Introduction
Post-menopausal osteoporosis (PMO) is one of the
most common types of osteoporosis. In 2008, the number of patients
with PMO in China was estimated to be 100 million. The key symptoms
of PMO include sex hormone disorder and immune imbalance, which
leads to an imbalance between bone formation and bone resorption,
where bone formation is significantly weakened (1). Clinical manifestations of PMO include
a decrease in bone mass and deterioration of the bone
microstructure, as well as an increase in bone brittleness,
resulting in a higher fracture risk. The pathogenesis of PMO is
well established; the disease is induced by estrogen deficiency as
a result of ovarian function failure in post-menopausal women
(2).
The regulatory effects of estrogen on bone
metabolism occur through numerous mechanisms. For example, estrogen
mediates the balance of bone resorption and bone formation by
regulating parathyroid hormone. In addition, estrogen integrates
with osteoblast receptors to promote bone formation. There are
several growth factors involved in the regulatory effects of
estrogen on bone metabolism, including interleukin (IL)-1, IL-6,
IL-7, tumor necrosis factor (TNF)-α, transforming growth factor-β
and interferon (IFN)-γ (3–6). Estrogen deficiency may directly or
indirectly increase the number of B lymphocytes by increasing the
expression levels of IL-7, of which promotes the expression of
proinflammatory cytokines by T lymphocytes and macrophages, leading
to bone mass loss (7,8). The inflammatory cytokines expressed
by CD4+T lymphocytes have the most significant effects
on bone cell function. CD4+T lymphocytes are divided
into four main subpopulations: T helper (Th)1, Th2, Th17 cells
promote osteoclast growth via the expression of receptor IFN-γ,
IL-6, and IL-17 respectively (9).
Among these four osteoclastogenic cytokines, RankL has a
particularly important role in bone resorption: In vitro
co-culture of murine bone marrow-derived pre-osteoclasts with
CD4+T lymphocytes leads to osteoclast differentiation
and maturation (10). Emerging
evidence has indicated an important role for estrogen in balancing
osteoblasts and osteoclasts, through regulation of Fas/Fas ligand
(FasL) signaling pathways. Fas is activated in response to FasL
integration with the surface of cells expressing Fas. The activated
Fas subsequently results in accumulation of adaptin in the
cytoplasm by interaction of its intracellular death domain (DD)
with the carboxyl terminal DD of the Fas-associated protein with
death domain (FADD). The accumulated FADD integrates with caspase-8
through its death effect domain to form the death-inducing
signaling complex, which activates the downstream caspase family
and cell apoptosis (11,12). It has been revealed that estrogen
can regulate the expression of FasL on the surface of osteoblasts
and osteoclasts, causing osteoclast apoptosis by disrupting the
bone dynamic balance (13,14).
Bone marrow mesenchymal stem cells (BMMSCs) are
osteoblast precursor cells. It has been demonstrated that BMMSCs
exert regulatory effects on immunocytes (10). BMMSCs may inhibit the growth of T
lymphocytes at the G0/G1 stage of the cell
cycle and suppress T lymphocyte proliferation (15,16).
Furthermore, BMMSCs reduce the release of IFN-γ by activated T
lymphocytes, and inhibit the proliferation and differentiation of B
lymphocytes, natural killer cells and neutrophils (17). Previous research has indicated that
BMMSCs have the ability to generate a variety of terminally
differentiated mesenchymal cells, such as osteoblasts and
chondrocytes, which are key in bone construction (18).
MicroRNAs (miRNAs/miRs) have several target genes
and extensively participate in the gene regulatory network to exert
various biological effects. Research suggests that miRNAs are key
regulatory factors of the downstream network, to a greater extent
than other regulatory factors and/or ligands (19). Previous work has indicated that
miRNAs are key in the regulation of bone functional cell apoptosis.
For example, miR-21 regulates expression of the FasL gene;
therefore, the apoptotic rate of osteoclasts transfected with
miR-21 is reduced, leading to the development of osteoporosis
(20).
Our previous study demonstrated that BMMSCs induce
osteoclast apoptosis in vitro and in vivo, and the
addition of estrogen promotes BMMSCs-induced osteoclast apoptosis
by increasing FasL protein expression. Further investigation
revealed that estrogen maintains FasL protein accumulation by
downregulating miR-181a expression (21). In the present study,
CD4+T lymphocytes were selected as a cell model to
elucidate the regulatory effects of the signaling molecules
involved in the development of estrogen deficiency-induced
osteoporosis. Ovariectomized mice were used as a model of
osteoporosis, and BMMSCs and CD4+ T lymphocytes were
subsequently isolated from osteoporotic and control mice. The
apoptotic effect of BMMSCs isolated from the different groups on
CD4+ T lymphocytes was evaluated, and the effects of
estrogen on BMMSCs-induced CD4+ T lymphocyte apoptosis
were investigated. Subsequently, the regulatory effects of estrogen
on FasL expression in BMMSCs were evaluated. BMMSCs were
transfected with miR-181a and co-cultured with CD4+T
lymphocytes, both in vitro and in vivo, to reveal the
regulatory effect of miR-181a on FasL protein expression in BMMSCs
and the effects of miR-181aon CD4+T lymphocyte
apoptosis.
Materials and methods
Materials
α-Minimum Essential Media (MEM), RPMI-1640 and
trypsin were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Goat anti-mouse CD45 (cat. no. ab65952), CD105
(cat. no. ab2529), stem cell antigen-1 (Sca-1; cat. no. ab25323)
and CD29 (cat. no. ab17981) monoclonal antibodies were purchased
from Abcam (Cambridge, MA, USA) and used for testing mesenchymal
stem cells surface markers. CD3 (cat. no. 100243) and CD28 (cat.
no. 102109) antibodies were purchased from BioLegend, Inc. (San
Diego, CA, USA) and used for activation of T lymphocytes. Rabbit
anti-mouse FasL (cat. no. sc-19988) and β-actin (cat. no. sc-69879)
antibodies from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA)
for FasL expression detection and normalization. Reagents for the
induction of BMMSC differentiation, including dexamethasone,
vitamin C, phosphate, β-phosphoglycerol,
3-isobutyl-1-methylxanthine (IBMX), insulin and transferrin were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
TRIzol reagent, Lipofectamine® 2000 transfection
reagent, miR-181a mimics, miR-181a inhibitor and miR-181a control
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
GoldView™nucleic acid gel stain (cat. no. 10201ES03) was purchased
from Beijing SBSGenetech Co., Ltd. (Beijing, China). SYBR Green I
Premix ExTaq™ (Cat. RR42LR), λ DNA-HindIII digest (cat. no.
3403), 50 bp DNA ladder (cat. no. 3421A), DL2000 DNA marker (cat.
no. 3427A) and the miRNA assay kit (cat. no. 631435) were purchased
from Takara Biotechnology Co., Ltd. (Dalian, China).
Mice osteoporosis model
All the experiments were performed on a total of 40
8-week-old female C57 mice (22–25 g), which were obtained from the
Laboratory Animal Center of the Fourth Military Medical University
(Xi'an, China). Bilateral ovaries were resected to establish the
osteoporosis model within 20 mice (OVX group). The control group
(n=20) were subjected to a similar surgery, with the exception that
instead of ovary removal, a small amount of adipose tissue was
removed (sham group). All mice were housed in a specific
pathogen-free (SPF) environment (22°C, 12 h light/dark cycle, and
50–55% humidity) with free access to food and water. The present
study was approved by the ethics committee of Chongqing Medical
University (Chongqing, China).
Microtomography (Micro-CT)
After 2 months maintenance in a SPF room, mice in
the OVX and sham groups, or mimic BMMSCs, inhibitor BMMSCs and
control BMMSCs groups were anaesthetized by intraperitoneal
injection of pentobarbital sodium and placed on Micro-CT apparatus
for an intravital scan of the proximal femoral area. The micro-CT
scan was performed using a scanning angle of 360° and a resolution
of 9 µm along the long axis of the femur. Following the Micro-CT
scan, an area of 1 mm below the growth plate and 1.5 mm from the
distal femur was selected as the range of interest and a
three-dimensional image of the trabecular bone was reconstructed.
