Introduction
Bone cysts and osteosarcoma are tumor-like lesions
of the bone. Bone cysts are mainly treated with surgery, which is
associated with good prognosis; however, osteosarcoma is the most
common malignant bone tumor that has poor prognosis, often
resulting in metastatic disease (1–3). It
represents 15% of all primary bone tumors and 0.2% of all malignant
tumors in children and young adults (4–7).
Currently, the main treatment for osteosarcoma is primary surgical
control combined with systemic chemotherapy. Although the 5-year
survival rate in patients with localized osteosarcoma is improved
to ~60% with this treatment, it is difficult for patients with
osteosarcoma at advanced stage to be cured (8,9).
Brain type glycogen phosphorylase (PYGB), which is
encoded by the PYGB gene, catalyzes the rate-determining step in
glycogen degradation (10,11). It is upregulated by adenosine
monophosphate, and downregulated by adenosine triphosphate and
adenosine diphosphate (12,13).
Previous studies reported that PYGB was overexpressed in various
types of cancers, including colorectal, gastrointestinal and
non-small cell lung cancer (14–16).
Due to the positive regulation of PYGB during the transitional
process of adenoma cells to carcinoma cells, PYGB may be a useful
biomarker to detect malignancy potential in precancerous lesions.
Thus, the present study attempted to explore the role served by the
PYGB gene in human osteosarcoma in order to identify a potential
molecular marker for early diagnosis and treatment in clinical
practice.
In the present study, the human osteosarcoma cell
lines MG63 and HOS, with overexpressed PYGB, were transfected with
PYGB small interfering (si)RNA. MG63 and HOS with PYGB knocked down
were evaluated for cell proliferation, cell apoptosis, cell cycle
distribution, invasion, migration and associated protein
expression. The aim of the present study was to investigate the
role of PYGB in the progression of osteosarcoma and explore novel
therapeutic methods for the treatment of osteosarcoma.
Materials and methods
Tissue samples collection
Between January 2014 to December 2014, 15 patients
with bone cysts (9 males and 7 females, age range: 5–59 years) and
35 patients with osteosarcoma (20 males and 15 females, age range:
8–55 years) were enrolled in the present study. The exclusion
criteria were bone metastasis, rheumatoid arthritis and
unwillingness to participate in the study. The study protocol was
approved by the independent Ethical Committee of Zhongnan Hospital
of Wuhan University (Hubei, China) and written informed consent was
obtained from all participants. The bone cysts or osteosarcoma
tissues were collected from all participants during routine surgery
at Zhongnan Hospital of Wuhan University and kept at −80°C until
use.
Cell culture and transfection
MG63, HOS, U-20S, SaoS-2 and SW1353 cells were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). All the cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal calf
serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
100X mycillin in 5% CO2 at 37°C. Cell viability was
evaluated by trypan blue staining at room temperature for 1 min and
observed using a light microscope (Olympus Corporation, Tokyo,
Japan) when the cells reached 90% confluence. Cells with 95% cell
viability were digested with 0.25% trypsin (Beijing Solarbio
Science & Technology Co., Ltd.) and seeded into 6-well plates
(5×105 cells/well) prior to transfection. A total of 3
PYGB siRNAs (Shanghai Genepharma, Co., Ltd., Shanghai, China) with
different interference sites and control siRNA (NC; Shanghai
Genepharma, Co., Ltd.) were used for transfection (Table I). Lipofectamine 2000™ (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
transfect 5 µl PYGB siRNA or NC siRNA into MG63 and HOS cells.
Cells without any treatment were served as a Control cells. At 48 h
following transfection, gene knockdown was confirmed by western
blotting as described below. Cells with the PYGB gene knocked down
were collected for subsequent experiments.
 | Table I.Sequences of the 3 interference sites
of Brain type glycogen phosphorylase small interfering RNA. |
Table I.
Sequences of the 3 interference sites
of Brain type glycogen phosphorylase small interfering RNA.
| Interference
site | Position (bp) | Sequence (5′-3′) |
|---|
| 1 | 941–963 |
GGUCCUGUAUCCAAAUGAU |
| 2 | 1,864–1,886 |
CCCUGUACAAUCGAAUCAA |
| 3 | 431–453 |
CUGCGAUGAAGCCAUCUAU |
| NC |
|
CCUAAGGUUAAGUCGCCCUCG |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA expression of PYGB in the 50 tissue samples
and the cell lines was measured by RT-qPCR. TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
and quantify total RNA from tissue samples or cultured cells. A
Revert-aid reverse transcription kit (Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA) and a SYBR-Green PCR kit
(Fermentas; Thermo Fisher Scientific, Inc.) were used to perform
RT-qPCR according to the manufacturer's protocol. The temperature
protocol for RT was as follows: 37°C for 60 min, 85°C for 5 min and
4°C for 5 min. The thermocycling conditions for qPCR were as
follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec
and 60°C for 45 sec. Table II
presents the primers used for the amplification of PYGB and the
reference gene, GAPDH. Quantification was performed using the
2−∆∆Cq method as previously described (17).
 | Table II.Sequences of the primers used in for
reverse transcription-quantitative polymerase chain reaction. |
Table II.
