Introduction
Cervical cancer is the fourth most common cancer
diagnosed among women, with ~520,000 novel cases each year
worldwide. It has been predicted that there will be 13,000 novel
cases of cervical cancer diagnosed each year in the United States,
with 5,000 more mortalities due to disease progression (1). Metastasis to pelvic and aortic lymph
nodes is a common characteristic of cervical cancer (2). Therefore, inhibition of metastasis is
essential to improve prognosis and to design effective therapeutic
methods. Recently, a variety of molecular and biochemical factors
have been identified to influence metastasis and the outcome of
cervical cancer (3). Site-specific
therapy provides a novel approach for tumor treatment; however, the
associated side effects cause injury in the adjacent tissues, and
limit the effectiveness and safety of this treatment method
(4). Currently, more effective
therapies with fewer side effects are required. Gene therapy is one
approach used to treat cervical cancer, which inhibits tumor cell
proliferation and also sensitizes tumor cells to chemotherapy
(5).
Survivin (molecular weight, 16.5 kDa), the smallest
member of the inhibitory apoptotic protein family, has demonstrated
a dual role in the control of apoptosis and regulation of cell
division (6). As an antiapoptotic
protein, survivin is expressed in a number of human neoplasm
tissues; however, it is undetectable in terminally differentiated
normal tissues with the exception of the thymus, basal colonic
epithelium and endothelial cells during angiogenesis (7–9).
Survivin expression is associated with apoptosis, tumor aggression
and recurrence, poor survival and drug resistance (10,11).
The distinct expression and functional profiles of survivin in
tumor tissues compared with normal tissues make it a potential
target for cervical cancer treatment (12). Inhibition of survivin has been
extensively investigated using different approaches, including
antisense oligonucleotide, dominant negative mutants, RNA
interference and cancer vaccines (13–15).
Downregulation of survivin effectively prevents tumor growth and
increases the susceptibility to chemotherapy (16,17).
Survivin expression is thought to be a standard method to evaluate
therapeutic efficacy (18).
However, whether liposome-plasmid DNA encoding mutant survivin-T34A
could prevent cervical cancer growth remains to be elucidated. In
the present study, a plasmid with mutant survivin-T34A was
synthesized and a liposome was constructed using
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP). The present study
aimed to evaluate the anti-cancer activity of a liposome-plasmid
complex in cervical cancer in vivo.
Materials and methods
Preparation of plasmids with
survivin-T34A
A pVITRO2 plasmid (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) expressing mutant survivin-T34A
was synthesized. Briefly, cDNA clone encoding mutant survivin-T34A
was a gift from Li Pan (West China Medical School, Sichuan
University) and amplified by polymerase chain reaction (PCR) (DNA
polymerase: cat. no. D7220; Beyotime Institute of Biotechnology,
Haimen, China) with the following primers: Forward,
5′-GATCACGCGTCACCATGGGAGC-3′ and reverse,
5′-GGCGGTCGACAGCATTAGGCAG-3′. The PCR protocol was as follows: 95°C
initial denaturation 5 min, 95°C denaturation 30 sec, 58°C
annealing 16 sec, 72°C extension 30 sec for 36 cycles.
Subsequently, the clone was digested with SalI/MluI and then
inserted into the pVITRO2 plasmid. In a previous study, Pan et
al (18) demonstrated that
DOTAP-chol liposome encoding survivin-T34A could down-regulate
survivin expression and pVITRO2 plasmid without mutant T34A
(pVITRO2-null) did not affect survivin expression. Colonies of
E. coli were cultured in Luria-Bertani broth (BW30620044;
Bioworld Technology, Inc., St. Louis Park, MN, USA) containing 100
µg/ml ampicillin. Large-scale plasmid survivin-T34A was purified
using a Qiagen EndoFree Plasmid Giga kit (cat. no. 12391; Qiagen,
Inc., Valencia, CA, USA) according to the manufacturer's protocol.
The purity (optical density 260/280) values for the prepared
plasmid DNA were equal to 1.8–2.0. Plasmids were stored at −20°C
for subsequent experimentation.
Preparation of liposome DOTAP
Liposome was prepared according to the procedure
described previously (19). DOTAP
and cholesterol (1:1; both Avanti Polar Lipids, Inc., Alabaster,
AL, USA) were dissolved in chloroform in a 100 ml-round-bottomed
flask and rotated to obtain a thin lipid layer. Subsequently, the
mixture was dried and treated with a vacuum for 2 h at 4°C in order
to remove the organic solvent. The lipid layer was rehydrated using
5% dextrose (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
water to obtain a final concentration of 10 mg/ml and eddied for 30
min at 60°C. Finally, the film was extruded through a 100-nm
polycarbonate filter using a Mini-Extruder (Avanti Polar Lipids,
Inc.). Liposomes were stored at 4°C until further use. Prior to
use, the liposome-plasmid complex was freshly prepared at a ratio
of 10:1 (liposome:plasmid) and incubated for 20 min at room
temperature prior to injection.
Cell culture
The human cervical cancer cell line (HeLa) was
purchased from the Cell Center, Institute of Basic Medical
Sciences, Xiehe Medical University (Beijing, China). Cells were
cultured at 37°C, in an atmosphere of 5% CO2 in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal calf serum (Hyclone;
GE Healthcare, Chicago, IL, USA), 100 U/ml penicillin and 100 mg/ml
streptomycin.
