Introduction
Functional recovery after cerebral infarction is a
complex phenomenon that is dependent on brain plasticity. Studies
have demonstrated that endogenous neural stem cells (NSCs)
proliferate, migrate and differentiate following cerebral
infarction, and are involved in regeneration of nervous tissue and
recovery of brain function (1–3).
NSCs have been the focus of much research; however, the
neurobiological mechanisms that control proliferation and
differentiation of endogenous NSCs after cerebral ischemia remain
unclear.
Physical exercise is widely used as a rehabilitation
treatment for promoting sensory and motor function recovery in
patients after stroke. The recovery mechanisms are generally
believed to be associated with neural plasticity (4). In rats, physical exercise has been
demonstrated to reduce infarct volume, promote angiogenesis and
induce neurogenesis (5,6); in addition, Luo et al
(7) reported that physical
exercise can promote proliferation of NSCs or precursor cells in
rat brain tissue. Most studies to date have only observed the
effect of physical exercise on NSCs in the hippocampus following
cerebral infarction; therefore, further studies are required to
better understand the underlying molecular mechanisms.
The role of various signal transduction pathways in
the mechanism of cerebral ischemic injury has garnered much
attention. Notably, activation of the extracellular
signal-regulated kinase (ERK) pathway appears to be implicated in
ischemic brain injury (8).
Ischemia, hypoxia, growth factors and other factors can lead to
activation of ERK, which in turn translocates to the nucleus, and
increases the expression of genes associated with cell
proliferation and differentiation (9,10).
It has previously been identified that pre-exercise training can
upregulate the ERK signaling pathway and improve neurological
function (11); however, it is
unknown whether physical exercise following cerebral ischemia can
still activate the ERK signaling pathway. At present, there are
different views on the timing and mechanism of physical exercise,
and physical exercise following cerebral infarction is more in line
with clinical practice. Therefore, the present study investigated
the effects of physical exercise 24 h after cerebral infarction in
rats. The aim was to investigate whether physical exercise could
promote proliferation and differentiation of NSCs in the dentate
gyrus of rats with cerebral infarction, and improve neurological
function by activating the ERK signaling pathway.
Exploring the role of physical exercise and ERK
signaling in endogenous NSCs will aid in determining the molecular
mechanisms associated with physical exercise, and may explain the
specific regulatory mechanism of the ERK signaling pathway. The
results may also provide novel targets for clinical rehabilitation,
further promote the application of physical exercise in the clinic
and provide a more solid theoretical foundation of
kinesiotherapy.
Materials and methods
Animals
Normal adult male Sprague Dawley rats (250–300 g,
3–4 months old) were provided by the Experimental Animal Center of
Southern Medical University (Guangzhou, China). The rats were given
enough water and food every day at 22°C, 3–5 rats per cage.
Procedures involving animals and their care were conducted in
accordance with National Institute of Health (NIH) guidelines (NIH
pub. no. 85-23, revised 1996), and the present study was approved
by the Animal Care and Use Committee of the Southern Medical
University (Guangzhou, China).
Middle cerebral artery occlusion
(MCAO) model
Rat cerebral ischemia reperfusion injury was
performed according to the Longa method of MCAO (12). Rats were anesthetized by
intraperitoneal injection of 10% chloral hydrate (400 mg/kg). The
skin on the neck was shaved, sterilized and a 2 cm midline incision
was made. The right carotid artery, external carotid artery and
internal carotid artery were identified and carefully separated.
Subsequently, the external carotid artery and common carotid artery
near the heart were ligated. Briefly, suture thread was inserted
through the carotid artery into the internal carotid artery for a
distance of 19±0.5 mm until resistance was felt, to occlude the
origin of the MCA. Following 2 h, the suture was withdrawn to allow
reperfusion. In the sham surgery group, rats were processed in the
same way as the MCAO group, however vessels were not ligated and no
occlusion suture was inserted. Following completion of the
operation, rats were placed in clean housing for recovery. All
procedures were performed under aseptic conditions.
