Introduction
Infantile hemangioma (IH) is the most common benign
tumor affecting infants (1), with
an incidence of 5–10% 1 year post-birth (2,3). IH
is more prevalent in African, American and Asian populations
compared with Caucasian populations. IH occurs more frequently in
females (male:female ratio, 1:3) (1,4). The
head and neck are the most commonly-affected bodily areas (60%),
which are followed by the trunk (25%) and the extremities (15%)
(1,5). IH is typically characterized by
dramatic postnatal growth followed by spontaneous regression, with
a complete regression rate of 90% by the age of 9 years (1,6).
Therefore, the majority of patients with IH do not require
treatment, as tumors naturally regress over time; however, during
the dramatic postnatal growth phase, ~10% of cases, dependent upon
their anatomical location, may exhibit severe symptoms of
ulceration and hemorrhage (7).
Occasionally, potentially life-threatening complications such as
airway impairment may develop (7).
Previous studies have suggested numerous pharmacological agents for
the treatment of problematic IHs, including oral corticosteroids,
interferon-α and bleomycin A5; however, long-term use of these
agents may have harmful side effects (8). Léauté-Labrèze and Taïeb (9) revealed that propranolol exhibits a
therapeutic effect against IH, which has provided potential new
treatment options for complicated IH (10). Following this discovery, numerous
studies have investigated the clinical application of propranolol
for the treatment of IH, and have demonstrated that propranolol is
an effective and safe therapeutic agent (8,9,11–13).
In recent years, propranolol has become the fist-line treatment for
IH in many major medical centers globally (14,15).
Despite widespread use of propranolol as a
therapeutic agent for the treatment of IH, the mechanism underlying
its therapeutic effects has not yet been determined (1,16).
Vasoconstriction, inhibition of angiogenesis and induction of
apoptosis are three possible mechanisms that propranolol may be
associated with that result in the inhibition of IH growth
(17–19). Several molecules have been revealed
to regulate the interactions between pericytes and
hemangioma-derived endothelial cells (HemECs) (20–22).
Mancini and Smoller (21) revealed
that the apoptosis rate of HemECs during the proliferation stage of
IH is enhanced compared with during the involution stage.
Furthermore, the expression of Bcl-2 (an inhibitor of apoptosis)
during the proliferation stage of IH is significantly suppressed
compared with during the regression stage (22,23).
These findings suggest a close association between the regression
of IH and the apoptosis of HemECs. Furthermore, a previous study
(24) demonstrated the
contribution of p53-dependent apoptosis to the therapeutic effect
of pingyangmycin against IH. Therefore, in the present study, an
enhanced p53-expression model was established via transient
transfection, and a serum-starved p53-expression model was
established using a p53 inhibitor to inhibit the function of p53
during the induction of apoptosis in HemECs, following treatment
with propranolol. The results demonstrated that enhanced p53
expression increases the apoptosis rate of HemECs, and that the
p53-BAX mediated mitochondrial pathway has an important role in
this process.
Materials and methods
Ethical approval
The present study was approved by the Ethical Board
of The Second Affiliated Hospital of Xi'an Jiaotong University
(Xi'an, China). Clinical diagnoses of each patient were confirmed
in the Department of Pediatric Surgery, The Second Affiliated
Hospital of Xi'an Jiaotong University. Written informed consent was
obtained routinely from the families of each patient, in accordance
with the treatment protocol of the associated hospital.
Additionally, written informed consent was obtained regarding the
handling of samples according to the Declaration of Helsinki.
Isolation and culture of HemECs
The HemEC cell line was same as in a previous study
(24). HemECs were isolated from a
proliferating IH at the Department of Pediatric Surgery of The
Second Affiliated Hospital of Xi'an Jiaotong University. Resected
tissue of a proliferating IH were subjected to enzymatic digestion
by trypsin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and then centrifuged at 100–200 × g at 37°C for 30 sec. HemECs
were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS), 100 µg/ml streptomycin and 100 µg/ml penicillin
(all Invitrogen; Thermo Fisher Scientific, Inc.) and incubated at
37°C with 5% CO2. The culture medium was replaced with fresh medium
every 2 days, and the confluent cells were isolated using
trypsin-EDTA solution (0.05%; Invitrogen; Thermo Fisher Scientific,
Inc.). HemECs at passages 6–8 were used in the present study.
HemECs at passage 6 were harvested and fixed in 2.5%
glutaraldehyde at 4°C for 2 h and coated using 1% osmium tetroxide,
then observed under both inverted phase contrast microscope and
scanning electron microscope (SEM; magnificatiom, ×8,000).
Immunocytochemical staining
Cell climbing slices were made using HemECs at sixth
passage and then treated with paraformaldehyde for 30 min at room
temperature. The cells were treated with 5% normal goat serum
(Abcam, Cambridge, UK) for 10 min at rrom temperature and incubated
with anti-factor VIII primary antibody (1 mg/ml; cat. no. ab6190;
Abcam) at 4°C overnight. Following three washes in PBS, sections
were incubated with streptavidin-peroxidase (S-P)-conjugated
secondary antibodies. The cells were visualized using DAB-chromogen
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) according
to the manufacturer's protocol. Then after gradient alcohol
treatment, the cells were treated with dimethylbenzene for 1 min
and sealed with neutral resin. Cells were observed using converted
microscope at a magnification of ×400. Yellow-stained particles
indicate the presence of clotting factor VIII.
Sequencing of p53 gene
The full-length sequence of the target gene was
determined using Illumina HiSeq 2000 platform (Illumina, Inc., San
Diego, CA, USA), as previously described (25) and sequence alignment was done with
Quality software (maq.sourceforge.net; version number:
Bwa-05.0.tar.bz2), which was corresponding with the sequence of
p53, confirming that false positive clones or mutations had not
been established (26).
Construction of high p53 expression
model of HemECs
The coding region of human wild-type p53 gene was
amplified and inserted into the Bg1II site of the 1 µg plasmid
p3XFlag-CMV7.1 (Fermentas; Thermo Fisher Scientific, Inc.)
(27). As the resultant plasmid
p3XFlag-CMV-p53 did not express green fluorescent protein (GFP),
the 1 µg pEGFP plasmid (Fermentas; Thermo Fisher Scientific, Inc.)
was co-transfected. HemECs were transferred to 12-well plates,
seeded at 2×106 cells/well and incubated overnight at 37°C.
Transfection with p3XFlag-Cmv7.1 or pEGFP was performed using
EndoFectin™ (GeneCopoeia, Inc., Rockville, MD, USA). A total of 0.5
µg plasmid DNA was mixed with 1.5 µl EndoFectin™ to generate a
final concentration of 0.33 µg DNA/ml, which is then dissolved in
serum-free RPMI-1640. The resulting complex was incubated at 37°C
for 3 h and then added to cells in 12-well plates. The cells were
incubated at 37°C for 3 h and then washed using RPMI-1640. The
cells were incubated at 37°C for a further 32 h in RPMI-1640 with
10% FBS prior to further experimentation. Fluorescence microscopy
was performed to determine transfection efficiency (magnification,
×400).
Construction of a low p53 expression
model of HemECs
Pifithrin-α (PFT-α; Merck KGaA, Darmstadt, Germany)
was used to inhibit p53-induced apoptosis pathways. Serum-starved
HemECs were treated with PFT-α at gradient concentrations (100 and
500 nM; and 1, 10, 5, 100 and 200 µM) for 3 h at 37°C followed by
treatment with propranolol (100 µmol/l at 37°C for 24 h) (8,9,28,29).
Cells were harvested according to morphological alterations and
investigated for apoptosis using an Annexin V-fluorescein
isothiocyanate (FITC) kit (Trevigen, Inc., Gaithersburg, MD, USA),
according to the manufacturer's protocol. Morphological alterations
associated with apoptosis were observed under a fluorescent
microscope at a magnification of ×400.
Verification of HemEC models via
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RNA from HemEC models and a blank control group was
isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and its purity and concentration were
investigated using ultraviolet spectrophotometry and A260/A280
ratio of 1.8–2.1 was considered as acceptable purity of the RNA.
Complimentary DNA synthesis was performed using reverse
transcriptase and oligo(dT) primers (Thermo Fisher Scientific,
Inc.). RevertAid M-MulV Reverse Transcriptase (200 u/µl; 1 µl),
oligo(dT) primer 1 µl; 5X reaction buffer 4 µl were used for
reverse transcription. The temperature protocol of reverse
transcription was 42°C for 60 min and then 70°C for 5 min. Primer
sequences used for PCR are detailed in Table I. SYBR-Green Super Mix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used for qPCR. The
following thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 10 min; 40 cycles of 95°C for 15 sec and
60°C for 30 sec. Expression levels were quantified using the
2−ΔΔCq method (30).
All expression levels were normalized to GAPDH.
 | Table I.Gene and primer sequence. |
Table I.
