Introduction
Esophageal carcinoma (EC) is a common type of cancer
that is associated with millions of cases of mortality per year
worldwide (1). The 5-year relative
survival rate is ~40% for localized tumors and 4% for advanced
distal metastastic tumors. Environmental and lifestyle factors,
including smoking and alcohol consumption, are the risk factor for
esophageal squamous cell carcinoma (ESCC) in Western countries, and
the consumption of hot beverages is a major risk factor in East
countries (2). The proliferation
of cancer cells and metastasis are the common risk factor for ESCC
development (3). ESCC can be
treated with various techniques, including chemotherapy,
radiotherapy and surgical resection. Surgery is suitable for early
stage of EC. Chemotherapy and radiotherapy is the strategy for
advanced stage of EC (4). In
China, the histological subtype of most EC cases is ESCC (5). In recent years, EC treatments have
advanced, but further improvement is required (6).
MicroRNAs (miRNAs/miRs) are highly conserved, small
non-coding RNAs, which regulate the expression of several oncogenes
and tumor suppressor genes (7).
miRNAs bind the mRNA of their target genes in the 3′-untranslated
region (UTR) to downregulate protein expression (8). It has been demonstrated that miRNAs
have important roles in many biological cellular processes,
including proliferation, differentiation, migration and apoptosis
(9,10). In addition, aberrant miRNA
expression is associated with the development of several types of
cancer (11,12). It has been suggested that
miR-449a-5p may suppress the proliferation of various cancer cells
by negatively regulating the expression of several oncogenes
(13). However, the effects of
miR-449a-5p on ESCC remain to be elucidated. In the present study
the aim was to determine the effects of miR-449a-5p and its target
gene on proliferation of ESCC cells. The present study demonstrated
that miR-449a-5p regulated ESCC cell proliferation via targeting
cyclin D1.
Materials and methods
ESCC tissues and cell lines
Paired ESCC tissues and adjacent normal esophageal
squamous tissues were collected from patients with ESCC (n=7; age,
56–72 years; mean age, 68 years; sex: Males, 3 and females, 4) that
under went esophagogastrostomy at the Fourth Hospital of Hebei
Medical University (Shijiazhuang, China) between 2014/1-2015/10.
Patients had not received any preoperative chemotherapy or
radiotherapy, and all clinicopathological information was recorded.
All tissue samples were flash-frozen in liquid nitrogen and stored
at −80°C. The present study was approved by the Ethical Review
Committee of the Fourth Hospital of Hebei Medical University
(Shijiazhuang, China). Written informed consent was obtained from
each patient.
The human ESCC cell line Eca-109 was purchased from
American Type Culture Collection (Manassas, VA, USA). Eca-109 cells
were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 80 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of ESCC cells and tissues was extracted
with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was reverse transcribed from 1 µg total RNA sample mixing with 1 µl
(500 nM) miRNA-specific loop RT-primers and 2 µl dNTP (10 mM;
Takara Biotechnology Co., Ltd., Dalian, China), then added
RNase-free water to 10 µl, mixed well. 70°C for 10 min, then placed
on ice for 5 min. Then 0.5 µl Recombinant RNase inhibitor (40 U/µl;
Takara Biotechnology Co., Ltd., Dalian, China), 0.5 µl MMLV Reverse
Transcriptase (200 U/µl; New England BioLabs, Inc., Ipswich, MA,
USA), 2 µl 10X Transcriptase Buffer, added RNase-free water to 10
µl, mix well. The thermocycling conditions were: 42° for 60 min,
95°C for 5 min, 4°C forever. The primer sequences used for reverse
transcription were as follows (5′-3′): miR-449a-5p,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCTTTG-3′ and U6,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATATG-3′.
Stem-loop RT-qPCR was performed to analyze miR-449a-5p levels. PCR
was performed with the SYBR Green master mix (Takara Biotechnology
Co., Ltd., Dalian, China) and iQ5 system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The procedures of the PCR are described
as follows: 95°C for 30 sec, followed by 40 cycles at 95°C for 5
sec and 60°C for 20 sec. The relative expression levels of
miR-449a-5p were normalized to U6 and were calculated with the
2−∆∆Cq method (14).
All reactions were performed in triplicate. The primer sequences
used for PCR were as follows: miR-449a-5p forward,
5′-ATAGTGGCAGTGTATTGTTAG-3′; U6 forward, 5′-GCGCGTCGTGAAGCGTTC-3′;
universal reverse primer, 5′-GTGCAGGGTCCGAGGT-3′.