Animal model
A total of 40 female BALB/c nude mice (16±1 g;
5–6-weeks old) were purchased from Vital River Laboratories Co.,
Ltd. (Beijing, China). Mice were housed and maintained in specific
pathogen-free conditions at a temperature of 23±2°C, a relative
humidity of 45–65%, and a controlled 12/12 h light/dark cycle.
Animals had ad libitum access to food and water. All
protocols were approved and supervised by the State Key Laboratory
of Biotherapy Animal Care and Use Committee of Sichuan University
(Chengdu, China). HeLa cells (1×107) were diluted in 0.2
ml PBS and administered to BALB/c nude mice by intraperitoneal
injection (i.p.) on day 0. Three days following inoculation, the 40
mice were randomly divided into four groups (n=10/group): i) The
normal saline group (NS; 100 µl sterile saline once/3 days for 15
days); ii) the DOTAP control group (100 µg DOTAP once/3 days for 15
days); iii) the plasmid encoding mutant survivin-T34A (PST34A)
group (10 µg PST34A once/3 days for 15 days); and iv) the
PST34A+DOTAP group (10 µg PST34A+100 µg DOTAP once/3 days for 15
days). The treatment was administered via i.p. injection once every
three days in 100 µl volume saline; four injections were
administered in total over 15 days. The general health of the mice
was monitored daily. On day 15, all mice were sacrificed by
dislocation of the neck and metastasis was evaluated. At the time
of sacrifice, tumor tissue, ascitic fluid and the vital organs of
the mice were harvested, and body weight, ascitic fluid volume,
tumor weight and the number of tumor nodules were counted. Ascitic
fluid, and tumor and normal tissues were used for further study.
Tumor specimen were fixed by paraformaldehyde (4%, pH 7.4) at 4°C
overnight and embedded in paraffin for tissue sectioning (4 µm)
vital organs (spleen, liver, kidneys, heart and lungs) were also
harvested and stored at −80°C for later assessment of tissue
toxicity.
Flow cytometry
Ascitic fluid from the NS, DOTAP, PST34A and
PST34A+DOTAP treatment groups was collected. A total of 5 ml normal
saline solution was injected into abdomen cavity and withdrawn from
mice with ascitic fluid. All specimens were washed with PBS,
resuspended in propidium iodide/RNase A solution (5 ml; Beyotime
Institute of Biotechnology) and incubated in the dark at 4°C for 15
min. Samples were analyzed by flow cytometry using the
NovoCyte® Flow Cytometer System which included the
analysis software (ACEA Biosciences, San Diego, CA, USA).
Hematoxylin and eosin (H&E) and
immunohistochemical staining
Immunohistochemical analyses of proliferation marker
protein Ki-67 (Ki67) and microvessel density (MVD) were determined
using rabbit anti-human Ki67 (cat. no. NB500; Novus Biologicals,
LLC, Littleton, CO, USA) and rabbit anti-mouse CD34 (cat. no.
ab81289; Abcam, Cambridge, MA, USA) antibodies using the labeled
streptavidin-biotin method. Briefly, sections (4 µm) were sliced
from paraffin-embedded tumor tissue and deparaffinized by
sequential washing with xylene, and 100, 95, 85 and 75% ethanol.
Endogenous peroxide activity was blocked by 3%
H2O2 for 10 min at room temperature. The
sections were stained with H&E for 20–30 sec at room
temperature. Representative images were captured under a light
microscope (magnification, ×400) in at least 5 random selected
fields.
For immunohistochemical staining, antigen retrieval
was conducted by heating the slices in a steam cooker (100°C) in 10
mM sodium citrate buffer (pH 6.0). The sections were blocked in 5%
goat serum (Hyclone; GE Healthcare) for 2 h at room temperature.
Following washing with PBS, slices were incubated with the primary
antibodies (both antibodies in 1:300) overnight at 4°C. Following
washing with PBS for three times, peroxidase conjugated goat
anti-mouse IgG (1:100; cat. no. TA130004; OriGene Technologies,
Inc., Beijing, China) was added and incubated for 2 h at room
temperature. This was followed by staining using a
3,3′-Diaminobenzidine substrate kit (Fuzhou Maixin Biotech Co.,
Ltd., Fuzhou, China) for 30–45 sec at room temperature. Cells were
counterstained with H&E for 20–30 sec at room temperature.
Control slices were exposed to the secondary antibody alone and did
not demonstrate specific staining (data not shown). Representative
images were captured under a light microscope (magnification, ×400)
in at least 5 randomly selected fields.
All slices were observed and counted by two
pathologists (Department of Obstetrics and Gynecology, West China
Second University Hospital, Sichuan, China) in a blind manner. CD34
is as a cell surface antigen selectively expressed on hematopoietic
progenitor cells and vascular endothelial cells. Weidner's method
was used to determine the density of the tumor-associated
microvasculature (19).
Microvessel density within tumors was calculated under a light
microscope in 10 randomly selected fields. Ki67-positive cells were
counted.
Statistical analysis
All data are presented as the mean ± standard
deviation with 10 repeats. Statistical analysis was performed by
SPSS version 19.0 (IBM Corp., Armonk, NY, USA) using one-way
analysis of variance followed by Bonferroni correction. P<0.05
was considered to indicate a statistically significant
difference.
Results
General observation of anti-tumor
activity of liposome-PST34A in vivo
Mice in all groups survived the experimental period,
although mice with tumors are not in healthy condition (ascites,
reduced activity, no eating). Following sacrifice, tumor tissues,
ascitic fluid and vital organs of mice were harvested.