Gene and primer sequence.
| Gene | Primer
sequences |
|---|
| p53 | F:
GAGGTTGGCTCTGACTGTACC |
|
| R:
TCCGTCCCAGTAGATTACCAC |
| GAPDH | F:
ACCCACTCCTCCACCTTTG |
|
| R:
CACCACCCTGTTGCTGTAG |
Drug treatment and apoptosis
analysis
HemECs were cultured overnight at 37°C and then
assorted into six groups, including the blank control group,
dosing+transfection group, transfection group, dosing+inhibitor
group, inhibitor group and dosing group. Blank control group,
HemECs untreated; Dosing Group, HemECs treated with Propranolol 100
µM/l; Inhibitor group, HemECs treated with PFT-alpha (10 µM/l);
Dosing + Inhibitor Group, HemECs treated with PFT-α (10 µM/l) for 3
h and then treated with propranolol (100 µM/l) for 3 h;
Transfection Group, high p53 expression model of HemECs untreated;
and Dosing + Transfection group, high p53 expression model of
HemECs treated with propranolol (100 µM/l) for 3 h. All treatments
were at 37°C. The Dosing and transfection group were incubated with
propranolol (100 µM/l) for 3 h following transfection. In addition,
HemECs in Dosing + Inhibitor group were treated with propranolol
for 3 h following addition of PFT-α (10 µm). Following this
treatment, cells were collected, washed and subjected to apoptosis
analysis using an Annexin V-FITC kit (Trevigen, Inc.), according to
the manufacturer's protocol. The cells were analyzed on a FACScan
flow cytometer with Cell Quest software (version 5.1; BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Total proteins were extracted using an ultrasonic
method (22) following treatment
with 1X SDS Sample Buffer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min at 0°C. Bicinchoninic acid was used for protein
determination. Protein samples (10–30 µg) were separated by 10%
SDS-PAGE and then transferred to polyvinylidene fluoride membranes
(Ameresco, Inc., Framingham, MA, USA). The protein determination
method we uses was BCA method. The membranes were incubated at 37°C
with Ponceau staining solution (0.1%) for 2 min followed by
incubation at 37°C with Tris buffered saline containing Tween-20.
The membranes were blocked with 5% Bovine Serum Albumin (HyClone;
GE Healthcare Life Sciences, Logen, UT, USA) at 4°C for 24 h. The
membranes were incubated overnight at 4°C with the following
primary antibodies: Anti-β-actin (cat. no. ab8226; dilution, 1
mg/ml, molecular weight 45 kDa), antibody against tumor necrosis
factor receptor superfamily member 6 (FAS; cat. no. ab133619,
dilution 1:1,000), p53-induced death domain-containing protein
(PIDD; cat. no. ab78389, dilution 1 µg/ml), death receptor 5 (DR5;
cat. no. ab199357, dilution 1:1,000), apoptosis regulator BAX (BAX;
cat. no. ab53154, dilution 1:1,000), BH3-interacting domain death
agonist (BID; cat. no. ab32060, dilution 1:1,000), p53 unregulated
modulator of apoptosis (PUMA; cat. no. ab33906, dilution 1:1,000),
insulin-like growth factor-binding protein 3 (IGF-BP3; cat. no.
ab77635, dilution 0.03 µg/ml) and phosphatidylinositol-glycan
biosynthesis class S protein (PIG-S; cat. no. ab157211, dilution
1:1,000; all Abcam). Following this process, membranes were
incubated at 20°C for 2 h with secondary antibodies. For IGF-BP3
the secondary antibody used was donkey anti-goat IgG (dilution,
1:300; cat. no. ab6566). Goat anti-rabbit IgG (dilution, 1:3,000;
cat. no. ab6721; all Abcam) was the secondary antibody used for all
other primary antibodies.
Following washing, the proteins were visualized
using a western blot fluorography developer kit (Beyotime Institute
of Biotechnology, Haimen, China) and scanned as computer files.
Densitometry was analyzed using ImageJ software version 1.8.0
(National Institutes of Health, Bethesda, MD, USA) and β-actin
expression was used for normalization. All experiments were
performed in triplicate.
Statistical analysis
Statistical differences between two groups were
determined using the Student's t-test, and the statistical
differences between multiple groups was determined using one-way
analysis of variance followed by Tukey's post hoc test. All
statistical analyses were performed using SPSS 20.0 software for
Windows 7 operating system (IBM Corp., Armonk, NY, USA). Data were
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
HemECs are successfully transfected
with p53
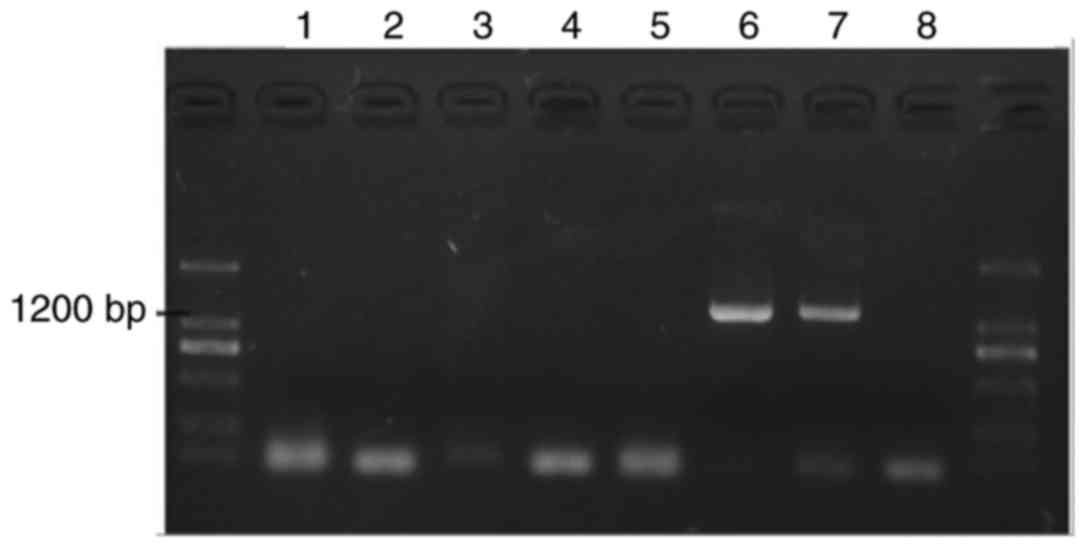
Colonies were identified via PCR. The results
revealed two positive colonies (Fig.
1, lanes 6 and 7).
Gene amplification
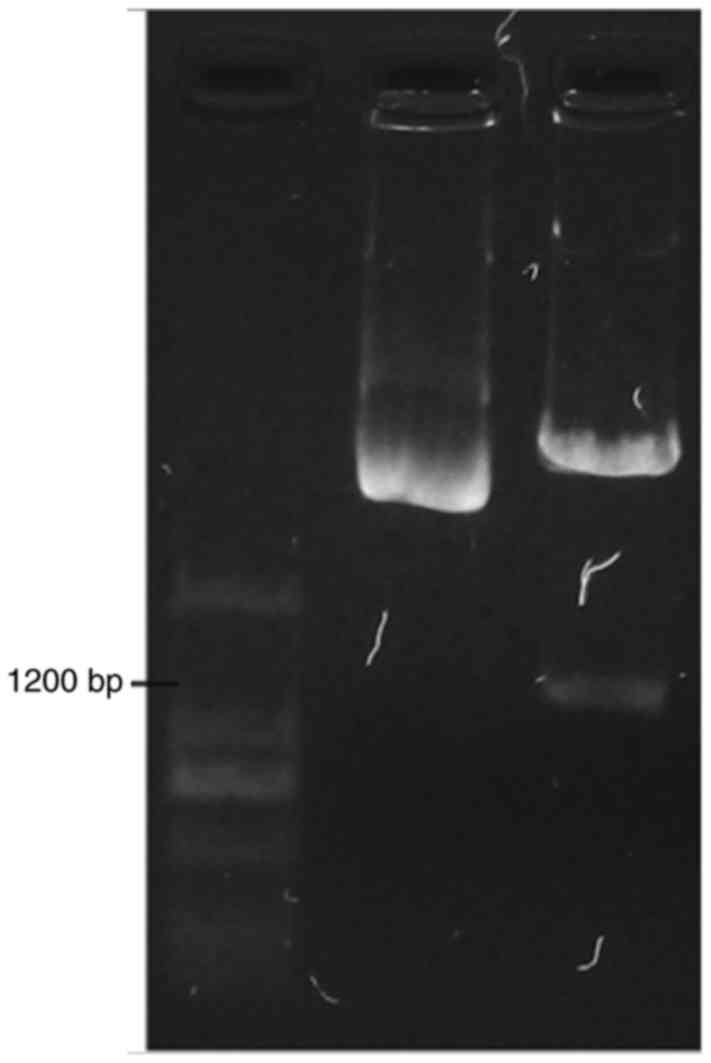
The positive colonies were propagated and the
plasmid extracted. Double enzyme digestion was performed to further
identify the colony. The results demonstrated that the colony
inserted into lane 6 had been successfully transfected with the
target sequence Fig. 2.