Finally, parameters representing the spatial structure of
trabecular bone, including bone volume relative to tissue volume
(BV/TV;%), trabecular thickness (Tb.Th; µm), trabecular spacing
(Tb.Sp; mm) and trabecula number (Tb.N; 1/mm) were analyzed and
determined using the built-in software (Inveon Research Workplace
2.2, Siemens Healthineers, Erlangen, Germany).
Isolation of CD4+T
lymphocytes
After 2 months maintenance in a SPF room, mice in
the OVX and sham groups were anaesthetized with 100 mg/kg
pentobarbital sodium, decapitated and immersed in 75% alcohol with
a temperature of 4°C for 5 min. The left abdomen skin of the mice
was cut, and the subcutaneous tissue and abdominal muscles were
separated to expose the spleen. Spleens were collected and
peripheral connective tissues were removed, after which spleens
were placed in PBS. Subsequently, the obtained spleens were minced
and squeezed using tweezers under sterile conditions, in order to
obtain a cell suspension. The cell suspension was transferred into
sterile tubes and centrifuged at 400 × g for 3 min at 4°C. The
supernatant was removed prior to the addition of 2 ml 1X Red Blood
Cell Lysis Buffer (Beyotime Institute of Biotechnology, Haimen,
China) at 4°C for 5 min for complete red blood cell lysis. The cell
suspension was centrifuged again at 400 × g for 3 min in 4°C after
which the supernatant was removed, and pellets were collected and
washed twice with PBS. CD4 antibody (cat. no. 100413, purity ≥95%
high-performance liquid chromatography; BioLegend, Inc.) was used
for isolation of CD4+ lymphocytes. Monocytes were
stained with fluorescent CD4+ antibody for 30 min at
room temperature and without light. Finally,
CD4+T lymphocytes were obtained. To determine
the concentration of CD4+T lymphocytes,
CD4+T lymphocytes were fixed with 4% paraformaldehyde
for 30 min at 4°C and a flow cytometer was used to count cell
number and analyzed by equipped software (CytExpert1.1, Beckman
Coulter, Inc., Brea, CA, USA).
Isolation and subculture of
BMMSCs
After 2 months maintenance in a SPF room, mice in
the OVX and sham groups were anaesthetized, decapitated and
immersed in 75% alcohol at 4°C for 3 min. Mice were subsequently
placed in a sterile glass dish, anterior and posterior limbs were
separated, and surgery was performed to obtain the marrow cavity of
the backbone. This was repeatedly washed in α-MEM until the color
of the backbone became pale; the collected cell suspension
contained the BMMSCs.
The obtained BMMSCs were seeded in 10-cm plastic
petri dishes, containing α-MEM, 20% fetal bovine serum (FBS;
Sijiqing Bioengineering Materials Co., Ltd, Hangzhou, China), 1%
streptomycin and penicillin, cultured in an atmosphere of 37°C and
5% CO2. After 9 days, when BMMSCs had reached 70–80%
confluence, cells were treated with 0.25% trypsin and subcultured
at a ratio of 1:2. In the following passages, cell medium was
refreshed every 2–3 days. When cells reached a confluence of
70–80%, cells were again treated with 0.25% trypsin and subcultured
at a ratio of 1:2 or 1:3.
Determination of the immunophenotype
of BMMSCs
The obtained third generation BMMSCs were dispersed
in 200 µl PBS solution in an Eppendorf (EP) tube with a final
concentration of 1×105 cells per EP tube. Then,
fluorescein isothiocyanate labeled anti-mouseSca-1 and CD29, and
PE-labeled anti-mouseCD45 were added to the BMMSCs cell suspension
and incubated in the dark at 4°C for 1 h. Subsequently, BMMSCs were
washed 3 times with PBS solution containing FBS with a
concentration of 30 ml/l, centrifuged at 400 × g for 5 min at 4°C
Finally, the fluorescence-labeled BMMSCs were analyzed by flow
cytometry. The positive rates of the cell surface antigen of BMMSCs
were then analyzed and determined using the built-in software with
the unit of %. (CytExpert 1.0.135.2, Beckman Coulter, Inc.).
Differentiation induction of
BMMSCs
The obtained third generation BMMSCs were seeded in
a 6-well plate with a concentration of 1×105 cells/well.
For the induction of differentiation, BMMSCs were either cultured
in α-MEM with the addition of 20% FBS, 100 U/ml penicillin, 100
U/ml streptomycin or in osteoblast inducing conditional medium with
10 mM sodium glycerophosphate, 8M dexamethasone and 50 mg/ml
vitamin C.
Alkaline phosphatase staining
After 7 days of differentiation induction, the cell
medium was removed and the cells were washed 3 times with PBS. Then
4% paraformaldehyde solution was added to fix the cells for 20 min,
followed by adding ALP staining solution (33 µl BCIP, 66 µl NBT and
10 ml PBS) and incubated in the dark for 30 min to 24 h. Following
two washes with PBS, BMMSCs were imaged by phase-contrast
microscopy. Note that the above protocols were strictly followed
the instructions of the BCIP/NBT Alkaline Phosphatase Color
Development kit (Beyotime Institute of Biotechnology,).
Calcified nodules staining
Following osteogenic induction for 21 days, BMMSCs
were washed 3 times with PBS, fixed with 4% paraformaldehyde
solution for 1 h, were washed twice again with PBS and finally
stained with alizarin red for 15 min at room temperature. After
complete removal of the excess alizarin red by three repeated
washes with PBS, BMMSCs were imaged via phase-contrast microscopy
(magnification, ×400).
Adipogenic differentiation induction
of BMMSCs
The third generation BMMSCs were seeded in a 6-well
plate at a concentration of 1×105 cells/well and
incubated with α-MEM supplemented with 20%FBS 100 U/ml penicillin,
100 U/ml streptomycin, 8.9445 g indometacin, 27.8 g IBMX, 500 µl
insulin (50 g/l) and 250 µlDex (39.1 g/l) for 14 days in 37°C. The
cell culture medium was replaced every 2 days.
Oil red O staining
After 14 days following adipogenic differentiation,
the cell medium was removed and cells were washed three times with
PBS. Then, Oil Red O staining solution (0.5 g Oil Red O powder
dissolved in 100 ml isopropanol) was added and incubated in the
dark for 15 min. After removing the excess oil red O stain, cells
were redispersed in PBS and imaged by the phase-contrast
microscopy.
Co-culture of BMMSCs with
CD4+T lymphocytes
The primary CD4+T lymphocytes derived
from normal mice (lymphocytes derived from normal mice for
co-culture with sham, OVX and estrogen-treated BMMSCs to observe
the pro-apoptotic effect among the BMMSCs from three groups) were
seeded in 24-well plates containing equal volume of α-MEM and
RPMI-1640 medium at a final concentration of 1×106
cells/well in 37°C. Normal mice are the mice without sham or OVX
operation, which are additional mice to the 40 stated above; the 40
mice stated above were used for osteoporosis or sham models. A
total of 20 normal mice (8-weeks-old, 22–25 g, female) were
obtained from the Laboratory Animal Center of the Fourth Military
Medical University and housed under the same conditions as
aforementioned. BMMSCs from each group (OVX, sham and
estrogen-treated) were seeded at a concentration of
1×104 cells/well and co-cultured with CD4+T
lymphocytes in the same plate for 3 days. For estrogen treatment,
the obtained third generation BMMSCs were washed twice with PBS and
cultured in 10% FBS containing α-MEM without phenol red. When
BMMSCs reached a confluence of 70–80%, estrogen with concentrations
of 0, 0.1, 1, 10 and 100 nM were added to the cell medium for 24 h
for activation. The activated BMMSCs were kept for further use.
Estrogen solutions of various concentrations were prepared by
diluting β-estradiol with 50 µl anhydrous ethanol.