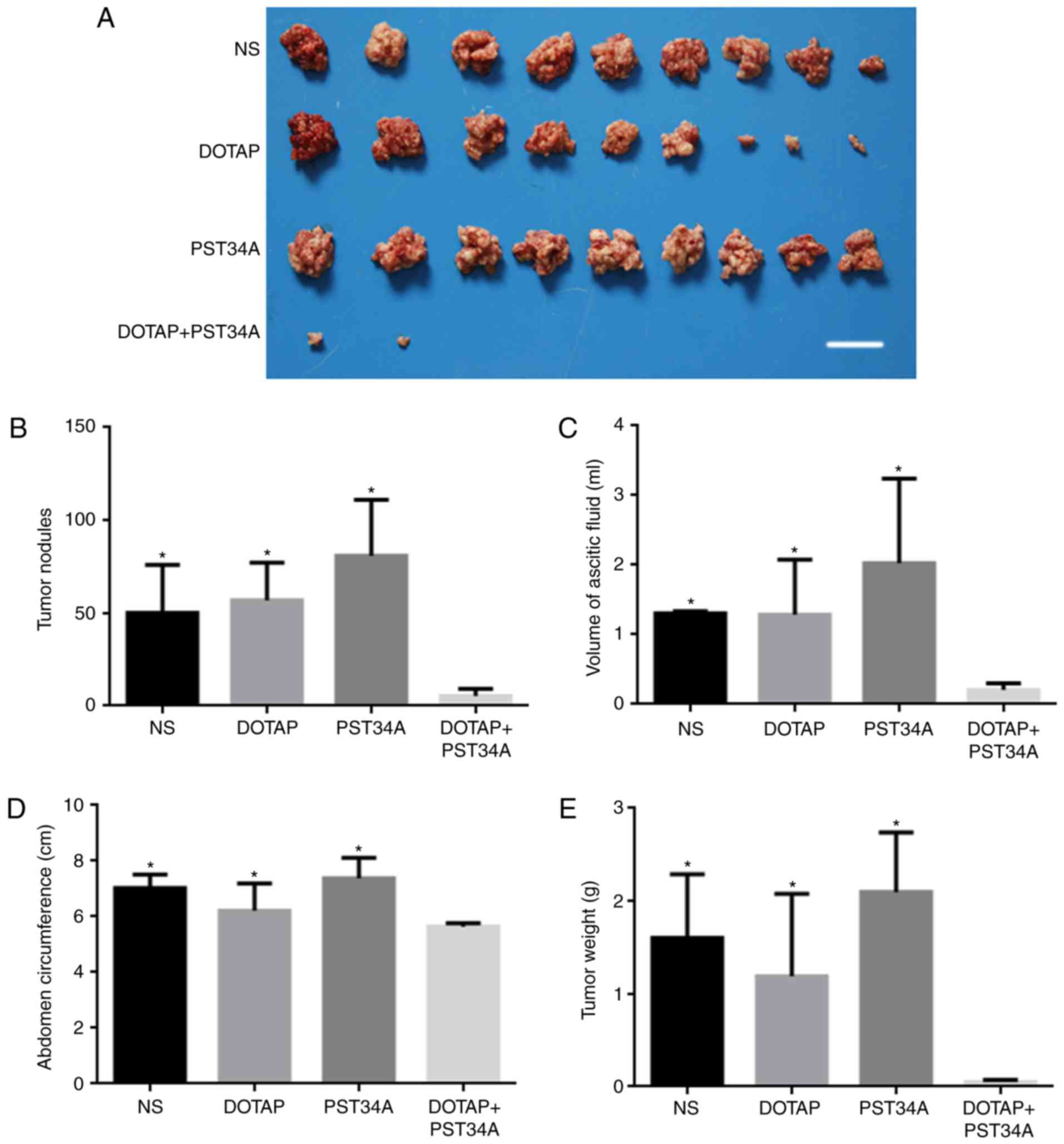
Administration of DOTAP+PST34A significantly inhibited tumor growth
when compared with the other groups (NS, DOTAP alone and PST34A
alone; all P<0.05; Fig. 1).
There were no statistical differences in body weight among the
different groups (NS, 18.03±1.37 g; DOTAP, 17.89±2.04 g; PST34A,
19.02±2.72 g; DOTAP+PST34A, 16.98±1.55 g). Liposome-plasmid complex
significantly decreased the number of tumor nodules to 5.33±3.80
compared with the other groups (NS, 50.19±25.78; DOTAP,
56.89±20.13; PST34A, 80.92±30.12; all P<0.05; Fig. 1B). The mean volume of ascitic fluid
collected from mice in the DOTAP+PST34A group (0.21±0.09 ml)
significantly decreased compared with the other groups (NS,
1.31±0.03 ml; DOTAP, 1.29±0.79 ml; PST34A, 2.03±1.21 ml; all
P<0.05; Fig. 1C). Furthermore,
a decrease in abdomen circumference and tumor weight were observed
in the DOTAP+PST34A group compared with all other groups [(NS,
7.03±0.49 cm; DOTAP, 6.22±0.98 cm; PST34A, 7.38±0.74 cm;
DOTAP+PST34A, 5.64±0.13 cm; all P<0.05; Fig. 1D) and (NS, 1.60±0.69 g; DOTAP,
1.19±0.89 g; PST34A, 2.10±0.64 g; DOTAP+PST34A, 0.05±0.02 g; all
P<0.05; Fig. 1E),
respectively].