Sequences of the primers used in for
reverse transcription-quantitative polymerase chain reaction.
| Primer | Direction | Sequence (5′-3′) |
|---|
| PYGB | Forward |
ACGCAGCAGCACTACTAC |
|
| Reverse |
TCGCAGGCATTCTGAAGG |
| GAPDH | Forward |
CACCCACTCCTCCACCTTTG |
|
| Reverse |
CCACCACCCTGTTGCTGTAG |
Western blot assay
Transfected cells were washed twice with 1X PBS,
followed by radioimmunoprecipitation assay lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.) containing a 0.01%
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany at 4°C. Lysed cells were centrifuged at 12,000 × g for 15
min at 4°C and the supernatant was collected. Proteins were
quantified using a Bicinchoninic Acid protein quantification kit
(Thermo Fisher Scientific, Inc.) and run on a 12% SDS-PAGE (30
µg/lane). Proteins were then transferred to a nitrocellulose filter
membrane (EMD Millipore, Billerica, MA, USA) electrophoretically
and blocked with 5% skim milk at room temperature for 1 h. The
membrane was then incubated with antibodies against PYGB (1:1,000;
ab154969; Abcam, Cambridge, MA, USA), E-cadherin (1:1,000; cat. no.
14472; CST Biological Reagents Co., Ltd., Shanghai, China), Twist
(1:500; ab175430), matrix metalloproteinase 9 (MMP9; 1:500;
ab119906), MMP2 (1:1,000; ab92536; all Abcam), B-cell lymphoma 2
(Bcl-2; 1:400; sc-492), Bcl-2-associated X protein (Bax; 1:500;
sc-493; both Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
GAPDH (1:1,500; 5174, CST Biological Reagents Co., Ltd.) at 4°C
overnight. Following further incubation with horseradish
peroxidase-conjugated goat anti-mouse (1:1,000; A0206) or goat
anti-rabbit (1:1,000; A0208) secondary antibodies (Beyotime
Institute of Biotechnology, Shanghai, China) at room temperature
for 1 h, the blots were observed visually using enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). ImageJ version
1.4.3 (National Institutes of Health, Bethesda, MD, USA) was used
for densitometry analysis.
Cell proliferation assay
Cultured and transfected cells were trypsinized with
0.25% trypsin (Beijing Solarbio Science & Technology Co.,
Ltd.), and diluted to 1–5×104 cells/ml. Each 1 ml of
cells was seeded into each well of 96-well plates. Plates were
incubated at 37°C for 0, 24, 48 and 72 h, hand mixed with 100 µl
DMEM containing 10% Cell Counting kit (CCK)-8 reagent (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan), then further
incubated for 1 h. Cell proliferation was evaluated using a
spectrophotometer at the optical density of 450 nm.
Cell apoptosis assay
Cultured and transfected cells were lightproof
stained using an Annexin V-fluorescein isothiocyanate apoptosis
detection kit (BD Biosciences, Franklin Lakes, NJ, USA) for 10 min
at room temperature. Cell apoptosis was then evaluated using a flow
cytometer (Accuri C6) and analyzed with Accuri C6 software, version
1.0.264 (both BD Biosciences).
Cell cycle assay
Cultured and transfected cells were fixed with 70%
pre-cooled ethanol at 4°C for 12 h following digestion with 0.25%
trypsin and stained with propidium iodide at 4°C for 10 min. DNA
content was measured using a flow cytometer (Accuri C6) and
analyzed with Accuri C6 software, version 1.0.264 (both BD
Biosciences).
Cell invasion and migration assay
using Transwell
Transfected cells were cultured in serum-free medium
for 24 h prior to inoculation. Cells were digested with 0.25
trypsin (Beijing Solarbio Science & Technology Co., Ltd.) and
diluted to 1×105 cells/ml using DMEM (Hyclone; GE
Healthcare Life Sciences) containing 1% fetal bovine serum (FBS,
Thermo Fisher Scientific, Inc.). In the cell invasion assay, the
upper chamber was coated with 80 µl Matrigel prior to the transfer
of cells. A total of 0.5 ml cell suspension was added into the
upper chamber; the lower chamber was filled with 0.75 ml DMEM
containing 10% FBS in each well. Following incubation at 37°C for
48 h, cells were fixed with 1 ml 4% methyl alcohol in each well at
room temperature for 10 min, and then stained with 1 ml 0.5%
crystal violet for at room temperature 30 min. The 24-well plates
were washed three times using 1X PBS and the number of invading
cells were counted in 3 randomly selected fields using a light
microscope (Olympus Corporation, Tokyo, Japan) under magnification,
×200.
Statistical analysis
Experiments were repeated three times. Data were
expressed as the mean ± standard deviation and were analyzed using
analysis of variance and Tukey's post hoc test. GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA) was used to
perform and analyze the data. RT-qPCR data was analyzed using ABI
Prism 7300 SDS Software (Applied Biosystems; Thermo Fisher
Scientific, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Screening of an osteosarcoma cell line
with a high expression level of PYGB
The gene expression level of PYGB was significantly
different between normal (bone cyst; n=15) and tumor tissues from
35 patients with osteosarcoma (P<0.01; Fig. 1A). In addition, MG63 and HOS cells
exhibited significantly higher expression levels of PYGB than the
other osteosarcoma cell lines including U-20S, SaoS-2 and SW1353
detected by the western blot assay (P<0.001; Fig. 1B and C). Therefore, the human
osteosarcoma cell lines MG63 and HOS were selected for the
subsequent experiments.
