Introduction
Cervical cancer is one of the most common
destructive tumors of obstetrics and gynecology, severely
endangering the health of women; however, an effective method for
gene therapy is yet lacking (1,2).
Despite that the advanced surgery and chemotherapy can enhance the
therapeutic effect, there is still a high death rate owing to the
recurrence of the disease in patients as well as drug resistance in
chemotherapy (3,4). Vaccines cannot treat or protect all
types of cancer patients or human papillomavirus (HPV) infection
(5,6). The occurrence of cervical cancer is a
complex, multifaceted process; a hazardous HPV infection has been
recognized as the crucial factor for cervical cancer (7–9). In
China, ~62,000 new cervical cancer cases are identified, and
~28,000 women are deceased each year (10). Therefore, the development of
advanced medical therapy against the disease is of vital
significance.
Asparaginase like 1 (ASRGL1) also termed as CRASH,
is an enzyme that hydrolyzes asparagine or glutamine into aspartic
acid and glutamic acid (11); it
has been used to treat acute lymphoblastic leukemia (ALL) for
decades (12–14). However, the role of ASRGL1 in
gynecological tumors has a different perspective. Studies on
endometrial cancer found that ASRGL1 destitution is related to
aggressive disease and meager survival (15,16).
On the contrary, a high level of ASRGL1 was identified in mammary
and ovarian cancers (17,18). Furthermore, there is no report
about ASRGL1 expression and function in cervical cancer to
date.
In this study, a high expression of ASRGL1 is shown
to be present in cervical cancer tissue samples. Then, ASRGL1-short
hairpin (sh) RNA-expressing lentivirus was adopted to explore the
influence of ASRGL1 knockdown on human cervical cancer SiHa cells
in vitro.
Materials and methods
Tissue samples and Cell lines
32 cervical cancer tissue specimens and
paracancerous tissue specimens were obtained from patients who
underwent surgical at the Gynecology and Obstetrics Department of
the Third Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) from January 2017 to October 2017. All patients were not
receiving radiotherapy or chemotherapy before the surgical. All
patients involved in the study were informed, in addition to being
provided with informed consent documentation, which they
subsequently signed. Experiments were approved by the Ethics
Committee of clinical trials of the Third Affiliated Hospital of
Zhengzhou University. SiHa (cervical squamous cell carcinoma) and
HeLa (cervical adenocarcinoma) cell lines were bought from the
Shanghai Institute for Biology Sciences of the Chinese Academy of
Sciences. The cell lines were cultured in DMEM medium with 10%
(v/v) prenatal bovine serum, which is not heat-activated (all from
Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 mg/ml streptomycin, and 100 U/ml penicillin (Sangon Co.,
Ltd, Shanghai, China) at 37°C in a humidified incubator with 5%
CO2.
Immunohistochemistry
Tissues were fixed in formalin and embedded in
paraffin wax. The paraffin-embedded samples were cut into
4-mm-thick, then were deparaffinized and rehydrated in xylene and
serially diluted alcohol solutions. Slides were heated by
microwaving in 0.01 M sodium citrate solution for 15 min to
retrieve antigen. Subsequently, the slides were incubated with
ASRGL1 rabbit polyclonal antibody (1:100; Abcam, Cambridge, UK) at
4°C overnight. Then, each slice was incubated with second antibody
for 30 min. Finally, all slides were stained with diaminobenzidine
followed by the hematoxylin was applied to counterstain. As a
negative control (NC), the primary antibody was replaced with PBS.
All the images were analyzed by conventional optical
microscopy.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cellular RNA from SiHa cells was extracted by
TRIzol (Invitrogen, Shanghai, China). Subsequently, 2 µg mRNA was
reverse transcribed into cDNA. The primers were designed as
follows: ASRGL1 forward, 5′-CGAGTTCAACGCAGGTTGTG-3′ and
reverse, 5′-GGGATTTGCTATACACTGGACTG-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and 5′-CACCCTGTTGCTGTAGCCAAA-3′. qPCR
was performed using the SYBR-Green kit (Takara Biotechnology Co.,
Ltd., Dalian, China) and the ABI 7500 Real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Relative expression as
determined using the 2−∆∆Cq method as described
previously (19). The RT-qPCR
reaction was as follows: 95°C for a period of 15 sec, followed by
45 cycles including denaturation at 95°C for 5 sec, annealing at
45–62°C for 30 sec. The PCR amplification of GAPDH and
ASRGL1 provided an amplicon of 121 and 127 bp,
respectively.
Recombinant lentiviral vector
production and cell infection
GeneChem Co., Ltd. (Shanghai, China) made the
blueprint of the mutually complementary DNA sequence
(5′-CAGTCCAGTGTATAGCAAA-3′) of ASRGL1. The knockdown efficiencies
of these oligonucleotides were tested by incorporating into the
lentivirus-based psc14173 (GeneChem Co., Ltd.) via
AgeI/EcoRI sites. The lentivirus particles functioned
as described previously (20).
