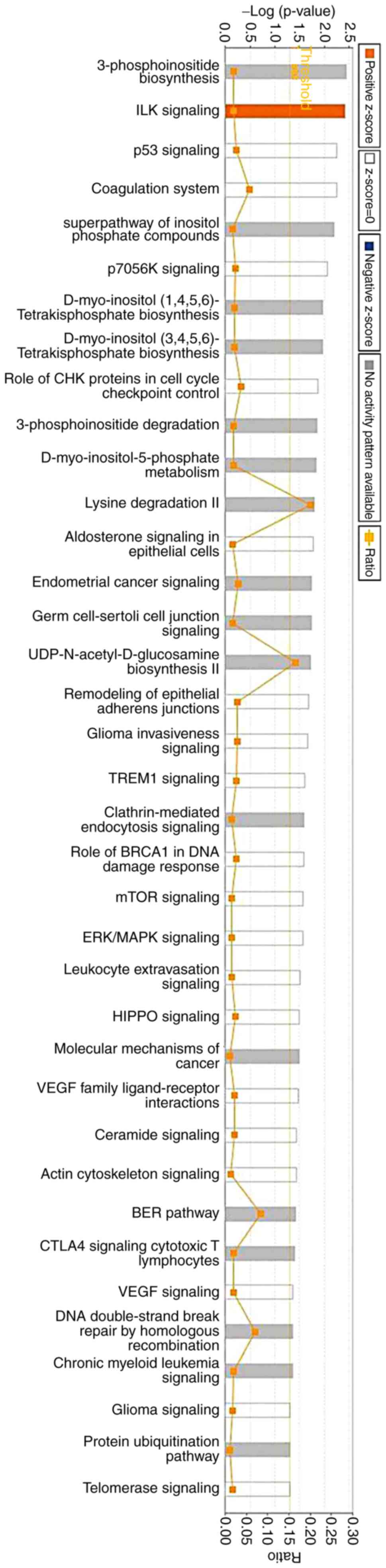
molecular pathways. IPA was used to investigate the involvement of
signaling pathways. The results indicated that 213 signaling
pathways were enriched, which were involved in the low dose
sinomenine-treated group compared with in the model group. The 37
most enriched signaling pathways are listed in Fig. 2.
 | Table X.Top 12 up- and downregulated
differentially expressed proteins between the sinomenine-treated
group and model group. |
Table X.
Top 12 up- and downregulated
differentially expressed proteins between the sinomenine-treated
group and model group.
| Gene name | Accession no. | Fold change |
|---|
| Amot | A0A067XG49 | −1.8538 |
| Phf20l1 | Q6P7V2 | −1.6297 |
| Myom1 | Q62234 | −1.5777 |
| Rbl1 | Q64701 | −1.5508 |
| Pla2g4d | Q14CI2 | −1.5336 |
| Slc4a3 | Q68EG4 | −1.5128 |
| Dcaf5 | Q80T85 | −1.4726 |
| Dido1 | Q8C9B9 | −1.4686 |
| Speer1 | J3QMX3 | −1.4320 |
| Hfm1 | F6XQ35 | −1.4088 |
| Trcg1 | Q58Y74 | −1.4086 |
| Tmem131 | O70472 | −1.3927 |
| Dnah7c | A0A087WR13 | 1.6626 |
| Dnah7a | E9Q0T8 | 1.6626 |
| Ptprf | A2A8L5 | 1.7042 |
| Ankrd27 | Q3UMR0-2 | 1.7052 |
| Fgfr2 | A1YYM7 | 1.8464 |
| Nup155 | Q6ZQ45 | 1.8588 |
| Ctnna1 | Q545R0 | 1.8978 |
| Tpr | Q8BK71 | 2.0978 |
| Rictor | Q6QI06-2 | 2.2287 |
| Dnajc8 | F6TQL3 | 2.2831 |
| Prokr2 | Q8K458 | 3.6995 |
| Antxr2 | Q6DFX2 | 6.0189 |
 | Table XI.Functional analysis between the
sinomenine-treated group and model group. |
Table XI.