Evaluation of apoptosis in the ascitic
fluid
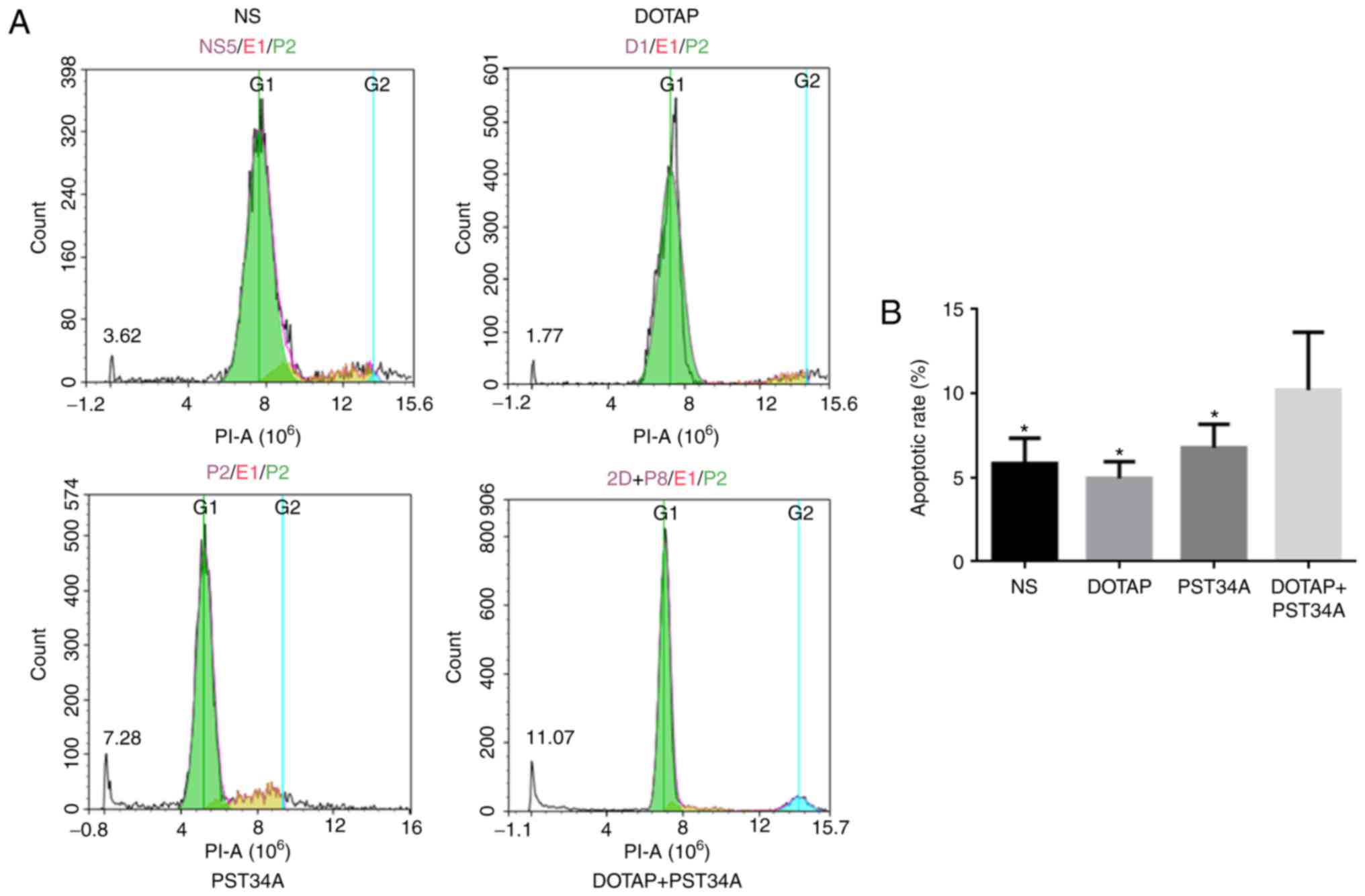
Flow cytometry was performed to detect the rates of
apoptosis in ascitic fluid following the administration of the
liposome-plasmid complex. DOTAP+PST34A significantly increased the
number of cells in the sub-G1 phase (apoptotic cells; 10.21±3.43%)
compared with all other treatments (NS, 5.83±1.51%; DOTAP,
4.96±0.98%; PST34A, 6.78±1.39%; all P<0.05; Fig. 2).