With respect to the lentivirus infection, SiHa cells were seeded in
6-well plates. Then, ASRGL1-shRNA-lentivirus or NC lentivirus was
added according to the multiplicity of infection (MOI). The cells
were examined using fluorescence microscope (Olympus, Tokyo, Japan)
after three days.
Western blot analysis
After several washes with cold phosphate-buffered
saline (PBS), the cells were solubilized in precooled 2X lysis
buffer (100 mM Tris, pH 6.8, 10% glycerol, 4% SDS, 2% 2-ME, 10 mM
EDTA) for 30 min on ice. Then, the supernatants were collected
after the lysates were centrifuged at 14,000 × g, 4°C for 10 min.
An equivalent amount of protein is separated by 12% SDS-PAGE and
transferred on PVDF membrane (Millipore, Bedford, MA). Then, the
membrane was blocked in 5% fat-free milk, in TBST buffer, followed
by probing with the anti-ASRGL1 (1:1,000), Bcl-2-associated X
protein (anti-Bax; 1:1,000), anti-B-cell lymphoma 2 (Bcl-2;
1:1,000), anti-cyclin dependent kinase (CDK2; 1:1,000), anti-cyclin
A2 (1:1,000; all Abcam) antibodies at 4°C overnight. GAPDH
(1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) is an
endogenous control. After 3 washes in TBST, the membrane was
incubated with the HRP-conjugated goat anti-mouse and goat
anti-rabbit secondary antibody (1:5,000; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Finally, it was detected using
ECL reagent (ECL-Plus kit; Amersham, Piscataway, NJ, USA).
Cell proliferation experiment
SiHa cells in the logarithmic stage were digested
and resuspended; 2,000 cells/well were seeded into 96-well plates.
Each experiment was performed three times independently. Since day
2, Cellomics ArrayScan VT1 Readers (Cellomics, Inc., Pittsburgh,
PA, USA) calculated the cell number once a day at an interval of
five days. By adjusting the analysis settings of input parameters,
the number of cells with green fluorescence in each scan orifice
were calculated accurately. Finally, the cell proliferation curve
was plotted.
Analysis of cell cycle
Cells were infected with ASRGL1 shRNA
lentivirus or control and were cultured to 80% confluency.
Subsequently, the cells were digested, resuspended, and centrifuged
at 1,300 rpm for 5 min, washed in chilled PBS, and fixed in 75%
alcohol for 1 h, followed by staining with propidium iodide (PI; 50
µg/ml, Sigma-Aldrich®, St. Louis, MO, USA) in the
presence of RNase A (100 µg/ml; Fermentas®, Shanghai,
China). The cell cycle was analyzed using BD FACSCalibur flow
cytometer (FCM; BD Biosciences, San Diego, CA, USA).
Apoptotic assay
Cells were seeded into 6-well plates and transfected
with ASRGL1-shRNA or NC lentivirus for 72 h. Then, the cells were
digested, resuspended, centrifuged at 1,300 rpm for 5 min, washed
in cold PBS and 1X binding buffer, followed by resuspension in 1 ml
1X staining buffer, and 5 ml Annexin V-APC (eBioscience, San Diego,
CA, USA) into 100 ml cell suspension. The reaction was incubated in
the dark for 15 min. The cells were analyzed by FCM.
Data analysis
The cell cultures were analyzed in triplicate using
the SPSS version 21.0 software (IBM SPSS, Corp., Armonk, NY, USA).
The original data were presented as mean ± standard deviation (SD).
