Introduction
Cancer multidrug resistance (MDR) is a major factor
affecting chemotherapy efficacy, which may ultimately lead to the
failure of chemotherapy (1).
Cancer stem cells (CSCs) represent a cancer cell population with
stem cell-like properties. Following the development of the cancer
stem cell hypothesis as well as increasing research of stem cells
in the oncology field, numerous studies have demonstrated that drug
resistance exhibited by CSCs is an important factor affecting
cancer MDR (2,3). Therapy targeting CSCs provides a
novel therapeutic approach for clinical cancer therapy (4). Therefore, establishing an adaptable
and feasible animal xenograft model bearing human colon CSCs is
essential for the study of the underlying mechanisms of CSCs in
order to develop novel and effective anticancer drugs.
At present, there is no agreement regarding the best
method to acquire colon CSCs for the establishment of an animal
model. There are three main methods routinely performed for the
isolation and identification of colon CSCs. Firstly, specific
expression of surface markers for CSCs can be determined,
predominantly by flow cytometry (FCM) (5) and immunomagnetic beads sorting
(6). In this method, isolation and
identification are performed using expressed cell surface markers
present on colon CSCs, including cluster of differentiation (CD)44,
CD166, CD133, Nanog and transcription factor sox-2 (SOX2). This
method has been widely used to identify and define CSCs. Secondly,
Hoechst 33342 staining can be performed to identify a population of
cells with high ATP-binding cassette sub-family G member 2 (ABCG2)
expression in colon cancer, as CSCs exhibiting enhanced ABCG2
expression can promote Hoechst 33342 efflux and decrease
fluorescence intensity of intracellular DNA dye Hoechst 33342
(7). Lastly, a culture screening
method based on the unique microenvironment required for the growth
of colon CSCs can be performed to isolate and identify colon CSCs,
of which serum-free suspension culture is the major method. CSCs
can be isolated by creating an environment conducive for
self-renewal and differentiation, as well as selective elimination
of non-CSC cancer cells. This method has been widely applied for
enriching CSCs (8). A nude mouse
model for colon cancer can be established via subcutaneous
injection or transplantation of xenograft tumor tissues (9,10);
however, the methods required for the establishment of a CSC mouse
model require further investigation.
Salvianolic acid B (SalB) is a water-soluble
phenolic compound and is extractable from Salvia
miltiorrhiza (11). SalB has
previously been demonstrated to reduce the toxicity and enhance the
efficacy of radiochemotherapy for the treatment of colon cancer,
and its role in reversing tumor MDR has generated increasing
attention (12,13). Stem cell factors, including SOX2,
CD24, organic cation/carnitine transporter 4 (OCT4), CD29, CD44 and
ABCG2; serve an important role in maintaining the morphology and
function of colon CSCs, and are closely associated with the
proliferation, drug resistance, invasion and migration of colon
cancer cells (14–17). Investigation into the regulatory
effects of SalB on CSC marker expression will further the
understanding of the mechanisms underlying the effect of SalB on
MDR reversal.
Two colon cancer cell lines (LoVo and HCT-116) were
used in the present study to investigate the effect and underlying
mechanism of SalB. The present study aimed to isolate and identify
colon CSCs, establish a nude xenograft mouse model bearing colon
CSCs, perform H&E staining, investigate the MDR of the
xenografts and determine the efficacy of chemotherapy drugs on the
mouse model. Furthermore, the present study aimed to investigate
the effect of SalB on drug resistance exhibited by xenografts,
determine the expression levels of CD44, CD133, SOX2 and ABCG2
following treatment with SalB, and investigate the underlying
mechanism of this effect.
Materials and methods
Cell lines
Human colon cancer cell lines LoVo and HCT-116 were
purchased from the Cell Resource Center, Shanghai Institutes for
Biological Science (Shanghai, China).
Animals
A total of 100 specific pathogen free (SPF) BALB/c
male nude mice were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. [Shanghai, China; license no. SCXK (Hu) 2007-005]. Mice
were aged 4 weeks and had a body weight of 18±2 g. All animal
experiments were performed according to the guidelines of the
Chinese Experimental Animals Administration Legislation and were
approved by the Ethics Committee for Animal Experiments of Shanghai
University of Traditional Chinese Medicine (Shanghai, China;
reference no. SZY 201504023). Mice were fed in separate cages under
specific pathogen-free conditions in a laminar flow chamber in the
Lab Animal Center, Shanghai University of Traditional Chinese
Medicine (Shanghai, China). Standard water in drinking bottles and
pelleted food were freely available to the mice. The temperature
was maintained at 18–25°C at a relative humidity of 40–60% and a
12/12 h light/dark cycle. The cages, padding, feed and water were
autoclaved at 121°C for 30 min. The padding was replaced at least
twice a week. Animals were acclimatized for 1 week prior to the
initiation of the experiments.
Drugs and reagents
SalB was purchased from Shanghai Winherb Medical
Technology Co., Ltd. (Shanghai, China). A total of 100 mg of
oxaliplatin (L-OHP) was purchased from Jiangsu Hengrui Medicine
Co., Ltd. (Lianyungang, China; cat. no. H20040817). 5-Fluorouracil
(5-FU) was purchased from Shanghai Xudong Haipu Pharmaceutical Co.,
Ltd. (Shanghai, China; 25 g/l; cat. no. 20070802). PRMI-1640 and
DMEM/F12 were purchased from Hyclone; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Penicillin (5,000 IU/ml) and streptomycin
(5,000 µg/ml) were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum (FBS),
L-glutamine, β-mercaptoethanol and 2-hydroxyethyl methacrylate were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Recombinant human basic fibroblast growth factor, recombinant human
epidermal growth factor, KnockOut Serum Replacement and 1% Non
Essential Amino Acid (NEAA) were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). FcR Blocking Reagent
was purchased from Miltenyi Biotec GmbH (Bergisch Gladbach,
Germany). Anti-human CD133-PE and Mouse IgG2b kappa isotype control
were purchased from eBioscience; Thermo Fisher Scientific, Inc.. A
bicinchoninic acid assay (BCA) kit, Hematoxylin and Eosin Staining
kit and an immunohistochemistry kit were purchased from Shanghai
Beyotime Biological Science & Technology Co., Ltd. (Shanghai,
China). Mouse monoclonal antibody ABCG2, mouse monoclonal antibody
CD24, rabbit monoclonal antibody CD133, rabbit monoclonal antibody
OCT-4, rabbit monoclonal antibody CD44, goat anti-mouse
immunoglobulin G (IgG) and goat anti-rabbit IgG were all purchased
from Abcam (Cambridge, MA, USA). Rabbit monoclonal antibody SOX2
was purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). RNA reverse kit and RNA amplification kit were purchased from
Takara Bio, Inc. (Otsu, Japan).
Cell culture and spheroid
formation
LoVo and HCT-116 cells were separately cultured in
F-12K and McCoy'5A medium, respectively. Both cultures contained
10% FBS, 100 µg/ml penicillin and 100 µg/ml streptomycin at 37°C,
in a humidified 5% CO2 incubator. Tumor cell spheres
were isolated using the serum-free suspension culture method:
Logarithmic growth phase LoVo and HCT-116 cells were trypsinized
and centrifuged at 300 × g for 5 min at room temperature. The
supernatant was then discarded and the precipitate was re-suspended
with serum-free medium [Dulbecco's Modified Eagle Medium (DMEM)/F12
+ KnockOut Serum Replacement (10–20%) + 1% NEAA + 1% L-glutamine +
0.1 mM β-mercaptoethanol + 20 µg/l recombinant human basic
fibroblast growth factor + 20 µg/l recombinant human epidermal
growth factor] and the cell suspension was then added to a
petridish coated with 2-hydroxyethyl methacrylate in a drop-wise
manner (8). Cells were incubated
at 37°C in 5% CO2. Following 5–6 days of incubation,
suspended CSCs spheroids were visible under a light microscope
(magnification, ×200).
Identification of colon CSCs
FCM was performed to determine CD133-positive
expression. A single cell suspension (100 µl) containing
106 cells/ml was prepared and treated with mouse IgG2b
kappa FcR blocking reagent in an ice bath for 10 min and then
incubated in the dark at 4°C for 30 min with phycoerythrin tagged
CD133 (1:100; cat. no. 12-1339-41; eBioscience; Thermo Fisher
Scientific, Inc.) and mouse IgG2b kappa isotype control (1:100;
cat. no. 12-4732-81; eBioscience; Thermo Fisher Scientific, Inc.).
Subsequently, the cells were washed twice with PBS and centrifuged
at 300 × g for 10 min at 4°C. The supernatant was aspirated and 500
µl PBS (cat. no. C0221A; Shanghai Beyotime Biological Science &
Technology Co., Ltd.) was added for analysis of the cells using a
FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to investigate the expression
levels of CD90, CD24, CD44, CD133, SOX2, ABCG2, OCT4 and Nanog.