Results of H&E and
immunohistochemical staining
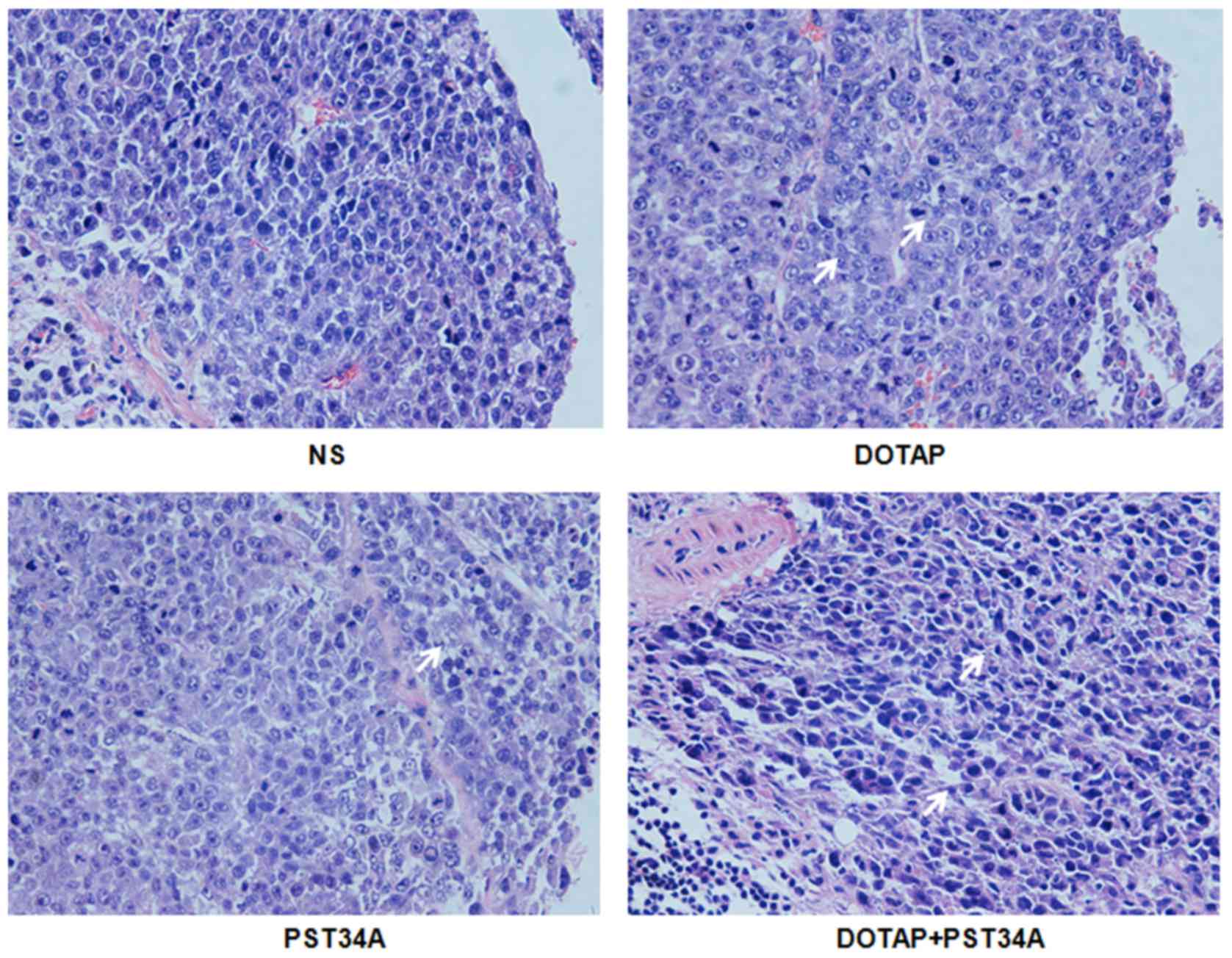
Morphology of tumor tissue was observed in the
present study. Characteristic morphological alterations, including
cell shrinkage, chromatin condensation and nuclear fragmentation
were observed in the DOTAP+PST34A group compared with other groups
(Fig. 3). Apoptotic cell and
certain lymphatic cell infiltrations were observed in the
DOTAP+PST34A group. By contrast, there were more tumor cells with
apparently enlarged atypical nuclei in the NS group. There were no
significant pathological alterations in the heart, liver, spleen,
lung and kidney samples following treatments (data not shown).
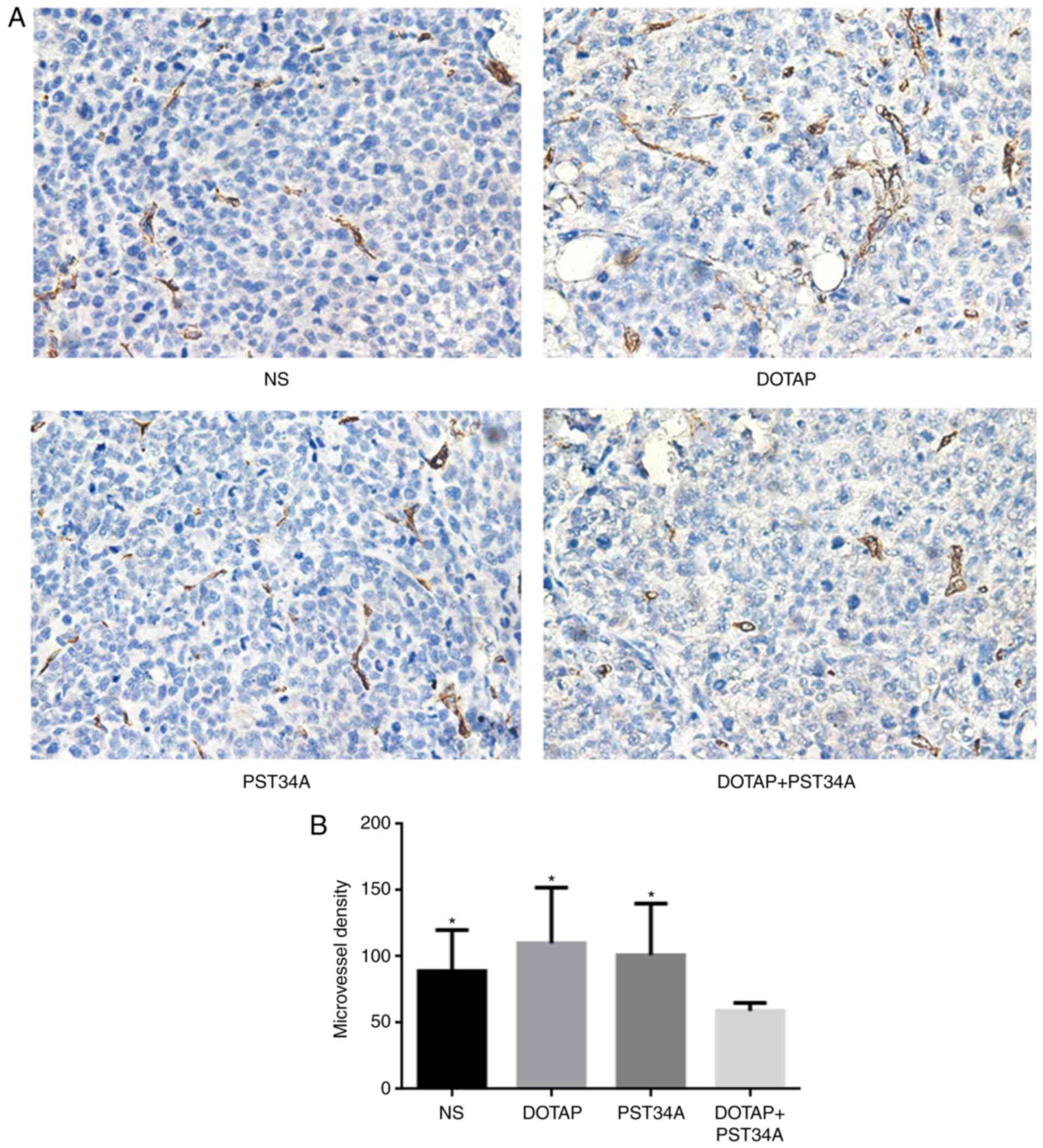