Functional analysis between the
sinomenine-treated group and model group.
| Comparison | Name | P-value | Molecules |
|---|
| MVD (Model group
vs. Sinomenine-treated group) | Cell cycle |
6.66×10−3-8.91×10−6 | 14 |
|
| Cell
morphology |
6.43×10−3-2.82×10−4 | 24 |
|
| Cellular function
and maintenance |
6.43×10−3-4.52×10−4 | 22 |
|
| Cellular assembly
and organization |
6.43×10−3-7.78×10−4 | 24 |
|
| Post-translational
modification |
3.22×10−3-7.78×10−4 | 6 |
Identification of proteins involved in
the inflammation-associated pathway
The present study used IPA software to investigate
the association between differentially expressed proteins by
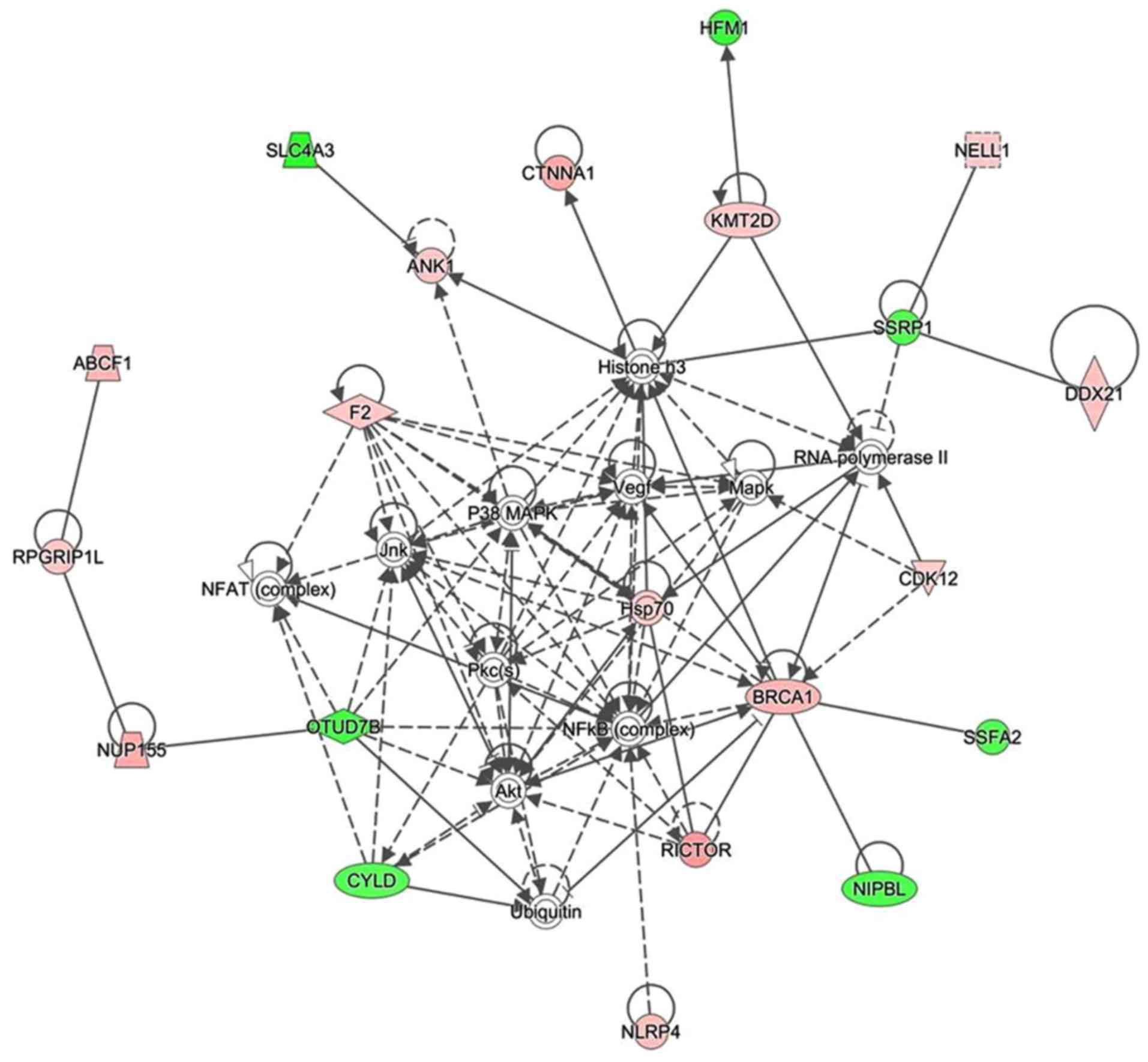
analyzing enrichment. A protein-protein interaction network was
constructed by calculating the score, which indicated that 33
proteins were associated with other proteins and led to 60 paired
relationships. These proteins were primarily involved in
immunomodulatory and inflammatory reactions. In addition, nuclear
factor (NF)-κB, histone H3, heat shock protein (Hsp)70 and protein
kinase B (Akt) were the main proteins to regulate the network
(Fig. 3). Among them, 14 proteins
were upregulated and 7 proteins were downregulated. For example, if
histone H3 was chosen, 14 paired relationships were connected
(Fig. 3).