Transfection
miR-449a-5p mimic (449AM), miR-449a-5p inhibitor
(449AI), microRNA mimic negative control (NC) and microRNA
inhibitor negative control (NCI) were obtained from Shanghai
GenePharma Co., Ltd., (Shanghai, China). The day prior to
transfection, 8×105 Eca-109 cells per well
were cultured in 6-well plate. NC; sense 5′-UUCUCCGAACGUGUCACGU-3′
and antisense 5′-ACGUGACACGUUCGGAGAA; 449AM, sense
5′-UGGCAGUGUAUUGUUAGCUGGU-3′ and antisense
5′-CAGCUAACAAUACACUGCCAUU-3′, NCI, sense
5′-CAGUACUUUUGUGUAGUACAA-3′; 449AI, sequence
5′-ACCAGCUAACAAUACACUGCCA-3′) were transfected into Eca-109 cells
using HiPerFect transfection reagent (Qiagen China Co., Ltd.,
Shanghai, China). A total of 150 ng miRNA oligonucleotides and 3 µl
HiPerFect transfection reagent were mixed in 100 µl RPMI-1640 and
the mixture was added to the cell culture medium. At 48 h
post-transfection, cells were harvested for miR-449a-5p
detection.
Cyclin D1-specific siRNA transfection was performed
by using HiPerFect transfection. Cyclin D1-specific siRNA (SI) and
negative control siRNA (siNC) were obtained from Shanghai
GenePharma Co., Ltd. The sequences were described as following:
Cyclin D1-specific siRNA (sense 5′-CCUCGGUGUCCUACUUCAAAUGUGU-3′ and
anti-sense ACACAUUUGAAGUAGGACACCGAGG-3′); and negative control
siRNA (sense 5′-UUCUCCGAACGUGUCACGU-3′ and antisense
5′-ACGUGACACGUUCGGAGAA). The day before transfection,
8×105 Eca-109 cells per well were cultured in 6-well
plate. A total of 150 ng miRNA oligonucleotides and 3 µl HiPerFect
transfection reagent were mixed in 100 µl RPMI-1640 and the mixture
was added to the cell culture medium. At 48 h post-transfection,
cells were harvested for detecting cyclin D1 protein and mRNA
levels.
Prediction of target genes
Target Scan (http://www.targetscan.org/), miR and a (http://34.236.212.39/microrna/home.do)
and PicTar (http://pictar.mdc-berlin.de/) were used to predict the
target genes of miR-449a-5p. Cyclin D1 was predicted as putative
target gene.
Luciferase assay
To confirm cyclin D1 as a target of miR-449a-5p, a
luciferase assay was performed. The binding region of miR-449a-5p
in the 3′-UTR of cyclin D1 was cloned using the following primers;
the restriction sites are underlined: Cyclin D1-F-SacI,
TCGAGCTCCTGTTTGGCGTTTCCCAGAG; cyclin D1-R-XbaI,
GCTCTAGAACTACTATGATGCTACGCCCC. Eca-109 cell genomic DNA was used as
a template. PCR was performed by using Q5 DNA polymerase (New
England BioLabs, Inc.). The PCR thermo cycling conditions were
used: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec,
55°C for 45 sec and 72°C for 30 sec. The PCR product was digested
by the endonucleases SacI and XbaI, and inserted into
the pmiRGLO vector (Promega Corporation, Madison, WI, USA). For the
luciferase reporter assay, Eca-109 cells were cultured in a 96-well
plate at 5,000 cells/well in 100 µl culture medium. Subsequently,
the recombinant luciferase vector (0.1 mg) and miR-449a-5p mimic or
inhibitor (5 ng) were transfected into Eca-109 cells with Effectene
reagent (Qiagen China Co., Ltd.) for 48 h. A dual-luciferase
reporter assay system (Promega Corporation) was subsequently used
to detect the luciferase activity of cells. Luciferase activity was
normalized to Renilla luciferase activity. A total of six samples
were measured for each group. The experiment was repeated three
times.
Cell proliferation assay
AMTT assay was performed to analyze cell
proliferation. Eca-109 cells were seeded into a 96-well plate
(1×104 cells/well) for 24, 48 and 72 h. MTT (20 µl; 10
mg/ml) was subsequently added to each well and incubated for 4 h at
37°C. The supernatant was removed and 150 µl dimethyl sulfoxide was
added for 15–20 min. Absorbance was measured at a wavelength of 450
nm. All experiments were performed in sextuplicate.
Colony formation assay
A day following transfection, ~300 Eca109 cells were
cultured in each well of 6-well plate and incubated at 37°C for 2
weeks. The culture medium was removed once a week and replaced with
fresh medium containing the miR-449a-5p mimic or inhibitor
transfection mixture (150 ng miRNA oligonucleotides and 3 µl
HiPerFect transfection reagent). On day 14, the cells were washed
three times with PBS, fixed with 4% polymerized formaldeyde
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) for 30 min at room temperature and stained with 2.5% crystal
violet staining solution (Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min at room temperature. The 6-well plates were
washed with PBS three times and air-dried. The colonies that
contained >50 cells were counted with the naked eye in from each
well. The relative colony numbers were calculated as the ratio of
449aM to NC or 449aI to NCI. Experiments were carried out in
triplicate each time and repeated three times.