In the present study there were significantly fewer
(59±6) CD34-positive cells in the DOTAP+PST34A treated group,
compared with the 89±31 CD34-positive cells in the NS, 110±42 in
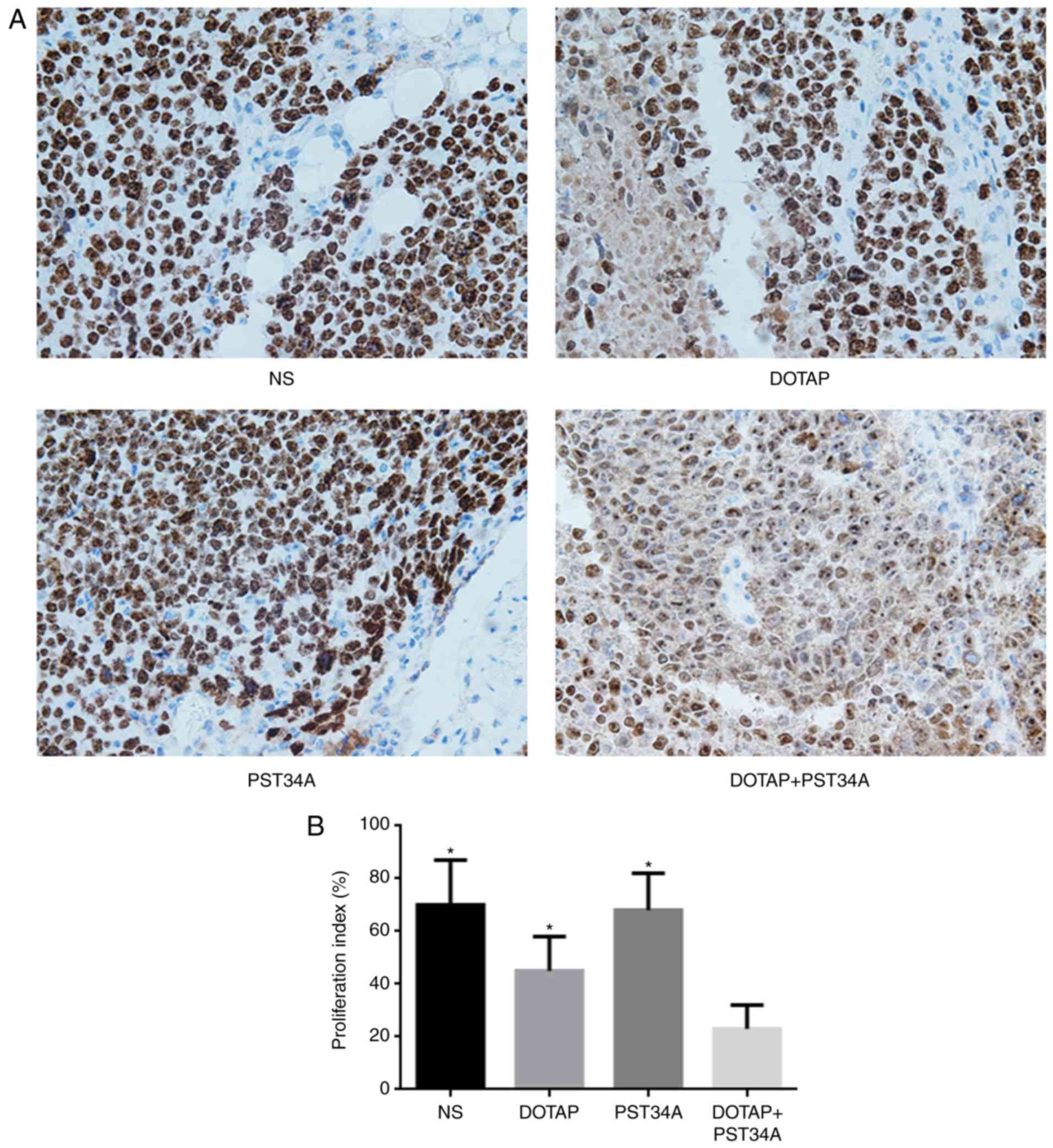
the DOTAP and 101±39 in the PST34A groups (all P<0.05; Fig. 4). Treatment with DOTAP+PST34A
resulted in significant inhibition of angiogenesis, compared with
the control group (all P<0.05). The effects of DOTAP+PST34A
treatment on cervical tumor cell proliferation were determined by
Ki67 staining. The positive/negative expression rate of Ki67 in the
DOTAP+ST34A group was 23±9%, which was significantly lower compared
with the other treatments (NS, 70±17%; DOTAP, 45±13%; PST34A,
68±14%; all P<0.05; Fig.