Following the culture of adherent LoVo and HCT-116 cells in
serum-free suspension media for ~5–6 days, suspended regular stem
cell spheroids were visible, and spheroid cell suspension and
adherent cells were simultaneously collected. Following this, total
RNA was extracted using the RNeasy extraction kit (cat. no. 9108Q;
Takara Biotechnology Co., Ltd., Dalian, China). Total RNA (1 µg)
was subjected to RT at 42°C for 15 min followed by 95°C for 3 min
using a FastQuant RT Kit (cat. no. KR106; Tiangen, Biotech Co.,
Ltd., Beijing, China). qPCR was performed using SYBR Green PCR
Master Mix (cat. no. FP302; Tiangen, Biotech Co., Ltd.) in a ABI
7000 PCR instrument (Eppendorf, Hamburg, Germany) under the
following thermocycling conditions: Initial denaturation at 95°C
for 2 min; followed by 40 cycles of 95°C at 10 sec and 60°C at 30
sec. The threshold cycle (Cq) values of each sample were calculated
using the 2−ΔΔCq method (18). GAPDH was used as an internal
control for the normalization of mRNA expression. Experiments were
performed in triplicate. Primer sequences for the targeting of the
selected genes are presented in Table
I.
 | Table I.DNA primer sequences. |
Table I.
DNA primer sequences.
|
| Sequence
(5′→3′) |
|---|
|
|
|
|---|
| Primer name | Forward | Reverse |
|---|
| CD24 |
CCAGGGCAATGATGAATGAGA |
GGGAGGCTGAGGCACGAGAAT |
| CD44 |
GGATGGACAAGTTTTGGTGGCACG |
GGTTACACCCCAATCTTCATGTCC |
| CD90 |
GACTGCCGCCATGAGAATAACACC |
CGCGCCCGAGACTTGAA |
| CD133 |
GATTCATACTGGTGGCTGGGTGG |
GCAGGTGAAGAGTGCCGTAAGT |
| SOX2 |
TTCGATCCCAACTTTCCAT |
ACATCGATTCTCGGCAGAC |
| ABCG2 |
GGTTTCCAAGCGTTCATTCAAA |
TAGCCCAAAGTAAATGGCACCTA |
| OCT4 |
CGAAGAGAAAGCGAACCAGTATC |
AGAACCACACTGGACCACATC |
| Nanog |
GCAAAAAAGGAAGACAAGGTCC |
CCTTCTGCGTCACACCATTG |
| GAPDH |
GGTGGTCTCCTCTGACTTCAACA |
CCAAATTCGTTGTCATACCAGGAAATG |
Western blot analysis
Western blot analysis was performed to determine the
expression levels of OCT4, CD24, CD44, CD133, SOX2 and ABCG2
proteins. Cytoplasmic and nuclear proteins were extracted using the
protein extraction kit (cat. no. P0013B; Shanghai Beyotime
Biological Science & Technology Co., Ltd.), and protein levels
were then determined using a BCA protein assay kit (cat. no. P0010;
Shanghai Beyotime Biological Science & Technology Co., Ltd.).
Protein samples (30 µg) were run on a 10% SDS-PAGE gel, and then
transferred to polyvinylidene fluoride membrane. Membranes were
then blocked with 5% bovine serum albumin (cat. no. W029; Shanghai
Bogu Biotechnology Co., Ltd., Shanghai, China) blocking solution
for 2 h at room temperature, and then incubated overnight at 4°C
with the following primary antibodies: Rabbit monoclonal antibody
OCT4 (1:1,000; cat. no. ab200834), rabbit monoclonal antibody CD44
(1:1,000; cat. no. ab51037), mouse monoclonal antibody CD24
(1:1,000; cat. no. ab76514), rabbit monoclonal antibody CD133
(1:1,000; cat. no. ab216323), rabbit monoclonal antibody SOX2
(1:1,000; cat. no. 3579) and mouse monoclonal antibody ABCG2
(1:1,000; cat. no. ab130244). Following overnight incubation, the
membranes were washed three times with Tris-buffered saline with
0.1% Tween-20 (TBST), and goat anti-mouse IgG (1:1,000; cat. no.
ab205719) and goat anti-rabbit IgG (1:1,000; cat. no. ab205718)
were incubated at 37°C for 2 h. The membrane was then washed three
times with TBST. and then visualized using enhanced
chemiluminescent reagents (cat. no. P0018; Shanghai Beyotime
Biological Science & Technology Co., Ltd.) according to the
previously published protocol (19). ImageJ software (version 1.48;
National Institutes of Health, Bethesda, MD, USA) was used for
densitometric analysis of target protein bands. Experiments were
performed in triplicate.
Establishment of the xenograft nude
mouse model
Spheroid cells generated from LoVo and HCT-116 cells
were labeled as LoVocsc and HCT-116csc, respectively. LoVo,
LoVocsc, HCT-116 and HCT-116csc cells were harvested, rinsed twice
with ice-cold PBS and then re-suspended with normal saline in order
to obtain cell suspensions with a concentration of 2×106
cells/ml, which were then placed on ice for subsequent injections.
A total of 0.2 ml of cell suspension of LoVo, LoVocsc, HCT-116 and
HCT-116csc groups were injected subcutaneously into the right fossa
axillaris of 12 randomly selected nude mice (each cell suspension
group injected into 3 nude mice). When the xenografts had grown to
~0.8–1.5 cm, the mice were sacrificed and the tumors were isolated.
The tumor tissue was then dissected into 1 mm3 blocks
and inoculated subcutaneously into the right fossa axillaris of
nude mice under sterile conditions. Tumors formed from LoVo and
HCT-116 cells were subsequently inoculated into 24 nude mice (12
mice per tumor type). Tumors formed from LoVocsc and HCT-116csc
cells were inoculated into 64 nude mice (32 mice per tumor type).
Every 2 days, major and minor axes of the tumors were measured
using a vernier caliper, denoted as a and b, respectively; to
determine the tumor volumes (V) using the following formula:
V(mm3)=1/2*a*b2(20)
When xenografts had grown to a size of 100
mm3, 8 nude mice bearing LoVo, HCT-116, LoVocsc and
HCT-116csc xenografts were randomly selected (2 mice per tumor
type). These mice were sacrificed via cervical dislocation, and the
tumor tissues were obtained for subsequent experimentation.
H&E staining
Tissues were collected, fixed with 4%
paraformaldehyde for 10 min at room temperature, paraffin-embedded
and sectioned (5 µm thick sections). Following dewaxing with xylene
and rehydrated using a descending ethanol series for 2 min per
concentration gradient (100, 90, 80 and 70%), sections were stained
using a H&E staining kit (cat. no. C0105; Shanghai Beyotime
Biological Science & Technology Co., Ltd.), in accordance with
the manufacturer's protocol, and subsequently dehydrated with
gradient ethanol for 10 sec per concentration gradient (70, 80, 90
and 100%). Sections were then mounted and histopathological changes
were observed under a light microscope (magnification, ×100 and
×400) (21).
Grouping and SalB dosing
The remaining mice bearing LoVocsc xenografts were
randomly assigned into 6 groups (5 mice per group): LoVocsc group,
LoVocsc + L-OHP group, LoVocsc + SalB-low dose (L) group, LoVocsc +
SalB-medium dose (M) group, LoVocsc + SalB-high dose (H) group and
LoVocsc + L-OHP + SalB-L group. The remaining mice bearing LoVo
xenografts were randomly assigned into 2 groups (5 mice per group):
LoVo group and LoVo + L-OHP group. The mice bearing HCT-116csc and
HCT-116 xenografts were grouped using the aforementioned protocol.
Mice in the LoVocsc + L-OHP and LoVo + L-OHP groups were
intraperitoneally injected with L-OHP (0.5 mg); Mice in the LoVocsc
+ SalB-L, LoVocsc + SalB-M and LoVocsc + SalB-H groups were
intragastrically administered low, medium and high doses of SalB
solution (0.36, 0.72 and 1.44 g, respectively), at a dosage of 0.4
ml; Mice in the LoVocsc + L-OHP + SalB-L group were
intraperitoneally injected with L-OHP (0.5 mg) combined with
intragastric administration of low dose of SalB (0.36 g).
Similarly, mice in the HCT-116csc + 5-FU and HCT-116 + 5-FU groups
were intraperitoneally injected with 5-FU (0.01 mg); and mice in
the HCT-116csc + SalB-L, HCT-116csc + SalB-M and HCT-116csc +
SalB-H groups were intragastrically administered low, medium and
high doses of SalB solution (0.36, 0.72 and 1.44 g, respectively),
at a dosage of 0.4 ml. Furthermore, the mice in the HCT-116csc +
5-FU + SalB-L group were intraperitoneally injected with 5-FU (0.01
mg) combined with intragastric administration of low dose of SalB
(0.36 g). Mice in the LoVo, LoVocsc, HCT-116 and HCT-116csc groups
were intragastrically administered 0.4 ml of normal saline. L-OHP
and 5-FU were administered to respective groups once every two
days, whereas SalB was administered every day. Treatment lasted for
a total duration of 28 days. The activity and skin color of nude
mice were recorded every day for 28 days and were then euthanized
by cervical dislocation when they reached the pre-determined
measureable end points: Weight loss exceeding 15%.
Determination of tumor volume, growth
curves and the rate of tumor inhibition
At 3 day time intervals, major and minor axes of the
tumors were determined using a vernier caliper to calculate the
tumor volume. Using these data, growth curves of the tumors were
determined. Furthermore, the inhibitory rate was determined using
the following formula: Inhibitory rate=(mean tumor volume of the
control group-mean tumor volume of the test group)/mean tumor
volume of the control group *100%. To investigate the interaction
between SalB with L-OHP and 5-FU chemotherapeutic agents, the
method described by Jin et al (22) was used. This method provides a ‘Q’
value, according to which the interaction between two drugs can be
classified as exhibiting an antagonistic effect (Q≤0.85), additive
effect (0.85≤Q<1.15) or synergistic effect (Q≥1.15). The formula
used to calculate the Q value is as follows:
Q=Ea+b/(Ea+Eb-Ea*Eb),
where Ea+b, represents the mean effect of combination
treatment, and Ea and Eb represent the effect of drug A and drug B
alone, respectively.