Detection of CD4+T
lymphocyte apoptosis
CD4+T lymphocyte apoptosis was detected
using the Annexin V, 7-aminoactinomycin D (7AAD) and anti-mouse CD4
staining method. Briefly, after 3 days of BMMSCs and
CD4+T lymphocyte co-culture, supernatants were collected
and centrifuged for 5 min at 400 × g (4°C). The resultant cell
pellets were collected, washed and dispersed in PBS solution
containing 3% FBS to a concentration of 1×106 cells/ml.
Subsequently, fluorescent anti-mouse CD4 antibody (1:50; BioLegend,
Inc., cat. no. 100413) was added and incubated at 4°C in the dark
for 1 h. AnnexinV (5 µl) and 7AAD (5 µl) were subsequently added
and the whole system was incubated in the dark at room temperature
for 15 min. Finally, the apoptotic ratio of CD4+T
lymphocytes was determined by flow cytometry and analyzed by
equipped software (CytExpert1.1, Beckman Coulter, Inc.).
Western blot analysis of FasL
expression in BMMSCs
BMMSCs from each group (OVX, sham and
estrogen-activated BMMSC groups) were washed with 2–3 ml PBS at 4°C
by gentle mixing for 1–2 min. The washing steps were repeated three
times and the supernatant was subsequently removed. Washed BMMSCs
were placed in an ice-bath, whereas attached BMMSCs were gently
scraped and redispersed in PBS at 4°C and centrifuged for 5 min at
400 × g. Cell pellets were subsequently treated with 1X cell lysis
buffer (Beyotime Institute of Biotechnology) according to the
instructions provided within the Nuclear and Cytoplasmic Protein
Extraction kit (Beyotime Institute of Biotechnology). Suspensions
were centrifuged for 12,000 × g with a duration of 5 min at 4°C and
the supernatant was collected for western blot analysis. Proteins
were quantified with a Bicinchoninic Acid protein assay. Equal
amounts (40 µg) of proteins were loaded on 10% SDS-PAGE. Following
separation, proteins were transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% bovine serum albumin (BSA) blocking buffer
(Sigma-Aldrich, Merck KGaA) and then were incubated overnight at
4°C with the primary antibodies (FasL and β-actin, 1:50 dilution).
The membranes were then incubated with goat anti-rabbit IgG (H+L)
secondary antibody (unconjugated) (Wuhan Boster Biological
Biological Technology, Ltd., Wuhan, China) at room temperature for
2 h. The blots on the membranes were analyzed using imaging system
(WD-9423C, Beijing Liuyi Biotechnology, Co., Ltd., Beijing, China)
under treatment of an enhanced chemiluminescence kit (GE
Healthcare, Chicago, IL, USA).
Microarray
Total RNAs were isolated from BMMSCs of Sham and OVX
mice, and were analyzed using microRNA microarray (uParaflo
platform, Sanger 17.0, LC Science Inc. Shanghai, China). Raw data
were normalized by Quantile algorithm (22). Prediction of target genes was
analyzed using the online database miRbase, release 22.0
(www.mirbase.org).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from OVX, Sham and
estrogen-treated BMMSCs. Total RNA was isolated using TRIzol
reagent according to the manufacturer's protocols. miR-181aprimers
were designed and synthesized by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China); sequences are listed in Table I. The PCR reaction mixture
included: SYBR® Green Mix (10 µl), RT template (2 µl),
Bulge-Loop™ miRNA forward (1 µl; 5 µM), Bulge-Loop™ miRNA reverse
(1 µl; 5 µM) and RNase-free H2O (5 µl). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 3 min, followed by 40 cycles of denaturation at 95°C for
30 sec, annealing for 30 sec and extension at 72°C for 30 sec. The
fluorescent signals were recorded during the extension process and
the data were analyzed using the dissolution curve (95°C for 15
sec, 60°C for 23 sec and 95°C for 15 sec) at the end of all cycles.
The data were normalized using 2−∆∆Cq (23).
 | Table I.Oligonucleotide primers used for
polymerase chain reaction. |
Table I.
Oligonucleotide primers used for
polymerase chain reaction.
| Primer | Sequence | Product size
(bp) | Tm |
|---|
| MicroRNA-181a | F:
5′-AACATTCAACGCTGTCGGTGAGT-3′ | 118 | 62°C |
|
| R:
5′-CTCCTTAGAATCTGTTTGCTCTCATA-3′ |
|
|
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ | 88 | 60.6°C |
|
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
|
|
RNA extraction
A total of 1 ml TRIzol was added to each well;
2×106 cells were incubated for 6 min. The lysis solution
was then transferred to 1.5 ml EP tube with the addition of 0.2 ml
chloroform. The tube was shaken vigorously for 15 min and incubated
at 15–30°C for 2–3 min, followed by centrifugation at 10,000 × g
for 15 min at 4°C. After careful removal of the upper layer into a
new EP tube, an equal volume of isopropanol was added, and the EP
tube was placed in the −20°C fridge without disturbance for 1 h,
then centrifuged at 10,000 × g for 15 min at 4°C. After removing
the supernatant, 500 µl 75% ethanol was added to disperse the
precipitates and gently mixed for 30 sec, followed by
centrifugation at 10,000 × g for 5 min at 4°C. The resultant
supernatant was removed and the precipitates in the tube were dried
under RNase free condition for 3–5 min. Finally, 20 µl DEPC was
added to dissolve the dried precipitates and the RNA solution was
saved in a fridge under −80°C.
RNA detection
A total of 2 µl of the aforementioned obtained RNA
solution with 1 µl buffer and then 1.5% agarose gel electrophoresis
was used for RNA detection at a voltage of 80 V. After 30 min run
in a fresh 1X TBE buffer, band 185 and 285 representing RNA sample
in the gel were detected by UV transmission detector. Finally, the
RNA concentration was determined using an EP RNA detector.
RNA reverse transcription
The reaction solution was prepared according to the
instructions of Bulge-loop™ miRNA-qRT-PCR Primer containing 10 µl
2X miRNA Reaction Buffer Mix (RT), 2 µl 0.1%BSA, 2 µl miRNA
PrimeScriptRT Enzyme Mix, 1 µl total RNA, and 5 µl RNase Free
ddH2O. The prepared reaction solution was placed in the
PCR amplifier for RT: 42°C, 60 min and 70°C, 10 min for 40 cycles.
Subsequently, cDNA derived from sham, OVX and estrogen-treated
BMMSCs was collected and placed on ice for microRNA detection.
miR-181a cell transfection
A total of 1 day prior to transfection, the
appropriate number of BMMSCs was seeded into the wells of a culture
plate, and cells were cultured in α-MEM without antibiotics until
cell confluence reached 30–50%. Cell density is a key factor that
affects transfection efficiency, and cell overgrowth weakens their
activity and reduces transfection efficiency. miRNA mimic
(miR10000858-1-5), inhibitor (miR20000858-1-5), control
(miR30017138-1-10) (1.25 µl) and Lipofectamine® 2000
were separately diluted with 50 µl serum-free α-MEM, mixed gently
and incubated at room temperature for 5 min for further use. The
diluted solutions were mixed gently and incubated at room
temperature for 20 min. The prepared transfection solution was
added to the bottom of each well of the 12-well plate and
5×104 cells/well were added. The cells were incubated at
37°C for 24 h. miR-181a mimic, inhibitor or control-transfected
BMMSCs were also used for intravascular injection at a dose of
5×105 cells. After 5 days following the administration
of transfected BMMSCs, the in vivo lymphocytes were
collected and used for apoptosis analysis by flow cytometry as
aforementioned.
Statistical analysis
Statistical analysis was performed using SPSS11.0
software (SPSS, Inc., Chicago, IL, USA). Comparison of multiple
groups was performed using one way analysis of variance followed by
a Tukey's post-hoc test. The means of two independent samples were
compared using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isolation and identification of
BMMSCs
A mouse model of osteoporosis was established and
verified by Micro-CT analysis (Table
II). BMMSCs were subsequently isolated from the mice in the OVX
and sham groups, and their immunophenotype and multi-potent
differentiation abilities were examined. The obtained BMMSCs were
revealed to possess stem cell-like characteristics and multi-potent
differentiation ability (data not shown).
 | Table II.Microtomography results of the sham
and OVX groups. |
Table II.