5).
Discussion
Survivin expression is associated with inhibition of
apoptosis, increased tumor aggressiveness and poor survival
(20). Survivin gene repression
can induce tumor cell apoptosis and increase sensitivity to
radiotherapy and chemotherapy in human cervical cancer lines
(21). In the present study,
liposome-plasmid DNA encoding mutant survivin-T34A was constructed
and revealed that DOTAP+PST34A exhibited antitumor abilities in
cervical cancer cells. CD34 staining also revealed that
DOTAP+PST34A treatment markedly decreased MVD, which indicated that
the plasmid together with the liposome could result in inhibition
of tumor growth by preventing the formation of novel vessels.
Furthermore, decreased level of Ki67 staining following treatment
with the plasmid-liposome complex, demonstrated that the
plasmid-liposome complex could inhibit the proliferation of tumor
cells.
Survivin is an inhibitor of apoptosis that regulates
cell proliferation. To exert its function, its threonine 34 (Thr34)
residue is phosphorylated by the cyclin-dependent kinase p34-cyclin
B1 protein complex (22). Loss of
Thr34 phosphorylation results in dissociation of the
caspase-9-survivin protein complex, leading to caspase-dependent
apoptosis (21). Adenoviruses
encoding mutant survivin-T34A can promote spontaneous apoptosis in
breast carcinoma (23).
Furthermore, liposome-plasmid DNA PST34A can sensitize Lewis lung
carcinoma cells to cisplatin-based chemotherapy and to radiation
(24,25). The present study demonstrated that
i.p. injection with liposome-plasmid DNA PST34A could inhibit the
tumor growth of cervical cancer in vivo. By contrast, PST34A
did not affect the viability of normal human cells, including
fibroblasts, endothelium and smooth muscle cells (21). The above observations together
implicate that liposome-plasmid DNA PST34A is a potential candidate
for cancer therapy. Phosphorylated Thr34 and p34 kinase have not
been previously detected in normal tissues, though they were
upregulated in the advanced stage cancer tissues (26). Survivin-dependent apoptosis could
improve the efficacy of a number of agents used to treat cancer.
The above results supported the previously reported
apoptosis-inducing ability of PST34A (27). The present study also demonstrated
that inhibition of tumor growth was associated with suppression of
angiogenesis as previously demonstrated (28,29).
Similar to other targeted therapies used in recent
years, there remain several limitations for gene therapies. The
relatively low efficiency of integration is a disadvantage. In
order to improve the efficiency, cationic liposome delivery system
was used in the present study, which represents the most common
tool in gene therapy (30). The
other limitation may result from a diversity of mutations in tumor
cells and a variety of histological types of tumors. Additionally,
an empty vector control was not used in the present study and this
is a limitation of the study, as the background effects of the
pVITRO2 plasmid could not be ascertained. In the future study, a
pVITRO2 plasmid should be included in the study to confirm the
specific function of PST34A delivered by DOTAP.
In conclusion, the present study demonstrated that
the liposome-plasmid complex PST34A can efficiently inhibit the
growth of cervical cancer in vivo. The mechanism potentially
involves two aspects: Induction of apoptosis of tumor cells and
inhibition of tumor-associated angiogenesis. In the present study,
the targeted strategy using survivin dominant negative mutant
mediated by liposome represents an alternative approach to survivin
gene therapeutic strategies.
Acknowledgements
The authors of the present study would like to thank
Dr Huilan Liu (School of Statistics, Guizhou University) for
performing the statistical analysis and Dr Yu Meng (Guiyang
University) for translation support.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kabekkodu SP, Chakrabarty S, Ghosh S,
Brand A and Satyamoorthy K: Epigenomics, pharmacoepigenomics and
personalized medicine in cervical cancer. Public Health Genomics.
20:100–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menderes G, Black J, Schwab CL and Santin
AD: Immunotherapy and targeted therapy for cervical cancer: An
update. Expert Rev Anticancer Ther. 16:83–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamer S, Ren Q and Dicker AP: Differential
radiation sensitization of human cervical cancer cell lines by the
proteasome inhibitor velcade (bortezomib, PS-341). Arch Gynecol
Obstet. 279:41–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, He L, Liu Y, Xia S, Fang A, Xie Y,
Gan L, He Z, Tan X, Jiang C, et al: Promising nanocarriers for PEDF
gene targeting delivery to cervical cancer cells mediated by the
over-expressing FR α. Sci Rep. 6:324272016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salvesen GS and Duckett CS: IAP proteins:
Blocking the road to death's door. Nat Rev Mol Cell Biol.