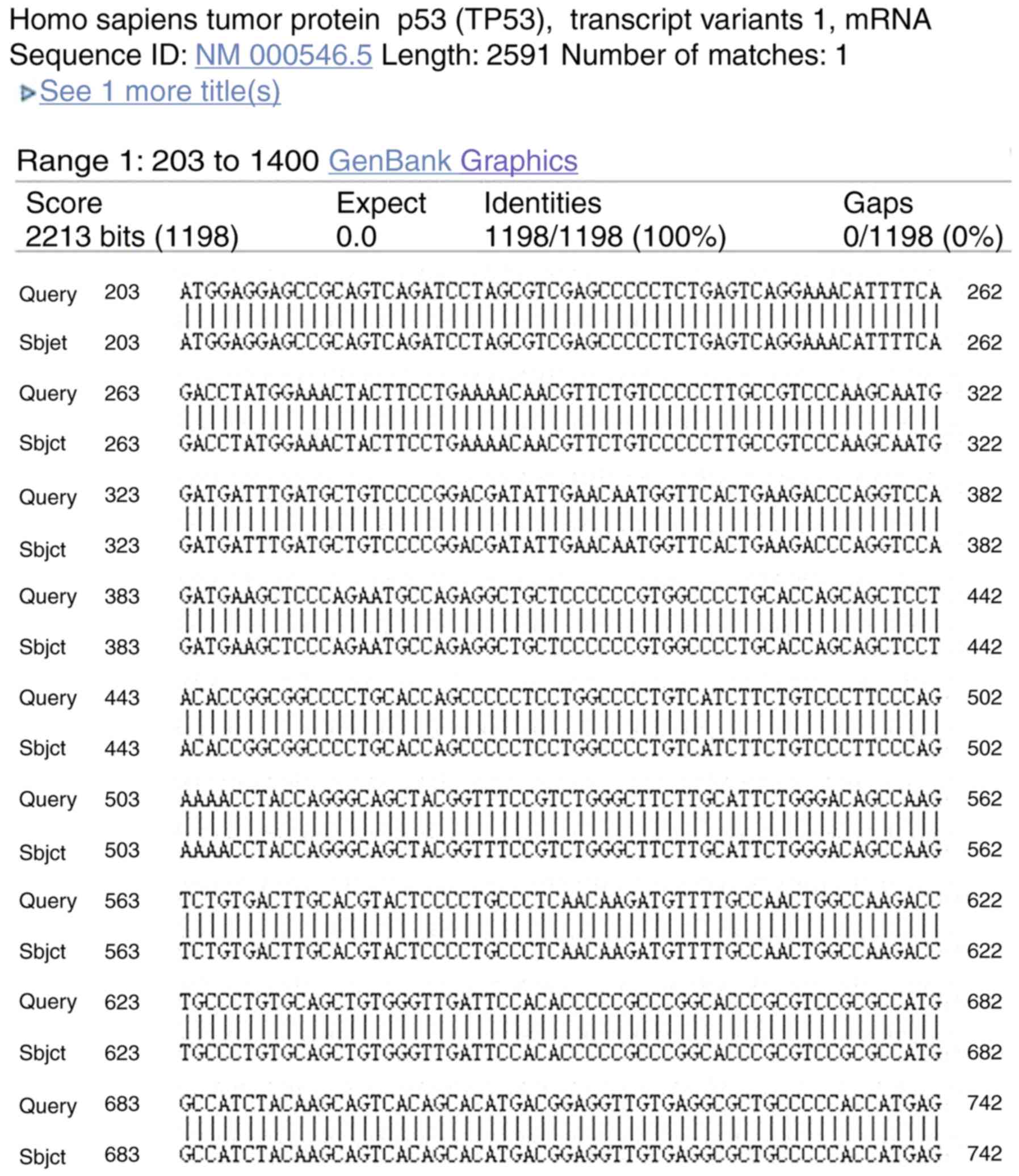
Sequencing of Gene
Sequencing of the positive colony of target gene was
done. Fig. 3 revealed that the
sequence of the target gene in the positive colony was exactly same
with that of p53, which confirmed that there were no false positive
clones or mutations (Fig. 3)
(26).
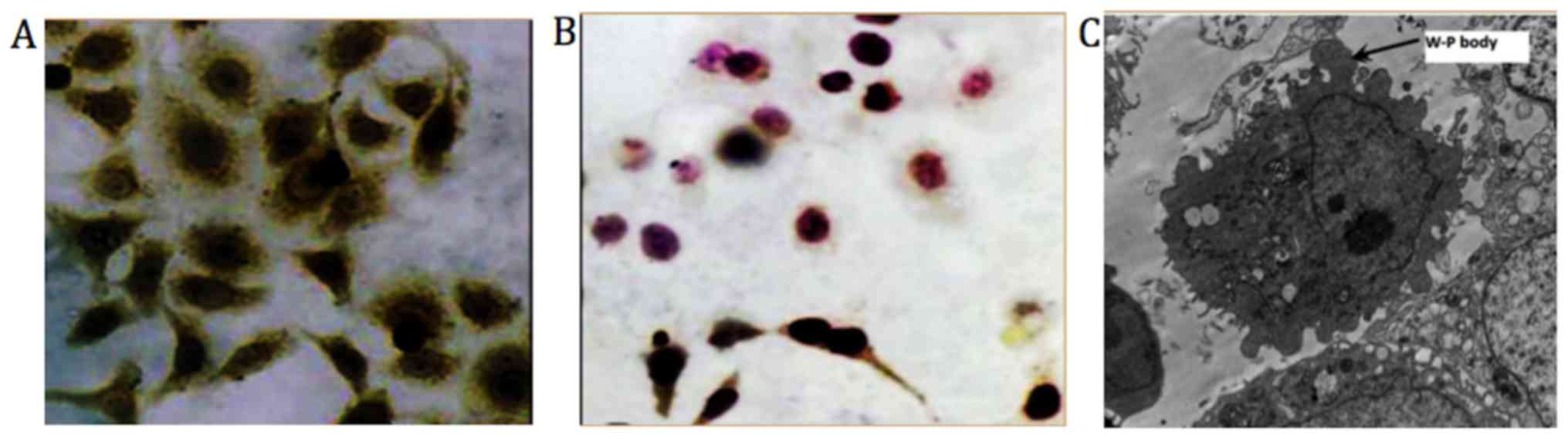
Immunocytochemical staining
Immunocytochemical staining results exhibited yellow
dyed particles demonstrating the presence of clotting factor VIII
related antigen in HemECs. SEM revealed presence of Weibel-Palade
bodies (magnification, ×8,000; Fig.
4) (31).
Transfection with p53 is optimal
following 24 h of incubation
Gene transfection revealed morphological alterations
associated with apoptosis, typically rounding and floating of the
HemECs (Fig. 5).
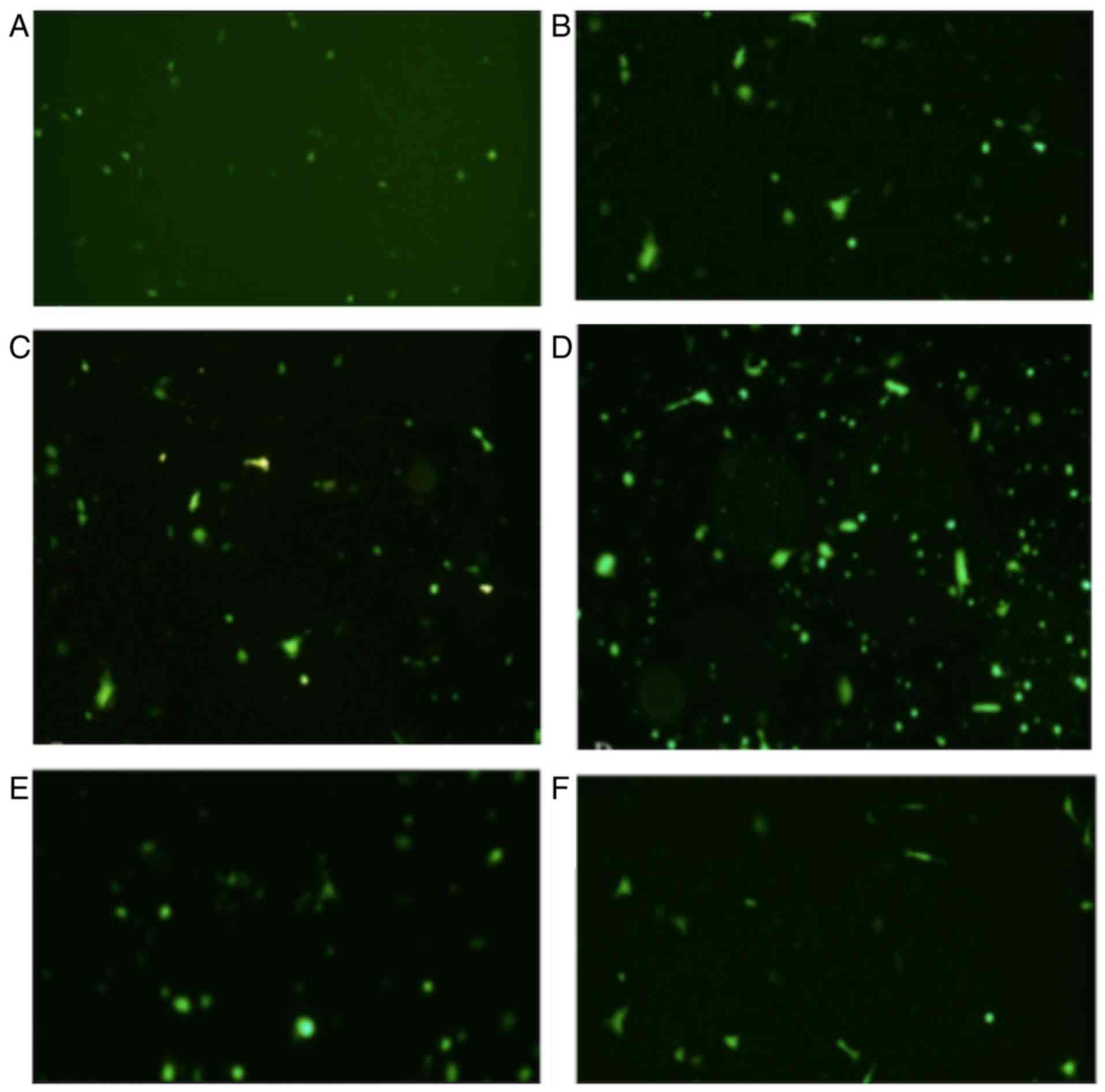
Fluorescence microscopy
The expression of fluorescence was investigated at
6, 12, 18, 24 and 32 h time intervals. The results revealed that
the expression of fluorescence increased in a time-dependent manner
and at the 24 h time interval, the expression of fluorescence
reached a maximum cell transfection ratio ~60% (Fig. 6). The values were calculated using
cell-counting chamber. Total cell number was counted using an
inverted microscope followed by the counting of fluorescent cell
number using a fluorescence microscope. The transfection
ratio=fluorescent cell number/total cell number. Three fields of
view were observed. The magnification used was ×400.