Tumor CD44, CD133, SOX2 and ABCG2
expression levels determined by western blot analysis
Tumor tissues were lysed in radioimmunoprecipitation
assay buffer (1 mg tumor tissue for 10 µl lysis buffer; cat. no.
P0013B; Shanghai Beyotime Biological Science & Technology Co.,
Ltd.), and liquid nitrogen was quickly added to grind the tissues
to extract protein. This was followed by centrifugation at 10,000 ×
g for 5 min at 4°C, and the supernatant was collected. Procedures
for western blot were performed according to the aforementioned
protocol. β-actin was used as an interval control.
Statistical analysis
Statistical analyses were performed using SPSS
software (v. 18.0; SPSS, Inc., Chicago, IL, USA). Data are
presented as mean ± standard deviation for at least three separate
experiments. Comparisons between two groups were performed using a
Student's t-test, and multiple comparisons between groups was
performed using one-way analysis of variance followed by the
Newman-Keuls post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Growth of spheroid CSCs
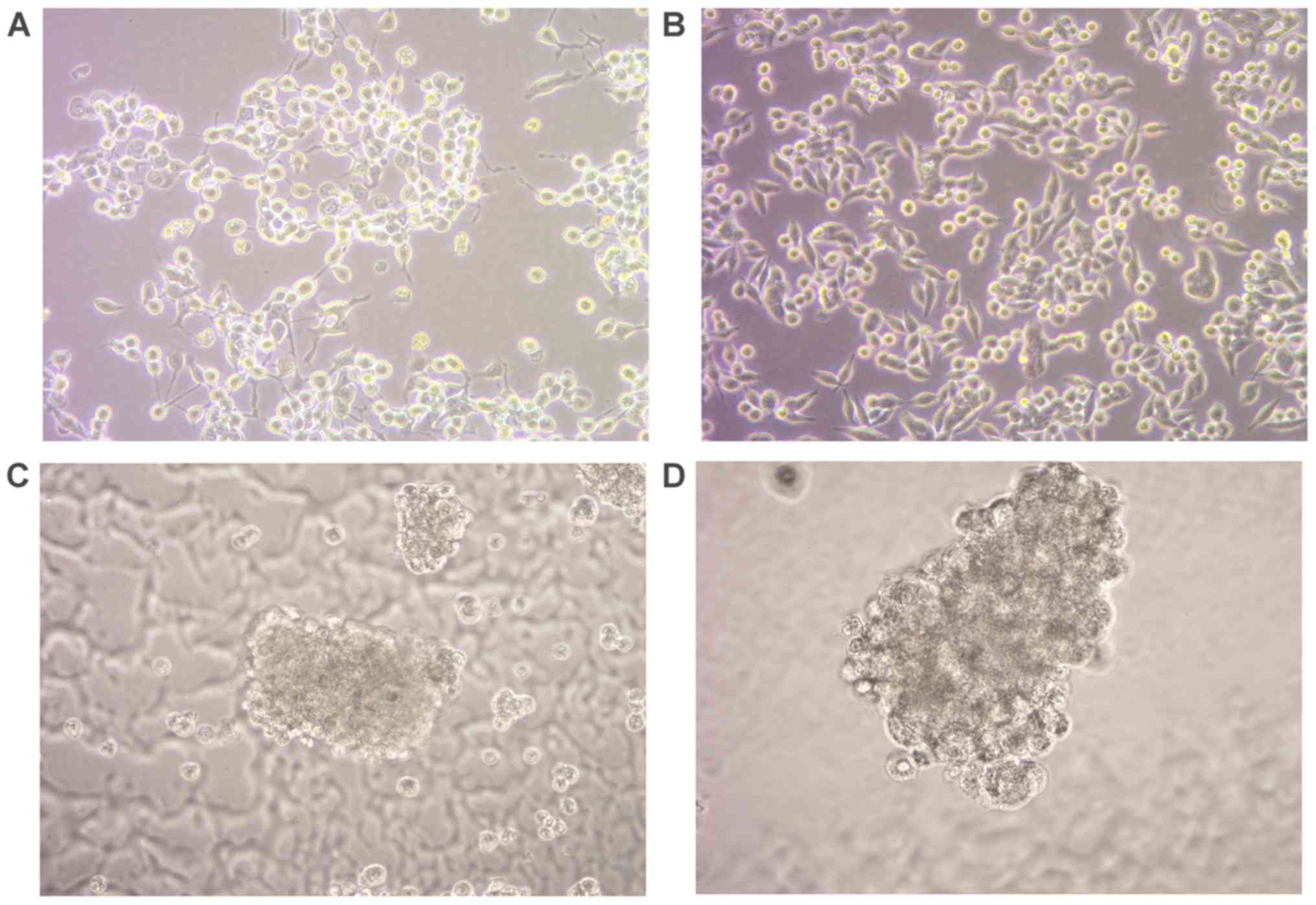
Human colon cancer cell lines LoVo and HCT-116 were
cultured for 2 days (Fig. 1A and
B) and then cultured in stem cell culture medium ES. Following
suspension culture for 2–3 days, only a small portion of cells
survived. At the day 4 time interval, a small number of floating
cell spheroids were visible, with a number of cells closely
clustered. These cell spheroids were oval shaped and exhibited a
clustered appearance, with a clear intercellular space (data not
shown). The spheroids increased in size in a time-dependent manner
and at days 5–6 time interval, suspended regular stem cell
spheroids were visible and an increased number of cells, blurred
intercellular space and strong refractivity was observed using a
light microscope (Fig. 1C and D).
Necrosis was observed in the center of the spheroids at the >10
day time intervals (data not shown). Spheroids were collected on
days 5 and 6 for further experimentation.
Verifying CSC formation
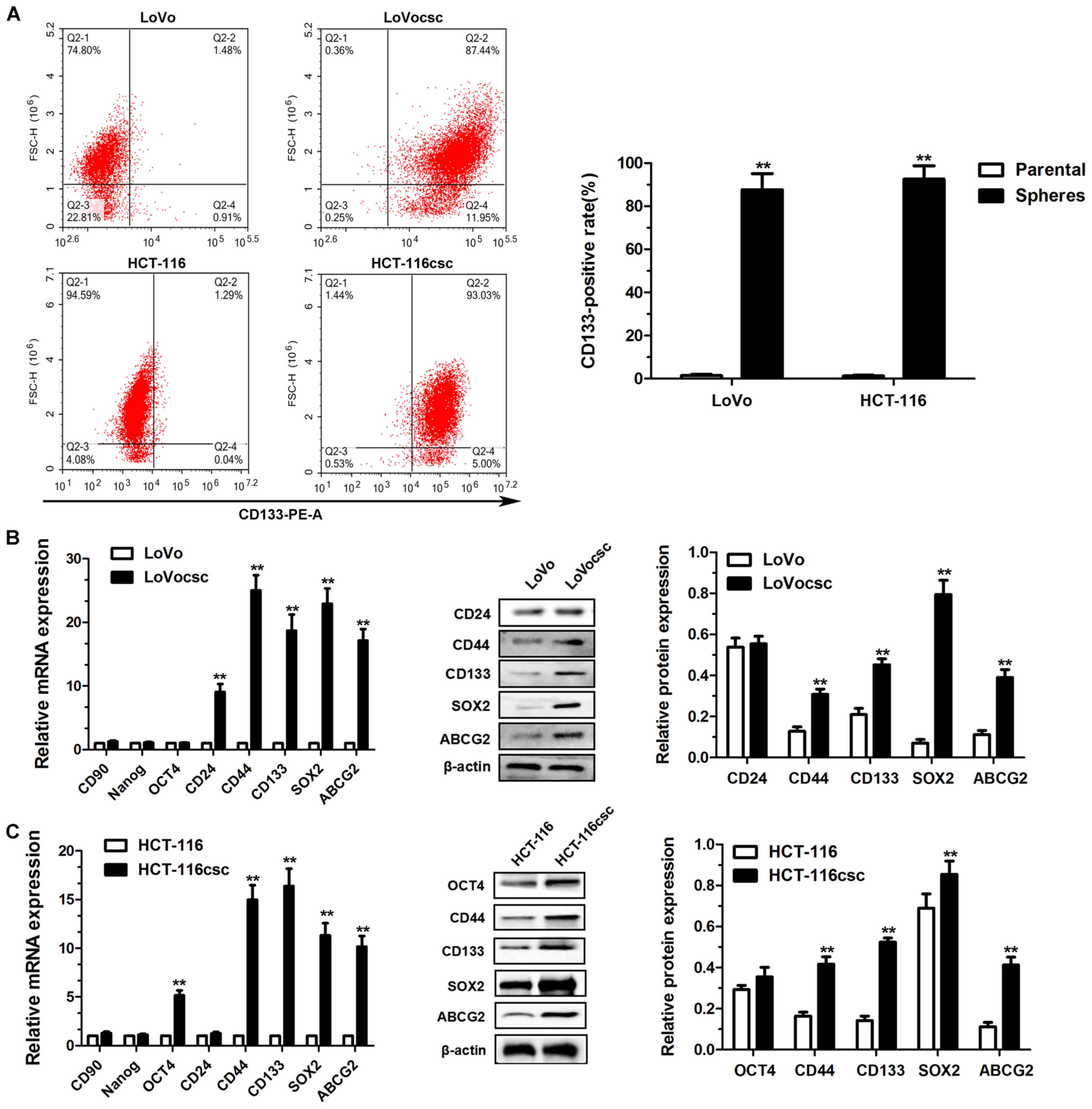
FCM was performed to determine the expression of
cell surface marker CD133 in populations of adherent and spheroid
primary colon cancer cell populations. CD133 has been previously
used as a marker for stem cells and to isolate colon CSCs from
spheroids (23,24). The results demonstrated that there
were significant differences in CD133 expression levels between the
adherent LoVo (1.48±0.30%) and LoVocsc spheroid cells (87.44±4.32%;
P<0.01; Fig. 2A). The levels of
CD133+ cells in the adherent HCT-116 cells (1.25±0.24%)
was also significantly decreased compared with HCT-116csc spheroid
cells (92.53±3.56%; P<0.01; Fig.