Identification of upstream
regulators
To identify the key upstream regulators governing
molecular status following sinomenine treatment, an upstream
regulator analysis with IPA was applied. The top seven upstream
regulators that were predicted to be activated or inhibited in the
sinomenine treated group are presented in Table XII. E2F1, TCF7L2, NUPR1, TGFβ1
and lipopolysaccharide were most top upstream regulators predicted
to be induced following treatment with sinomenine; whereas CST5 and
AHR were the top upstream regulators predicted to be inhibited
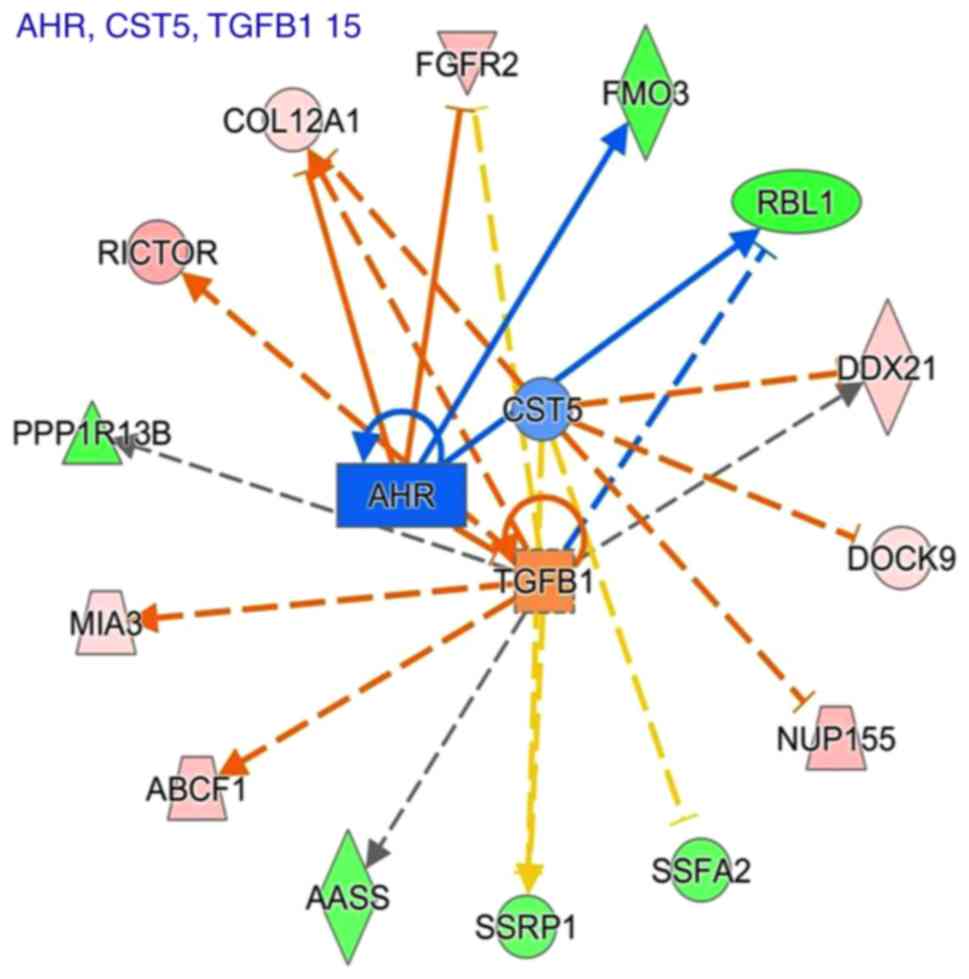
following treatment with sinomenine (Table XII). Fig. 4 showed a graphical representation
of the molecular networks that may exert important roles following
sinomenine treatment, which revealed that the most significant
upstream regulators were transforming growth factor (TGF)-β1, aryl
hydrocarbon receptor (AHR) and cystatin D (CST5).