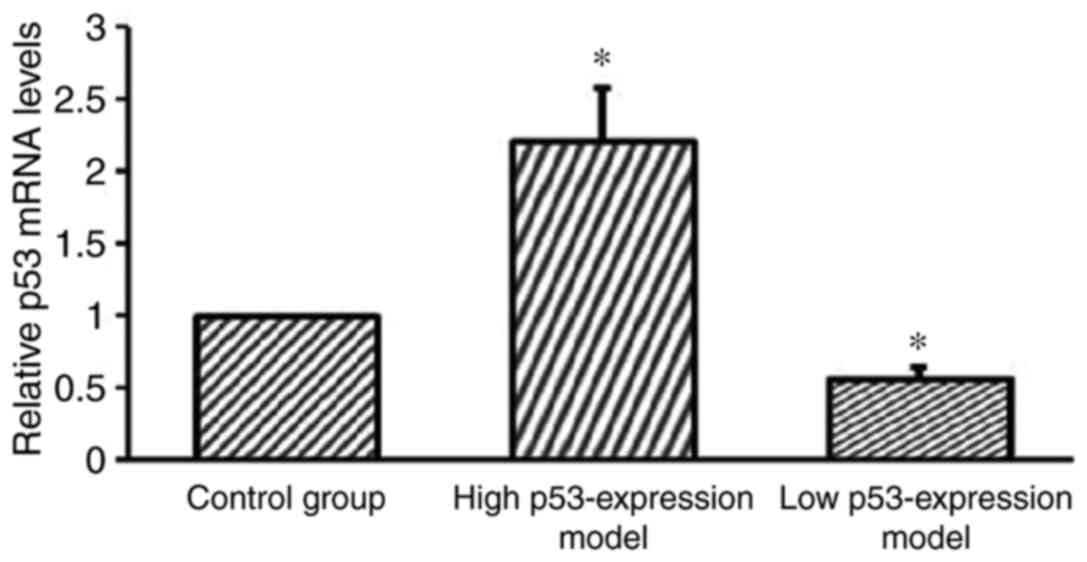
RT-qPCR analysis reveals the
successful establishment of a low p53 expression model group and a
high p53 expression model group
RT-qPCR analysis was performed to further verify the
successful establishment of the cell models. The high p53
expression model group, the low p53 expression model group and the
blank control groups were investigated. The results revealed that
the low p53 expression model group demonstrated significantly
suppressed p53 expression compared with the control group
(P<0.05; Fig. 7). Furthermore,
the results revealed that the high p53 expression model group
demonstrated significantly enhanced p53 expression compared with
the control group (P<0.05; Fig.
7).
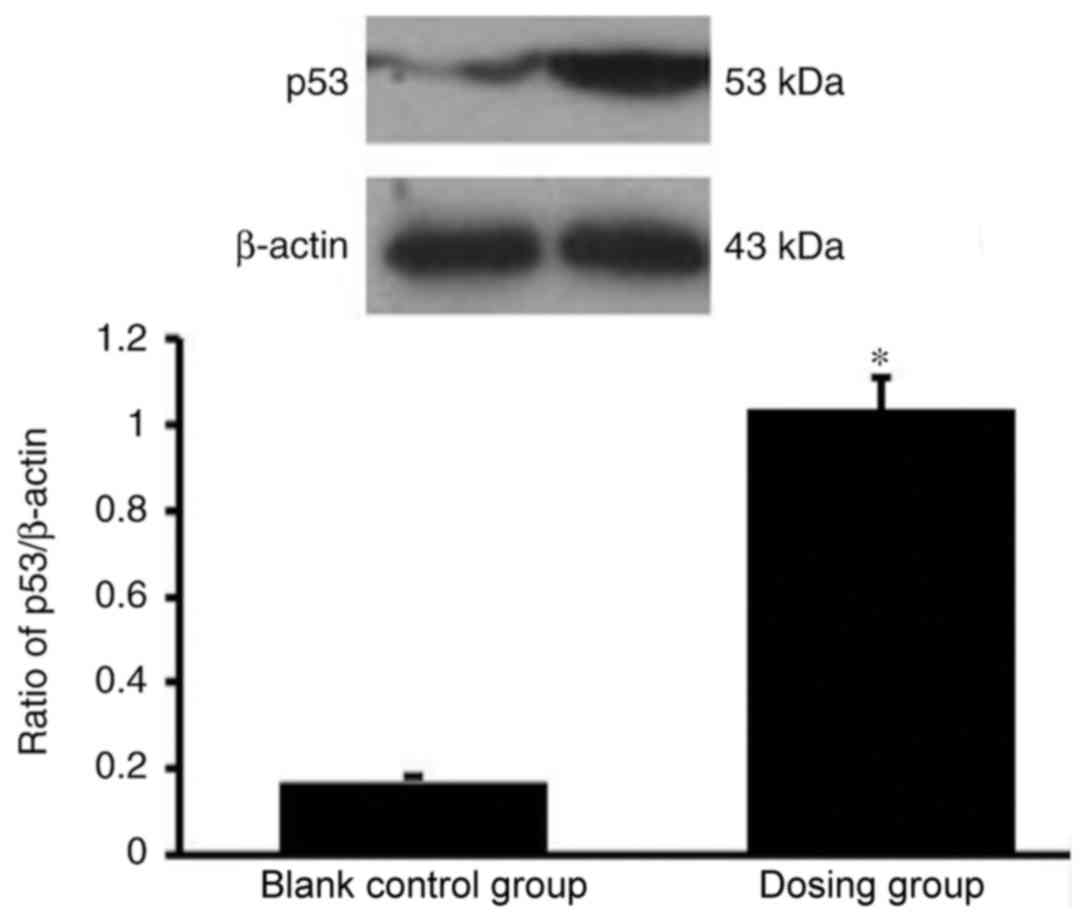
p53 expression is significantly
enhanced in HemECs following treatment with propranolol
The western blot analysis results demonstrated that
p53 expression was significantly enhanced in HemECs following
treatment with propranolol (100 µmol/l; Fig. 8).