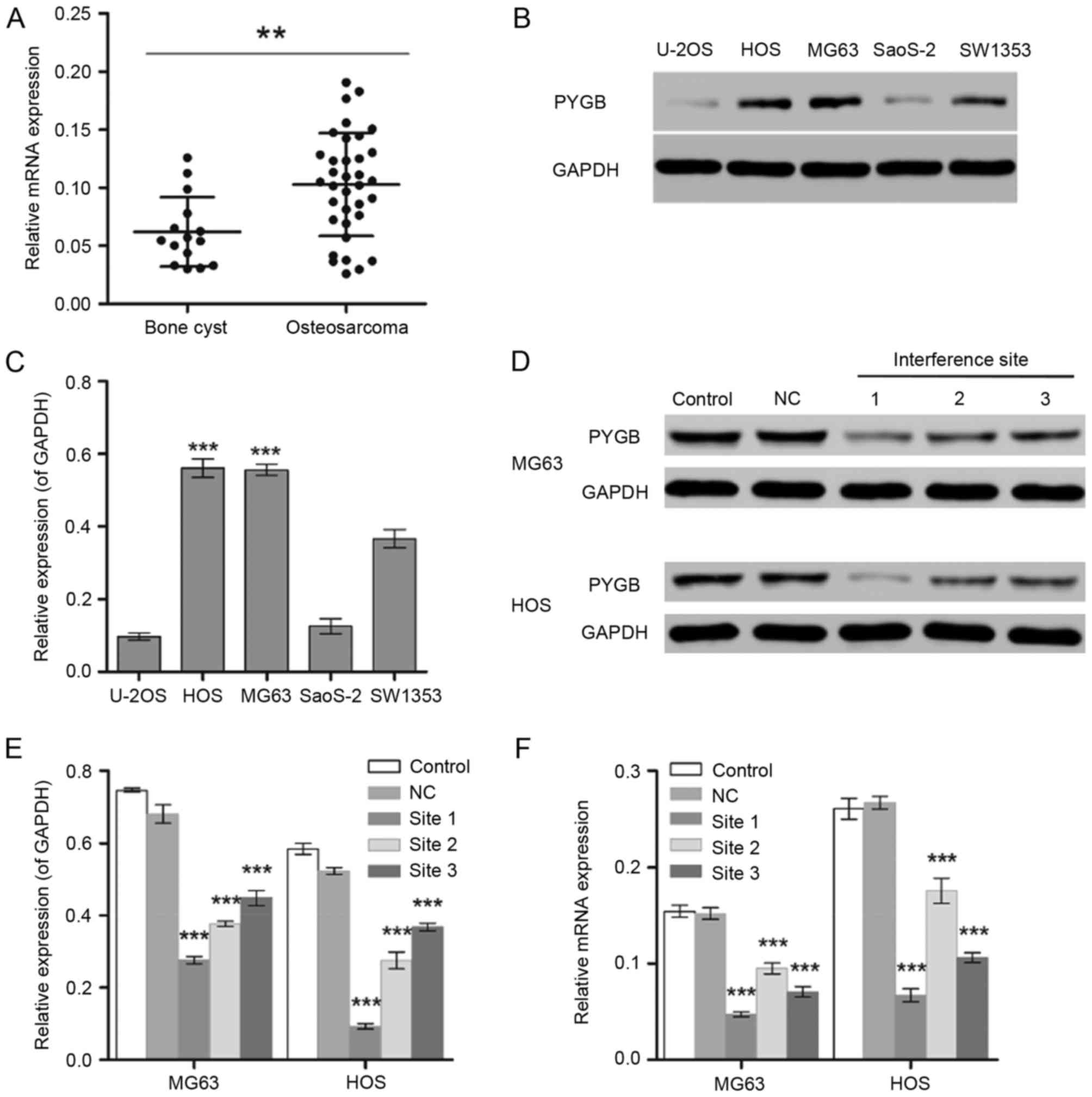 | Figure 1.Screening of osteosarcoma cell lines
with high expression level of PYGB. (A) RT-qPCR was used to measure
the mRNA expression levels of PYGB in 15 bone cyst samples and 35
osteosarcoma samples. **P<0.01, as indicated. (B and C) The
protein expression levels of PYGB in the human osteosarcoma cell
lines U-20S, HOS, MG63, SaoS-2 and SW1353 were detected by western
blot analysis. ***P<0.001 vs. U-2OS, SaoS-2 and SW1353. (D)
Western blotting was also used to measure the PYGB protein
expression in (E) MG63 and HOS cells following the transfection of
PYGB siRNA at 3 interference sites. (F) In addition, the mRNA
expression level of PYGB was also measured using RT-qPCR.
***P<0.001 vs. the NC group. Data are expressed as the mean ±
standard deviation (n=3). PYGB, Brain type glycogen phosphorylase;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; NC, negative control; siRNA, small interfering RNA. |
PYGB siRNA inhibits cell
proliferation
The interference effect of PYGB siRNA, at 3
interference sites, on MG63 and HOS cells was determined using
western blotting and RT-qPCR. The protein expression level of PYGB
at all 3 interference sites declined significantly in MG63 and HOS
cells (P<0.001; Fig. 1D and E).