 | Table XII.Upstream analysis. |
Table XII.
Upstream analysis.
| Upstream
regulator | Molecule type | Activation
z-score | P-value of
overlap | Target molecules in
dataset |
|---|
| CST5 | Other | −0.816 | 0.000331 | COL12A1, DDX21,
DOCK9, NUP155, SSFA2, SSRP1 |
| E2F3 | Transcription
regulator |
| 0.00154 | FGFR2, NCAPG2,
PPP1R13B, RBL1 |
| SP100 | Transcription
regulator |
| 0.00202 | BRCA1, HSPA8 |
| SMARCE1 | Transcription
regulator |
| 0.00223 | BRCA1, CYLD |
| E2F2 | Transcription
regulator |
| 0.0201 | FGFR2, RBL1 |
| E2F1 | Transcription
regulator | 0.077 | 0.0212 | BRCA1, FGFR2,
HSPA8, PPP1R13B, RBL1 |
| TCF7L2 | Transcription
regulator | 1 | 0.0225 | CTNNA1, DOCK9,
FGFR2, OTUD7B |
| Mmp | Group |
| 0.0232 | PLG |
| NUP107 | Transporter |
| 0.0232 | TPR |
| TMPO | Other |
| 0.0232 | COL12A1 |
| ZNF423 | Transcription
regulator |
| 0.0232 | BRCA1 |
| PGK1 | Kinase |
| 0.0232 | PLG |
| GW 5074 | Chemical-kinase
inhibitor |
| 0.0232 | FGFR2 |
| Boc-D-FMK | Chemical
reagent |
| 0.0232 | RBL1 |
| Rb | Group |
| 0.0243 | FGFR2, RBL1 |
| AHR | Ligand-dependent
nuclear Receptor | −1.982 | 0.0243 | COL12A1, FGFR2,
FMO3, RBL1 |
| TBX2 | Transcription
regulator |
| 0.0262 | NCAPG2, RBL1 |
| PHF8 | Enzyme |
| 0.0265 | RBL1 |
| S100A10 | Other |
| 0.0265 | PLG |
| LIMS1 | Other |
| 0.0265 | CTNNA1 |
| GRIP1 | Transcription
regulator |
| 0.0265 | FREM2 |
| ALCAM | Other |
| 0.0265 | AMOT |
| TNRC6A | Other |
| 0.0265 | RBL1 |
| NUPR1 | Transcription
regulator | 0.447 | 0.0271 | ANK1, BRCA1, DIDO1,
SHROOM3, SYNE2 |
|
N-Ac-Leu-Leu-norleucinal | Chemical-protease
inhibitor |
| 0.0289 | BRCA1, RBL1 |
| TIP60 | Complex |
| 0.0298 | RBL1 |
| SHOX | Transcription
regulator |
| 0.0298 | RBL1 |
| RBL2 | Other |
| 0.0436 | BRCA1, RBL1 |
| RRP1B | Other |
| 0.0444 | BRCA1, RBL1 |
| COL9A1 | Other |
| 0.0459 | COL12A1 |
| PHB | Transcription
regulator |
| 0.0459 | RBL1 |
|
Gamma-tocotrienol | Chemical drug |
| 0.0459 | OTUD7B |
| CTGF | Growth factor |
| 0.046 | ABCF1, MIA3 |
| TGFB1 | Growth factor | 0.956 | 0.0482 | AASS, ABCF1,
COL12A1, DDX21, FGFR2, MIA3, PPP1R13B, RBL1, RICTOR, SSRP1 |
| Dactolisib | Chemical drug |
| 0.0491 | RICTOR |
| TNFAIP2 | Other |
| 0.0491 | RBL1 |
| RGS1 | Other |
| 0.0491 | RBL1 |
| NDN | Transcription
regulator |
| 0.0491 | RBL1 |
|
Lipopolysaccharide | Chemical drug | 1.969 | 1 | COL12A1, F2, HSPA8,
PLG |
Discussion
To gain an insight into the mechanism underlying the
effects of sinomenine on RA, the present study used an animal
experimental arthritis model. Rats were administered with
sinomenine, methotrexate or vehicle. In the CIA study, the
protective effects of sinomenine against arthritis were confirmed,
as evidenced by the decreased incidence and severity of arthritis
following CII immunization. Sinomenine also exerted an
anti-inflammatory effect, as revealed by the suppression of CRP
expression. These results are consistent with those of previous
studies. Yang et al (23)
reported that sinomenine exerts protective effects against
lipopolysaccharide-induced inflammation in piglets. Additionally,
Xu et al (9) reported that
sinomenine and NSAID treatment regulates CRP and improved clinical
conditions of RA. Furthermore, in the present study, sinomenine did
not exert liver damage or bone marrow inhibition compared with in
the positive control group. To the best of our knowledge, no