The discrepancies in ASRGL1 knockdown and control cells were
comparison using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of ASRGL1 in clinical
cervical carcinoma tissues
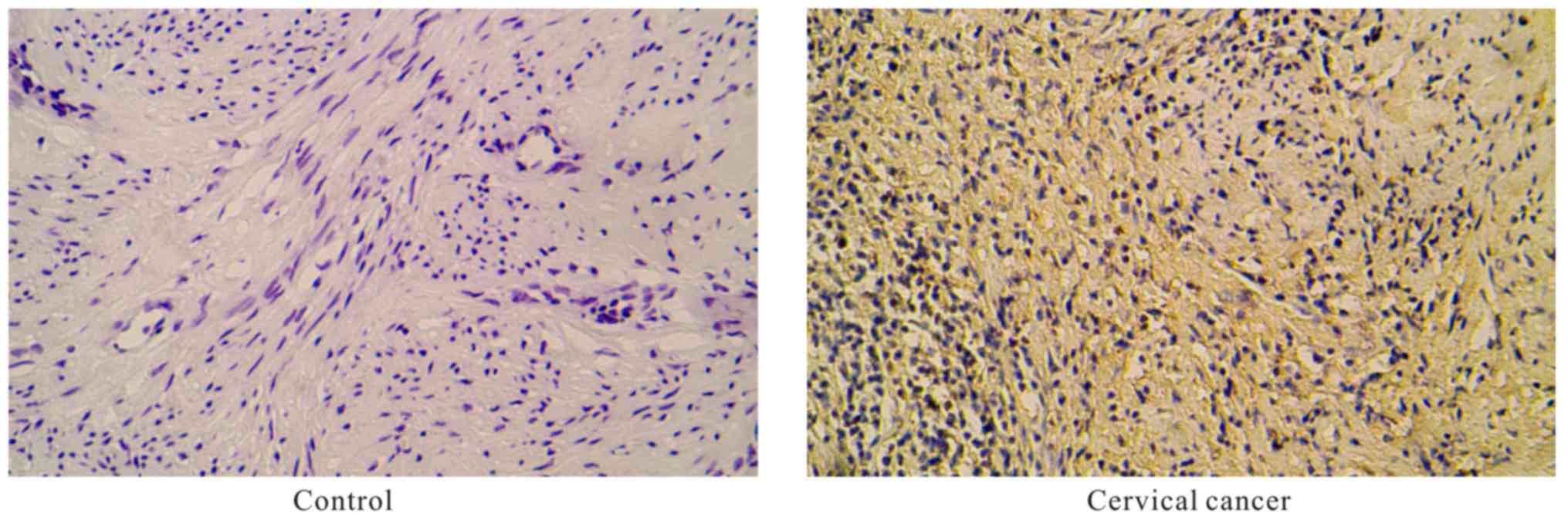
The ASRGL1 proteins were mainly located in the
cytoplasm and was found to be overexpressed in cervical tissues
from patients with cervical cancer when compared with the
paracancerous tissue specimens, as analyzed by immunohistochemistry
(Fig. 1) (P<0.05).
Knockdown efficiency of ASRGL1
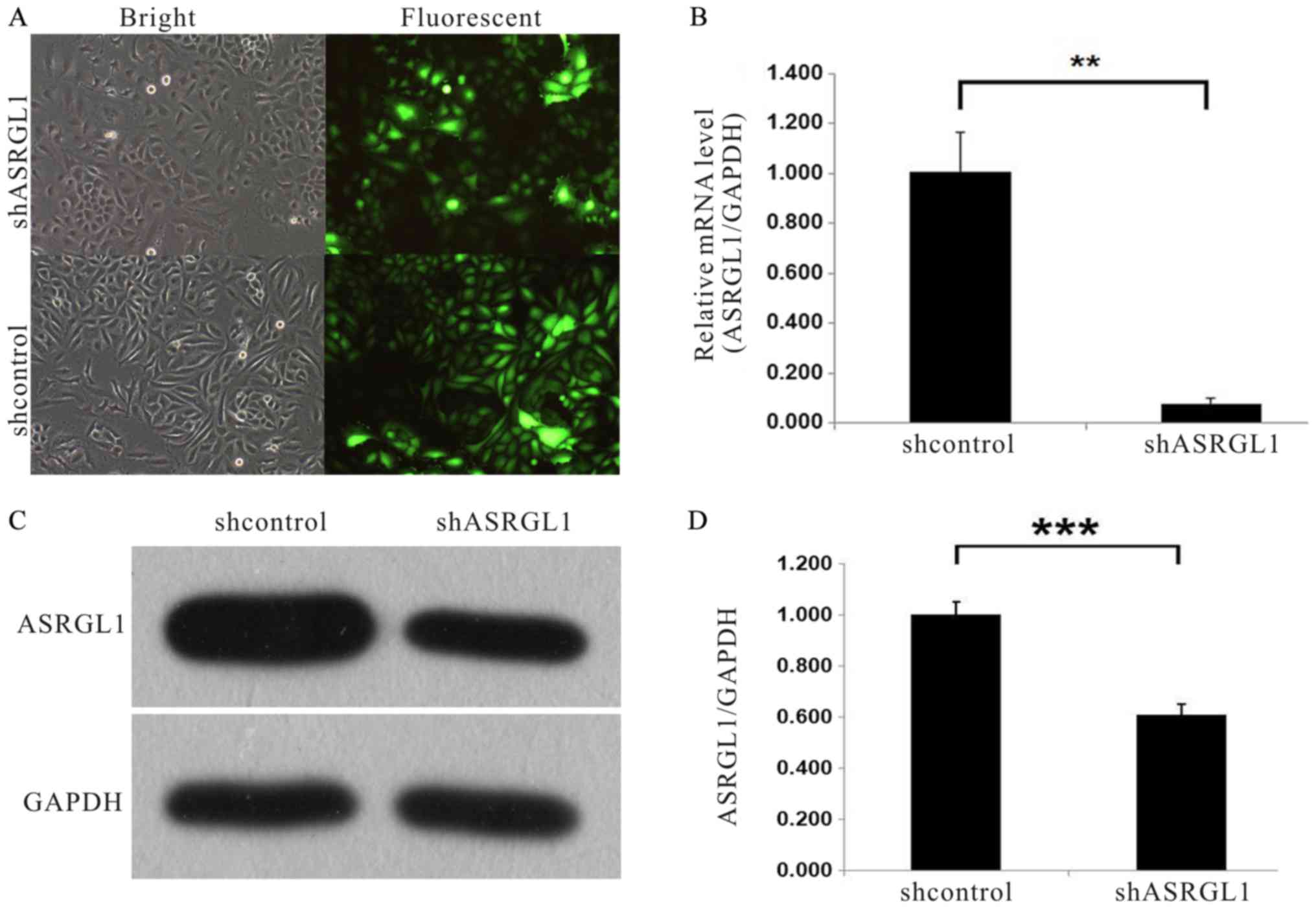
The efficiency of ASRGL1 in SiHa cervical cancer
cells was examined by infecting the cells with ASRGL1 shRNA
lentivirus and NC shRNA lentivirus-expressing GFP, respectively.