The mRNA expression of PYGB also decreased significantly when
compared with the negative control group (Fig. 1F), indicating the efficient
interference ability of PYGB siRNA at interference site 1. In
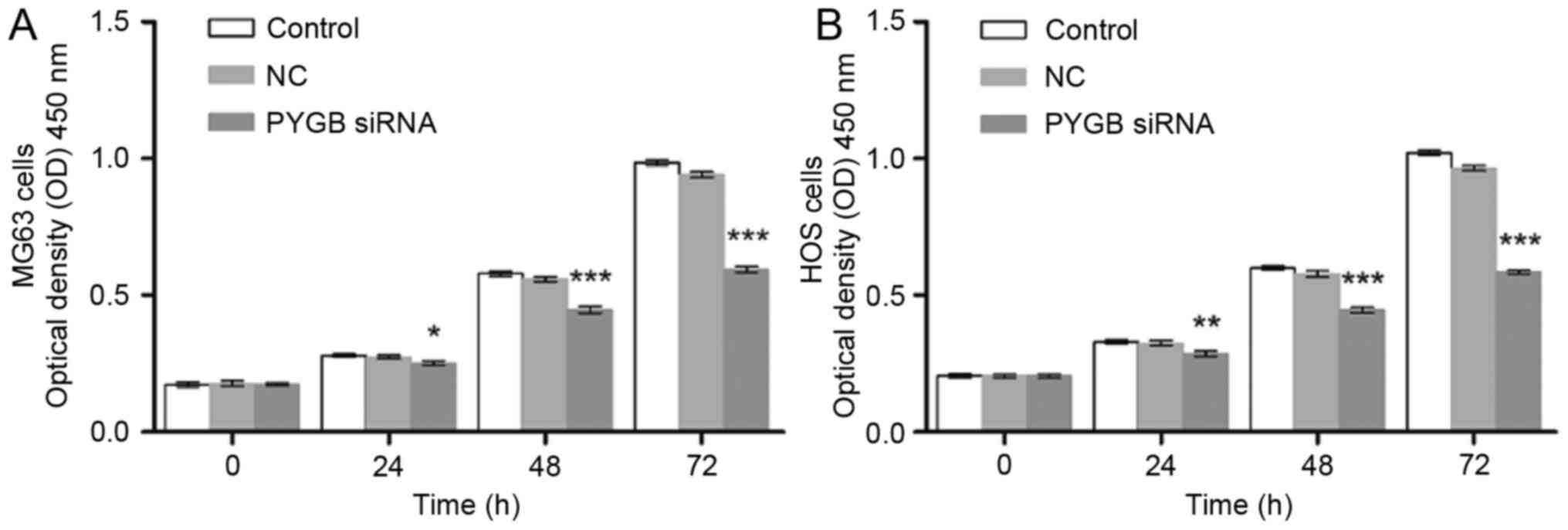
addition, CCK8 was employed to evaluate the cell viability of MG63
and HOS cells. As a result, the cell proliferation of PYGB siRNA
transfected MG63 and HOS cells declined in a time-dependent manner
(P<0.05, P<0.01 and P<0.001; Fig. 2) indicating that siPYGB inhibited
cell viability.
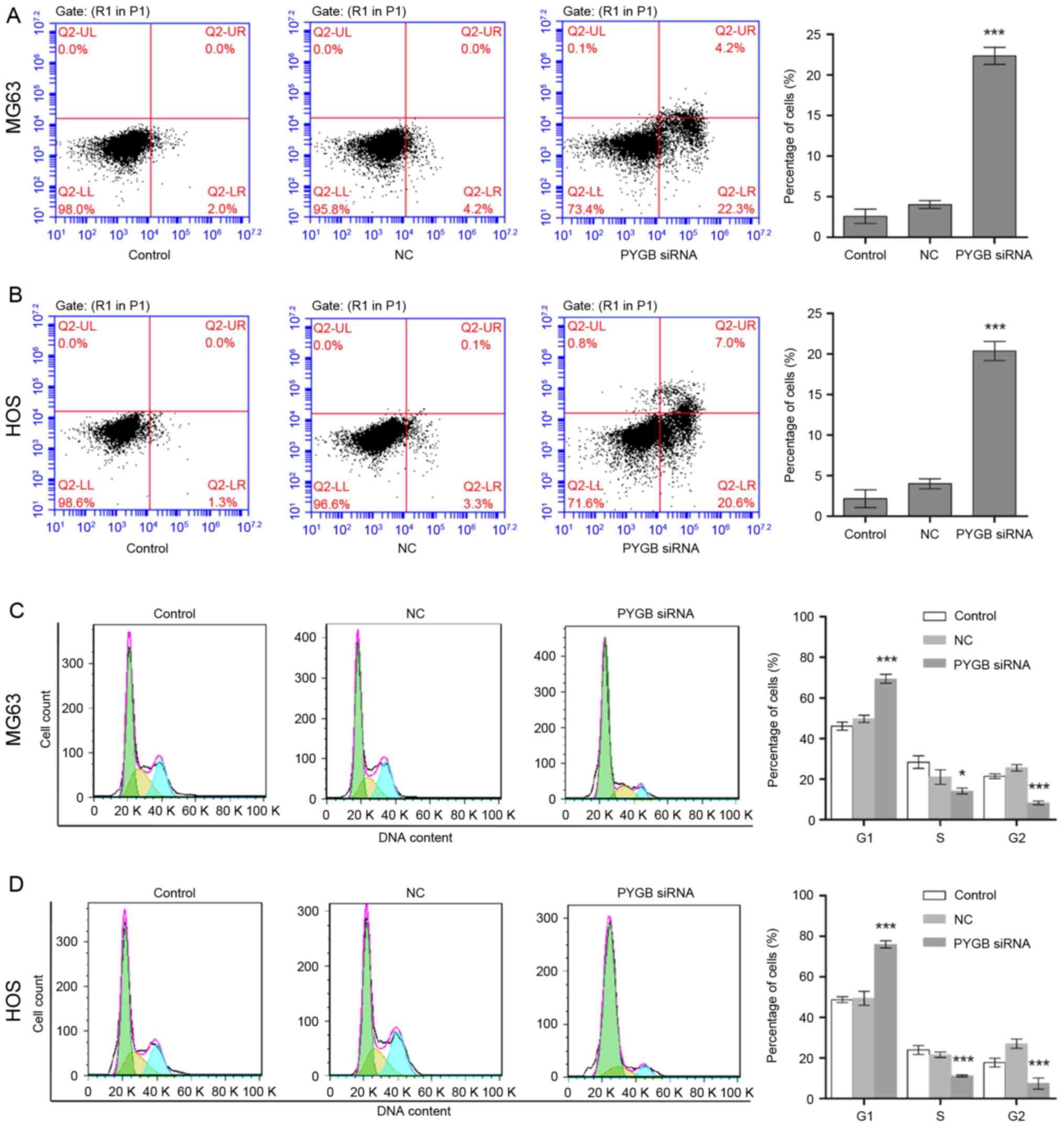
PYGB siRNA induces cell apoptosis
The apoptotic rate of MG63 and HOS cells was
measured through flow cytometry following transfection for 48 h.