previous studies have focused on proteomic analysis following
treatment of CIA rats with sinomenine. The current proteomic
analysis study revealed that NF-κB, histone, Hsp70 and Akt
interacted with other proteins, leading to 60 relationship pairs.
Taken together, these results indicated that the use of sinomenine
has the potential to treat RA, and the present study identified
pathways in the rats with CIA involved in the response to
sinomenine. These results provide information to suggest that
sinomenine may be used to treat RA.
In the present study, dual high-performance liquid
chromatography and MS were performed to identify the protein
profiles associated with sinomenine treatment in rats with CIA. A
total of 320 proteins were differentially expressed. In response to
treatment with low dose sinomenine, there were 79 differentially
expression proteins, among which 36 proteins were downregulated.
The differentially expressed proteins were involved in
tumorigenesis, developmental disorder, inflammatory, cell
morphology, lipid metabolism, cell cycle, amino acid metabolism,
gene expression and drug metabolism. The present study focused on
network analysis via IPA software to construct the protein network
in the low dose sinomenine treatment group. NF-κB, histone H3,
Hsp70 and Akt were involved in the enrichment networks as core
proteins, which was determined using Uniprot (http://www.uniprot.org/). In addition, the key
upstream regulators that governed molecular status following
sinomenine treatment were predicted; the most significant upstream
regulators were TGF-β1, AHR and CST5.
Akt is involved in the phosphoinositide 3-kinase
(PI3K)/Akt signaling pathway. Akt kinase activity is induced
following activation of PI3K in growth factor receptor-mediated
signaling cascades (24). Akt is
involved in tumor formation, and is also involved in higher brain
function, cell size matters, cell cycle regulation and metabolic
functions, and serves various roles in diseases and biological
functions (25). In the present
study, the proteomic analysis results revealed that enriched
proteins are associated with Akt expression, thus suggesting that
Akt may function as an inducer of these proteins. Therefore, the
present study provided evidence to suggest that Akt is a target of
the far-reaching physiological effects regulated by sinomenine in
RA, as in tumor and brain disease.
IPA upstream regulator analysis predicted that the
most significant upstream regulators associated with sinomenine
treatment are TGF-β1, AHR and CST5. Previously, Sugiura et
al (26) reported that TGF-β1
is highly expressed in joints in RA, and it is considered an
anti-inflammatory regulator in RA. AHR activation may induce the
production of inflammatory cytokines and RA synoviocytes (27). The present findings suggested that
TGF-β1 and AHR serve a key role in the anti-arthritic effects of
sinomenine. Therefore, it was hypothesized that sinomenine exerted
its anti-arthritic effects via inhibition of TGF-β1 and AHR. To the
best of our knowledge, there are no published studies investigating
the association between RA and the expression level of CST5. In the
future, the authors of the present study aim to study the
association between CST5 and RA. In the present study, Akt
expression enrichment was inhibited by sinomenine in CIA rats.