Verification of the MCAO model
To verify the reliability and reproducibility of the
MCAO model using the Longa method, triphenyl tetrazolium chloride
(TTC) staining was performed to detect brain lesions in two rats
that were randomly selected from the MCAO group. Firstly, rats were
anesthetized with 10% chloral hydrate (500 mg/kg) and the heart was
then exposed. An infusion needle was inserted through the left
ventricle to cannulate the aorta and cut the right atrium, the
blood was flushed by perfusion with ~500 ml saline. Once the liquid
was clear, the rats were decapitated; the brain was dissected,
placed in the refrigerator of −20°C for quick freezing 20–30 min
and placed in a customized slicer to cut 2 mm coronal sections.
Sections were incubated in 2% TTC solution at 37°C in the dark for
30 min and then removed. The stained sections were photographed
immediately.
Inclusion criteria and experimental
groups
Rat neurological findings following MCAO were
evaluated using the Longa scoring method (12): 0, no neurological deficit; 1,
failure to extend left forepaw fully indicating mild neurological
deficit; 2, circling to the left indicating moderate focal
neurological deficit; 3, falling to the left indicating severe
focal deficit; and 4, no spontaneous walking and depressed level of
consciousness. Animals with scores of 1–3 were included in the
present study.
Rats were randomly divided into the physical
exercise group (E; n=27), the physical exercise group receiving
U0126, a mitogen-activated protein kinase kinase (MEK) 1/2
inhibitor that blocks ERK signaling (EU; n=27), the control group
(MCAO but untreated) (C, n=27), the control group treated with
U0126 (CU; n=27), and the sham surgery group (S; n=27). These five
groups were further divided into three subgroups with time points
of 7, 14 and 21 days after MCAO.
Physical exercise
The E and EU groups underwent treadmill adaptation
exercise training on an electric treadmill (length, 45 cm), 2 days
prior to the MCAO surgery. Rats in each group were housed in
standard cages after surgery (n=3–5 rats/cage). Rats in the C, CU
and S group were fed ad libitum, and allowed to move freely
in the cage. Rats in the E and EU group began exercise training 24
h after MCAO surgery. The treadmill was not inclined and was set to
a speed of 10–20 m/min; and the rats ran 30 min/day five times a
week. Animals were sacrificed at the respective time points for
tissue analysis.
Neurological severity scores
Modified neurological severity score (mNSS) tests
were used to assess the neurological function of the rats in each
group at 7, 14 and 21 days after MCAO surgery (13). There were six items in the mNSS
test: Spontaneous activity test, a paresis test, a forelimb motor
function test, a motor function test, and tests for pain sensation
and deep sensation, with a total score of 18. The neurological
function was graded on a scale of 0–18: 13–18 indicated severe
injury, 7–12 indicated moderate injury and 1–6 indicated mild
injury. The tests were performed blind and in triplicate, and the
average score was recorded.
5-bromodeoxyuridine (BrdU)
administration and tissue collection
A total of 3 days prior to tissue collection, rats
in each group were intraperitoneally injected with BrdU (50 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) three times a day at
8 h intervals (for 2 days). Animals were sacrificed 24 h after the
last injection. U0126 (Cell Signaling Technology, Inc., Danvers,
MA, USA) was dissolved in dimethyl sulfoxide, diluted to 0.5 mg/ml
in PBS, and injected into the tail vein of CU and EU rats (0.5
mg/kg) 30 min prior to MCAO surgery.
Rats were anesthetized with 500 mg/kg 10% chloral
hydrate via intraperitoneal injection. The chest was opened to
expose the heart, and the brain was fixed by cardiac perfusion with
4% paraformaldehyde in PBS for 24 h at 4°C. Brains were dissected
following decapitation, and the right hemispheres were
paraffin-embedded. Paraffin blocks were serially sliced in the
coronal plane into 5-µm sections; every fifth section was used for
staining.
Hematoxylin and eosin (H&E)
staining
H&E staining was used to detect MCAO-induced
lesions at the respective time points. Deparaffinization of tissue
sections was achieved by conventional xylene dewaxing, ethanol
removal of xylene and washing in distilled water for 2 min. The
sections were placed in hematoxylin for 1 min, washed in water for
1 min, differentiated in 1% hydrochloric acid ethanol for 10–30
sec, washed in water for 20 min, placed in eosin for 5–10 min all
at room temperature and then dehydrated using an ethanol gradient.