2A).
RT-qPCR and western blot analyses were performed to
determine the expression levels of the following stem cell markers
in the parental and CSCs: CD24, CD44, CD90, SOX2, ABCG2, OCT4 and
Nanog. As revealed in Fig. 2B,
mRNA expression levels of CD24, CD44, CD133, SOX2 and ABCG2 were
significantly increased in LoVocsc cells compared with LoVo cells
(P<0.01; Fig. 2B). In addition,
the protein expression levels of CD44, CD133, SOX2 and ABCG2 were
significantly increased in LoVocsc cells compared with LoVo cells
(P<0.01; Fig. 2B); whereas the
expression of CD24 did not exhibit a significant difference
(Fig. 2B). Furthermore, mRNA
expression levels of OCT4, CD44, CD133, SOX2 and ABCG2 were
significantly increased in HCT-116csc cells compared with HCT-116
cells (P<0.01; Fig. 2C), and
their protein expression levels were also significantly higher in
HCT-116csc cells compared with HCT-116 cells, with the exception of
OCT4 (P<0.01; Fig. 2C).
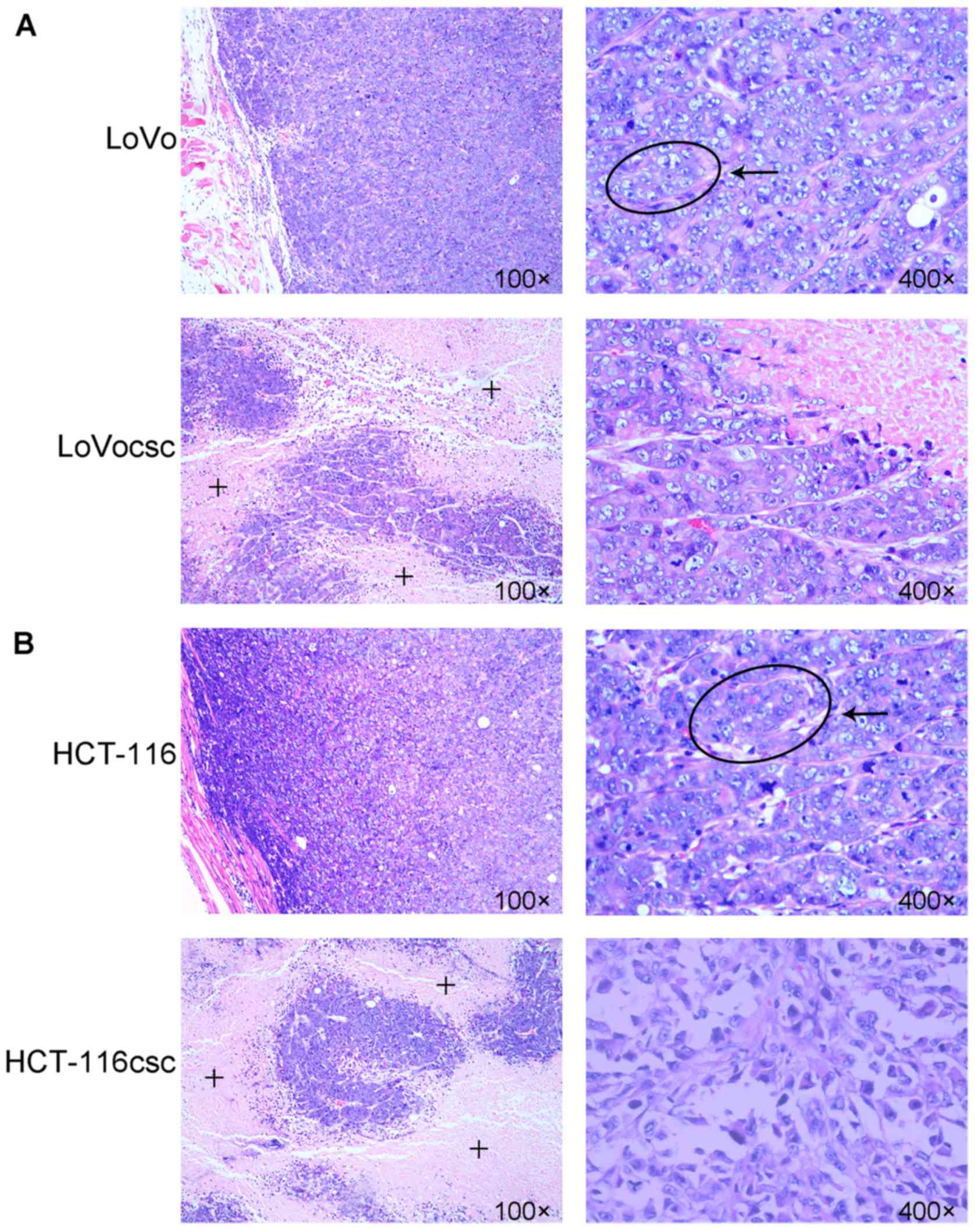
Histopathology of xenografts
10-15 days following the subcutaneous injection of
LoVo and HCT-116 cells, grain-like bulges in the right axillary
appeared. By contrast, LoVocsc and HCT-116csc cells exhibited
decreased oncogenic time, grain-like bulges appeared ~6–8 days
post-injection (data not shown). When the xenografts grew to
~0.8–1.5 cm (measured at the major axis of the tumor), whole tumors
were harvested, cut into sections and transplanted into the right
armpit of the remaining nude mice. Soft, grain-like solid bulges
were visible 4–5 days post-transplant, and the xenografts grew to a
size of 100 mm3 2 weeks post-injection (data not shown).
No nude mice died during the modeling.
The results of HE staining revealed glandular
cavities in the xenografts of the LoVo and HCT-116 group, whereas
irregular growth was observed in the LoVocsc and HCT-116csc groups.
In addition, large areas of necrotic tissue were observed in the
core regions of the tumors in the LoVocsc and HCT-116csc groups.
Compared with the LoVo and HCT-116 groups, xenografts of the
corresponding LoVocsc and HCT-116csc groups exhibited an increased
level of irregular structures, a decreased level of
differentiation, a marked increase in nuclear atypia and an
increased level of mitosis (Fig.
3), all of which indicated a higher malignancy in the tumors in
the LoVocsc and HCT-116csc groups.
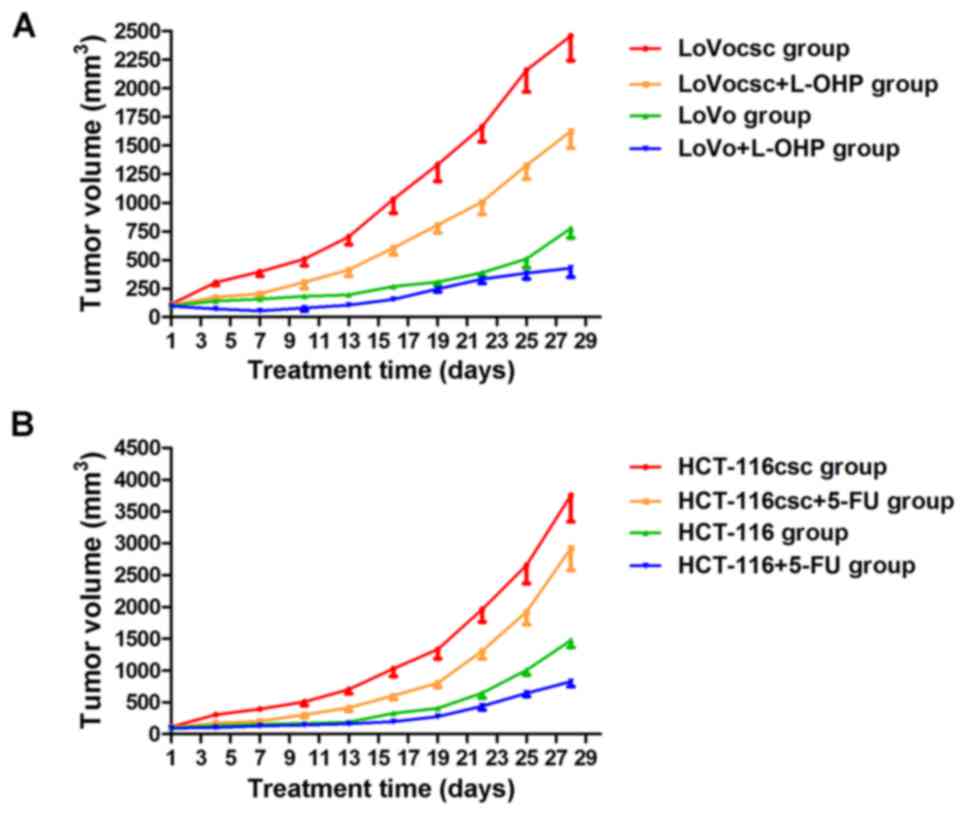
Treatment with L-OHP and 5-FU inhibits
rate of tumor growth
No mice died during drug administration. Nude mice
in the LoVocsc + L-OHP, HCT-116csc and HCT-116csc+5-FU groups
gradually lost weight, with the HCT-116csc+5-FU group exhibiting
the greatest weight loss. Furthermore, these groups exhibited lower
activity as well as skin color deterioration; however, these
conditions were not observed in the other groups (data not shown).