Therefore, it was hypothesized that TGF-β1 may mediate Akt activity
in RA, and both were downregulated by sinomenine. It has previously
been reported that TGF-β1 enhances Akt phosphorylation in MC3T3-E1
cells (28) and A549 cells
(29). The present data are
consistent with these studies; however, further investigations are
required to confirm this hypothesis.
The present study explored the anti-arthritic and
anti-inflammatory effects of sinomenine in vivo. To the best
of our knowledge, the present study is the first to perform a
proteomic analysis for analyzing the effects of sinomenine against
RA using a CIA rat model. The present study aimed to elucidate the
associated proteins involved in sinomenine-treated RA via proteomic
analysis. The results of the present study revealed that sinomenine
exerted anti-arthritic effects via numerous targets during CIA. In
addition, the proteomic analysis provided a novel approach and
evidence for exploring the other biological effects of sinomenine.
Therefore, the findings may provide an insight into the anti-RA
mechanisms of sinomenine and proteomic analysis may be used to
explore its functions in other relevant diseases.
Acknowledgements
The authors would like to thank Jinjin Shang for
proof reading the manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XQ, ZZ and HC designed the study and performed the
experiments. WS, ZX and BZ analyzed and interpreted the
experimental data, and drafted the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Nanjing University of Chinese
Medicine (Nanjing, China).
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no competing
interests.
References
|
1
|
Gu X, Gu B, Lv X, Yu Z, Wang R, Zhou X,
Qiao W, Mao Z, Zuo G, Li Q, et al: 1, 25-dihydroxy-vitamin D3 with
tumor necrosis factor-alpha protects against rheumatoid arthritis
by promoting p53 acetylation-mediated apoptosis via Sirt1 in
synoviocytes. Cell Death Dis. 7:e24232016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boissier MC, Semerano L, Challal S,
Saidenberg-Kermanac'h N and Falgarone G: Rheumatoid arthritis: From
autoimmunity to synovitis and joint destruction. J Autoimmun.
39:222–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burmester GR, Bijlsma JWJ, Cutolo M and
McInnes IB: Managing rheumatic and musculoskeletal diseases - past,
present and future. Nat Rev Rheumatol. 13:443–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Fang Y, Huang W, Zhou X, Wang M,
Zhong B and Peng D: Effect of sinomenine on cytokine expression of
macrophages and synoviocytes in adjuvant arthritis rats. J
Ethnopharmacol. 98:37–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian L, Xu Z, Zhang W, Wilson B, Hong JS
and Flood PM: Sinomenine, a natural dextrorotatory morphinan
analog, is anti-inflammatory and neuroprotective through inhibition
of microglial NADPH oxidase. J Neuroinflammation. 4:232007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou B, Lu X, Tang Z, Liu D, Zhou Y, Zeng
P and Xiong H: Influence of sinomenine upon mesenchymal stem cells
in osteoclastogenesis. Biomed Pharmacother. 90:835–841. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HC, Liu MX, Wang EP, Lin Z, Lv GF
and Chen X: Effect of sinomenine on the expression of rheumatoid
arthritis fibroblast-like synoviocytes MyD88 and TRAF6. Genet Mol
Res. 14:18928–18935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen XM, Huang RY, Huang QC, Chu YL and
Yan JY: Systemic review and meta-analysis of the clinical efficacy
and adverse effects of zhengqing fengtongning combined with
methotrexate in rheumatoid arthritis. Evid Based Complement
Alternat Med. 2015:9103762015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu M, Liu L, Qi C, Deng B and Cai X:
Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis:
A systematic review and meta-analysis. Planta Med. 74:1423–1429.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q and Li XK: Immunosuppressive and
anti-inflammatory activities of sinomenine. Int Immunopharmacol.