Then, we examined the fluorescence level of GFP protein to
determine the infection efficiency. As seen in Fig. 2A, 72 h after infection, >80%
cells were expressing GFP as assessed by fluorescence microscopy.
The mRNA and protein expression level of ASRGL1 after shRNA
infection was evaluated by quantitative PCR and western blot
analysis, respectively. As observed in Fig. 2B, the ASRGL1 mRNA level was
decreased by almost 90% in cell lines infected with ASRGL1
shRNA lentivirus as compared to the NC group. Furthermore, Fig. 2C showed the downregulation of
ASRGL1 protein expression in SiHa cells infected with ASRGL1
shRNA lentivirus. Fig. 2D, the
quantitative analysis of western blotting brands.
Knockdown of ASRGL1 in SiHa cells
inhibits cell multiplication
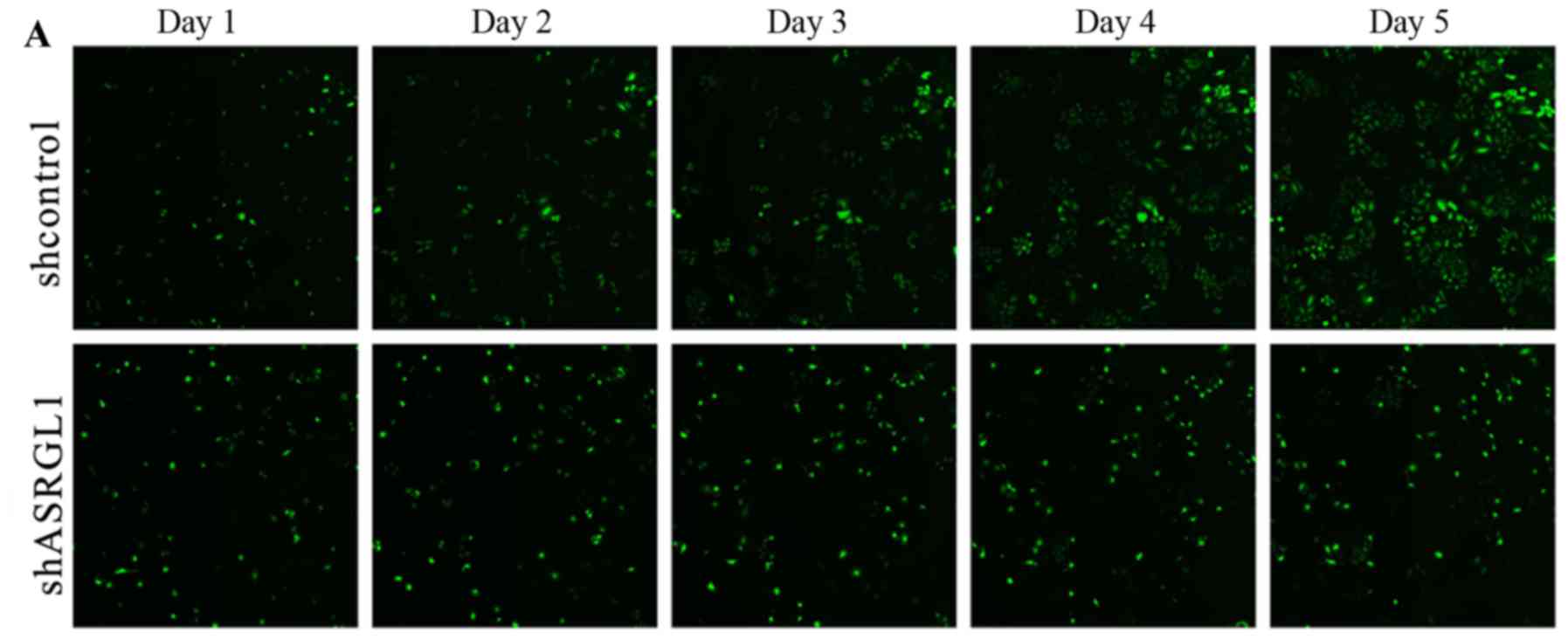
In order to evaluate the function of ASRGL1 in the
proliferation of cervical cancer cells, GFP-expressing cells were
infected with ASRGL1 shRNA lentivirus and NC shRNA
lentivirus, respectively using Cellomics ArrayScan VT1 Readers
continuously for 5 days. As shown in Fig. 3A, the cells transfected with NC
shRNA lentivirus were significantly more as compared to the
ASRGL1-shRNA-transfected cells on day 5 (P<0.05). Fig. 3B further confirmed that knockdown
of ASRGL1 had a dramatic impact on curbing the SiHa cells'
number.