Some of the mice suffered from ulcerations on the tumor surface
when reaching the end of drug intervention. Such mice did not
exhibit clinical evidence of pain or distress. In addition, the
depth and size of the ulcer is very limited and did not exhibit any
manifestation of infection or hemorrhage, and so no clinical
intervention regarding ulceration was performed. Mice belonging to
the LoVocsc and HCT-116csc groups demonstrated a reduced oncogenic
time (data not shown), and the growth rates of the tumors were
markedly increased compared with their corresponding parental
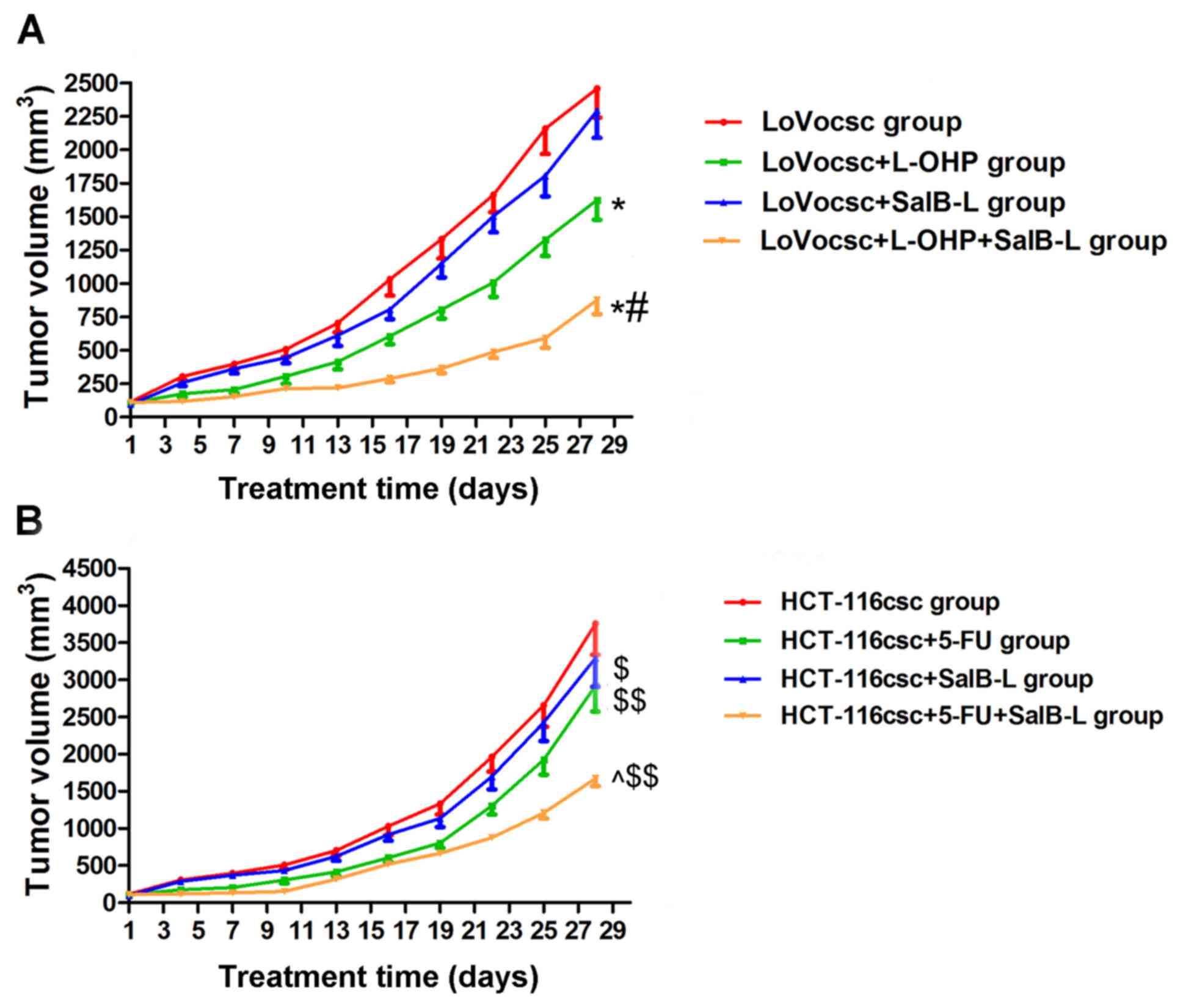
cells. Treatment with L-OHP was revealed to induce an inhibitory
effect on tumor volumes in the LoVo and LoVocsc groups, where its
inhibitory rate in the LoVo group was 44.98%, which was markedly
higher compared with that exhibited by the LoVocsc group (33.92%;
Fig. 4A and Table II). Treatment with 5-FU was also
demonstrated to induce an inhibitory effect on tumor volume in
HCT-116 and HCT-116csc groups compared with non-treated HCT-116 and
HCT-116csc groups, which demonstrated inhibitory rates of 43.92 and
22.18%, respectively (Fig. 4B and
Table III). These results
suggested that xenografts of the LoVocsc and HCT-116csc groups
exhibited drug resistance, thus suggesting that the model was
successfully established.
 | Table II.Inhibitory rate of L-OHP on LoVo and
LoVocsc xenografts (n=5). |
Table II.
Inhibitory rate of L-OHP on LoVo and
LoVocsc xenografts (n=5).
|
|
| Tumor volume
(mm3) |
|
|---|
|
|
|
|
|
|---|
| Group | n | Pre-treatment |
Post-administration | Tumor inhibition
rate (%) |
|---|
| LoVo | 5 | 99.06±5.09 | 776.42±82.72 | / |
| LoVo + L-OHP | 5 | 96.07±7.20 | 427.19±78.33 | 44.98 |
| LoVocsc | 5 | 112.69±13.89 |
2,456.82±216.35 | / |
| LoVocsc +
L-OHP | 5 | 106.07±17.10 |
1,623.58±147.86 | 33.92 |
 | Table III.Inhibitory rate of 5-FU on HCT-116
and HCT-116csc xenografts (n=5). |
Table III.
Inhibitory rate of 5-FU on HCT-116
and HCT-116csc xenografts (n=5).
|
|
| Tumor volume
(mm3) |
|
|---|
|
|
|
|
|
|---|
| Group | n | Pre-treatment |
Post-administration | Tumor inhibition
rate (%) |
|---|
| HCT-116 | 5 | 117.42±11.37 |
1,476.38±112.07 | / |
| HCT-116 + 5-FU | 5 | 114.83±9.70 | 827.95±78.24 | 43.92 |
| HCT-116csc | 5 | 108.54±10.62 |
3,756.20±416.92 | / |
| HCT-116csc +
5-FU | 5 | 110.69±9.06 |
2,923.17±347.33 | 22.18 |
SalB attenuates drug resistance
exhibited by colon CSCs xenografts
Prior to drug administration, tumor size was not
significantly different between the different groups (P>0.05;
Fig. 5 and Table IV). Following L-OHP and SalB
administration, tumor volumes exhibited by the LoVocsc + L-OHP and
LoVocsc + L-OHP + SalB-L groups were significantly reduced and
demonstrated statistically significant differences compared with
the LoVocsc group (P<0.01; Fig.
5A and Table IV). However,
the LoVocsc + SalB-L group and the LoVocsc group did not
demonstrate a significant difference in tumor volume post-drug
administration (P>0.05; Fig. 5A
and Table IV). In addition, the
tumor volume of the LoVocsc + L-OHP + SalB-L group was revealed to
be suppressed compared with that exhibited by the LoVocsc + L-OHP
group (P<0.01; Fig. 5A and
Table IV). Based on a previously
described method by Jin et al (22), the Q value was revealed to be 1.68
(data not shown), thus suggesting that SalB and L-OHP may exhibit a
synergistic effect on the suppression of tumor volume, and that
treatment with SalB may significantly reverse the drug resistance
exhibited by LoVocsc xenografts in nude mice (Fig. 5A and Table IV). Furthermore, the results
demonstrated that the tumor volumes of the LoVocsc + SalB-M and
LoVocsc + SalB-H groups were 1,638.22±130.41 and 1,270.15±108.58
mm3, respectively; which were significantly smaller than
that of the LoVocsc group (2,456.69±216.35 mm3) and
LoVocsc + SalB-L group (2,293.45±203.64) (P<0.01, data not
shown). These results suggested that medium and high doses of SalB
suppressed tumor volume in LoVocsc xenografts in a dose-dependent
manner, whereas low doses of SalB did not exhibit marked antitumor
activity.
 | Table IV.Inhibitory rate of tumor growth in
the L-OHP, low doses of SalB, and L-OHP combined with low doses of
SalB in xenografts obtained from the LoVocsc group (n=5). |
Table IV.