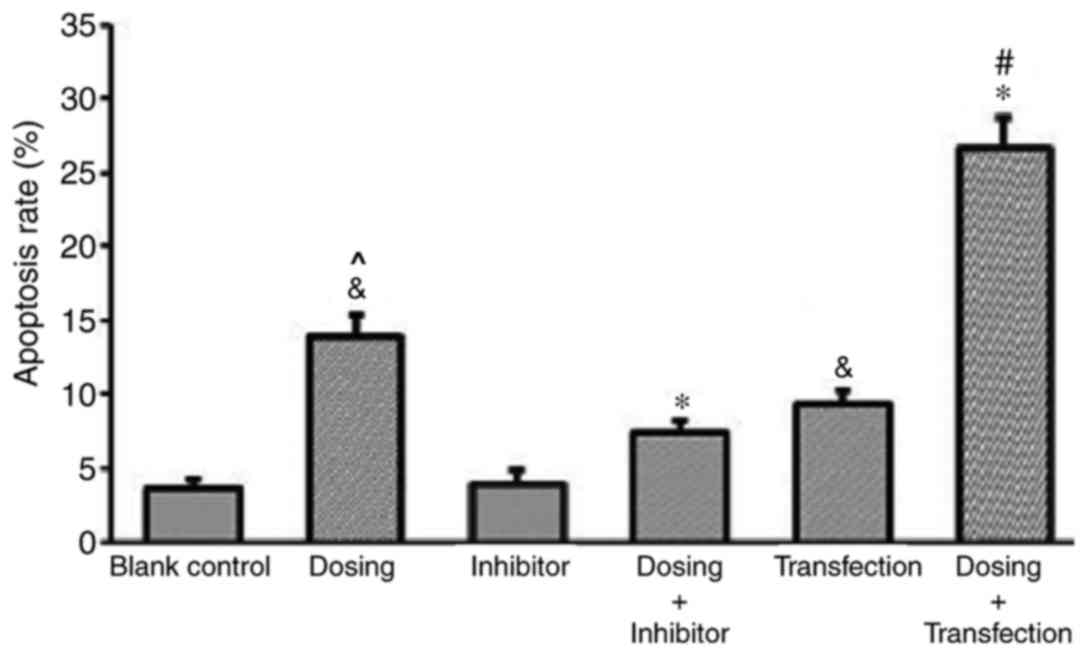
Enhanced p53 expression increases the
apoptosis rate of HemECs
The results of the flow cytometry and
Annexin/propidium iodide (PI) double staining analyses demonstrated
a significantly increased rate of apoptosis exhibited by the dosing
group compared with the blank control group, thus suggesting that
propranolol (100 µM) may induce apoptosis in HemECs. To further
investigate the effect of the administration of propranolol on
HemECs expressing different levels of p53, a control group and
different experimental groups were established, as previously
mentioned. Different experimental groups had different level of
apoptosis rate. Following treatment with propranolol, the apoptosis
rate of the transfection group was significantly enhanced compared
with the control group, and the apoptosis rate of the inhibitor
group was significantly suppressed compared with the control group.
This result demonstrated an association between p53 expression and
apoptosis rate (Fig. 9).
Furthermore, the apoptosis rate of HemECs dosed with
propranolol (Dosing group) was significantly enhanced compared with
the blank control group and that with the inhibitor group. The
apoptosis rate of the Dosing group was significantly increased
compared with the Inhibitor group. The apoptosis rate of
transfected HemECs treated with propranolol (Dosing + Transfection)
was significantly enhanced compared with Dosing group and
Transfection groups (Fig. 9).
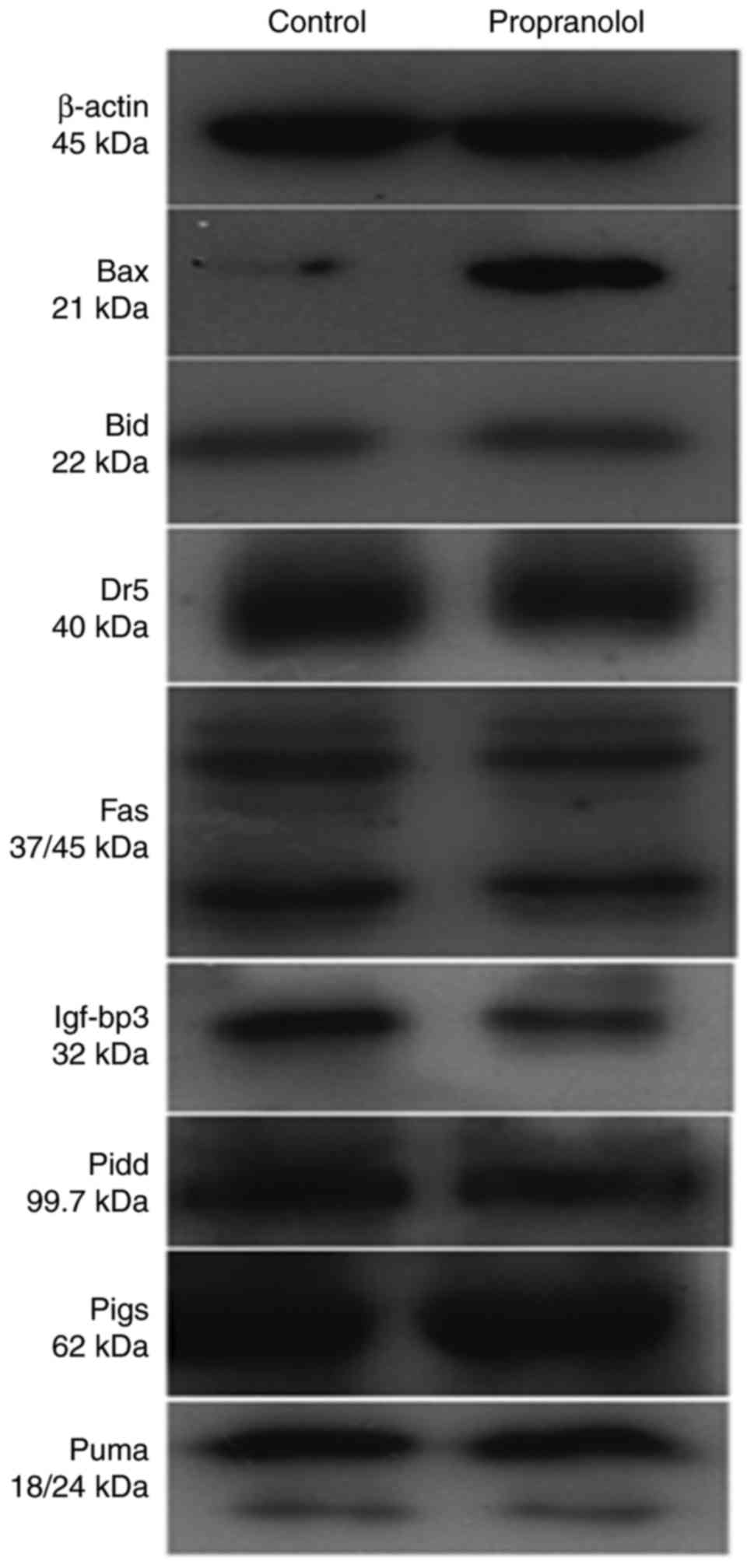
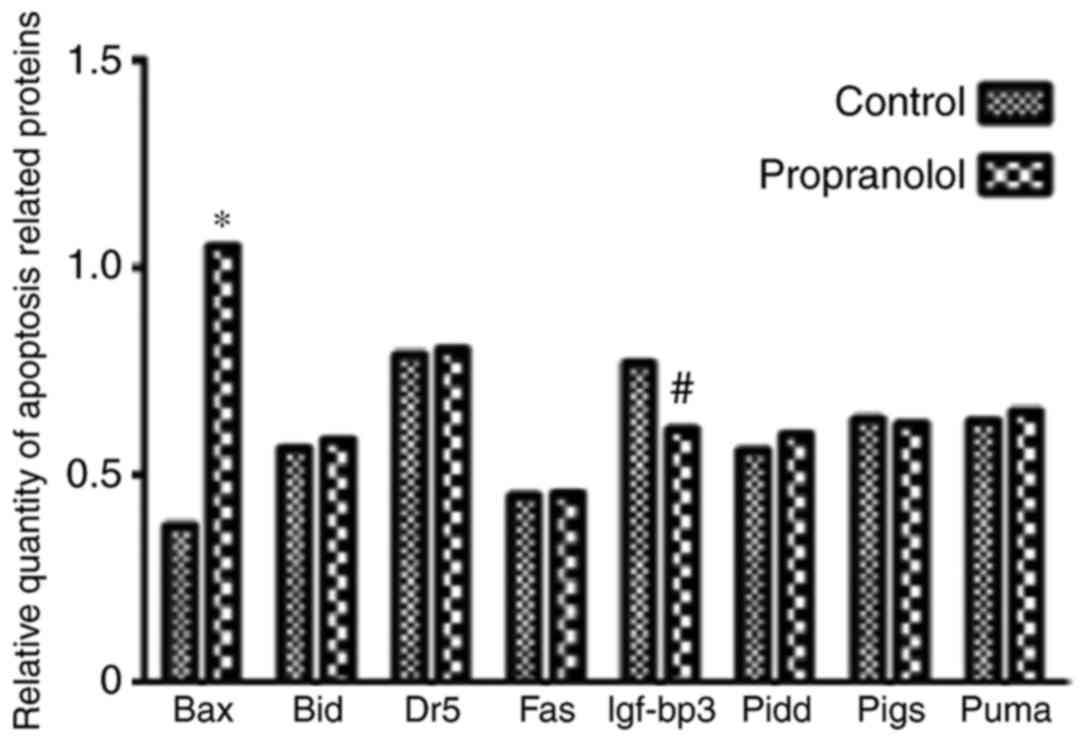
BAX expression is significantly
upregulated, and IGF-BP3 expression is significantly downregulated,
following treatment with propanolol
To determine the mechanism underlying the effect of