Apoptotic rate was calculated from the percentage of early
apoptotic cells shown in the lower right quadrant (Fig. 3A and B). The results demonstrated
that the apoptotic rate of MG63 cells increased to 22.33±2.14% in
comparison with the 4.03±0.77% of the negative control group (n=3;
P<0.001; Fig. 3A). Similarly,
the apoptotic rate of HOS cells rose from 4.00±0.68 to 20.37±1.68%
(n=3; P<0.001; Fig. 3B),
indicating cell apoptosis was induced due to siPYGB, which in turn
was suggestive of the important role of PYGB in osteosarcoma.
PYGB siRNA arrests cell cycle
Cell cycle distribution of MG63 and HOS cells was
identified using flow cytometry following transfection for 48 h.
Cell cycle distribution was calculated according to the cell count
at each stage (Fig. 3C and D). The
results revealed that the percentage of PYGB siRNA treated MG63
cells in the G1 phase increased significantly from 49.74±1.54 to
69.41±2.35% (n=3; P<0.01; Fig.
3C) when compared with the negative control. While the
percentage of cells in the S and G2 phases decreased from
21.04±2.59 to 14.15±0.98% (n=3; P<0.05) and from 25.62±0.64 to
8.19±0.11% (n=3; P<0.01), respectively. In addition, a decline
in cell count was also observed in the S phase of HOS cells from
21.62±0.99 to 11.23±0.16% (n=3; P<0.001), and in the G2 phase
from 27.03±0.89 to 7.42±2.11% (n=3; P<0.001; Fig. 3D). However, an increase in the
percentage of G1 phase HOS cells was observed from 49.52±2.44 to
75.95±0.64%, suggesting that the cell cycle distribution was
arrested in the G1 phase.
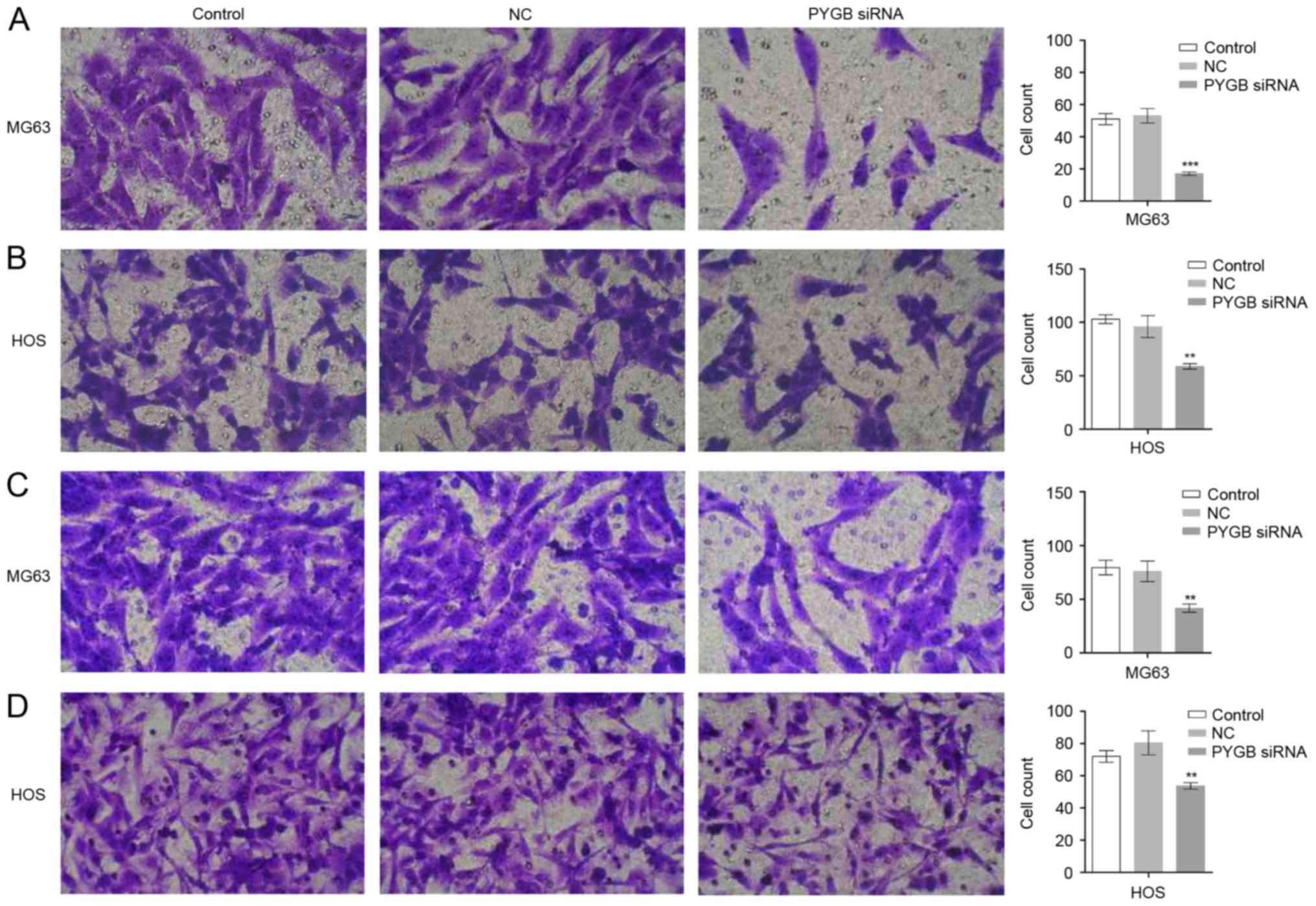
PYGB siRNA inhibits cell invasion and
migration
The invasion and migration ability of MG63 and HOS
cells was evaluated using a Transwell assay. As the results show in
Fig. 4A and B, the invaded MG63
and HOS cells observed were fewer in number in the PYGB siRNA group
than in the control and negative control groups. The cell count
also markedly declined in the PYGB siRNA group in MG63 and HOS
cells (P<0.01 and P<0.001), indicating that there was
significantly inhibited cell invasion in the two cell lines due to
siPYGB. Suppressed migration rate of MG63 and HOS cells was also
detected. A decreased number of migrated cells was observed in
Fig. 4C and D, and a decline in
cell count indicated that there was a significantly suppressed
migration rate in MG63 and HOS cells (Fig. 4B and D; P<0.01).