3:401–410. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka K, Iwamoto S, Gon G, Nohara T,
Iwamoto M and Tanigawa N: Expression of survivin and its
relationship to loss of apoptosis in breast carcinomas. Clin Cancer
Res. 6:127–134. 2000.PubMed/NCBI
|
|
8
|
Altieri DC: The molecular basis and
potential role of survivin in cancer diagnosis and therapy. Trends
Mol Med. 7:542–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Connor DS, Schechner JS, Adida C, Mesri
M, Rothermel AL, Li F, Nath AK, Pober JS and Altieri DC: Control of
apoptosis during angiogenesis by survivin expression in endothelial
cells. Am J Pathol. 156:393–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moriai R, Asanuma K, Kobayashi D, Yajima
T, Yagihashi A, Yamada M and Watanabe N: Quantitative analysis of
the anti-apoptotic gene survivin expression in malignant
haematopoietic cells. Anticancer Res. 21:595–600. 2001.PubMed/NCBI
|
|
11
|
Kato J, Kuwabara Y, Mitani M, Shinoda N,
Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J and
Fujii Y: Expression of survivin in esophageal cancer: correlation
with the prognosis and response to chemotherapy. Int J Cancer.
95:92–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy: Fulfilled promises and open
questions. Carcinogenesis. 28:1133–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanwar JR, Shen WP, Kanwar RK, Berg RW and
Krissansen GW: Effects of survivin antagonists on growth of
established tumors and B7-1 immunogene therapy. J Natl Cancer Inst.
93:1541–1552. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang G, Li J, Zeng Z and Xian L:
Lentivirus-mediated gene therapy by suppressing survivin in BALB/c
nude mice bearing oral squamous cell carcinoma. Cancer Biol Ther.
5:435–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pisarev V, Yu B, Salup R, Sherman S,
Altieri DC and Gabrilovich DI: Full-length dominant-negative
survivin for cancer immunotherapy. Clin Cancer Res. 9:6523–6533.
2003.PubMed/NCBI
|
|
16
|
Ma WH, Liu YC, Xue ML, Zheng Z and Ge YL:
Downregulation of survivin expression exerts antitumoral effects on
mouse breast cancer cells in vitro and in vivo. Oncol
Lett. 11:159–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan Y and Chen J: Clinicopathological
significance of survivin expression in patients with cervical
cancer: A systematic meta-analysis. Bioengineered. 8:511–523. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan L, Peng XC, Leng F, Yuan QZ, Shan Y,
Yu DD, Li ZY, Chen X, Xiao WJ, Wen Y, et al: Therapeutic effects of
survivin dominant negative mutant in a mouse model of prostate
cancer. J Cancer Res Clin Oncol. 137:19–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song H, Xin XY, Xiao F, Wang DT, Han X and
Guo HL: Influence of survivin gene repression by RNA interference
on the radiosensitivity and chemosensitivity to cisplatin of
cervical cancer cell HeLa. Zhonghua Fu Chan Ke Za Zhi. 41:554–558.
2006.(In Chinese). PubMed/NCBI
|
|
20
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
21
|
Mesri M, Wall NR, Li J, Kim RW and Altieri
DC: Cancer gene therapy using a survivin mutant adenovirus. J Clin
Invest. 108:981–990. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Connor DS, Grossman D, Plescia J, Li F,
Zhang H, Villa A, Tognin S, Marchisio PC and Altieri DC: Regulation
of apoptosis at cell division by p34cdc2 phosphorylation of
survivin. Proc Natl Acad Sci USA. 97:13103–13107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wall NR, O'Connor DS, Plescia J, Pommier Y
and Altieri DC: Suppression of survivin phosphorylation on Thr34 by
flavopiridol enhances tumor cell apoptosis. Cancer Res. 63:230–235.
2003.PubMed/NCBI
|
|
24
|
Yu DD, Wang CT, Shi HS, Li ZY, Pan L, Yuan
QZ, Leng F, Wen Y, Chen X and Wei YQ: Enhancement of cisplatin
sensitivity in Lewis Lung carcinoma by liposome-mediated delivery
of a survivin mutant. J Exp Clin Cancer Res. 29:462010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan QZ, Wang CT, Mao YQ, Zhang P, Shi HS,
Li ZY, Pan L, Yu DD, Leng F, Chen X, et al: Enhanced tumor
radiosensitivity by a survivin dominant-negative mutant. Oncology
Reports. 23:97–103. 2010.PubMed/NCBI
|
|
26
|
Zhou S, Li L, Jian X, Ou X, Jiang H, Yao
Z, Xu C and Peng J: The phosphorylation of survivin Thr34 by
p34cdc2 in carcinogenesis of oral submucous fibrosis. Oncol Rep.
20:1085–1091. 2008.PubMed/NCBI
|
|
27
|
Grossman D, Kim PJ, Schechner JS and
Altieri DC: Inhibition of melanoma tumor growth in vivo by survivin
targeting. Proc Natl Acad Sci USA. 98:635–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang R, Mizutani N, Luo Y, Chiodoni C,
Zhou H, Mizutani M, Ba Y, Becker JC and Reisfeld RA: A DNA vaccine
targeting survivin combines apoptosis with suppression of
angiogenesis in lung tumor eradication. Cancer Res. 65:553–561.
2005.PubMed/NCBI
|
|
29
|
Peng XC, Yang L, Yang LP, Mao YQ, Yang HS,
Liu JY, Zhang DM, Chen LJ and Wei YQ: Efficient inhibition of
murine breast cancer growth and metastasis by gene transferred
mouse survivin Thr34->Ala mutant. J Exp Clin Cancer Res.
27:462008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirko A, Tang F and Hughes JA: Cationic
lipid vectors for plasmid DNA delivery. Curr Med Chem.
10:1185–1193. 2003. View Article : Google Scholar : PubMed/NCBI
|