Western blot analysis
A total of 48 h following transfection, the cells
were washed by ice PBS and harvested by centrifuging at 200 × g for
10 min at 4°C. Cell protein was extracted by Lysis Buffer (CST
Biological Reagents Co., Ltd., Danvers, MA, USA). Protein
concentration was determined with a bicinchoninic acid protein
assay kit. Protein samples (15 µg/lane) were separated by 10%
SDS-PAGE and electrotransferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MD, USA). Membranes were
subsequently blocked with 5% non-fat milk for 2 h at room
temperature and incubated with cyclin D1 (1:1,000; cat. no. 2978;
CST Biological Reagents Co., Ltd.) and GAPDH (1:1,000; cat. no.
5174; CST Biological Reagents Co., Ltd.) primary antibodies
overnight at 4°C. Blots were washed five times in Tris-buffered
saline (TBS) with 0.1% Tween-20 (TBST) followed by incubation with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (1:5,000; cat. no. 7074; CST Biological Reagents Co.,
Ltd.) for 1 h at room temperature. Membranes were washed in TBST
for 10 min three times and bands were visualized using an enhanced
chemiluminescence kit (EMD Millipore). Relative protein levels were
calculated as the ratio of cyclin D1 band intensity to that of
GAPDH using ImageJ version 1.42 (National Institutes of Health,
Bethesda, MD, USA). Experiments were carried out in triplicate each
time and repeated three times.
Statistical analysis
Statistical analysis was performed with the SPSS
13.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
Data are expressed as the means ± standard error of the mean. The
non-parametric Spearman's rank-order correlation was used to
determine the correlation between miR-449a and cyclin D1 in ESCC
tissues. The non-parametric Mann-Whitney U test was used to compare
two groups, and one-way analysis of variance followed by a Tukey's
post-hoc test was used to compare three or more groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-449a-5p expression is reduced in
ESCC tissues
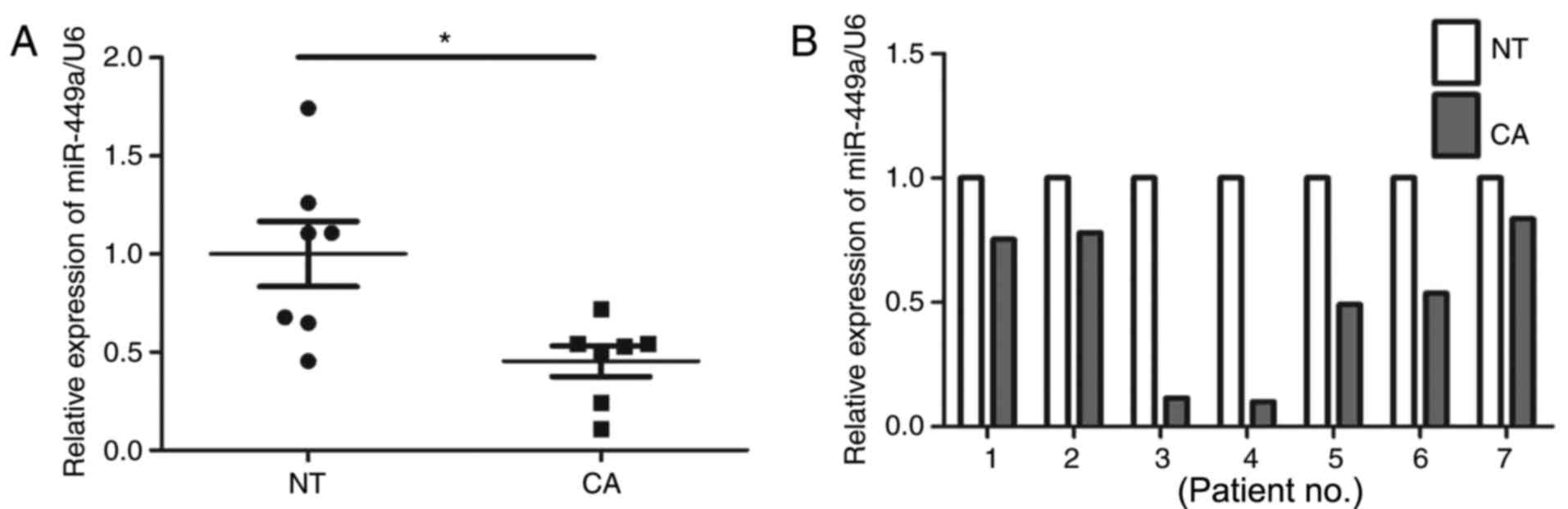
Changes in miR-449a-5p expression were analyzed in
ESCC (n=7) and adjacent normal tissues (n=7) by RT-qPCR. The
results revealed that the expression levels of miR-449a-5p were
significantly reduced in the ESCC tissues (P<0.01; Fig. 1) compared with in the adjacent
normal tissues. These findings suggested that decreased miR-449a-5p
may be associated with ESCC.