Effect of ASRGL1 shRNA on cell
cycle
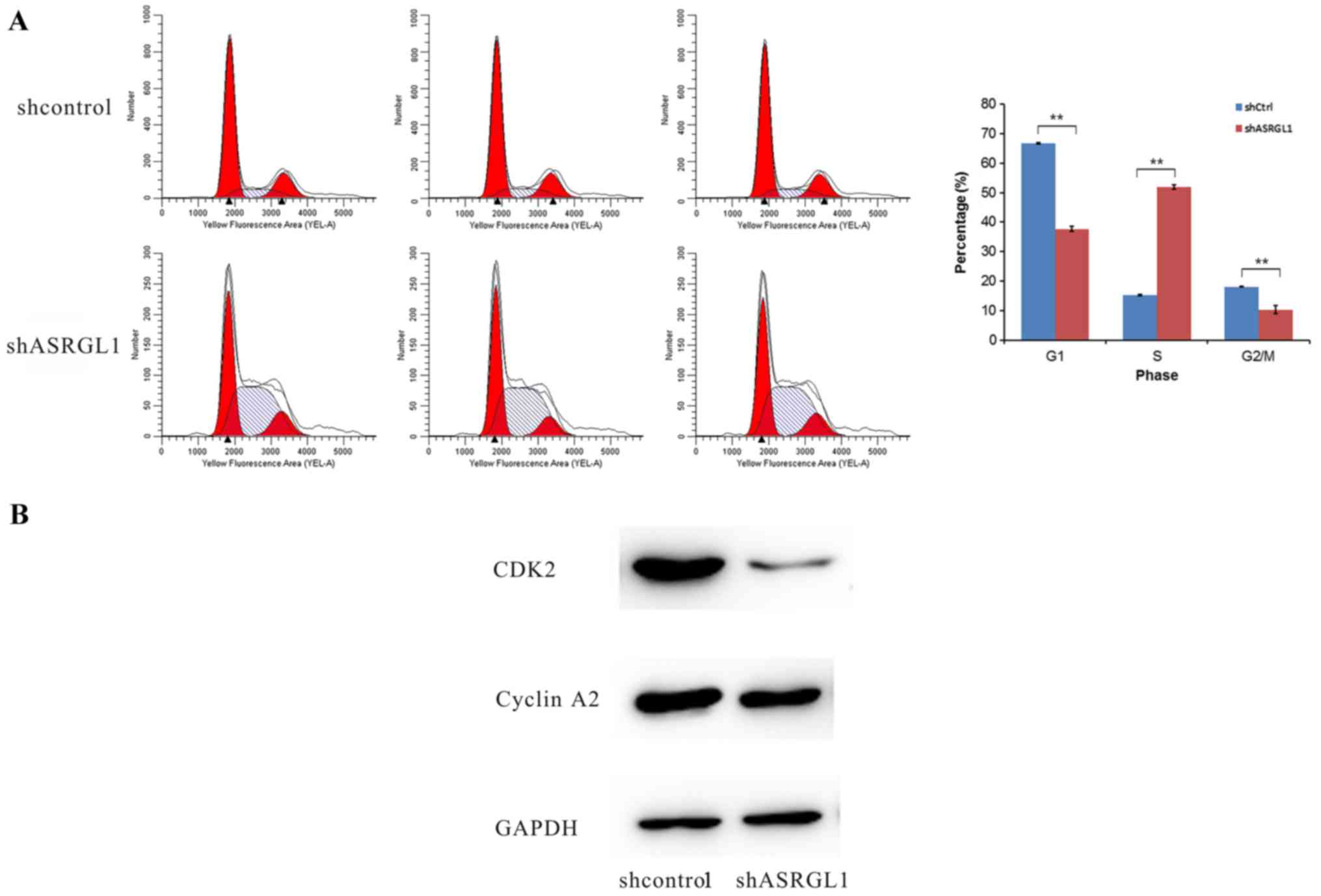
FCM was used to analyze whether the knockdown of
ASRGL1 will affect the cell cycle progression in SiHa cells. As
illustrated in Fig. 4A, the
ASRGL1-shRNA-transfected cells were distributed in G1 phase
(37.71±0.86%, P<0.01); S phase (51.95±0.68%, P<0.01), and
G2/M (10.34±1.31%, P<0.01). However, the NC-shRNA-transfected
cells were distributed in G1 phase (66.62±0.27%, P<0.01); S
phase (15.30±0.26%, P<0.01), and G2/M (18.08±0.19%, P<0.01).