Microtomography results of the sham
and OVX groups.
| Group | BV/TV (%) | BS/BV (1/mm) | Tb.Th (mm) | Tb.N (1/mm) | Tb.Sp (mm) | BMD (mg/cc) |
|---|
| Sham | 21.15±3.42 | 25.34±3.12 | 0.840±0.08 | 2.01± 0.4 | 0.41±0.11 | 408.80±21.55 |
| OVX |
5.32±1.65a |
8.98±4.05b |
0.051±0.02a |
.42±0.6b |
0.82±0.16b | 60.45±18.79 |
Comparison of CD4+T
lymphocyte numbers in each group
A previous study indicated that estrogen deficiency
activates T lymphocytes and disrupts the dynamic balance between
bone resorption and formation (8).
Therefore, in the present study, CD4+T cells were
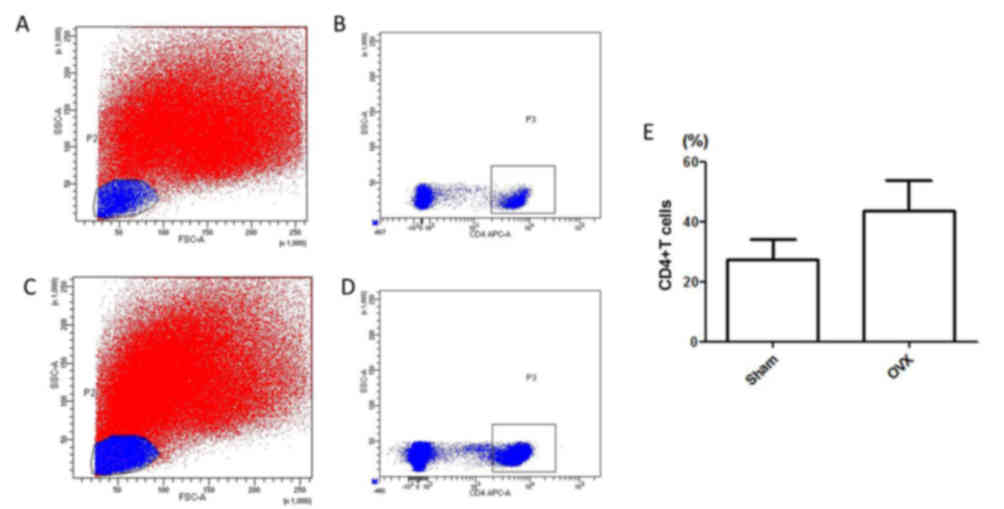
isolated from mice in the sham (Fig.
1A and B) and OVX groups (Fig. 1C
and D), and cell numbers were determined by flow cytometry. As
shown in Fig. 1E, the percentage
of CD4+T lymphocytes was determined in total
spleen cells. The number of CD4+T lymphocytes in the OVX
group was markedly higher compared within the Sham group, thus
suggesting that estrogen deficiency may increase the number of
CD4+T lymphocytes.
Apoptotic effects of estrogen-treated
BMMSCs on CD4+ T lymphocytes
Our previous work demonstrated that BMMSCs induce
osteoclast apoptosis and estrogen promotes BMMSCs-induced
osteoclast apoptosis (18). In the
present study, the apoptotic effects of BMMSCs on CD4+T
lymphocytes were examined. Isolated BMMSCs from the OVX group were
activated by treatment with estrogen at various concentrations.
Estrogen-treated BMMSCs were co-cultured with CD4+T
lymphocytes for 3 days (OVX+ estrogen group) and CD4+T
lymphocyte apoptosis in the co-culture system was subsequently
determined. As a control, CD4+T lymphocytes were
co-cultured with untreated BMMSCs from the OVX and sham groups, and
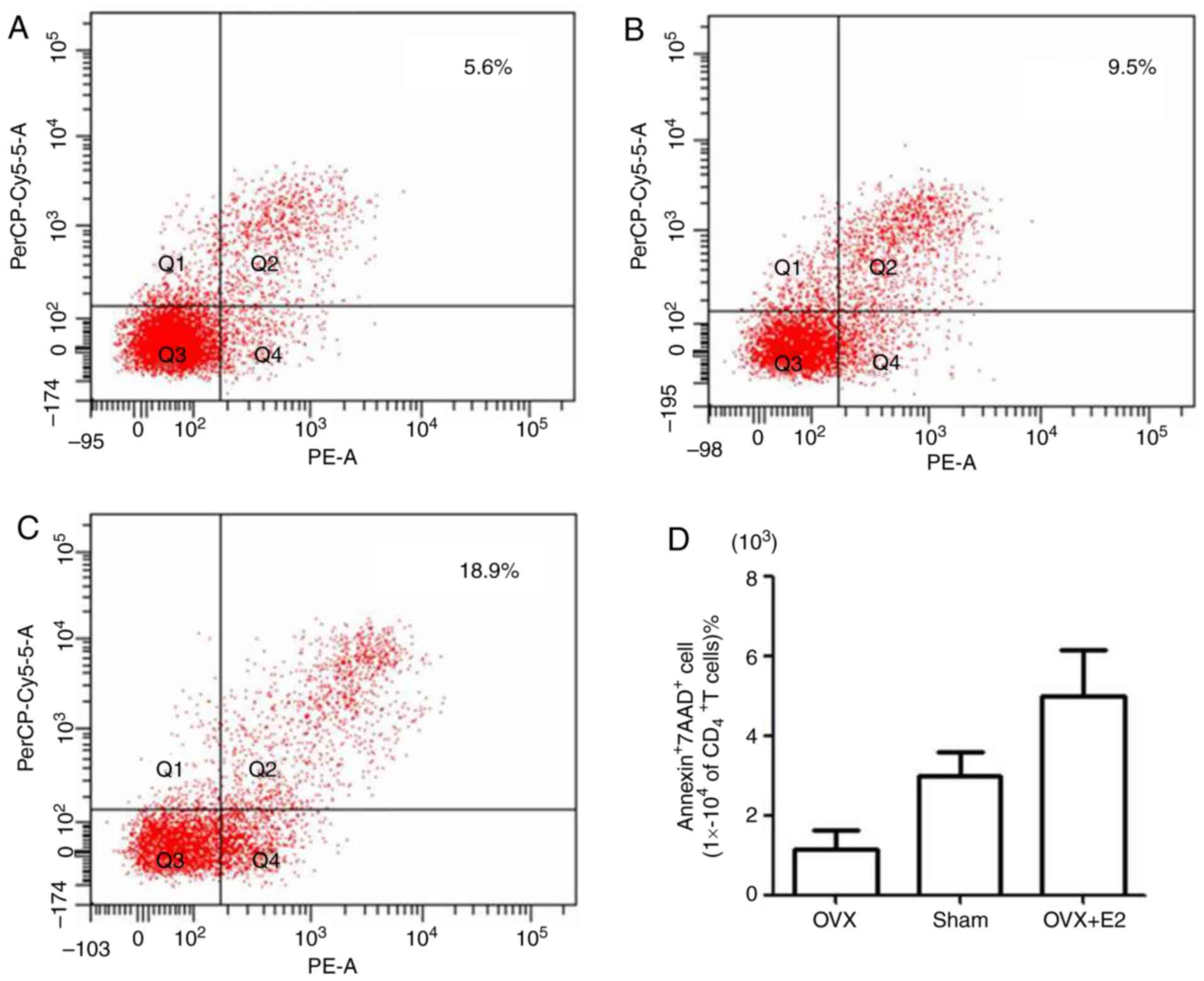
the apoptotic rate was determined. Annexin V was used to label
early apoptotic and necrotic cells, 7AAD was used to label late
apoptotic and necrotic cells, and anti-mouse CD4 antibody was used
to specifically investigate CD4+T lymphocytes. The flow
cytometry results of the OVX (Fig.
2A), sham (Fig. 2B) and OVX+E2
groups (Fig. 2C) revealed that the
apoptotic rate of CD4+T lymphocytes in the OVX+E2 group
was markedly higher, compared with that in the sham or OVX groups.