miR-449a-5p regulates ESCC cell
proliferation
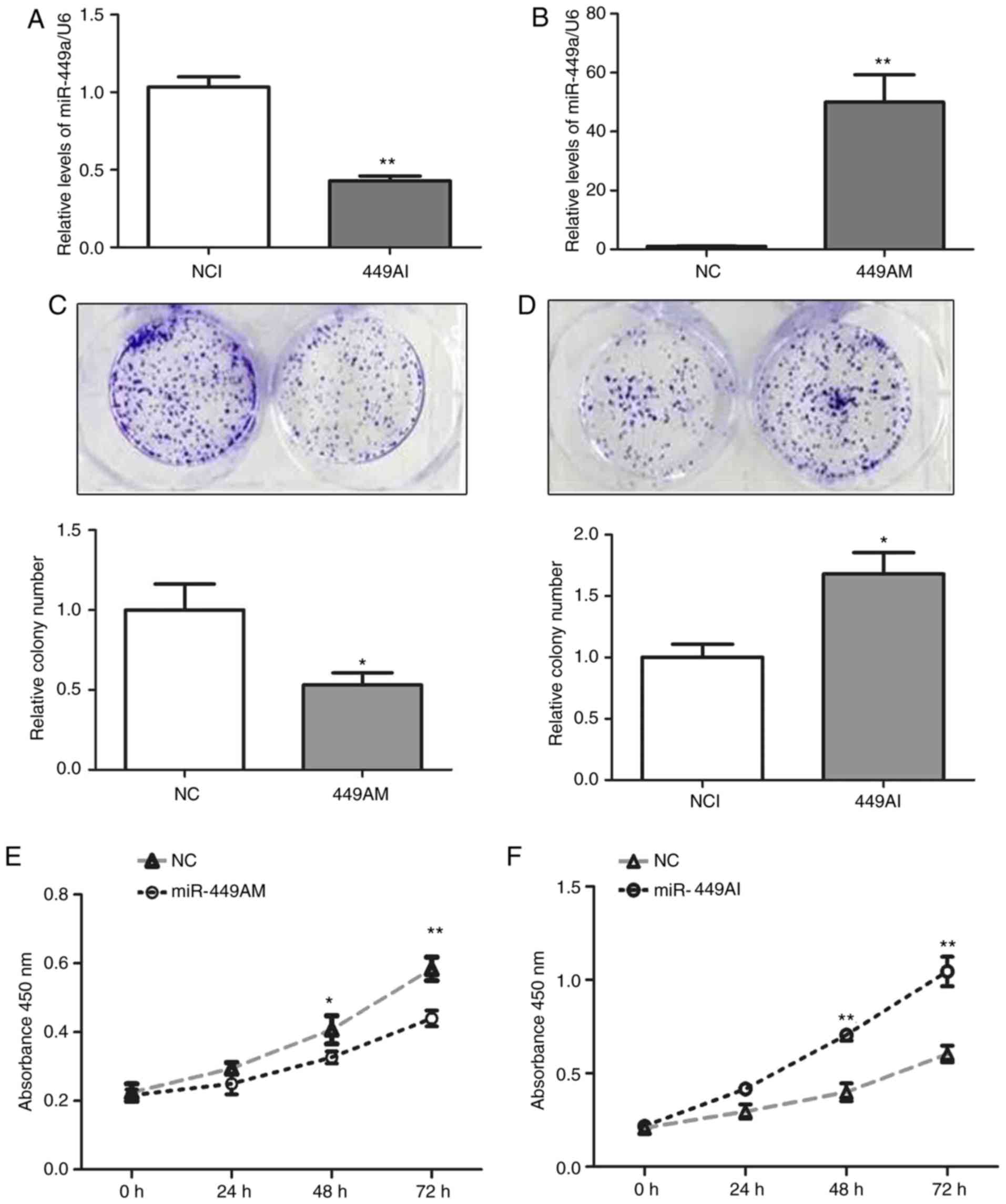
In order to investigate the effects of miR-449a-5p
on ESCC cell proliferation, 499AM and 499AI were transfected into
Eca-109 cells. After 48 h, the expression levels of miR-449a-5p
were increased by ~50-fold in Eca-109 cells transfected with 499AM,
whereas miR-449a-5p expression was reduced to 40% of that in the
NCI group (P<0.01; Fig. 2A and
B), indicating successful transfection. Colony number was
significantly decreased in cells transfected with 499AM, whereas
499AI significantly increased colony number (P<0.05; Fig. 2C and D). Additionally, 499AM
inhibited the proliferation of Eca-109 cells, whereas 499AI
increased cell proliferation (P<0.05; Fig. 2C and D). These results suggested
that miR-449 may regulate Eca-109 cell proliferation.