PYGB siRNA suppresses cell viability
through the Bcl/Caspase and cyclin dependent kinase (CDK1)
signaling pathway
Protein expression of the cell apoptosis associated
proteins Bcl-2 and Bax, as well as the invasion and migration
associated proteins E-cadherin, Twist, MMP9 and MMP2 were measured
by western blotting (Fig. 5). The
results revealed that Bax was significantly upregulated and Bcl-2
was significantly downregulated in MG63 and HOS cells, indicating
that cell apoptosis was induced, and in turn, that the Bcl/Caspase
signaling pathway was activated (n=3; P<0.001). In addition, the
expression levels of Twist, MMP9 and MMP2 were significantly
downregulated in MG63 and HOS cells when compared with the negative
control (n=3; P<0.05, P<0.01 and P<0.001). The protein
levels of E-cadherin increased in MG63 and HOS cells following
treatment with PYGB siRNA (n=3; P<0.001), indicating that cell
invasion and migration was inhibited.
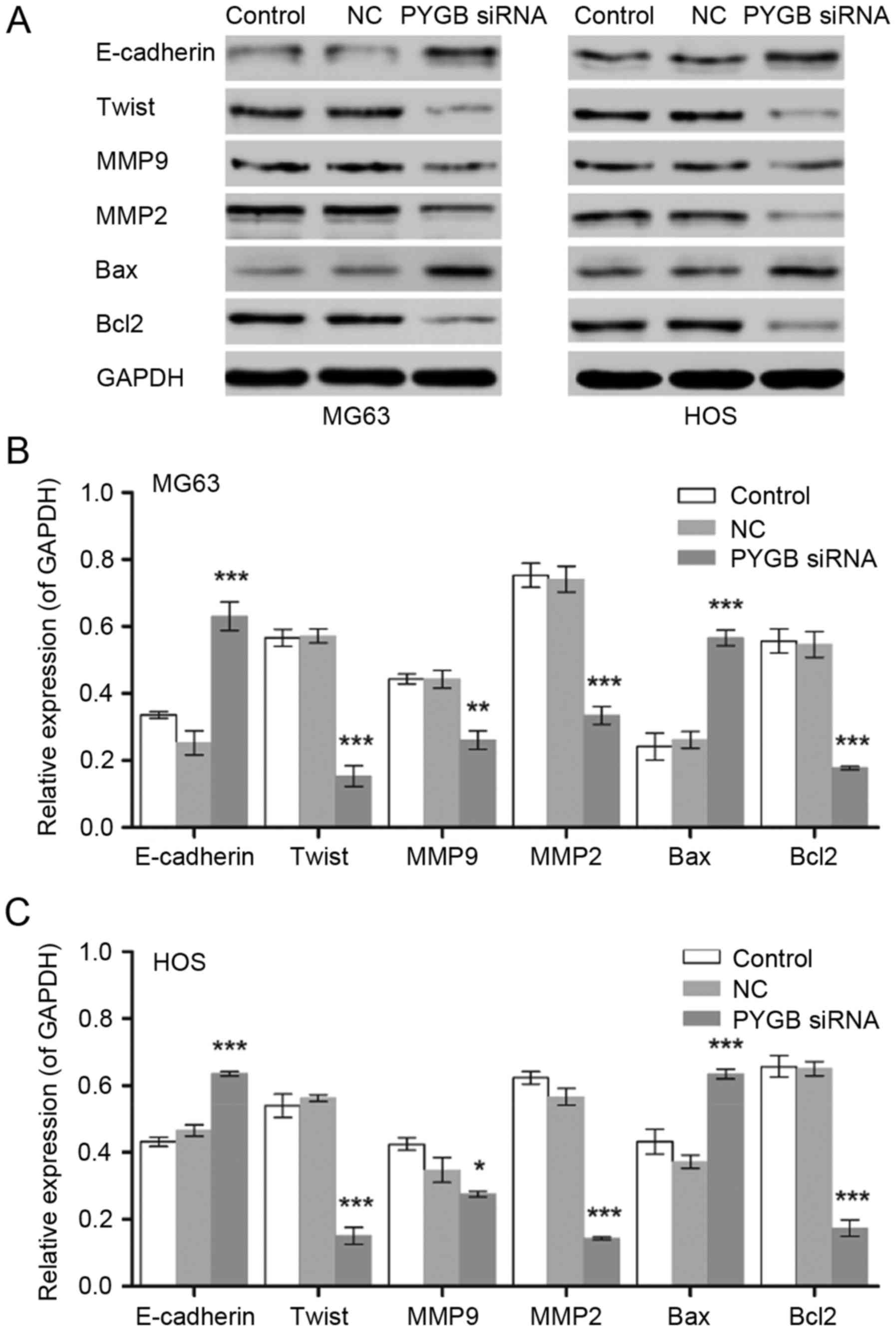 | Figure 5.Effect of PYGB siRNA on the expression
of E-cadherin, Twist, MMP9, MMP2, Bax and Bcl2 in the osteosarcoma
cell lines MG63 and HOS. (A) The blots show the results of western
blot analysis. The protein expression levels in (B) MG63 and (C)
HOS cells were measured by western blot analysis 48 h following
treatment with PYGB siRNA. *P<0.05, **P<0.01 and
***P<0.001 vs. the NC group. Data are expressed as the mean ±
standard deviation (n=3). PYGB, Brain type glycogen phosphorylase;
NC, negative control; siRNA, small interfering RNA; MMP, matrix
metalloproteinase; Bcl2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
Discussion
In the present study, a greater expression of PYGB
was detected in osteosarcoma samples when compared with bone cyst
samples. Furthermore, as the regulatory effect of siPYGB on the
cell proliferation, apoptosis, cell cycle, and cell invasion and
migration of the osteosarcoma cell lines MG63 and HOS, the
identification function of PYGB in osteosarcoma was demonstrated.