The sections were fixed with neutral balata for 12–24 h at room
temperature and visualized under a light microscope.
Immunofluorescence staining
Immunofluorescence staining was used to detect
BrdU+/neuronal nuclei (NeuN)+ and
BrdU+/glial fibrillary acidic protein (GFAP)+
cells in the hippocampal dentate gyrus at 7, 14 and 21 days after
MCAO surgery. Deparaffinized sections were incubated in 3%
H2O2 in methanol solution for 10 min and then
trypsin-digested for 10 min at 37°C. Subsequently, the sections
were microwaved in citrate buffer (pH 6.0) for antigen retrieval
and blocked with normal goat serum (Boster Biotechnology, Inc.,
Wuhan, China) for 10 min at 37°C. The sections were incubated at
4°C overnight with the following primary antibodies: Mouse
anti-BrdU (1:100; cat. no. B2531; Sigma-Aldrich; Merck KGaA),
rabbit anti-NeuN (1:200; cat. no. ab177487) and rabbit anti-GFAP
(1:500; cat. no. ab7260; both Abcam, Cambridge, MA, USA). Following
a wash step in PBS, the sections were incubated with secondary
antibodies for 1 h at 37°C: Cy3 goat anti-mouse IgG (1:300; cat.
no. TA130012; OriGene Technologies, Inc., Beijing, China) and Alexa
Fluor 488 goat anti-rabbit IgG (1:400; cat. no. ab150077; Abcam).
Lastly, DAPI (cat. no. D9542; Sigma-Aldrich; Merck KGaA) was added
for 10 min at room temperature, sections were washed in PBS and
mounted in 50% glycerol. Negative controls were generated by
replacing the primary antibodies with PBS. Sections were examined
using an Olympus inverted microscope, and five non-overlapping
visual fields were selected at random. Images were analyzed using
Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA), and the number of BrdU+/NeuN+ and
BrdU+/GFAP+ cells/mm2 of
hippocampal dentate gyrus slice was counted.
Western blot analysis
Western blotting was used to detect cyclin-dependent
kinase 4 (CDK4), Cyclin D1, retinoblastoma protein (p-Rb), P-16,
phosphorylated (p)-ERKl/2 and c-Fos protein expression in the
hippocampus 7, 14 and 21 days after MCAO surgery. To extract
proteins, the hippocampus was incubated in RIPA lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). The sample
was centrifuged and the supernatant was harvested for protein
quantification using bicinchoninic acid assay. A total of 50 µg
proteins were separated by 12% polyacrylamide gel electrophoresis
and transferred onto a polyvinylidende difluoride membrane. 5%
skimmed milk powder was incubated with the membrane at room
temperature for 2 h. Membranes were incubated at 4°C overnight with
antibodies against: CDK4 (1:1,000; cat. no. ab199728; Abcam),
Cyclin D1 (1:1,000; cat. no. 2978), p-Rb (1:1,000; cat. no. 8516;
both Cell Signaling Technology, Inc.), P-16 (1:1,000; cat. no.
ab54210; Abcam), ERK (1:1,000; cat. no. 4695), p-ERK (1:2,000; cat.
no. 4370; both Cell Signaling Technology, Inc.), c-Fos (1:4,000;
cat. no. ab134122; Abcam) or β-actin (1:1,000; cat. no. 4970; Cell
Signaling Technology, Inc.). Subsequently, the membranes were
washed and incubated with HRP-conjugated goat anti-rabbit IgG
antibody (1:2,500; cat. no. TA140003; OriGene Technologies, Inc.
Beijing, China) for 1 h at 37°C. Proteins were visualized and
analyzed by the Bio-Rad ChemiDoc™ XRS+ gel imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the software is ImageJ
v1.8 (National Institutes of Health, Bethesda, MD, USA). Protein
expression was normalized against the internal reference protein,
β-actin.