miR-449a-5p negatively regulates
cyclin D1 expression by binding to its 3′-UTR
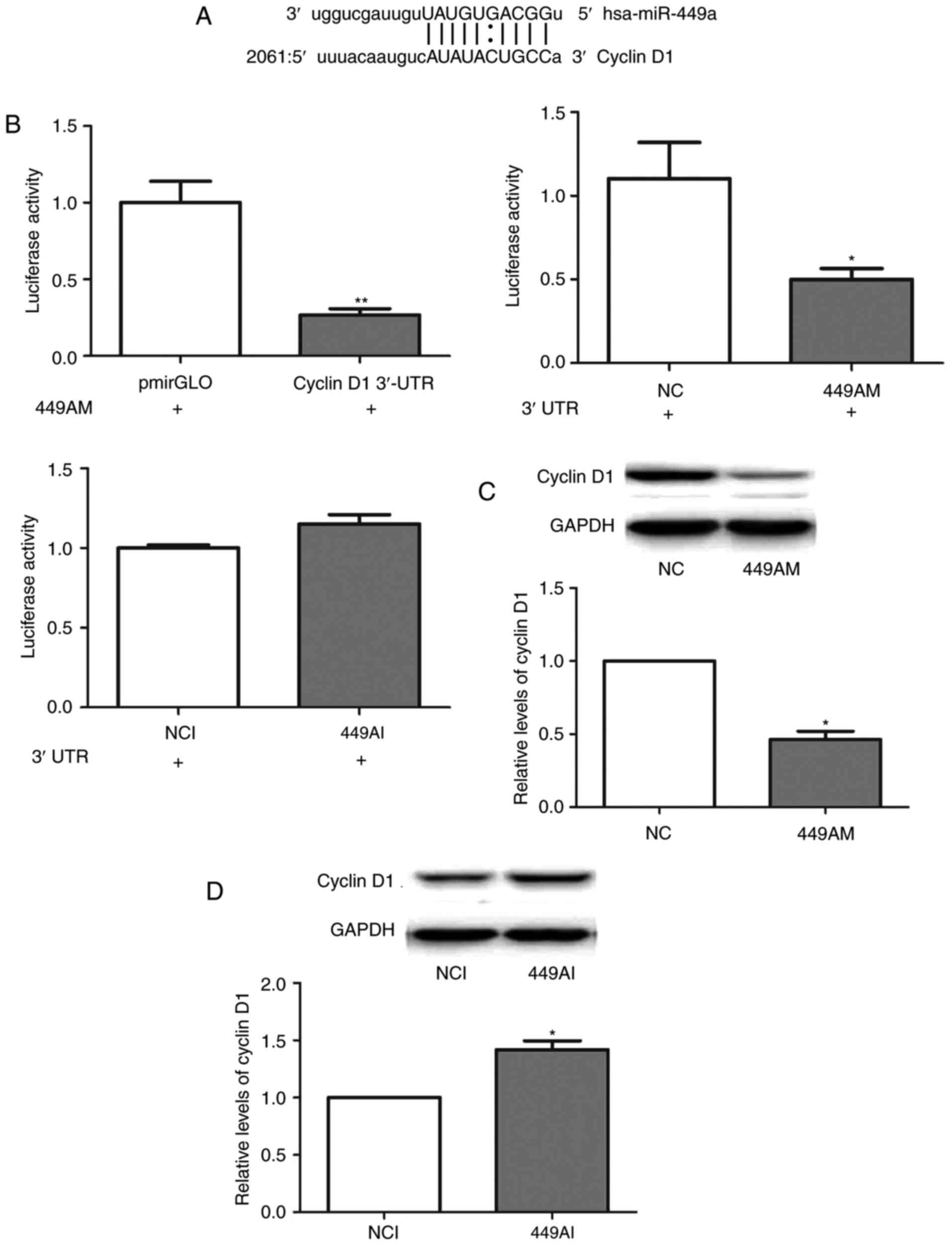
miRanda, TargetScan and PicTar were used to predict
the potential target genes of miR-449a-5p. Cyclin D1was identified
as a potential target gene; a miR-449a-5p binding site was revealed
to be present at nucleotides 2021–2080 in the cyclin D1 3′-UTR
(Fig. 3A). This region of the
cyclin D1 3′-UTR was subsequently cloned and inserted into a
pmiRGLO vector. Transfection with 499AM significantly decreased the
luciferase activity of Eca-109 cells (Fig. 3B). However, 499AI transfection did
not significantly alter luciferase activity (Fig. 3B). Additionally, cyclin D1 protein
levels were reduced in Eca-109 cells transfected with 499AM
(Fig. 3C). By contrast, 499AI
upregulated cyclin D1 protein levels (Fig. 3D). These results suggested that
miR-449a-5p may negatively regulate cyclin D1 expression by binding
to its 3′-UTR.