Bax and Bcl-2 were investigated in order to obtain further insight
into the potential mechanisms involved in cell apoptosis induced by
siPYGB in osteosarcoma cells. Bcl-2 belongs to the Bcl/B-cell
leukemia-2 family and is antagonistic towards Bax (18,19).
Bcl-2 has an anti-apoptosis function that inactivates the
pro-apoptotic proteins Caspase-3, Bax and Bcl-2 antagonist/killer,
and inhibits the release of apoptosis-promoting substances from the
mitochondria. In agreement with the cellular function of Bcl-2 and
Bax, induced cell apoptosis in PYGB siRNA treated MG63 and HOS
cells was accompanied by increased Bax and declined Bcl-2 protein
levels, which indicated that apoptosis in siPYGB transfected cells
was induced via the Bcl/Caspase signaling pathway; this in turn
suggested the vital role of PYGB in the survival of osteosarcoma
cells.
Tumor metastasis involves epithelial-mesenchymal
transition (EMT) and mesenchymal-epithelial transition (MET)
(20). MET is coupled with the
degradation of the extracellular matrix (ECM) by the MMP family
(21,22). EMT is accompanied by the
degradation of the basement membrane, which is normally responsible
for tissue organization maintenance; cell structural support is an
essential step for tumor metastasis (23). Type IV collagen is the most
abundant component of the basement membrane, and can easily be
degraded by MMP2 and MMP9 (21,22).
The declined expression levels of MMP2 and MMP9 in the present
study, suggested there may be stabilization of the ECM and a
decreased invasion rate of osteosarcoma cells.
E-cadherin belongs to the cadherin superfamily,
which serve an important role in the switch between EMT and MET.
Epithelial cells are held together tightly via a crucial type of
cell to cell adhesion. It has been reported to act as an invasion
suppressor gene in pre-invasive lobular breast carcinoma (24,25).
Mutations in this gene have been associated with gastric, breast,
colorectal, thyroid and ovarian cancer. Loss of E-cadherin
expression leads to the release of β-catenin into the cytoplasm and
results in the expression of EMT-inducing transcription factors
(26). However, cancer cells in
the mesenchymal state may undergo MET in certain favorable
microenvironments following migration to novel sites by E-cadherin
during metastasis (27). In
addition, upregulated E-cadherin induced cell-cell adhesions
between cancer cells at differentiated epithelial cell features in
order to form a novel tumor lesion (28). Twist is a highly conserved
transcription factor that belongs to the alkaline
spiral-ring-spiral protein family. In contrast to E-cadherin, it
serves an important role in the occurrence and development of
embryos as a key regulation factor in the process of
epithelium-interstitial change (29). Twist was demonstrated to be a
potential cancer gene protein that promotes the occurrence,
invasion, metastasis and tolerance, and inhibits the apoptosis, of
tumor cells (30). In the present
study, the upregulation of E-cadherin and downregulation of Twist
in the siPYGB group corresponded to the suppressed invasion and
migration rate, which indicated that there may be tight cell-cell
adhesion and relocation of tumor cells during the metastasis of
MG63 and HOS cells. In view of the decreased Twist and increased
E-cadherin expression levels, the cell invasion and migration of
the human osteosarcoma cell lines MG63 and HOS may be inhibited by
PYGB siRNA via the CDK1 signaling pathway.
In conclusion, in the present study PYGB
interference resulted in inhibited cell proliferation, arrested
cell cycle, induced cell apoptosis, and suppressed cell invasion
and migration, potentially via the Caspase/Bcl and CDK1 signaling
pathway. Thus, the significant inhibitory effect of PYGB siRNA on
the cell viability of the human osteosarcoma cell lines MG63 and
HOS was demonstrated. The findings of the present study suggested
that PYGB may considered as a therapeutic target for
osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SZ, JL and WJ conceived and designed the study. SZ,
YCZ, YYZ, YY and LW performed the experiments. SZ and WJ wrote the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the independent
Ethical Committee of Zhongnan Hospital of Wuhan University (Hubei,
China) and written informed consent was obtained from all
participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anoop T, Geetha N, Babanrao SA and
Jayasree K: Primary osteosarcoma of rib mimicking lung mass with
secondary aneurysmal bone cyst formation. J Thorac Oncol.