Furthermore, the ratios of S stage cells in ASRGL1-shRNA
transfected group were significantly larger than those in the
negative shRNA control group (P<0.01). Additionally, the levels
of CDK2 and cyclin A2 were significantly reduced in the ASRGL1
siRNA-transfected cells (Fig. 4B).
Cyclin A2 appeared in the late Go, can combine with CDK2 and
promote the synthesis of DNA in S phase. Consequently, the
knockdown of ASRGL1 might capture the cell cycle during the S stage
in SiHa cells.
Knockdown of ASRGL1 in SiHa cells
increases cellular apoptosis
FCM was employed to analyze whether the knockdown of
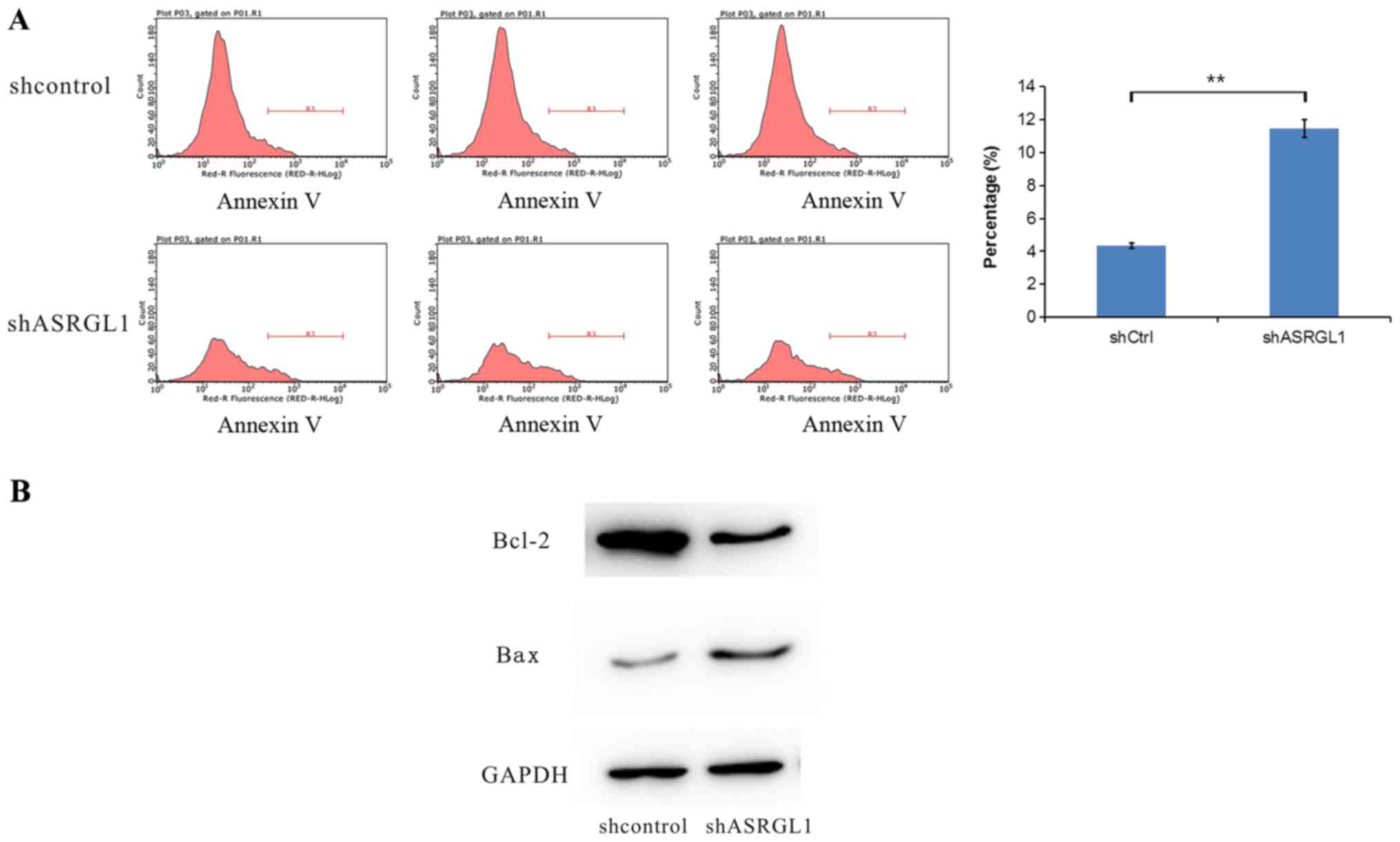
ASRGL1 affected the cell apoptosis in SiHa cells (Fig. 5A). The rate of apoptosis in the
ASRGL1-shRNA exhibited a dramatic increase as compared to the
negative shRNA control group (ASRGL1-shRNA 11.483±0.55% vs.