miR-449a-5p regulates Eca-109 cell
proliferation via cyclin D1 targeting
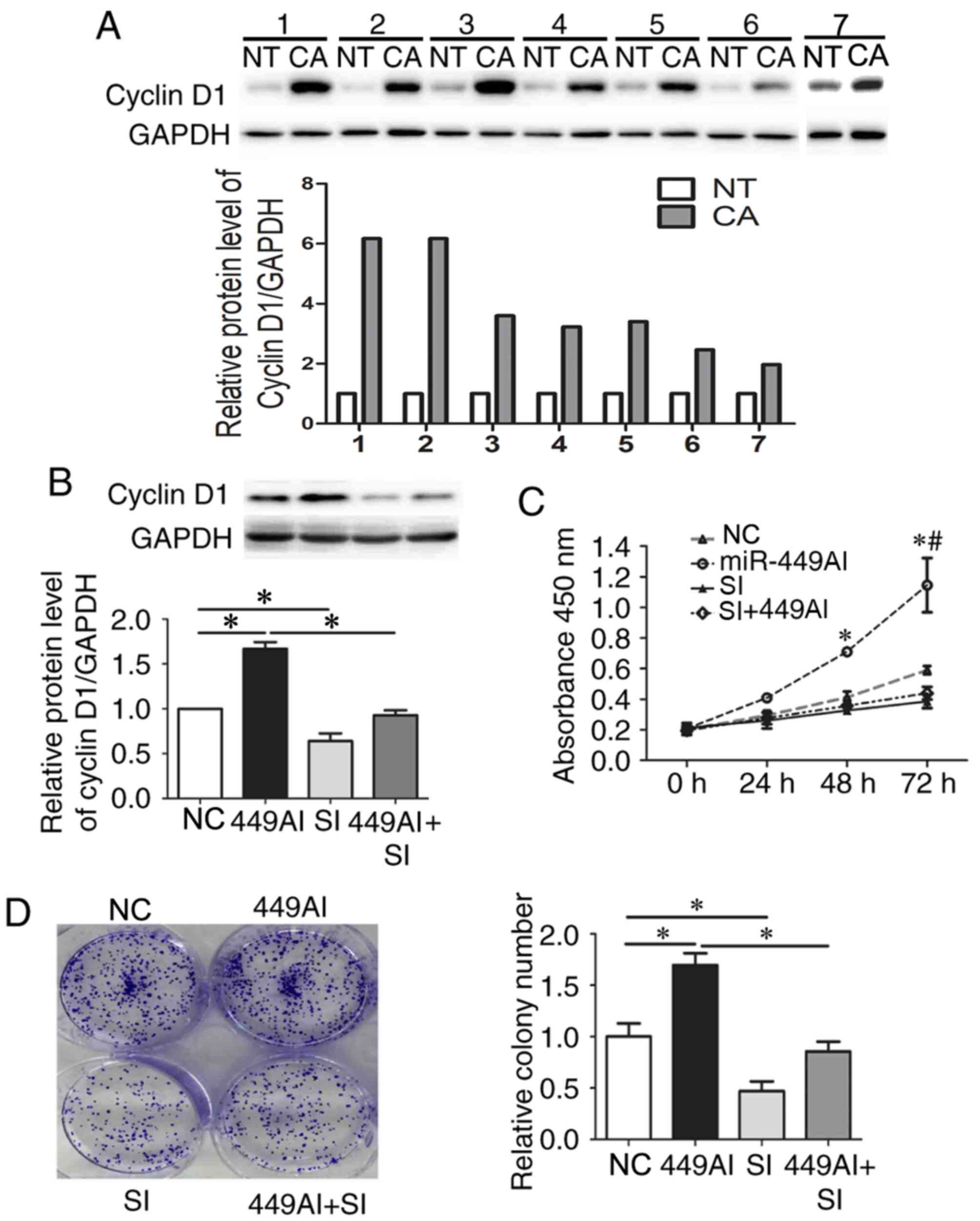
Cyclin D1 protein expression was increased in ESCC
tissues (Fig. 4A). To further
determine the role of cyclin D1 in 499AI-induced Eca-109 cell
proliferation, small interfering siRNA targeting cyclin D1 mRNA and
499AI were co-transfected into Eca-109 cells. Cyclin D1 expression
was decreased by ~50% compared with in cells transfected with siNC
(Fig. 4B). Downregulation of
cyclin D1 expression rescued the effects of 449AI on Eca-109
proliferation (Fig. 4C) and colony
formation (Fig. 4D). The
non-parametric Spearman's correlation test was used to determine if
a correlation existed between the levels of miR-449a-5p and cyclin
D1. The results revealed that in ESSC tissues miR-449a levels were
not correlated with cyclin D1 (r=−0.406; P=0.425; data not shown);
however, this may be due to the small sample size. Taken together,
these results indicated that miR-449a-5p may regulate the
proliferation of Eca-109 cells by targeting cyclin D1.
Discussion
Although the treatment of ESCC has advanced, disease
prognosis remains poor, as most patients are diagnosed at an
advanced stage; consequently, the 5-year survival rate of patients
is <30% post-surgery (15). In
addition, ~10% of patients were diagnosed at an advanced and the
tumor had spread to other organs. So, the patients do not have the
opportunity to undergo surgery (16,17).