11:373–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A and Snyder M: Protein complexes
take the bait. Nature. 415:123–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wepf A, Glatter T, Schmidt A, Aebersold R
and Gstaiger M: Quantitative interaction proteomics using mass
spectrometry. Nat Methods. 6:203–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pflieger D, Gonnet F, de la Fuente van
Bentem S, Hirt H and de la Fuente A: Linking the
proteins-elucidation of proteome-scale networks using mass
spectrometry. Mass Spectrom Rev. 30:268–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gollapalli K, Ghantasala S, Kumar S,
Srivastava R, Rapole S, Moiyadi A, Epari S and Srivastava S:
Subventricular zone involvement in Glioblastoma-A proteomic
evaluation and clinicoradiological correlation. Sci Rep.
7:14492017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ray S, Patel SK, Venkatesh A, Chatterjee
G, Ansari NN, Gogtay NJ, Thatte UM, Gandhe P, Varma SG, Patankar S
and Srivastava S: Quantitative proteomics analysis of plasmodium
vivax induced alterations in human serum during the acute and
convalescent phases of infection. Sci Rep. 7:44002017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh R, Park SG, Ju JH, Chi SW, Kim S, Lee
CK, Kim JH and Park BC: Comparative proteomic analyses of synovial
fluids and serums from rheumatoid arthritis patients. J Microbiol
Biotechnol. 24:119–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Chen Y, Sun X, Li Y, Huang C,
Deng H and Li Z: Identification of potential serum biomarkers for
rheumatoid arthritis by high-resolution quantitative proteomic
analysis. Inflammation. 37:1459–1467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanagida M, Kawasaki M, Fujishiro M, Miura
M, Ikeda K, Nozawa K, Kaneko H, Morimoto S, Takasaki Y, Ogawa H, et
al: Serum proteome analysis in patients with rheumatoid arthritis
receiving therapy with tocilizumab: An anti-interleukin-6 receptor
antibody. Biomed Res Int. 2013:6071372013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones-Bolin S: Guidelines for the care and
use of laboratory animals in biomedical research. Curr Protoc
Pharmacol Appendix 4: Appendix 4B. 2012. View Article : Google Scholar
|
|
21
|
Feng L, Huang Q, Huang Z, Li H, Qi X, Wang
Y, Liu Z, Liu X and Lu L: Optimized animal model of
cyclophosphamide-induced bone marrow suppression. Basic Clin
Pharmacol Toxicol. 119:428–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Safaei F, Mehrzadi S, Haghighian Khadem H,
Hosseinzadeh A, Nesari A, Dolatshahi M, Esmaeilizadeh M and
Goudarzi M: Protective effects of gallic acid against
methotrexate-induced toxicity in rats. Acta Chir Belg. 25:1–9.
2017.
|
|
23
|
Yang H, Jiang C, Chen X, He K and Hu Y:
Protective effects of sinomenine against LPS-induced inflammation
in piglets. Microb Pathog. 110:573–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Butler MG, Dasouki MJ, Zhou XP,
Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton
R, Pilarski R and Eng C: Subset of individuals with autism spectrum
disorders and extreme macrocephaly associated with germline PTEN
tumour suppressor gene mutations. J Med Genet. 42:318–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franke TF: PI3K/Akt: Getting it right
matters. Oncogene. 27:6473–6488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugiura Y, Niimi T, Sato S, Yoshinouchi T,
Banno S, Naniwa T, Maeda H, Shimizu S and Ueda R: Transforming
growth factor beta1 gene polymorphism in rheumatoid arthritis. Ann
Rheum Dis. 61:826–828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen NT, Nakahama T, Nguyen CH, Tran TT,
Le VS, Chu HH and Kishimoto T: Aryl hydrocarbon receptor antagonism
and its role in rheumatoid arthritis. J Exp Pharmacol. 7:29–35.
2015.PubMed/NCBI
|
|
28
|
Suzuki E, Ochiai-Shino H, Aoki H, Onodera
S, Saito A, Saito A and Azuma T: Akt activation is required for
TGF-β1-induced Osteoblast differentiation of MC3T3-E1
pre-osteoblasts. PLoS One. 9:e1125662014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jo E, Park SJ, Choi YS, Jeon WK and Kim
BC: Kaempferol suppresses transforming growth factor-β1-induced
epithelial-to-mesenchymal transition and migration of A549 lung
cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3
at Threonine-179. Neoplasia. 17:525–537. 2015. View Article : Google Scholar : PubMed/NCBI
|