The apoptotic rate of CD4+T lymphocytes in the OVX group
was the lowest among the three groups (Fig. 2D). These results suggested that
BMMSCs may have an apoptotic effect on CD4+T lymphocytes
and that estrogen significantly increased this effect.
Regulatory effects of estrogen on FasL
expression in BMMSCs
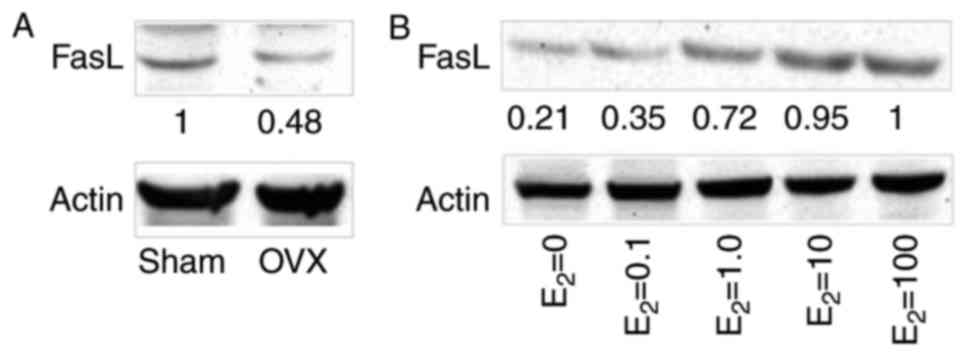
BMMSCs have been reported to induce T-cell apoptosis
via FasL expression (24). To
investigate the regulatory effects of estrogen on the protein
expression levels of FasL in BMMSCs, western blot analysis was
performed in the three co-culture systems. The results revealed
that FasL protein expression was markedly lower in BMMSCs from the
OVX group compared with in the sham group (Fig. 3A). Gradually increasing
concentrations of estrogen resulted in a marked increase in FasL
expression (Fig. 3B), whereas
>10 mM estrogen may not further increase the expression of FasL.
These findings indicated that estrogen may promote CD4+T
lymphocyte apoptosis by increasing FasL protein expression in
BMMSCs.
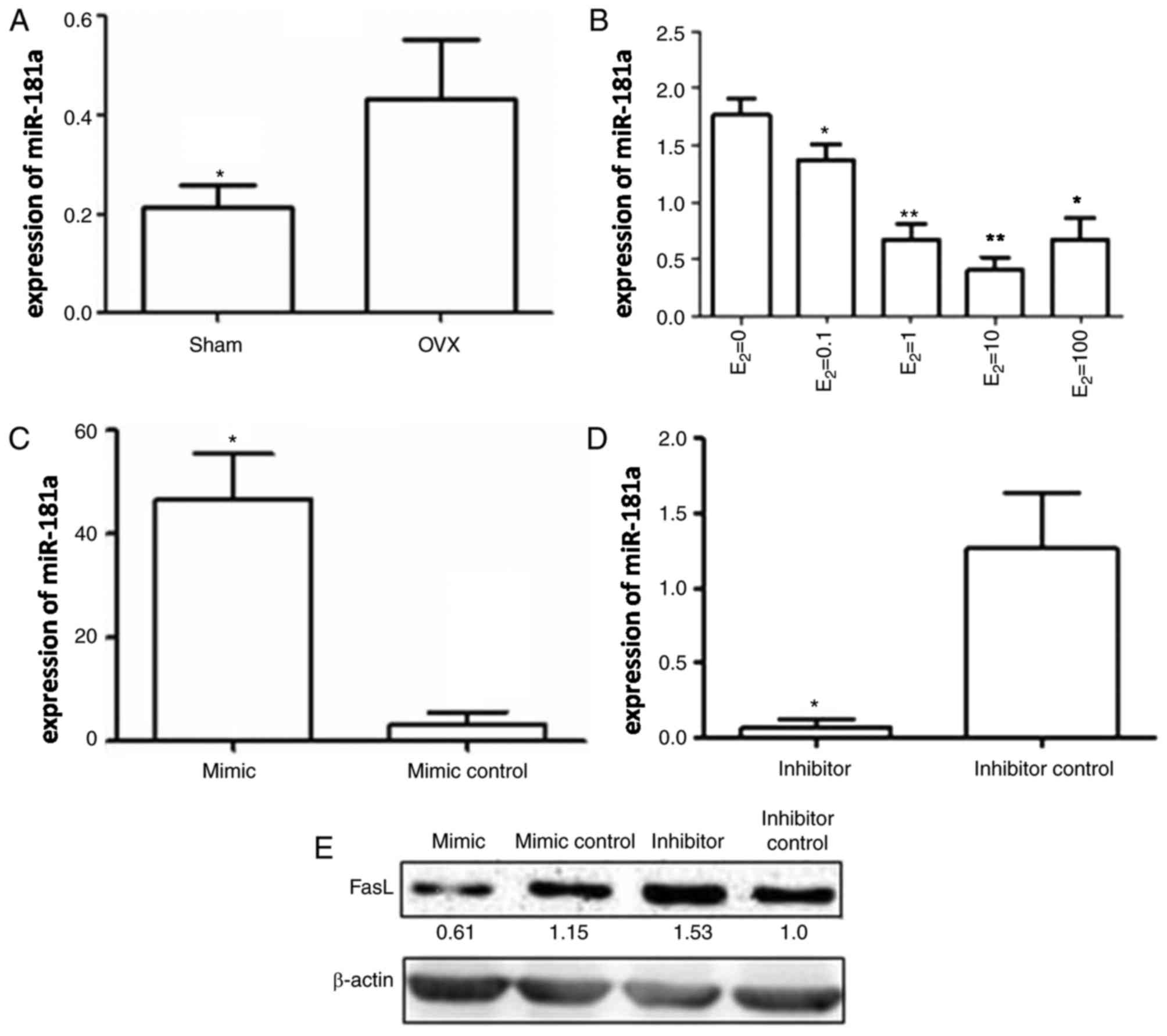
miR-181a expression in BMMSCs
It has previously been reported that miRNAs are
important in the regulation of BMMSC function. According to a
previous study it was demonstrated that miRNA-21 expression is
decreased in BMMSCs following surgical ovariectomy (25). In addition, it has been
demonstrated that miR-181a expression is significantly increased in
an OVX group (18). In the present
study, the expression levels of miR-181a in the three different
co-culture systems were determined by RT-qPCR. miR-181a expression
was significantly higher in the OVX group compared with in the sham
group (Fig. 4A). Conversely,
estrogen treatment decreased miR-181a expression in BMMSCs in a
concentration-dependent manner; however, when estrogen
concentration reached 100 nM, miR-181a expression was increased to
some extent compared with the levels detected following treatment
with 10 nM estrogen, thus indicating that excessive estrogen may
exert an inverse effect (Fig.
4B).
Effects of miR-181a transfection on
FasL expression in BMMSCs
miR-181a was transfected into BMMSCs to determine
the regulatory effects of miR-181a on FasL protein expression in
BMMSCs. BMMSCs were transfected with miR-181a mimic, miR-181a
inhibitor, mimic control or inhibitor control. The transfection
efficiency of the miR-181a mimic and the miR-181a inhibitor was
confirmed by RT-qPCR. Compared with in the mimic control group, the
expression levels of miR-181a were increased by ~50-fold in BMMSCs
transfected with the miR-181a mimic (Fig. 4C), whereas miR-181a expression was
~15 times lower in BMMSCs transfected with the miR-181a inhibitor
compared with the inhibitor control (Fig. 4D). These data suggested that the
miR-181a mimic and miR-181a inhibitor were transfected into BMMSCs
with high efficiency.
Western blot analysis was subsequently performed to
detect FasL protein levels in each group. FasL protein expression
in BMMSCs transfected with the miR-181a mimic was markedly
inhibited compared with in the mimic control group, whereas a
significant increase in FasL protein expression was detected in
BMMSCs transfected with the miR-181a inhibitor compared with the
inhibitor control (Fig. 4E).