Statistical analysis
Statistical analyses were conducted using SPSS
version 20.0 software (IBM Corp., Armonk, NY, USA). All values were
expressed as the means ± standard deviation. The experiments were
repeated three times. One-way analysis of variance was used to
determine whether there was a statistically significant difference
between multiple groups. The Levene's test was used to assess the
equality of variances. If the variance was homogeneous, the
Fisher's least significant difference test was used and if the
variance was not homogeneous the Dunnett's test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
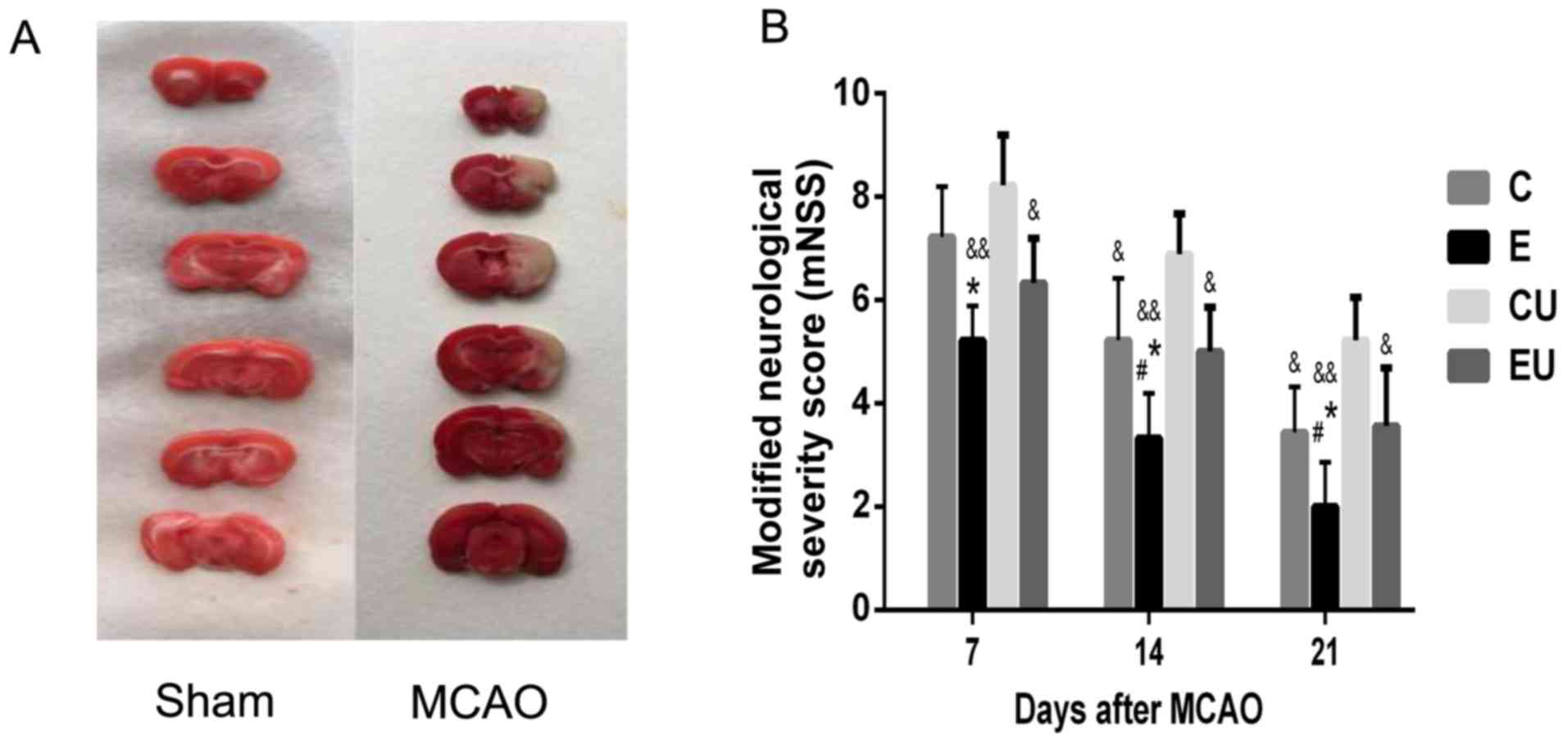
Preparation of the MCAO model
TTC staining of the rat brains confirmed that the
MCAO was successfully established. As shown in Fig. 1A, MCAO generated infarction in the
brain, which was indicated by a pale white color. Conversely,
normal brain tissue was pink in color, indicating living
tissue.