propranolol on HemECs, the expression of eight proteins associated
with the p53 downstream pathway were investigated via western
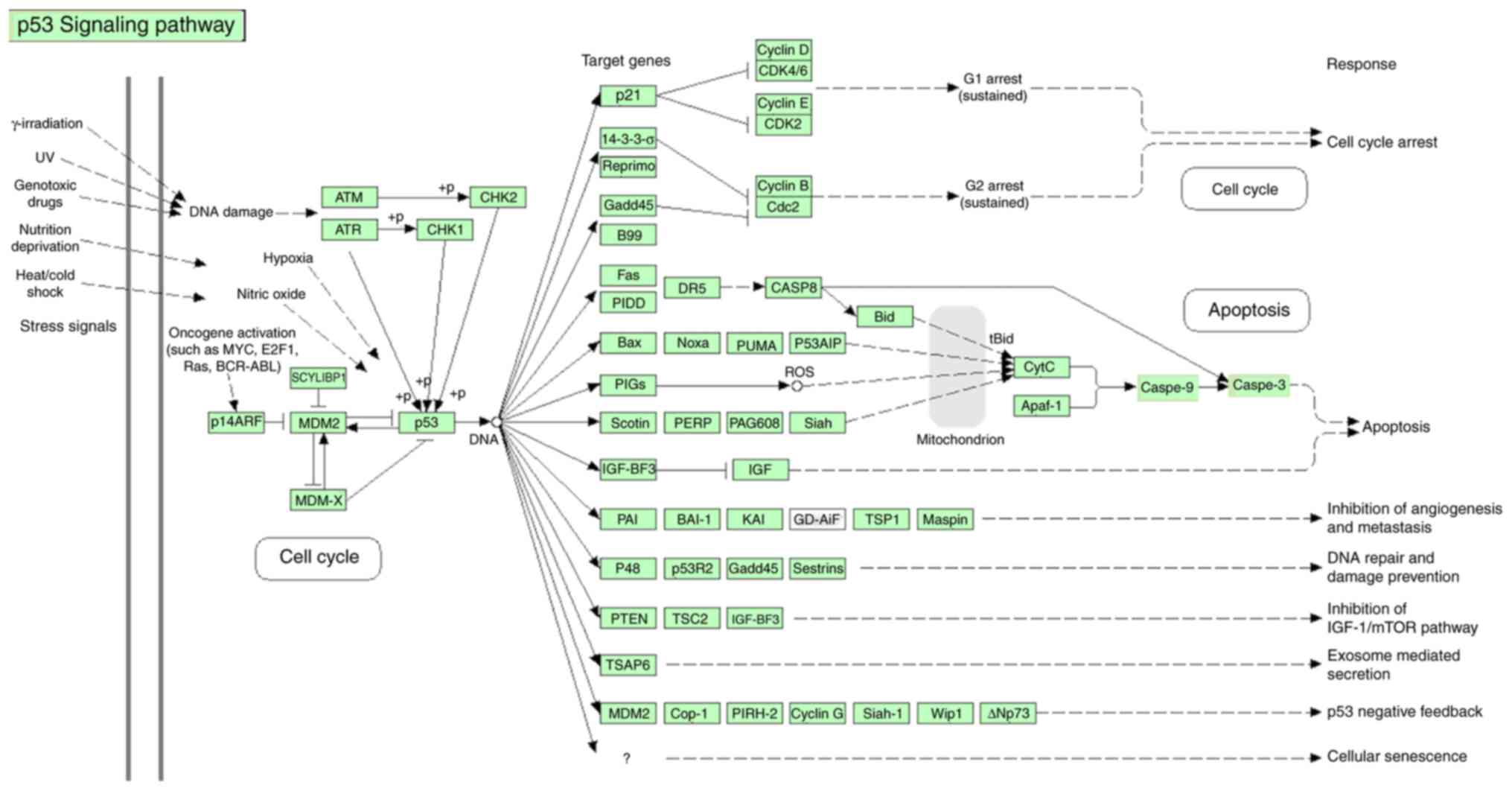
blotting (Fig. 10). The signaling
pathway of p53 gene has been shown in Fig. 11 (Fig. 11) which was taken from DAVID
Database (david.ncifcrf.gov/home.jsp) (32).
As presented in Fig.
12, the expression of BAX in the propranolol group was
significantly increased compared with the control group
(P<0.05). Furthermore, the expression of IGF-BP3 in the
propranolol group was significantly decreased compared with the
control group (P<0.05). No significant differences were
exhibited by any of the other proteins investigated.
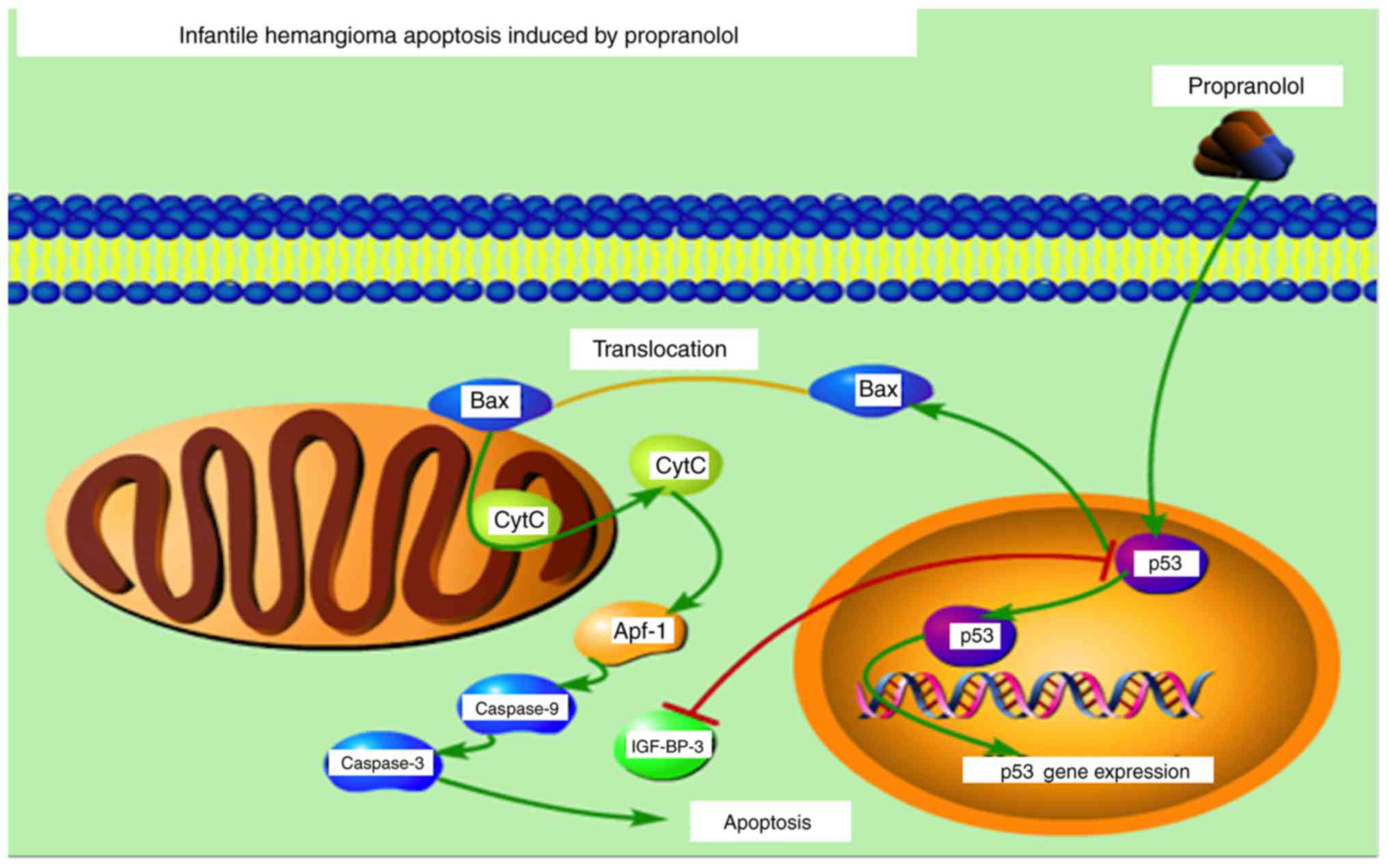
BAX activates the release of Cytochrome C via
membrane remodeling and increasing membrane permeability, which
subsequently results in the activation of caspases Signaling
pathway of propranolol inducing apoptosis in HemECs in vitro
is presented in Fig. 13. The
figure was constructed using Portable Pathway Builder 2.0.
(www.proteinlounge.com/PathwayBuilder.aspx).
Discussion
Despite IH being widely studied, the disease is not
completely understood due to differences in therapeutic options and
patient ages, in addition to tumor location, stage and size
(33,34). Treatment is typically initiated
during the early proliferative stage of the tumor, at which point
numerous treatment options are available (35–39).
Propranolol, a nonselective β-blocker, has been
demonstrated to be the most effective treatment option available
with few adverse side effects (40). Propranolol has become the
first-line treatment option in a number of tertiary healthcare
centers around the world (34).