In vitro regulatory effect of miR-181a
on CD4+T lymphocyte apoptosis
Our previous work revealed a negative regulatory
effect of miR-181a on BMMSCs-induced osteoclast apoptosis (18). Therefore, the regulatory effects of
miR-181a on BMMSC-induced CD4+T lymphocyte apoptosis
were examined in the present study. BMMSCs were transfected with
miR-181a mimic, mimic control and miR-181a inhibitor. The
transfected BMMSCs were subsequently co-cultured with
CD4+T lymphocytes, in order to determine their apoptotic
effect on CD4+T lymphocytes. Flow cytometric analysis
revealed that miR-181a mimic transfection resulted in a significant
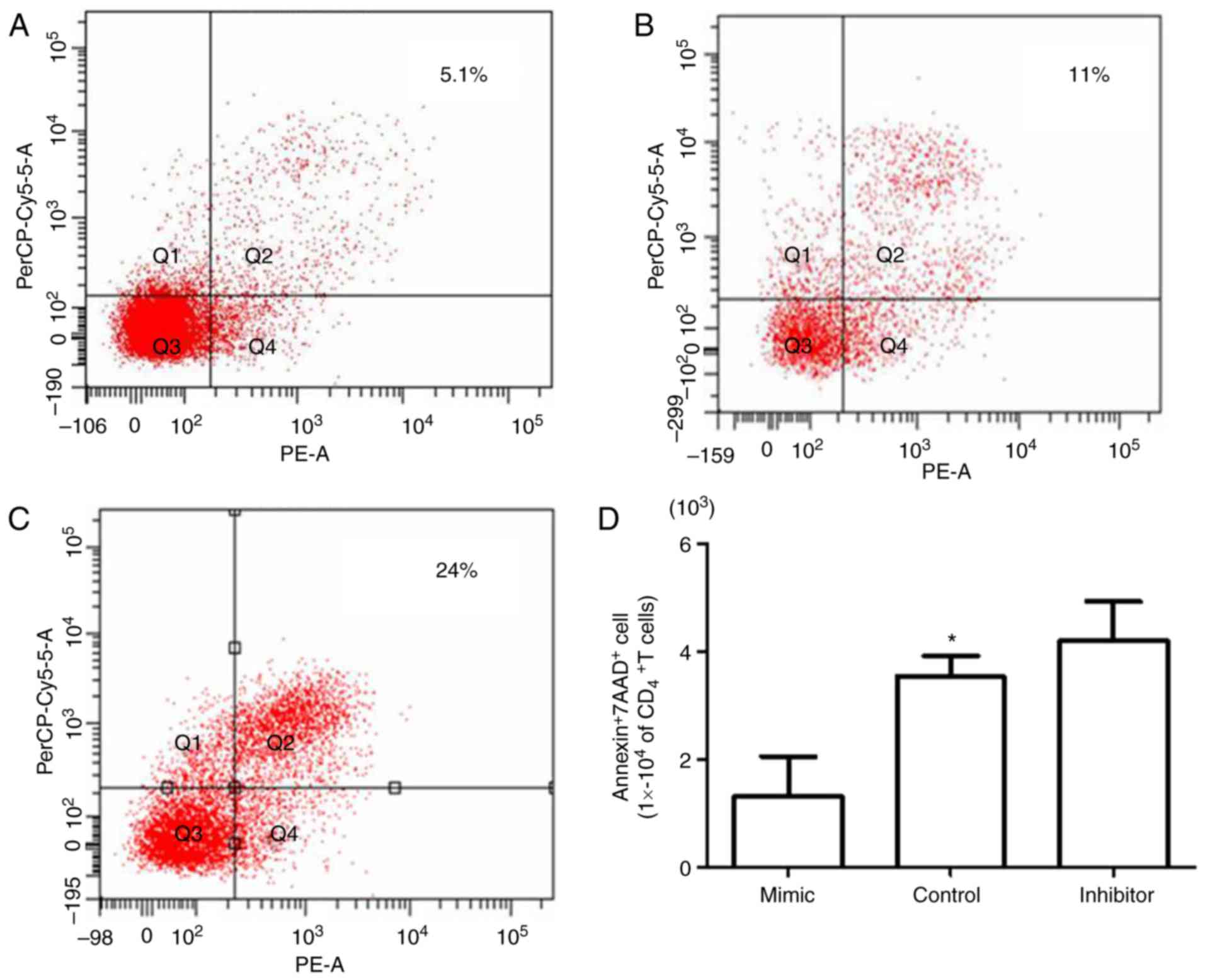
reduction in the proportion of apoptotic cells (5.1%; Fig. 5A) compared with the control group
(11.0%; Fig. 5B); however,
miR-181a inhibitor transfection resulted in an increased number of
apoptotic cells (24.0%; Fig. 5C),
thus indicating the regulatory effect of miR-181a on BMMSC-induced
CD4+T lymphocyte apoptosis (Fig. 5D).
In vivo regulatory effect of miR-181a
on CD4+ T lymphocyte apoptosis
To validate the in vivo regulatory effects of
miR-181a on CD4+T lymphocyte apoptosis, BMMSCs
transfected with miR-181a mimic, mimic control and miR-181a
inhibitor were intravenously injected into mice. After 7 days of
treatment, alterations in mouse bone content were determined by
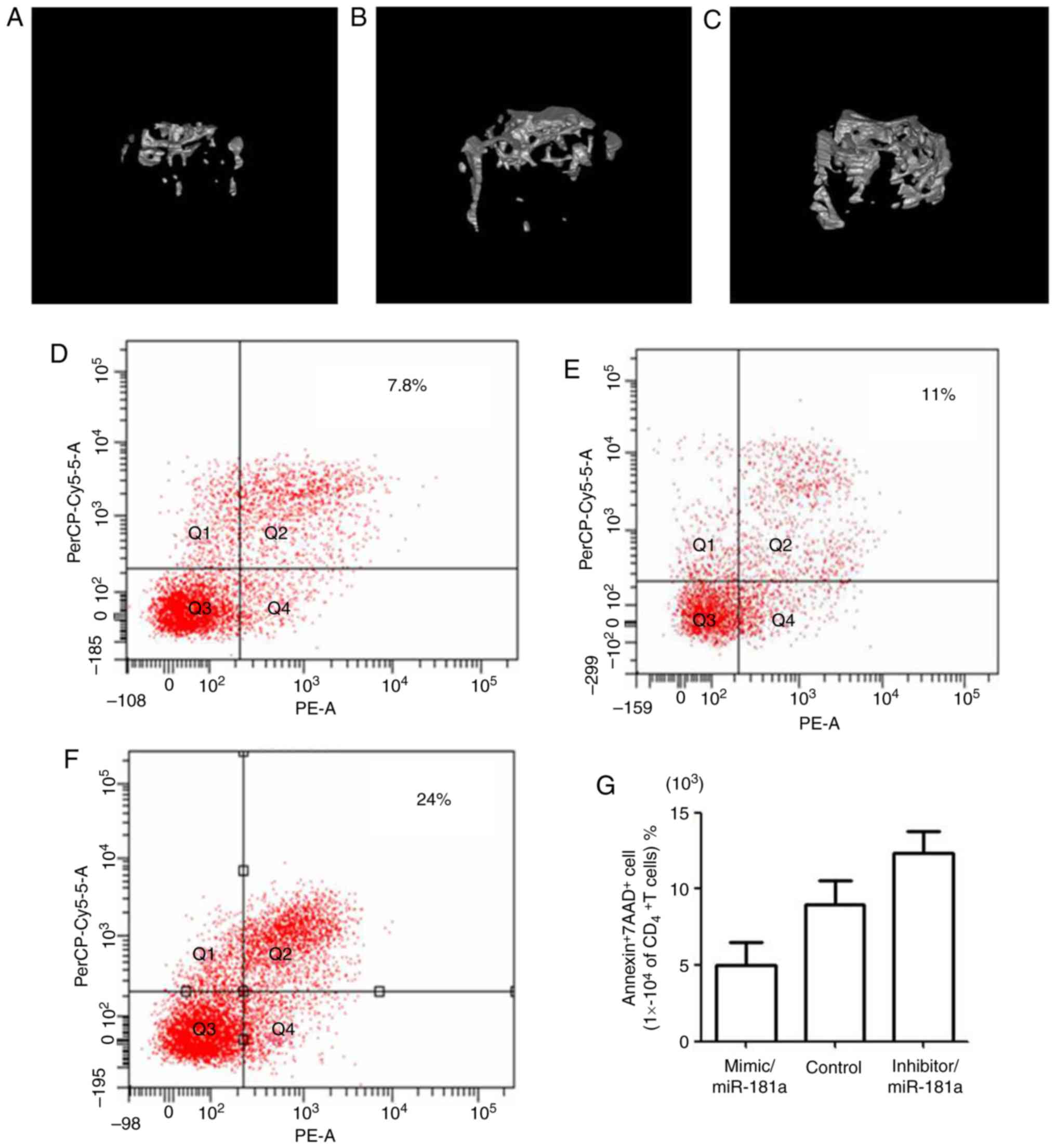
micro-CT. The bone volume of mice treated with the miR-181a mimic
(Fig. 6A) was markedly lower
compared with in the control group (Fig. 6B). Conversely, mice in the miR-181a
inhibitor group (Fig. 6C) had a
visibly higher bone volume than the control group. The derivate
parameters from the micro-CT measurements (Table III) further confirm these
findings; bone mass measurements, including BV/TV, BS/BV and BMD,
were significantly decreased in the miR-181a mimic-treated group
compared with in the control group. Conversely, the opposite effect
was observed in the group treated with the miR-181a inhibitor.