Molecular targeted therapy improves the 5-year survival rate of
patients with ESCC (18).
miRNAs are a class of small non-coding RNAs, which
negatively regulate gene expression via binding to the 3′-UTR of
target mRNAs. It has been demonstrated that miRNAs participate in
the pathogenesis of various types of cancer by targeting numerous
oncogenes, and aberrant expression of miRNAs may contribute to
carcinogenesis (19–22). It has been verified that ~50% of
miRNAs are involved in the development of human cancer (23); miRNAs can regulate the development
of various human cancers by acting as both oncogenes and tumor
suppressors (24–26). miR-449a-5p expression is reduced in
various cancer cells, including prostate (27), gastric (28), bladder (29) and lung cancer (30). Furthermore, miR-449a-5p is involved
in G1 cell cycle arrest, apoptosis and senescence via
the regulation of key factors in cell cycle and apoptosis
regulation, including histone deacetylase 1 (30), cyclin-dependent kinase 6 (31–33),
cell division cycle 25A (31,33),
cyclin D1 (34) and nicotinamide
adenine dinucleotide-dependent protein deacetylase sirtuin-1
(35).
In the present study, it was demonstrated that
miR-449a-5p expression was significantly reduced in ESCC tissues
compared within adjacent normal esophageal squamous tissues. The
effects of miR-449a-5p on Eca-109 cell proliferation were
subsequently determined in vitro. miRNAs can
post-transcriptionally negatively regulate their target genes
(35), by binding to the 3′-UTR of
target mRNA (36). The present
study confirmed that cyclin D1 was a target gene of miR-449a-5p
using a luciferase assay. Transfection with 499AM decreased the
luciferase activity of Eca-109 cells. However, 499AI transfection
did not alter luciferase activity Cyclin D1. The level of
miR-449a-5p was increased by ~50 fold in cells transfected with
miR-449a-5p mimics. However, in cells transfected with the
miR-449a-5p inhibitor the level of miR-449a-5p was reduced to 40%
of NCI group. Cyclin D1is involved in the growth progression of
various cells, and is considered a proto-oncogene that is
overexpressed in several types of cancer. The results of the
current study revealed that cyclinD1 was downregulated in ESCC
cells transfected with 499AM, whereas cyclin D1 was upregulated in
ESCC cells transfected with 499AI. The results of the luciferase
assay confirmed that the 3′-UTR of cyclin D1 mRNA contained a
miR-449a-5p binding site. Inhibition of cyclin D1 reversed the
effects of 499AI on the proliferation of ESCC cells. However, the
results of the Spearman's rank correlation test did not demonstrate
a correlation between miR-449a and cyclin D1 expression; this is
likely due to the small sample size used in the present study. In
future experiments, we aim to collect more ESCC tissue samples.
In conclusion, miR-449a-5p expression was
significantly reduced in ESCC tissues compared with in the adjacent
normal tissues. In addition, inhibition of miR-449a-5p was able to
promote the proliferation of ESCC cells by upregulating cyclin D1
expression. Therefore, the findings of the present study indicated
that miR-449a-5p may be an effective biomarker and therapeutic
target for ESCC in the future.
Acknowledgements
The authors would like to thank Prof. Zhenlong Ge
(Fourth Hospital of Hebei Medical University, Xingtai, China) for
providing ESCC tissues.
Funding
This work is supported by Government Foundation
Grant from Hebei provincial Department of Education (grant no.
HBGX2005-52) and National Natural Science Foundation of China
(grant no. 30371413).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TJ and JM planned the experiments and wrote the
paper; TJ and JL performed the experiments; TJ analyzed data.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Committee of the Fourth Hospital of Hebei Medical University
(Shijiazhuang, China). Written informed consent was obtained from
each patient.
Consent for publication
Written informed consent was obtained from each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Otsubo T, Yamada K, Hagiwara T, Oshima K,
Iida K, Nishikata K, Toyoda T, Igari T, Nohara K, Yamashita S, et
al: DNA hypermethyation and silencing of PITX1 correlated with
advanced stage and poor postoperative prognosis of esophageal
squamous cell carcinoma. Oncotarget. 8:84434–84448. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaheen O, Ghibour A and Alsaid B:
Esophageal cancer metastases to unexpected site: A systematic
review. Gastroenterol Res Pract. 2017:16573102017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo Y, Mao Q, Wang X, Yu J and Li M:
Radiotherapy for esophageal carcinoma: Dose, response and survival.
Cancer Manag Res. 10:13–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu SG, Zhang WW, He ZY, Sun JY, Chen YX
and Guo L: Sites of metastasis and overall survival in esophageal
cancer: A population-based study. Cancer Manag Res. 9:781–788.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harada K, Baba Y, Ishimoto T, Shigaki H,
Kosumi K, Yoshida N, Watanabe M and Baba H: The role of microRNA in
esophageal squamous cell carcinoma. J Gastroenterol. 51:520–530.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan TM, Lam CS, Nq L, Chow AK, Wong SK, Li
HS, Man JH, Lo OS, Foo D, Cheung A, et al: The clinicopathological
significance of miR-133a in colorectal cancer. Dis Markers.