Despite its effectiveness, the underlying therapeutic mechanism
through which propranolol attenuates the regression of IH is not
completely understood (41). At a
molecular level, propranolol has been revealed to induce the
expression of apoptotic genes, such as BAX, p53, caspase-8 and
cytochrome C in HemECs (28,42).
Therefore, the p53-dependent apoptosis pathway may represent a
potential mechanism involved in the therapeutic effect of
propranolol against IH. In the present study, a high p53 expression
model and a low p53 expression model of HemECs was constructed. The
results demonstrated enhanced apoptotic activities in the high p53
expression model of HemECs in vitro. Furthermore, eight key
proteins downstream of the p53-dependent apoptosis pathway were
selected and subsequently detected using western blotting. The
results revealed a significant upregulation of BAX expression. In
addition, the results suggested that the mitochondrial apoptotic
pathway regulated by p53-BAX may be the underlying mechanism
responsible for the therapeutic effect of propranolol against IH.
However, this result is in contrast with findings of Kum and Khan
(43), which suggested that
propranolol inhibits the growth of HemECs without inducing
apoptosis. However, the results of the present study are somewhat
similar to those obtained by Wong et al (28), which suggested that propranolol
enhances the apoptosis of HemECs, although not hemangioma stem
cells (HemSCs), and enhances adipogenesis in HemSCs. The results of
the present study differ from the results obtained by Munabi et
al (44) who found that the
propranolol at doses of <10-4 M, had reduced cyclic adenosine
monophosphate (cAMP) level causing decreased proliferation and
viability of HemSC and propranolol at ≥10-5 M had reduced cyclic
adenosine monophosphate (cAMP) levels and increased extracellular
signal regulated kinase (ERK1/2) activation, suggesting induction
of HemSC apoptosis and cytotoxicity at ≥10-4 M propranolol.
Stimulation with isoprenaline, a β androgen receptor (βAR) agonist,
had promoted HemSC proliferation and rescued the antiproliferative
effects of propranolol, indicating that propranolol inhibited βAR
signaling in HemSCs (44). The
HemSC cell viability suppressed by propranolol was partially
rescued by treatment with a cyclic adenosine monophosphate (cAMP)
analog or a mitogen activated protein kinase (MAPK) inhibitor
(44). A selective β 2 androgenic
receptor (β2AR) antagonist mirrored propranolol's effects on HemSCs
in a dose-dependent manner and a selective β1AR antagonist had no
effect, supporting a role for β2AR signaling in IH pathobiology
(44). Therefore, Munabi et
al (44) demonstrated that
propranolol acts on HemSCs in infantile hemangioma to suppress
proliferation and promote apoptosis in a dose-dependent manner via
β2 adrenergic receptor activation, resulting in reduced cAMP and
MAP activation.
Transfection is the process of introducing nucleic
acids into cells and is widely used in gene therapy, model
construction and drug screening. In the present study, a plasmid
with amplified p53 sequences was inserted into HemECs. Flow
cytometry analysis, following treatment with propranolol, revealed
that the apoptosis rate of transfected HemECs was significantly
enhanced compared with the control. PFT-α is a p53 inhibitor that
is widely used to study p53 function (45), and it is able to specifically
inhibit the p53-dependent apoptotic pathway (45–47).
In the present study, the apoptosis rate of HemECs inhibited by
PFT-α was significantly suppressed compared with the control. In
conclusion, the results suggest that p53 has an important role in
propranolol-induced apoptosis.
Apoptosis is a process of programmed cell death in
multicellular organisms, and it has an important role in the
accommodation and adaptation of organisms. The mitochondrial
pathway and the receptor signaling pathway represent the two major
apoptosis pathways, and numerous proteins in these two pathways are
targets of the p53 gene, including FAS, PIDD, DR5, BID, BAX, PIGs,
PUMA and IGF-BP3. Considering this, the present study aimed to
investigate the expression level alterations of these proteins
between cells treated and untreated with propranolol using western
blotting analysis. The results demonstrated that the expression
level of BAX was significantly upregulated and the expression level
of IGF-BP3 was significantly downregulated following treatment with
propranolol, and the expression levels of the other investigated
proteins did not significantly alter following treatment with
propranolol. A further study (28)
revealed that p53 and BAX were upregulated in HemECs following
treatment with propranolol. BAX, a member of the Bcl-2 gene family,
may be activated by p53 to induce apoptosis (48,49).
In the present study, the expression level of BAX in HemECs treated
with propranolol was significantly upregulated compared with the
control, thus suggesting that BAX was activated following treatment
with propranolol.
IGF-BP3 is able to interact with the cell surface
proteins and is involved in the process of transduction of
extracellular signals into cells with corresponding ligands
(50). It has previously been
demonstrated that there is a negative association between IGF-BP3
and p53 (51,52). In the present study, the expression
level of IGF-BP3 was suppressed following treatment with
propranolol, thus suggesting that p53 expression is upregulated.
BAX activates the release of Cytochrome C via membrane remodeling
and increasing membrane permeability, which subsequently results in
the activation of caspases (Fig.
12).
A previous study (24) demonstrated that the p53-dependent
pathway is the mechanism underlying the propranolol-induced
apoptotic effect on HemECs in vitro so and the clinical use
of propranolol has been increased gradually (53,54).
In the present study, propranolol was revealed to have an apoptotic
effect on HemECs in vitro and, simultaneously, the
expression of BAX by the mitochondrial pathway was significantly
elevated. Furthermore, the results of the present study
demonstrated that enhanced p53 expression increased the apoptosis
rate of HemECs, in which the p53-BAX-mediated mitochondrial pathway
has an important role. Therefore, the results of the present study
suggested that p53-BAX-mediated apoptosis of HemECs is the
underlying mechanism responsible for the therapeutic effect of
propranolol administration against IH.
Acknowledgements
The authors would like to thank the Department of
Pediatric Surgery, The Second Affiliated Hospital of Xi'an Jiaotong
University for providing experimental materials and support. The
authors are grateful to Dr Zhong-Cheng Gong and Dr Bin Ling
(Oncology Department of Oral and Maxillofacial Surgery, The First
Affiliated Hospital of Xinjiang Medical University, Urumqi, China)
who helped to make detailed adjustments of the procedures during
the experiments.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 30700954), and the
Shaanxi Province Social Science and Technology Development and
Research Project (grant no. 2016SF-315).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
T-HY and PP participated in the design of the study
and conducted of the whole study. J-BT designed of the experiments
and provided funding. KPR designed of the study, wrote the
manuscript and was involved in word processing of data. X-WG and
Q-YL constructed of high p53 expression model of HemECs and low p53
expression model of HemECs. J-TD and S-MG performed isolation and
culture of HemECs and immunocytochemical staining.
Ethics approval and consent to
participate
The present study was approved by the Ethical Board
of The Second Affiliated Hospital of Xi'an Jiaotong University.
Written informed consent was obtained routinely from the families
of each patient, in accordance with the treatment protocol of the
associated hospital. Additionally, written informed consent was
obtained regarding the handling of samples according to the
Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IH
|
infantile hemangioma
|
|
HemECs
|
hemangioma-derived endothelial
cells
|
|
PFT-α
|
pifithrin-α
|
|
cDNA
|
complimentary DNA
|
|
PID
|
p53-induced protein with death
domain
|
|
DR5
|
death receptor 5
|
|
BAX
|
apoptosis regulator BAX
|
|
BID
|
BH3-interacting domain death agonist
(a pro-apoptotic protein)
|
|
PUMA
|
p53 unregulated modulator of
apoptosis
|
|
IGF-BP3
|
insulin-like growth factor-binding
protein 3
|
References
|
1
|
Starkey E and Shahidullah H: Propranolol
for infantile haemangiomas: A review. Arch Dis Child. 96:890–893.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drolet BA, Swanson EA and Frieden IJ:
Hemangioma Investigator Group: Infantile hemangiomas: An emerging
health issue linked to an increased rate of low birth weight
infants. J Pediatr. 153(712–715): 715.e12008.
|
|
3
|
Greenberger S and Bischoff J: Infantile
hemangioma-mechanism(s) of drug action on a vascular tumor. Cold
Spring Harb Perspect Med. 1:a0064602011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bowers RE, Graham EA and Tomlinson KM: The
natural history of the strawberry nevus. Arch Dermatol. 82:667–680.
1960. View Article : Google Scholar
|
|
5
|
Léauté-Labrèze C and Taïeb A: Efficacy of
beta-blockers in infantile capillary haemangiomas: The
physiopathological significance and therapeutic consequences. Ann
Dermatol Venereol. 135:860–862. 2008.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zimmermann AP, Wiegand S, Werner JA and
Eivazi B: Propranolol therapy for infantile haemangiomas: Review of
the literature. Int J Pediatr Otorhinolaryngol. 74:338–342. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz RA, Sidor MI, Musumeci ML, Lin RL
and Micali G: Infantile haemangiomas: A challenge in paediatric
dermatology. J Eur Acad Dermatol Venereol. 24:631–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buckmiller LM, Munson PD, Dyamenahalli U,
Dai Y and Richter GT: Propranolol for infantile hemangiomas: Early
experience at a tertiary vascular anomalies center. Laryngoscope.