Inhibitory rate of tumor growth in
the L-OHP, low doses of SalB, and L-OHP combined with low doses of
SalB in xenografts obtained from the LoVocsc group (n=5).
|
|
| Tumor volume
(mm3) |
|
|---|
|
|
|
|
|
|---|
| Group | n |
Pre-administration |
Post-administration | Tumor inhibition
rate (%) |
|---|
| LoVocsc | 5 | 112.69±13.89 |
2,456.82±216.35 | / |
| LoVocsc +
L-OHP | 5 | 106.07±17.10 |
1,623.58±147.86a | 33.92 |
| LoVocsc +
SalB-L | 5 | 95.14±7.58 |
2,293.45±203.64 | 6.65 |
| LoVocsc + L-OHP +
SalB-L | 5 | 110.29±8.43 |
875.30±106.21a,b | 64.37 |
Following 5-FU and SalB administration, tumor
volumes exhibited by the HCT-116csc + SalB-L, HCT-116csc + 5-FU and
HCT-116csc + 5-FU + SalB-L groups demonstrated statistically
significant differences compared with that exhibited by the
HCT-116csc group (P<0.05 and P<0.01; Table V). In addition, the tumor volume of
the HCT-116csc + 5-FU + SalB-L group was revealed to be suppressed
compared with that exhibited by the HCT-116csc + 5-FU group
(P<0.01; Fig. 5B and Table V). A Q value of 1.75 suggested that
SalB and 5-FU may exhibit a synergistic effect on the suppression
of tumor volume, and that treatment with SalB may significantly
reverse the drug resistance exhibited by HCT-116csc xenografts in
nude mice (Fig. 5B and Table V). Tumor volumes exhibited by the
HCT-116csc, HCT-116csc + SalB-L, HCT-116csc + SalB-M and HCT-116csc
+ SalB-H groups were 3,756.20±416.92, 3,289.15±383.58,
2,857.24±255.62 and 2,564.73±241.08 mm3 respectively,
indicating that SalB suppressed tumor volume in HCT-116csc
xenografts in a dose-dependent manner (P<0.01, data not
shown).
 | Table V.Inhibitory rate of tumor growth
following treatment with 5-FU, low doses of SalB, and 5-FU combined
with low doses of SalB in xenografts obtained from the HCT-116csc
group (n=5). |
Table V.
Inhibitory rate of tumor growth
following treatment with 5-FU, low doses of SalB, and 5-FU combined
with low doses of SalB in xenografts obtained from the HCT-116csc
group (n=5).
|
|
| Tumor volume
(mm3) |
|
|---|
|
|
|
|
|
|---|
| Group | n |
Pre-administration |
Post-administration | Tumor inhibition
rate (%) |
|---|
| HCT-116csc | 5 | 108.54±10.62 |
3,756.20±416.92 | / |
| HCT-116csc +
5-FU | 5 | 110.69±9.06 |
2,923.17±347.33b | 22.18 |
| HCT-116csc +
SalB-L | 5 | 107.33±10.14 |
3,289.15±383.58a | 12.43 |
| HCT-116csc + 5-FU +
SalB-L | 5 | 110.86±9.15 |
1,667.37±106.81b,c | 55.61 |
SalB was revealed to attenuate drug resistance
exhibited by colon CSCs xenografts in nude mice, thereby increasing
tumor sensitivity to chemotherapeutic agents. In addition, SalB was
revealed to inhibit tumor growth in a dose-dependent manner.
CD44, CD133, SOX2 and ABCG2 protein
expression is regulated by SalB
Western blot analyses were used to determine the
protein expression of stem cell markers CD44, CD133 and SOX2, as
well as the drug resistance marker ABCG2, in all experimental
groups. The results revealed that protein expression levels of
CD44, CD133, SOX2 and ABCG2 exhibited by the LoVocsc and HCT-116csc
groups were significantly increased compared with those exhibited
by the LoVo and HCT-116 groups (Fig.
6A and B; P<0.01). Protein expression levels of these four
markers exhibited by the LoVocsc + SalB-L group were significantly
decreased compared with the LoVocsc group (P<0.01; Fig. 6A); whereas the expression levels of
only three of these markers (CD44, SOX2 and ABCG2) were revealed to
be significantly decreased in the HCT-116csc + SalB-L group
compared with those exhibited by the HCT-116csc group (P<0.01;
Fig. 6B). These results
demonstrate that low doses of SalB may significantly suppress CD44,
SOX2 and ABCG2 protein expression in colon CSCs xenografts, which
may contribute to the attenuation or reversal of drug
resistance.
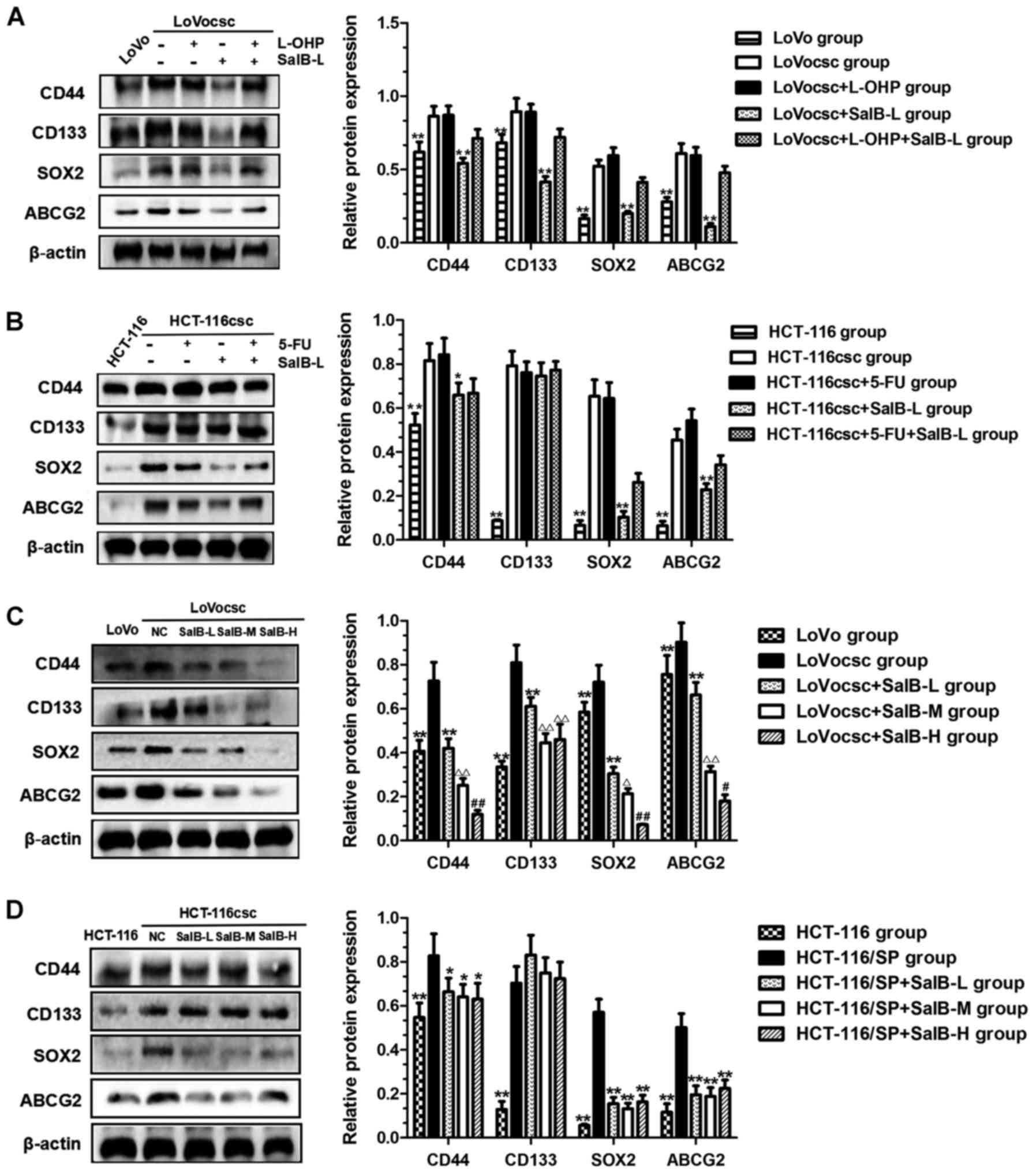 | Figure 6.SalB regulates the expression of
CD44, CD133, SOX2 and ABCG2 proteins. (A) Protein expression of
CD44, CD133, SOX2 and ABCG2 in LoVo, LoVocsc, LoVocsc + L-OHP,
LoVocsc + SalB-L and LoVocsc + L-OHP + SalB-L groups. (B) Protein
expression of CD44, CD133, SOX2 and ABCG2 in HCT-116, HCT-116csc,
HCT-116csc + 5-FU, HCT-116csc + SalB-L and HCT-116csc + 5-FU +
SalB-L groups. (C) Protein expression of CD44, CD133, SOX2 and
ABCG2 in LoVo, LoVocsc, LoVocsc+ SalB-L, LoVocsc + SalB-M and
LoVocsc + SalB-H groups. **P<0.01 vs. LoVocsc group;
ΔP<0.05 and ΔΔP<0.01 vs. LoVocsc +
SalB-L group; #P<0.05 and ##P<0.01 vs.