NC-shRNA 4.36±0.15%, P<0.05). Hence, silencing the expression of
ASRGL1 in SiHa cells induces apoptosis. Subsequently, we measured
the expression level of cell apoptosis-associated proteins Bax and
Bcl-2 after the knockdown of ASRGL1 (Fig. 5B). The proapoptotic protein, Bax,
was significantly enhanced, whereas the expression of
anti-apoptosis Bcl-2 protein was reduced compared with the
control.
Discussion
During the present study, the ASRGL1 expression was
significantly upregulated in cervical cancer tissue compared with
the paracancerous tissue. To explore the function of ASRGL1 in SiHa
cells, we used ASRGL1 shRNA lentivirus to inhibit the
expression of ASRGL1, which indicated that the loss of ASRGL1 could
significantly suppress the proliferation and promote apoptosis in
SiHa cells. However, converse results were observed in endometrial
carcinoma (16), but similar in
mammary and ovarian cancers (17,18).
This phenomenon suggested that ASRGL1 was closely associated with
the tumor growth and can function as the potential target of
cervical cancer gene therapy. There is a striking discrepancy
between the expression of ASGRL1 on mRNA and on protein level after
lentivirus. This is because the main role of shRNA is in RNA level,
and there is also a process of RNA transcription before protein
transcription, so the RNA level effect is more obvious. The
expression level of cell proapoptotic proteins Bax exhibited a
dramatic increase as compared to the negative shRNA control group,
indicate that the ASRGL1 may acts as an anti-apoptotic factor in
the cell apoptotic process. Moreover, after infection with
ASRGL1 shRNA lentivirus, the ratio of cells in G1 and G2/M
stages were declined significantly; however, that in the S stage
was elevated significantly. The cell cycle related proteins Cyclin
A2 and CDK2 are the important proteins that promote cells to
transform from S phase to G2 phase and contribute the synthesis of
DNA (21,22). In this study, the results of
western blot analysis show a decline in both CDK2 and Cyclin A2
protein expression compared with the control. This phenomenon
indicated that ASRGL1 could inhibit tumor growth by regulating the
cell cycle. Thus, the specific molecular mechanism underlying the
ASRGL1-regulated cervical cancer cell growth necessitates further
investigations in other cervical tumor cell lines.
Owing to the rapid advances in tumor molecular
biology and genetic engineering, the genetic treatment is rendered
as a cutting-edge therapy mode for tumors (23). RNA interference-based gene therapy
can accurately and effectively silence the expression of the target
gene. Therefore, identifying the novel biomarker for cervical
cancer intervention is essential. The gene encoding ASRGL1, one
part of the N-terminal nucleophile (Ntn) hydrolase group (24), occurs in duplicate, and the
corresponding transcriptional activation was assessed in
endometrial and breast cancers (16,18).
As the final step in the degradation of cell surface glycoproteins,
ASRGL1 can remove the carbohydrate side chains from asparagine
(25), thereby controlling the
signaling function from the cell surface, metabolism of tumor
cells, and cell growth (11).
However, the role t in cervical cancer has not yet been reported.
In the current study, the knockdown of ASRGL1 in SiHa cells by
ASRGL1-shRNA lentivirus can significantly inhibit cell
growth and enhance cellular apoptosis.
In conclusion, ASRGL1 had a close relationship with
growth and apoptosis in cervical cancer. The knockdown of
ASRGL1 can significantly inhibit the proliferation, promote
apoptosis, and arrest the cells in S phase. Thus, it is a potential
method to treat cervical cancer by downregulating ASRGL1 in these
overexpressed cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Department of Henan, China (grant no. 161100311100),
National Health and Family Planning Commission of Henan, China
(grant no. 201601010) and National Natural Science Foundation of
China (grant no. 81702967).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
X-FLV, H-QH and S-HC conceived and designed the
experiments. X-FLV, H-QH, LL, H-YL and C-CR performed the
experiments. X-FLV, X-AZ and L-DZ analyzed the data. T-XW, J-JL,
W-YX, S-JY and HF contributed reagents, materials and analysis
tools. T-XW, J-JL, W-YX, S-JY, HF, X-FLV and S-HC wrote the paper,
and critically revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients involved in the present study provided
written informed consent. Experiments were approved by the Ethics
Committee of Clinical Trials of The Third Affiliated Hospital of
Zhengzhou University.
Consent for publication
All patients involved in the study provided written
informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu W, Gao Q, Chen K, Xue X, Li M, Chen Q,
Zhu G and Gao Y: Hiwi facilitates chemoresistance as a cancer stem
cell marker in cervical cancer. Oncol Rep. 32:1853–1860. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yee GP, de Souza P and Khachigian LM:
Current and potential treatments for cervical cancer. Curr Cancer
Drug Targets. 13:205–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scatchard K, Forrest JL, Flubacher M,
Cornes P and Williams C: Chemotherapy for metastatic and recurrent
cervical cancer. Cochrane Database Syst Rev.