2014:9192832014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Daly SM, Abba ML and Gamal-Eldeen AM:
The role of microRNAs in photodynmic therapy of cancer. Eur J Med
Chem. 142:550–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Shan B, Du Y, Wang M, Liu L and
Ren FZ: Periplocin from Cortex periplocae inhibits cell growth and
down-regulates survivin and c-myc expression in colon cancer in
vitro and in vivo via beta-catenin/TCF signaling. Oncol Rep.
24:375–383. 2010.PubMed/NCBI
|
|
13
|
Yong-Ming H, Ai-Jun J, Xiao-Yue X,
Jian-Wei L, Chen Y and Ye C: miR-449a: A potential therapeutic
agent for cancer. Anticancer Drugs. 28:1067–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JF, Wang QZ and Hou J: Surgical
treatment for cancer of the esophagus and gastric cardia in Hebei,
China. Br J Surg. 91:90–98. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoner GD and Wang LS: Chemoprevention of
esophageal squamous cell carcinoma with berries. Top Curr Chem.
329:1–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mei LL, Qiu YT, Zhang B and Shi ZZ:
MicroRNAs in esophageal squamous cell carcinoma: Potential
biomarkers and therapeutic targets. Cancer Biomark. 19:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei W, Hu Z, Fu H, Tie Y, Zhang H, Wu Y
and Zheng X: MicroRNA-1 and microRNA-499 downregulate the
expression of the ets1 proto-oncogene in HepG2 cells. Oncol Rep.
28:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Voorhoeve PM, le Sage C, Schrier M, Gillis
AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A,
et al: A genetic screen implicates miRNA-372 and miRNA-373 as
oncogenes in testicular germ cell tumors. Cell. 124:1169–1181.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Detassis S, Grasso M, Del Vescovo V and
Denti MA: MicroRNAs make the call in cancer personalized medicine.
Front Cell Dev Biol. 5:862017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: C-Myc regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langevin SM and Christensen BC: Let-7
microRNA-binding-site polymorphism in the 3′UTR of KRAS and
colorectal cancer outcome: A systematic review and meta-analysis.
Cancer Med. 3:1385–1395. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pekarsky Y, Balatti V and Croce CM: BCL2
and miR-15/16: From gene discovery to treatment. Cell Death Differ.
25:21–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noonan EJ, Place RF, Pookot D, Basak S,
Whitson JM, Hirata H, Giardina C and Dahiya R: miR-449a targets
HDAC-1 and induces growth arrest in prostate cancer. Oncogene.
28:1714–1724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kheir Bou T, Futoma-Kazmierczak E,
Jacobsen A, Krogh A, Bardram L, Hother C, Grønbæk K, Federspiel B,
Lund AH and Friis-Hansen L: miR-449 inhibits cell proliferation and
is down-regulated in gastric cancer. Mol Cancer. 10:292011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Lin YW, Mao YQ, Wu J, Liu YF,
Zheng XY and Xie LP: MicroRNA-449a acts as a tumor suppressor in
human bladder cancer through the regulation of pocket proteins.
Cancer Lett. 320:40–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeon HS, Lee SY, Lee EJ, Yun SC, Cha EJ,
Choi E, Na MJ, Park JY, Kang J and Son JW: Combining
microRNA-449a/b with a HDAC inhibitor has a synergistic effect on
growth arrest in lung cancer. Lung Cancer. 76:171–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M
and Yu Q: MiR-449a and miR-449b are direct transcriptional targets
of E2F1 and negatively regulate pRb-E2F1 activity through a
feedback loop by targeting CDK6 and CDC25A. Genes Dev.
23:2388–2393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lizé M, Pilarski S and Dobbelstein M:
E2F1-inducible microRNA 449a/b suppresses cell proliferation and
promotes apoptosis. Cell Death Differ. 17:452–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng M and Yu Q: miR-449 regulates
CDK-Rb-E2F1 through an auto-regulatory feedback circuit. Cell
Cycle. 9:213–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noonan EJ, Place RF, Basak S, Pookot D and
Li LC: miR-449a causes Rb-dependent cell cycle arrest and
senescence in prostate cancer cells. Oncotarget. 1:349–358.
2010.PubMed/NCBI
|
|
35
|
Ni Y, Meng L, Wang L, Dong W, Shen H, Wang
G, Liu Q and Du J: MicroRNA-143 functions as a tumor suppressor in
human esophageal squamous cell carcinoma. Gene. 517:197–204. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Felekkis K, Touvana E, Stefanou Ch and
Deltas C: microRNAs: A newly described class of encoded molecules
that play a role in health and disease. Hippokratia. 14:236–240.
2010.PubMed/NCBI
|