LoVocsc + SalB-M group. (D) Protein expression of CD44, CD133, SOX2
and ABCG2 in HCT-116, HCT-116csc, HCT-116csc + SalB-L, HCT-116csc +
SalB-M and HCT-116csc + SalB-H groups. *P<0.05 and **P<0.01
vs. HCT-116csc group. Data are presented as mean ± standard
deviation. csc, cancer stem cells; 5-FU, fluorouracil; L-OHP,
oxaliplatin; SalB, salvianolic acid B; -L, low dose; -M, medium
dose; -H, high dose; NC, negative control; CD, cluster of
differentiation; SOX2, transcription factor sox-2; ABCG2,
ATP-binding cassette sub-family G member 2. |
In addition, treatment with SalB was revealed to
have an inhibitory effect on the protein expression levels of CD44,
SOX2 and ABCG2 in the LoVocsc xenografts in a dose-dependent manner
(Fig. 6C; P<0.05 and
P<0.01). As presented in Fig.
6C, protein expression levels of these three markers in the
LoVocsc + SalB-L, LoVocsc + SalB-M and LoVocsc + SalB-H groups were
significantly decreased in a dose-dependent manner and exhibited a
statistically significant difference compared with associated
expression levels exhibited by the LoVocsc group (Fig. 6C; P<0.05 and P<0.01). CD133
expression levels did not demonstrate a significant difference
between the LoVocsc + SalB-M and LoVocsc + SalB-H groups (Fig. 6C; P>0.05); however, it was
significantly decreased compared with the expression levels
exhibited by the LoVocsc and LoVocsc + SalB-L groups (Fig. 6C; P<0.01). Furthermore, there
were no significant differences in the expression levels of CD44,
CD133, SOX2 and ABCG2 proteins exhibited by the HCT-116csc +
SalB-L, HCT-116csc + SalB-M and HCT-116csc + SalB-H groups;
however, the expression levels of CD44, SOX2 and ABCG2 were
significantly suppressed compared with the HCT-116csc group
(Fig. 6D; P<0.05).
Discussion
Cancer MDR refers to the phenomenon that tumor cells
are resistant to the effects of numerous antineoplastic drugs,
regardless of their chemical structure or target. MDR in tumors is
a complex process and involves numerous mechanisms (25).
CSCs are specialized cell populations of cancer
cells with unlimited potential to proliferate, self-renew and
differentiate into numerous cell types (24). CSCs promote rapid proliferation of
tumors, and can induce tumor differentiation into malignancies that
are of greater maturity, both in function and phenotype (26). CSCs exhibit the primary
characteristics of drug resistance, as they express high levels of
ABC transporter families on their cell membrane, which tends to
enhance the efflux of chemotherapeutic drugs, thus resulting in
greater resistance of cancer cells to cytotoxicity and apoptotic
induction by a variety of chemotherapeutic agents (27,28).
ABCG2 is an important member of the ABC transporter family that is
directly involved in the induction of drug resistance; however, it
also maintains the stem cell characteristics of cancer cells. Thus,
ABCG2 may represent a marker for cancer stem cells in numerous
cancer types (29,30). Deng et al (31) demonstrated that CSCs present in
malignant fibrous fibrohistiocytoma expressing high levels of ABCG2
were able to self-renew and exhibited a strong resistance to
doxorubicin and cisplatin. SOX is a transcription factor protein
family characterized by a homologous sequence called the high
mobility group-box. SOX2 is a member of the SOX protein family, and
is an important stem cell marker for inducing stem cell formation,
maintaining the characteristics of stem cells as well as inhibiting
the differentiation of stem cells (32). The association between SOX2 and CSC
resistance has been extensively demonstrated (33,34).
In addition, CD44 is a transmembrane glycoprotein belonging to the
adhesion molecule family and serves a role in numerous
physiological and pathological processes. CD44 has been
demonstrated to serve a significant role in tumor invasion,
metastasis and drug resistance (35). Yan et al (36) and Bourguignon et al
(37) have demonstrated that CD44
is closely associated with drug resistance exhibited by colon
CSCs.
With the increasing interest and research regarding
CSCs, colon CSCs have been successfully isolated from colon cancer
solid tumors, colon cancer ascites and colon cancer cell lines
(38). Furthermore, a number of
studies have revealed that colon CSCs are closely associated with
primary and secondary multidrug resistance of colon cancer
(39,40). Despite the existence of CSCs having
been demonstrated in the 1970s, the gold standard for isolating
CSCs, as well as the development of animal xenograft models, have
not been fully established. LoVo and HCT-116 cells were selected
for investigation in the present study for the two reasons:
Firstly, cell lines are readily available as a pure population
compared with cells from colon cancer metastases; Second, the cells
exhibit stable biological characteristics; The results of the
present study suggested that colon CSCs LoVocsc and HCT-116csc
derived from LoVo and HCT-116 cells stably express stem cell-like
characteristics and xenografts generated by subcutaneous
inoculation of colon CSCs LoVocsc and HCT-116csc can be serially
passaged in mice. In addition, the results of the present study
demonstrated that xenograft tumors exhibited drug resistance, rapid
proliferation and other malignant biological characteristics.
During modeling and drug administration, none of the mice died,
however, some did exhibit weight loss. This suggested that the
technique used to isolate CSCs and the method used to establish the
nude mouse model was effective.
The results of the present study revealed that the
serum-free suspension culture method may be used to isolate colon
CSCs from LoVo and HCT-116 adherent cells. Furthermore, it was
revealed that the xenografts obtained by subcutaneous inoculation
of LoVocsc and HCT-116csc cells could be serially passaged in mice.
The growth rate of the tumors in the LoVocsc and HCT-116csc groups
was demonstrated to be increased compared with rates exhibited by
the LoVo and HCT-116 groups. Furthermore, the xenografts exhibited
a high malignancy as determined by H&E staining and were
resistant to chemotherapy drugs, such as L-OHP and 5-FU. In
addition, the derived CSCs from the LoVocsc and HCT-116csc groups
exhibited significantly increased expression levels of CD44, CD133,
SOX2 and ABCG2 proteins compared with corresponding LoVo and
HCT-116 groups. In vivo experiments revealed that treatment
with L-OHP or 5-FU combined with SalB had an inhibitory effect on
tumor growth and demonstrated a greater efficacy compared with
treatment with L-OHP or 5-FU alone. The determined Q values were
>1.15, which, based on the method by Jin et al (22), suggested that SalB, when used in
combination with L-OHP or 5-FU, exhibited a synergistic inhibition
of tumor growth. This suggested that SalB could significantly
attenuate the drug resistance of xenografts, and thus improve the
efficacy of chemotherapeutic agents. Furthermore, the results of
the present study demonstrated that SalB suppressed tumor growth in
a dose-dependent manner. In addition, western blot analysis
revealed that treatment with SalB significantly decreased the
expression levels of CD44, SOX2 and ABCG2 proteins in LoVocsc and
HCT-116csc xenografts, and this was exhibited in a dose-dependent
manner in LoVocsc xenografts.
In conclusion, the results of the present study
revealed that serum-free suspension cultures may be used to
effectively isolate colon CSCs from LoVo and HCT-116 cells. Nude
mice models bearing LoVocsc and HCT-116csc cells were successfully
established, and treatment with SalB was demonstrated to
effectively attenuate MDR exhibited by of colon CSCs xenografts via
the suppression of CD44, SOX2 and ABCG2 protein expression
levels.
A limitation of the present study was the absence of
non-cancerous cell line to be used as a control. Future studies
should focus on the observation of dynamic alterations of xenograft
growth in the orthotopic transplant tumor model of colon CSCs in
mice using in vivo optical imaging, as well as tumor
invasion and metastasis of liver, lung, brain and other organs.
Such investigations would serve to deepen the understanding of
in vivo SalB treatment as a novel therapeutic strategy for
the treatment of MDR in colon CSCs. In addition, in future studies
we will aim to further investigate the anti-MDR effect of SalB
using in vitro models as well as determine the effect of
SalB on morphological changes of colon CSCs, including the cell
refractive index, the cell cycle, drug resistance, proliferation
and apoptosis. Furthermore, we will aim to uncover the molecular
mechanism underlying the anti-MDR effect of SalB.
Acknowledgements
The authors would like to thank Dr Wenjing Wang
(Medical Department, Shandong Xiehe College, Shandong, China) and
Dr Li Min (Department of Anorectal, JiaDing Traditional Chinese
Medicine Hospital, Shanghai University of Traditional Chinese
Medicine, Shanghai, China) for their technical assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PG, YL, JW and WG contributed to the study design.
PG, YL, JW, WG, XL, SW, LX and BW performed the experiments. XL, SW
and BW analyzed data and contributed to the writing of the
manuscript. All authors reviewed the manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the guidelines of the Chinese Experimental Animals Administration
Legislation and were approved by the Ethics Committee for Animal
Experiments of Shanghai University of Traditional Chinese Medicine
(Shanghai, China; reference no. SZY 201504023).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vtorushin SV, Khristenko KY, Zavyalova MV,
Perelmuter VM, Litviakov NV, Denisov EV, Dulesova AY and
Cherdyntseva NV: The phenomenon of multi-drug resistance in the
treatment of malignant tumors. Exp Oncol. 36:144–156.