120:676–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Léauté-Labrèze C, de la Roque Dumas E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Handgretinger R: How an accidental
discovery paved the way for the treatment of complicated infantile
haemangiomas. Acta Paediatr. 103:896–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manunza F, Syed S, Laguda B, Linward J,
Kennedy H, Gholam K, Glover M, Giardini A and Harper JI:
Propranolol for complicated infantile haemangiomas: A case series
of 30 infants. Br J Dermatol. 162:466–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Missoi TG, Lueder GT, Gilbertson K and
Bayliss SJ: Oral propranolol for treatment of periocular infantile
hemangiomas. Arch Ophthalmol. 129:899–903. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moodley ST, Hudson DA, Adams S and Adams
KG: Shouldn't propranolol be used to treat all haemangiomas?
Aesthetic Plast Surg. 39:963–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schiestl C, Neuhaus K, Zoller S, Subotic
U, Forster-Kuebler I, Michels R, Balmer C and Weibel L: Efficacy
and safety of propranolol as first-line treatment for infantile
hemangiomas. Eur J Pediatr. 170:493–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vercellino N, Romanini MV, Pelegrini M,
Rimini A, Occella C and Dalmonte P: The use of propranolol for
complicated infantile hemangiomas. Int J Dermatol. 52:1140–1146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawley LP, Siegfried E and Todd JL:
Propranolol treatment for hemangioma of infancy: Risks and
recommendations. Pediatr Dermatol. 26:610–614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, Ma Q, Shen S and Hu H: Inhibition
of pancreatic cancer cell proliferation by propranolol occurs
through apoptosis induction: The study of beta-adrenoceptor
antagonist's anticancer effect in pancreatic cancer cell. Pancreas.
38:94–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phillips JD, Zhang H, Wei T and Richter
GT: Expression of β-adrenergic receptor subtypes in proliferative,
involuted, and propranolol-responsive infantile hemangiomas. JAMA
Facial Plast Surg. 19:102–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caporali A, Martello A, Miscianinov V,
Maselli D, Vono R and Spinetti G: Contribution of pericyte
paracrine regulation of the endothelium to angiogenesis. Pharmacol
Ther. 171:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mancini AJ and Smoller BR: Proliferation
and apoptosis within juvenile capillary hemangiomas. Am J
Dermatopathol. 18:505–514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong XY, Guo LL, Wei F, Li JF, Jiang ML,
Li GM, Zhao YD and Chen H: Some characteristics and functional
properties of rapeseed protein prepared by ultrasonication,
ultrafiltration and isoelectric precipitation. J Sci Food Agric.
91:1488–1498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahogo CK, Ezzedine K, Prey S, Colona V,
Diallo A, Boralevi F, Taïeb A and Léauté-Labrèze C: Factors
associated with the relapse of infantile haemangiomas in children
treated with oral propranolol. Br J Dermatol. 169:1252–1256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tu JB, Li QY, Jiang F, Hu XY, Ma RZ, Dong
Q, Zhang H, Pattar P and Li SX: Pingyangmycin stimulates apoptosis
in human hemangioma-derived endothelial cells through activation of
the p53 pathway. Mol Med Rep. 10:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye S, Zhao XY, Hu XG, Li T, Xu QR, Yang
HM, Huang DS and Yang L: TP53 and RET may serve as biomarkers of
prognostic evaluation and targeted therapy in hepatocellular
carcinoma. Oncol Rep. 37:2215–2226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Homo sapiens tumor protein p53 (TP53),
transcript variant 1, mRNA-Nucleotide-NCBI. 2017.
|
|
27
|
Li Y, Peart MJ and Prives C: Stxbp4
regulates DeltaNp63 stability by suppression of RACK1-dependent
degradation. Mol Cell Biol. 29:3953–3963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong A, Hardy KL, Kitajewski AM, Shawber
CJ, Kitajewski JK and Wu JK: Propranolol accelerates adipogenesis
in hemangioma stem cells and causes apoptosis of hemangioma
endothelial cells. Plast Reconstr Surg. 130:1012–1021. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji Y, Li K, Xiao X, Zheng S, Xu T and Chen
S: Effects of propranolol on the proliferation and apoptosis of
hemangioma-derived endothelial cells. J Pediatr Surg. 47:2216–2223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weibel ER and Palade GE: New cytoplasmic
components in arterial endothelia. J Cell Biol. 23:101–112. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frieden IJ, Eichenfield LF, Esterly NB,
Geronemus R and Mallory SB: Guidelines of care for hemangiomas of
infancy. American Academy of Dermatology Guidelines/Outcomes
Committee. J Am Acad Dermatol. 37:631–637. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haimowitz JE: Guidelines of care:
Hemangiomas of infancy. J Am Acad Dermatol. 39:6621998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lakshmi Chandrasekar K, Sankarapandiyan S
and Mohanarangam Pulivadula VS: Intramuscular haemangioma with
diagnostic challenge: A cause for strange pain in the masseter
muscle. Case Rep Dent. 2014:2858342014.PubMed/NCBI
|
|
36
|
Cho JK, Cha W and Sung MW: Intramuscular
hemangioma in the anterior scalene muscle diagnosed by core needle
biopsy. Clin Exp Otorhinolaryngol. 8:298–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Apfelberg DB, Maser MR and Lash H: Argon
laser treatment of cutaneous vascular abnormalities: Progress
report. Ann Plast Surg. 1:14–18. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Edgerton MT: The treatment of hemangiomas:
with special reference to the role of steroid therapy. Ann Surg.
183:517–532. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kveton JF and Pillsbury HC: Conservative
treatment of infantile subglottic hemangioma with corticosteroids.
Arch Otolaryngol. 108:117–119. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Léauté-Labrèze C, Hoeger P,
Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ,
Caceres H, Gutierrez Lopez JC, Ballona R, et al: A randomized,
controlled trial of oral propranolol in infantile hemangioma. N
Engl J Med. 372:735–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Drolet BA, Frommelt PC, Chamlin SL,
Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE,
Liberman L, et al: Initiation and use of propranolol for infantile
hemangioma: Report of a consensus conference. Pediatrics.
131:128–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin TT and He YJ: The advance of
β-blockers in the treatment of infantile hemangiomas. Zhonghua Yan
Ke Za Zhi. 49:1138–1144. 2013.(In Chinese). PubMed/NCBI
|
|
43
|
Kum JJ and Khan ZA: Propranolol inhibits
growth of hemangioma-initiating cells but does not induce
apoptosis. Pediatr Res. 75:381–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Munabi NC, England RW, Edwards AK,
Kitajewski AA, Tan QK, Weinstein A, Kung JE, Wilcox M, Kitajewski
JK, Shawber CJ and Wu JK: Propranolol targets hemangioma stem cells
via cAMP and mitogen-activated protein kinase regulation. Stem
Cells Transl Med. 5:45–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
El-Gibaly AM, Scheuer C, Menger MD and
Vollmar B: Improvement of rat liver graft quality by
pifithrin-alpha-mediated inhibition of hepatocyte necrapoptosis.
Hepatology. 39:1553–1562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leker RR, Aharonowiz M, Greig NH and
Ovadia H: The role of p53-induced apoptosis in cerebral ischemia:
effects of the p53 inhibitor pifithrin alpha. Exp Neurol.
187:478–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murphy PJ, Galigniana MD, Morishima Y,
Harrell JM, Kwok RP, Ljungman M and Pratt WB: Pifithrin-alpha
inhibits p53 signaling after interaction of the tumor suppressor
protein with hsp90 and its nuclear translocation. J Biol Chem.
279:30195–30201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Longobardi L, Torello M, Buckway C, O'Rear
L, Horton WA, Hwa V, Roberts CT Jr, Chiarelli F, Rosenfeld RG and
Spagnoli A: A novel insulin-like growth factor (IGF)-independent
role for IGF binding protein-3 in mesenchymal chondroprogenitor
cell apoptosis. Endocrinology. 144:1695–1702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ali O, Cohen P and Lee KW: Epidemiology
and biology of insulin-like growth factor binding protein-3
(IGFBP-3) as an anti-cancer molecule. Horm Metab Res. 35:726–733.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Grimberg A, Liu B, Bannerman P, El-Deiry
WS and Cohen P: IGFBP-3 mediates p53-induced apoptosis during serum
starvation. Int J Oncol. 21:327–335. 2002.PubMed/NCBI
|
|
53
|
Fernandez-Pineda I, Williams R,
Ortega-Laureano L and Jones R: Cardiovascular drugs in the
treatment of infantile hemangioma. World J Cardiol. 8:74–80. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tian Y, Xu DP, Tong S, Xi SL, Liu ZM and
Wang XK: Oral propranolol for the treatment of infantile
hemangiomas in the post-proliferative phase: A-single center
retrospective study of 31 cases. J Oral Maxillofac Surg.
74:1623–1629. 2016. View Article : Google Scholar : PubMed/NCBI
|