 | Table III.Microtomography results of the mice
treated with microRNA-181a mimic, inhibitor or control for 7
days. |
Table III.
Microtomography results of the mice
treated with microRNA-181a mimic, inhibitor or control for 7
days.
| Group | BV/TV (%) | BS/BV (1/mm) | Tb.Th (mm) | Tb.N (1/mm) | Tb.Sp (mm) | BMD (mg/cc) |
|---|
| Mimic | 5.15±3.02 | 25.34±3.12 | 0.040±0.08 | 2.01±0.4 | 0.91±0.11 | 150.80±21.55 |
| Control |
10.32±1.65a |
30.98±4.05b | 0.51±
0.02a |
4.42±0.6b |
0.71±0.16b |
250.45±16.09b |
| Inhibitor | 19.45±3.42 | 28.31±3.10 | 0.840±0.08 | 2.01± 0.4 | 0.41±0.11 | 370.80±21.55 |
Furthermore, alterations in the apoptotic rate of
CD4+T lymphocytes were detected after 2 days of
injection with BMMSCs transfected with miR-181a mimic, mimic
control and miR-181a inhibitor. The apoptotic rate of
CD4+T lymphocytes in mice treated with the
miR-181a mimic (Fig. 4D) was
markedly decreased compared with in the control group (Fig. 4E), whereas apoptosis was markedly
increased in the miR-181a inhibitor-treated group (Fig. 6F), which further demonstrated the
effects of miR-181a on CD4+ T lymphocyte apoptosis
(Fig. 6G).
Discussion
It has previously been reported that PMO is a bone
formation disorder caused by estrogen deficiency (14). Estrogen deficiency facilitates the
differentiation and maturation of osteoclasts by inducing the
expression of RankL/Rank in T lymphocytes (26). The results of the present study
demonstrated that estrogen affected BMMSC-induced apoptosis of
CD4+T lymphocytes. The present study also
suggested that the regulatory effects of estrogen on BMMSC-induced
CD4+ T lymphocyte apoptosis may be mediated by FasL
protein expression in BMMSCs, with estrogen increasing FasL protein
expression. However, no difference in FasL protein expression was
detected in BMMSCs when estrogen concentration reached 100 nM
compared with 10 nM treatment. This may be due to the presence of
an estrogen response element (ERE) on the promoter sequence of the
FasL gene, which integrates with the estrogen-activated estrogen
receptor (ER) to promote gene expression of FasL (27); when estrogen concentration is too
high, the integration ability between ERE and ER may be weakened,
thus reducing the regulatory effect of estrogen on FasL expression
(28).
Estrogen has been reported to affect miRNA
expression in zebrafish, mice and ACI rats (29,30).
It has been revealed that estrogen inhibits osteoblast apoptosis
through upregulation of miR-17-92a (31). In addition, estrogen treatment
results in the increased expression of miR-146a, miR-125a,
miR-125b, let-7e and miR-126 in T lymphocytes isolated from mouse
spleen (32). Previous studies
have suggested that miR-181a is involved in cell differentiation
and development, as well as immunological, cardiovascular and
central nervous system diseases (33–35).
In the present study, miRNAs that upregulate BMMSCs in mice with
osteoporosis were selected using genechips, and miR-181a was
selected as the miRNA of interest based on miRNA target gene
prediction software.
The present study observed that miR-181a expression
in BMMSCs was markedly increased in the OVX group, which may be due
to the decrease in estrogen during the development of osteoporosis
following ovary removal. Conversely, the expression levels of
miR-181a were significantly reduced in BMMSCs treated with estrogen
at a concentration of 0.1, 1 and 10 nM, thus demonstrating the
negative regulatory effect of estrogen on miR-181a expression
within a certain concentration range. In addition, a significant
decrease in CD4+T lymphocyte apoptosis was observed
following the upregulation of miR-181a expression; this may be due
to the inhibited expression of CD4+ T lymphocyte
apoptosis-associated factors in BMMSCs, including FasL. By
performing western blot analyses, it was revealed that the
expression levels of the apoptotic protein, FasL, were reduced in
BMMSCs following miR-181amimic transfection. In conclusion, it may
be suggested that miR-181 affects BMMSCs-mediated apoptosis of
CD4+T lymphocytes via the regulation of FasL protein
expression; this regulatory ability may be directly associated with
estrogen concentration. A previous study reported similar results,
that estrogen induces the expression of B-cell lymphoma 2, cyclin
D1 and survivin by inhibiting the expression of miR-6, miR-143 and
miR-203 in MCF-7 cells. In addition, the regulatory effects of
estrogen are eliminated by estrogen inhibitors (36). In addition, further study may be
conducted to investigate the downstream signaling underlying
miR-181a/FasL to understand mechanism of osteoporosis and to
develop a novel therapy for osteoporosis, by potentially targeting
the regulation of miR-181a.
Acknowledgements
The authors would like to thank the Chongqing
Municipal Key Laboratory of Oral Biomedical Engineering of Higher
Education and the Program from Innovation Team Building at
Institutions of Higher Education of Chongqing, 2016 for support of
basic medicine research. The authors would also thank to Miss Hua
Ni for molecular and cellular experiments assistance, and thank Mr.
Xiaolin Xu for assisting with the animal experiments (Research and
Development Center for Tissue Engineering, Fourth Military Medical
University (Xi'an, China).
Funding
This research was funded by the General Program of
National Natural Science Foundation of China (grant no. 31571508)
and the General Program of National Natural Science Foundation of
China (grant no. 31371473).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
BS made substantial contributions to the conception
and design, collection and assembly of data, data analysis and
interpretation, wrote the manuscript. XF made substantial
contributions to the design of the present study, collection,
analysis and interpretation of data; YY made contributions in
modifying the study design, assembly of data and data analysis and
interpretation. DY made substantial contributions to the conception
and design of the present study, data analysis and interpretation,
wrote the manuscript and gave final approval of version to be
published. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Chongqing Medical University (Chongqing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMMSCs
|
bone marrow mesenchymal stem cells
|
|
FasL
|
Fas ligand
|
|
PMO
|
post-menopausal osteoporosis
|
References
|
1
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weitzmann MN and Pacifici R: Estrogen
deficiency and bone loss: An inflammatory tale. J Clin Invest.
116:1186–1194. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roggia C, Gao Y, Cenci S, Weitzmann MN,
Toraldo G, Isaia G and Pacifici R: Up-regulation of TNF-producing T
cells in the bone marrow: A key mechanism by which estrogen
deficiency induces bone loss in vivo. Proc Natl Acad Sci USA.