10:CD0064692012.PubMed/NCBI
|
|
5
|
Gabrielli B, Bokhari F, Ranall MV, Oo ZY,
Stevenson AJ, Wang W, Murrell M, Shaikh M, Fallaha S, Clarke D, et
al: Aurora A is critical for survival in HPV-transformed cervical
cancer. Mol Cancer Ther. 14:2753–2761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabrizi SN, Brotherton JM, Kaldor JM,
Skinner SR, Liu B, Bateson D, McNamee K, Garefalakis M, Phillips S,
Cummins E, et al: Assessment of herd immunity and cross-protection
after a human papillomavirus vaccination programme in Australia: A
repeat cross-sectional study. Lancet Infect Dis. 14:958–966. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosch FX, Burchell AN, Schiffman M,
Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK and
Muñoz N: Epidemiology and natural history of human papillomavirus
infections and type-specific implications in cervical neoplasia.
Vaccine. 26 Suppl 10:K1–K16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ndiaye C, Mena M, Alemany L, Arbyn M,
Castellsagué X, Laporte L, Bosch FX, de Sanjosé S and Trottier H:
HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck
cancers: A systematic review and meta-analysis. Lancet Oncol.
15:1319–1331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doorbar J, Egawa N, Griffin H, Kranjec C
and Murakami I: Human papillomavirus molecular biology and disease
association. Rev Med Virol. 25 Suppl 1:S2–S23. 2015. View Article : Google Scholar
|
|
10
|
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu
S, Li Y, Wang L, Liu Y, Yin P, et al: Cause-specific mortality for
240 causes in China during 1990–2013: A systematic subnational
analysis for the Global Burden of Disease Study 2013. Lancet.
387:251–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Becker FF and Broome JD: L-asparaginase:
Inhibition of early mitosis in regenerating rat liver. Science.
156:1602–1603. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rigouin C, Nguyen HA, Schalk AM and Lavie
A: Discovery of human-like L-asparaginases with potential clinical
use by directed evolution. Sci Rep. 7:102242017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shrivastava A, Khan AA, Khurshid M, Kalam
MA, Jain SK and Singhal PK: Recent developments in L-asparaginase
discovery and its potential as anticancer agent. Crit Rev Oncol
Hematol. 100:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Den Boer ML, van Slegtenhorst M, De
Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, Van Zutven LJ,
Beverloo HB, Van der Spek PJ, Escherich G, et al: A subtype of
childhood acute lymphoblastic leukaemia with poor treatment
outcome: A genome-wide classification study. Lancet Oncol.
10:125–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edqvist PH, Huvila J, Forsström B, Talve
L, Carpén O, Salvesen HB, Krakstad C, Grénman S, Johannesson H,
Ljungqvist O, et al: Loss of ASRGL1 expression is an independent
biomarker for disease-specific survival in endometrioid endometrial
carcinoma. Gynecol Oncol. 137:529–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fonnes T, Berg HF, Bredholt T, Edqvist PD,
Sortland K, Berg A, Salvesen HB, Akslen LA, Werner HMJ, Trovik J,
et al: Asparaginase-like protein 1 is an independent prognostic
marker in primary endometrial cancer, and is frequently lost in
metastatic lesions. Gynecol Oncol. 148:197–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Evtimova V, Zeillinger R, Kaul S and
Weidle UH: Identification of crash, a gene deregulated in
gynecological tumors. Int J Oncol. 24:33–41. 2004.PubMed/NCBI
|
|
18
|
Weidle UH, Evtimova V, Alberti S, Guerra
E, Fersis N and Kaul S: Cell growth stimulation by CRASH, an
asparaginase-like protein overexpressed in human tumors and
metastatic breast cancers. Anticancer Res. 29:951–963.
2009.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song X, Li L, Shi Q, Lehmler HJ, Fu J, Su
C, Xia X, Song E and Song Y: Polychlorinated biphenyl quinone
metabolite promotes p53-dependent DNA damage checkpoint activation,
S-Phase cycle arrest and extrinsic apoptosis in human liver
hepatocellular carcinoma HepG2 cells. Chem Res Toxicol.
28:2160–2169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fotedar A, Cannella D, Fitzgerald P,
Rousselle T, Gupta S, Dorée M and Fotedar R: Role for cyclin
A-dependent kinase in DNA replication in human S phase cell
extracts. J Biol Chem. 271:31627–31637. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guinn BA and Mulherkar R: International
progress in cancer gene therapy. Cancer Gene Ther. 15:765–775.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Irani S, Crutchfield A, Hodge K,
Matthews W, Patel P, Zhang YJ and Stone E: Intramolecular cleavage
of the hASRGL1 homodimer occurs in two stages. Biochemistry.
55:960–969. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saarela J, Laine M, Tikkanen R, Oinonen C,
Jalanko A, Rouvinen J and Peltonen L: Activation and
oligomerization of aspartylglucosaminidase. J Biol Chem.
273:25320–25328. 1998. View Article : Google Scholar : PubMed/NCBI
|