2014.PubMed/NCBI
|
|
2
|
Di C and Zhao Y: Multiple drug resistance
due to resistance to stem cells and stem cell treatment progress in
cancer (Review). Exp Ther Med. 9:289–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeishi S and Nakayama KI: To wake up
cancer stem cells, or to let them sleep, that is the question.
Cancer Sci. 107:875–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanwar JR, Samarasinghe RM, Kamalapuram SK
and Kanwar RK: Multimodal nanomedicine strategies for targeting
cancer cells as well as cancer stem cell signalling mechanisms.
Mini Rev Med Chem. 17:1688–1695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greve B, Kelsch R, Spaniol K, Eich HT and
Götte M: Flow cytometry in cancer stem cell analysis and
separation. Cytometry A. 81:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin F, Li HS, Zhao L, Wei YJ, Zhang H, Guo
YJ, Pang R, Jiang XB and Zhao HY: Expression of anti-apoptotic and
multi-drug resistance-associated protein genes in cancer stem cell
isolated from TJ905 glioblastoma multiforme cell line. Zhonghua Yi
Xue Za Zhi. 88:2312–2316. 2008.PubMed/NCBI
|
|
7
|
Asuthkar S, Stepanova V, Lebedeva T,
Holterman AL, Estes N, Cines DB, Rao JS and Gondi CS:
Multifunctional roles of urokinase plasminogen activator (uPA) in
cancer stemness and chemoresistance of pancreatic cancer. Mol Biol
Cell. 24:2620–2632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin T, Wei H, Gou S, Shi P, Yang Z, Zhao G
and Wang C: Cancer stem-like cells enriched in Panc-1 spheres
possess increased migration ability and resistance to gemcitabine.
Int J Mol Sci. 12:1595–1604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cabang AB, De Mukhopadhyay K, Meyers S,
Morris J, Zimba PV and Wargovich MJ: Therapeutic effects of the
euglenoid ichthyotoxin, euglenophycin, in colon cancer. Oncotarget.
8:104347–104358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY,
Gong FR, Shen M, Liu L, Tao M, Shen B, et al: Ginsenoside Rg3
targets cancer stem cells and tumor angiogenesis to inhibit
colorectal cancer progression in vivo. Int J Oncol.
52:127–138. 2018.PubMed/NCBI
|
|
11
|
Guo P, Wang S, Liang W, Wang W, Wang H,
Zhao M and Liu X: Salvianolic acid B reverses multidrug resistance
in HCT-8/VCR human colorectal cancer cells by increasing ROS
levels. Mol Med Rep. 15:724–730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Sun G, Wu P, Chen R, Yao F, Qin M,
Luo Y, Sun H, Zhang Q, Dong X and Sun X: Salvianolic Acid B
prevents arsenic trioxide-induced cardiotoxicity in vivo and
enhances its anticancer activity in vitro. Evid Based Complement
Alternat Med. 2013:7594832013.PubMed/NCBI
|
|
13
|
Zhao Y, Hao Y, Ji H, Fang Y, Guo Y, Sha W,
Zhou Y, Pang X, Southerland WM, Califano JA and Gu X: Combination
effects of salvianolic acid B with low-dose celecoxib on inhibition
of head and neck squamous cell carcinoma growth in vitro and in
vivo. Cancer Prev Res (Phila). 3:787–796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Y, Jiang Z, Guan X, Chen Y, Tang Q,
Wang G and Wang X: miR-450b-5p suppresses stemness and the
development of chemoresistance by targeting SOX2 in colorectal
cancer. DNA Cell Biol. 35:249–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An Y and Ongkeko WM: ABCG2: The key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Stoica G, Tasca SI, Kelly KA and
Meininger CJ: Modulation of tumor angiogenesis by stem cell factor.
Cancer Res. 60:6757–6762. 2000.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Chen DQ, Chen L, Liu D, Zhao H,
Zhang ZH, Vaziri ND, Guo Y, Zhao YY and Cao G: Novel RAS inhibitors
poricoic acid zg and poricoic acid ZH attenuate renal fibrosis via
a Wnt/β-catenin pathway and targeted phosphorylation of smad3
signaling. J Agric Food Chem. 66:1828–1842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Li L and Wang Y: Effects of Per2
overexpression on growth inhibition and metastasis, and on MTA1,
nm23-H1 and the autophagy-associated PI3K/PKB signaling pathway in
nude mice xenograft models of ovarian cancer. Mol Med Rep.
13:4561–4568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Shou Q, Chen C, Cai H, Zhang J, Tang
S, Cai B, Tang D and Cao G: An herbal formula attenuates
collagen-induced arthritis via inhibition of JAK2-STAT3 signaling
and regulation of Th17 cells in mice. Oncotarget. 8:44242–44254.
2017.PubMed/NCBI
|
|
22
|
Jin ZJ: Addition in drug combination
(author's transl). Zhongguo Yao Li Xue Bao. 1:70–76. 1980.(In
Chinese). PubMed/NCBI
|
|
23
|
Zhou JY, Chen M, Ma L, Wang X, Chen YG and
Liu SL: Role of CD44(high)/CD133(high) HCT-116 cells in the
tumorigenesis of colon cancer. Oncotarget. 7:7657–7666.
2016.PubMed/NCBI
|
|
24
|
Dou J, Ni Y, He X, Wu D, Li M, Wu S, Zhang
R, Guo M and Zhao F: Decreasing lncRNA HOTAIR expression inhibits
human colorectal cancer stem cells. Am J Transl Res. 8:98–108.
2016.PubMed/NCBI
|
|
25
|
Hasan S, Taha R and Omri HE: Current
opinions on chemoresistance: An overview. Bioinformation. 14:80–85.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okamoto K, Ninomiya I, Ohbatake Y, Hirose
A, Tsukada T, Nakanuma S, Sakai S, Kinoshita J, Makino I, Nakamura
K, et al: Expression status of CD44 and CD133 as a prognostic
marker in esophageal squamous cell carcinoma treated with
neoadjuvant chemotherapy followed by radical esophagectomy. Oncol
Rep. 36:3333–3342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei L, Chen P, Chen Y, Shen A, Chen H, Lin
W, Hong Z, Sferra TJ and Peng J: Pien Tze Huang suppresses the
stem-like side population in colorectal cancer cells. Mol Med Rep.
9:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia M, Wei Z, Liu P and Zhao X: Silencing
of ABCG2 by MicroRNA-3163 inhibits multidrug resistance in
retinoblastoma cancer stem cells. J Korean Med Sci. 31:836–842.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu J, Li J, Yue X, Wang J, Liu J, Sun L
and Kong D: Expression of the cancer stem cell markers ABCG2 and
OCT-4 in right-sided colon cancer predicts recurrence and poor
outcomes. Oncotarget. 8:28463–28470. 2017.PubMed/NCBI
|
|
30
|
Yanamoto S, Yamada S, Takahashi H, Naruse
T, Matsushita Y, Ikeda H, Shiraishi T, Seki S, Fujita S, Ikeda T,
et al: Expression of the cancer stem cell markers CD44v6 and ABCG2
in tongue cancer: Effect of neoadjuvant chemotherapy on local
recurrence. Int J Oncol. 44:1153–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng L, Li D, Gu W, Liu A and Cheng X:
Formation of spherical cancer stem-like cell colonies with
resistance to chemotherapy drugs in the human malignant fibrous
histiocytoma NMFH-1 cell line. Oncol Lett. 10:3323–3331. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lundberg IV, Edin S, Eklöf V, Öberg Å,
Palmqvist R and Wikberg ML: SOX2 expression is associated with a
cancer stem cell state and down-regulation of CDX2 in colorectal
cancer. BMC Cancer. 16:4712016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chou MY, Hu FW, Yu CH and Yu CC: Sox2
expression involvement in the oncogenicity and radiochemoresistance
of oral cancer stem cells. Oral Oncol. 51:31–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carina V, Zito G, Pizzolanti G, Richiusa
P, Criscimanna A, Rodolico V, Tomasello L, Pitrone M, Arancio W and
Giordano C: Multiple pluripotent stem cell markers in human
anaplastic thyroid cancer: The putative upstream role of SOX2.
Thyroid. 23:829–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu B, Ma Y, Yang Y, Zhang L, Han H and
Chen J: CD44 promotes cell proliferation in non-small cell lung
cancer. Oncol Lett. 15:5627–5633. 2018.PubMed/NCBI
|
|
36
|
Yan Y, Zuo X and Wei D: Concise review:
Emerging role of CD44 in cancer stem cells: A promising biomarker
and therapeutic target. Stem Cells Transl Med. 4:1033–1043. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bourguignon LY, Shiina M and Li JJ:
Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA
functions, chemoresistance, and radiation resistance in cancer stem
cells leading to tumor progression. Adv Cancer Res. 123:255–275.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim MJ, Koo JE, Han GY, Kim B, Lee YS, Ahn
C and Kim CW: DDual-blocking of PI3K and mTOR improves
chemotherapeutic effects on SW620 human colorectal cancer stem
cells by inducing differentiation. J Korean Med Sci. 31:360–370.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wilson BJ, Schatton T, Frank MH and Frank
NY: Colorectal cancer stem cells: Biology and therapeutic
implications. Curr Colorectal Cancer Rep. 7:128–135. 2011.
View Article : Google Scholar : PubMed/NCBI
|