98:13960–13965. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duque G, Huang DC, Dion N, Macoritto M,
Rivas D, Li W, Yang XF, Li J, Lian J, Marino FT, et al:
Interferon-γ plays a role in bone formation in vivo and rescues
osteoporosis in ovariectomized mice. J Bone Min Res. 26:1472–1483.
2011. View
Article : Google Scholar
|
|
5
|
Cenci S, Toraldo G, Weitzmann MN, Roggia
C, Gao Y, Qian WP, Sierra O and Pacifici R: Estrogen deficiency
induces bone loss by increasing T cell proliferation and lifespan
through IFN-gamma-induced class II transactivator. Proc Natl Acad
Sci USA. 100:10405–10410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kudo O, Sabokbar A, Pocock A, Itonaga I,
Fujikawa Y and Athanasou NA: Interleukin-6 and interleukin-11
support human osteoclast formation by a RANKL-independent
mechanism. Bone. 32:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hartgring SAY, Bijlsma JWJ, Lafeber FPJG
and van Roon JAG: Interleukin-7 induced immunopathology in
arthritis. Ann Rheumatic Dis. 65:iii69–iii74. 2006. View Article : Google Scholar
|
|
8
|
Han X, Kawai T, Eastcott JW and Taubman
MA: Bacterial-Responsive B Lymphocytes Induce Periodontal Bone
Resorption. J Immunol. 176:625–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J and Paul WE: CD4 T cells: fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kikuta J, Wada Y, Kowada T, Wang Z,
Sun-Wada GH, Nishiyama I, Mizukami S, Maiya N, Yasuda H, Kumanogoh
A, et al: Dynamic visualization of RANKL and Th17-mediated
osteoclast function. J Clin Invest. 123:866–873. 2003.
|
|
11
|
Griffith TS, Brunner T, Fletcher SM, Green
DR and Ferguson TA: Fas ligand-induced apoptosis as a mechanism of
immune privilege. Science. 270:1189–1192. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegel RM, Frederiksen JK, Zacharias DA,
Chan FK, Johnson M, Lynch D, Tsien RY and Lenardo MJ: Fas
preassociation required for apoptosis signaling and dominant
inhibition by pathogenic mutations. Science. 288:2354–2357. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura T, Imai Y, Matsumoto T, Sato S,
Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, et
al: Estrogen prevents bone loss via estrogen receptor alpha and
induction of fas ligand in osteoclasts. Cell. 130:811–823. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krum SA, Miranda-Carboni GA, Hauschka PV,
Carroll JS, Lane TF, Freedman LP and Brown M: Estrogen protects
bone by inducing Fas ligand in osteoblasts to regulate osteoclast
survival. EMBO J. 27:535–545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krampera M, Glennie S, Dyson J, Scott D,
Laylor R, Simpson E and Dazzi F: Bone marrow mesenchymal stem cells
inhibit the response of naive andmemory antigen-specific T cells to
their cognate peptide. Blood. 101:3722–3729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosado MM, Bernardo ME, Scarsella M,
Conforti A, Giorda E, Biagini S, Cascioli S, Rossi F, Guzzo I,
Vivarelli M, et al: Inhibition of B-cell proliferation and antibody
production by mesenchymal stromal cells is mediated by T cells.
Stem Cells. 24:93–103. 2015. View Article : Google Scholar
|
|
17
|
Schurgers E, Kelchtermans H, Mitera T,
Geboes L and Matthys P: Discrepancy between the in vitro and in
vivoeffects of murine mesenchymal stem cells onT-cell proliferation
and collagen-induced arthritis. Arthritis Res Ther. 12:R312003.
View Article : Google Scholar
|
|
18
|
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z,
Zhao L, Nagy TR, Peng X, Hu J, et al: TGF-beta1-induced migration
of bone mesenchymal stem cells couples bone resorption with
formation. Nat Med. 15:757–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao H, Xu H and Xue L: Regulatory network
involving miRNAs and genes in serous ovarian carcinoma. Oncol Lett.
14:6259–6268. 2007.
|
|
20
|
Sugatani T and Hruska KA: Down-regulation
of miR-21 biogenesis by estrogen action contributes to osteoclastic
apoptosis. J Cell Biochem. 114:1217–1222. 2003. View Article : Google Scholar
|
|
21
|
Shao B, Liao L, Yu Y, Shuai Y, Su X, Jing
H, Yang D and Jin Y: Estrogen preserves Fas ligand levels by
inhibiting microRNA-181a in bone marrow-derived mesenchymal stem
cells to maintain bone remodeling balance. FASEB J. 29:3935–3944.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akiyama K, Chen C, Wang D, Xu X, Qu C,
Yamaza T, Cai T, Chen W, Sun L and Shi S:
Mesenchymal-stem-cell-induced immunoregulation involves
FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell.
10:544–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor α
suppresses the mesenchymal stem cell osteogenesis promoter miR-21
in estrogen deficiency-induced osteoporosis. J Bone Miner Res.
28:559–573. 2003. View Article : Google Scholar
|
|
26
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mor G, Kohen F, Garcia-Velasco J, Nilsen
J, Brown W, Song J and Naftolin F: Regulation of Fas ligand
expression in breast cancer cells by estrogen: Functional
differences between estradiol and tamoxifen. J Steroid Biochem Mol
Biol. 73:185–194. 2003. View Article : Google Scholar
|
|
28
|
Cohen A, Shmoish M, Levi L, Cheruti U,
Levavi-Sivan B and Lubzens E: Alterations in Micro-ribonucleic acid
expression profiles reveal a novel pathway for estrogen regulation.
Endocrinology. 149:1687–1696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kovalchuk O, Tryndyak VP, Montgomery B,
Boyko A, Kutanzi K, Zemp F, Warbritton AR, Latendresse JR,
Kovalchuk I, Beland FA and Pogribny IP: Estrogen-induced rat breast
carcinogenesis is characterized by alterations in DNA methylation,
histone modifications, and aberrant microrna expression. Cell
Cycle. 6:2010–2018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai R, Phillips RA, Zhang Y, Khan D,
Crasta O and Ahmed SA: Suppression of LPS-induced Interferon-gamma
and nitric oxide in splenic lymphocytes by select
estrogen-regulated microRNAs: A novel mechanism of immune
modulation. Blood. 112:4591–4597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo L, Xu J, Qi J, Zhang L, Wang J, Liang
J, Qian N, Zhou H, Wei L and Deng L: MicroRNA-17-92a upregulation
by estrogen leads to Bim targeting and inhibition of osteoblast
apoptosis. J Cell Sci. 126:978–988. 2003. View Article : Google Scholar
|
|
32
|
Naguibneva I, Ameyar-Zazoua M, Polesskaya
A, Ait-Si-Ali S, Groisman R, Souidi M, Cuvellier S and Harel-Bellan
A: The microRNA miR-181 targets the homeobox protein Hox-A11 during
mammalian myoblast differentiation. Nat Cell Biol. 8:278–284. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar S, Naqvi RA, Khanna N and Rao DN:
Disruption of HLA-DR raft, deregulations of Lck-ZAP-70-Cbl-b
cross-talk and miR181a towards T cell hyporesponsiveness in
leprosy. Mol Immunol. 48:1178–1190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YG, Zhang PP, Jiao KL and Zou YZ:
Knckdown of microRNA-181 by lentivirus mediated siRNA expression
vector decreases the arrhythmogenic effect of skeletal myoblast
transplantation in rat with mycardial infarction. Microvasc Res.
78:393–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX,
White RE, Sun X and Giffard RG: miR-181 regulates GRP78 and
influences outcome from cerebral ischemia in vitro and in vivo.
Neurobiol Dis. 45:555–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu X, Zhang X, Dhakal IB, Beggs M,
Kadlubar S and Luo D: Induction of cell proliferation and survival
genes by estradiol-repressed microRNAs in breast cancer cells. BMC
Cancer. 12:292012. View Article : Google Scholar : PubMed/NCBI
|