9:738–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Do T, Renker EK and Weber MA: Simulation
of teleangiectactic osteosarcoma by aneurysmatic bone cyst.
Orthopade. 42:1067–1070. 2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janevska V, Spasevska L, Samardziski M,
Nikodinovska V, Zhivadinovik J and Trajkovska E: From aneurysmal
bone cyst to telangiectatic osteosarcoma with metastasis in
inguinal lymph nodes-Case report. Med Pregl. 68:127–132. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aung L, Gorlick RG, Shi W, Thaler H,
Shorter NA, Healey JH, Huvos AG and Meyers PA: Second malignant
neoplasms in long-term survivors of osteosarcoma. Cancer.
95:1728–1734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bacci G, Ferrari C, Longhi A, Ferrari S,
Forni C, Bacchini P, Palmerini E, Briccoli A, Pignotti E,
Balladelli A and Picci P: Second malignant neoplasm in patients
with osteosarcoma of the extremities treated with adjuvant and
neoadjuvant chemotherapy. J Pediatr Hematol Oncol. 28:774–780.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagarajan R, Kamruzzaman A, Ness KK,
Marchese VG, Sklar C, Mertens A, Yasui Y, Robison LL and Marina N:
Twenty years of follow-up of survivors of childhood osteosarcoma: A
report from the Childhood Cancer Survivor Study. Cancer.
117:625–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaffe N: Historical perspective of the
treatment of osteosarcoma: An interview with Dr Norman Jaffe.
Interview by Margaret pearson. J Pediatr Oncol Nurs. 15:90–94.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longhi A, Ferrari S, Tamburini A, Luksch
R, Fagioli F, Bacci G and Ferrari C: Late effects of chemotherapy
and radiotherapy in osteosarcoma and ewing sarcoma patients: The
Italian Sarcoma Group Experience (1983–2006). Cancer.
118:5050–5059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Newgard CB, Hwang PK and Fletterick RJ:
The family of glycogen phosphorylases: Structure and function. Crit
Rev Biochem Mol Biol. 24:69–99. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Newgard CB, Littman DR, van Genderen C,
Smith M and Fletterick RJ: Human brain glycogen phosphorylase.
Cloning, sequence analysis, chromosomal mapping, tissue expression,
and comparison with the human liver and muscle isozymes. J Biol
Chem. 263:3850–3857. 1988.PubMed/NCBI
|
|
12
|
Philips KB, Kurtoglu M, Leung HJ, Liu H,
Gao N, Lehrman MA, Murray TG and Lampidis TJ: Increased sensitivity
to glucose starvation correlates with downregulation of glycogen
phosphorylase isoform PYGB in tumor cell lines resistant to
2-deoxy-D-glucose. Cancer Chemother Pharmacol. 73:349–361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimada S, Maeno M, Akagi M, Hatayama I,
Sato T and Sato K: Immunohistochemical detection of glycogen
phosphorylase isoenzymes in rat and human tissues. Histochem J.
18:334–338. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barbosa AJ and Castro LP: BGP expression
in gastric epithelium and early gastric cancer. Gastric Cancer.
5:123–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada S, Shiomori K, Honmyo U, Maeno M,
Yagi Y and Ogawa M: BGP expression in gastric biopsies may predict
the development of new lesions after local treatment for early
gastric cancer. Gastric Cancer. 5:130–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tashima S, Shimada S, Yamaguchi K, Tsuruta
J and Ogawa M: Expression of brain-type glycogen phosphorylase is a
potentially novel early biomarker in the carcinogenesis of human
colorectal carcinomas. Am J Gastroenterol. 95:255–263. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kirkin V, Joos S and Zörnig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manion MK and Hockenbery DM: Targeting
Bcl-2 related proteins in cancer therapy. Cancer Biol Ther. 2 4
Suppl 1:S105–S114. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waerner T, Alacakaptan M, Tamir I,
Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M and Beug
H: ILEI: A cytokine essential for EMT, tumor formation, and late
events in metastasis in epithelial cells. Cancer Cell. 10:227–239.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halbleib JM and Nelson WJ: Cadherins in
development: Cell adhesion, sorting, and tissue morphogenesis.
Genes Dev. 20:3199–3214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rothberg Gould BE and Bracken MB:
E-cadherin immunohistochemical expression as a prognostic factor in
infiltrating ductal carcinoma of the breast: A systematic review
and meta-analysis. Breast Cancer Res Treat. 100:139–148. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shibata T and Hirohashi S: E-cadherin cell
adhesion system in human cancer. Seikagaku. 78:647–656. 2006.(In
Japanese). PubMed/NCBI
|
|
26
|
Tycko B, Li CM and Buttyan R: The
Wnt/beta-catenin pathway in Wilms tumors and prostate cancers. Curr
Mol Med. 7:479–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christofori G and Semb H: The role of the
cell-adhesion molecule E-cadherin as a tumour-suppressor gene.
Trends Biochem Sci. 24:73–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leptin M: Twist and snail as positive and
negative regulators during Drosophila mesoderm development. Genes
Dev. 5:1568–1576. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vesuna F, van Diest P, Chen JH and Raman
V: Twist is a transcriptional repressor of E-cadherin gene
expression in breast cancer. Biochem Biophys Res Commun.
367:235–241. 2008. View Article : Google Scholar : PubMed/NCBI
|