Functional assessment
The mNSS scores were zero for the S group at all
time points. The mNSS score for the E group was significantly lower
compared with in the C and CU groups 7 days following MCAO surgery
(P<0.05), and although the score was also lower than in the EU
group, significance was not reached. At 14 and 21 days after MCAO,
the E group had a significantly lower mNSS score compared with all
other experimental groups (P<0.05). The mNSS score of the C
group was significantly lower compared with in the CU group 14 and
21 days after MCAO (P<0.05; Fig.
1B).
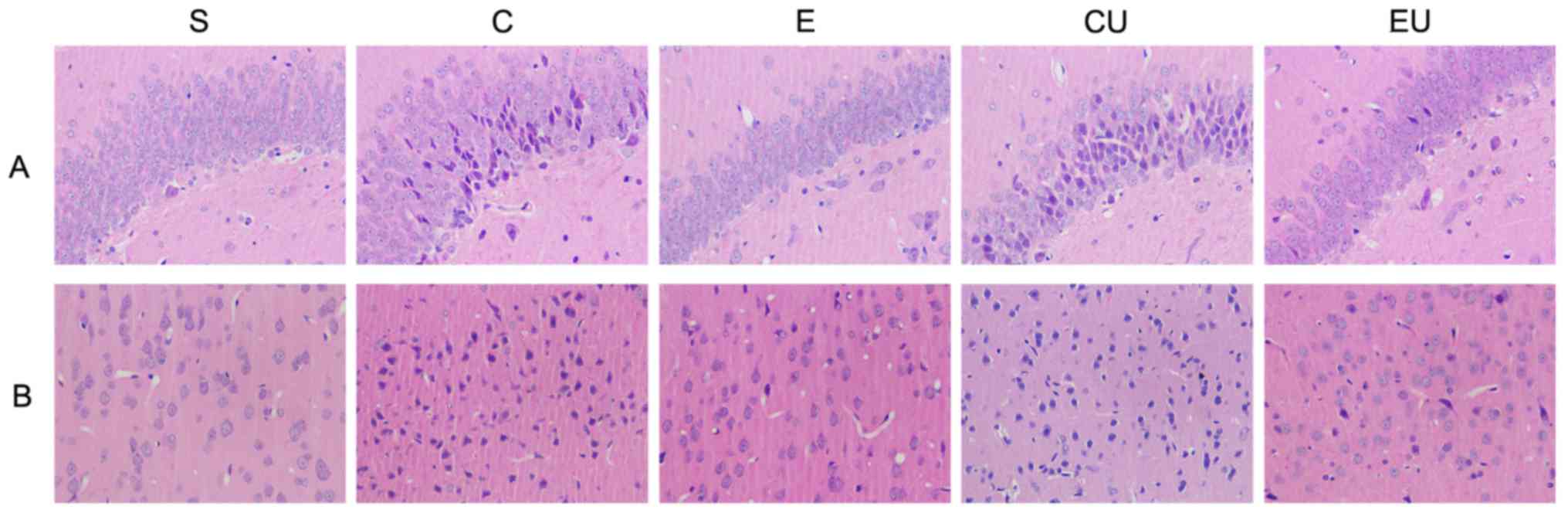
Pathological alterations
H&E staining (Fig.
2) revealed that the cells in the cerebral cortex and
hippocampus of the S group were large and neatly arranged, with
distinct nuclear membranes and nucleoli. In addition, the nucleus
was located in the center of the cells, and the tissue was
intact.
In the C, CU and EU groups, the right cerebral
cortex and hippocampal area contained ischemic lesions, tissue
edema, cell degeneration and necrosis. The normal morphology of
some neurons was lost. The cytoplasm, nuclear membrane and nucleoli
appeared abnormal, with deeply stained nuclei and nuclear
condensation.
Pathological alterations were alleviated in the E
group. The nerve cells were generally neatly arranged, with normal
cell size and morphology. The cytoplasm was stained blue and the
nucleoli were clear.
Expression of
BrdU+/NeuN+ cells in the hippocampal dentate
gyrus
The majority of cells in the S group were
BrdU−/NeuN+, indicating that proliferation was minimal;
only a small number of BrdU+/NeuN+ cells were observed in some
brain slices. The number of BrdU+/NeuN+ cells in the E group was
significantly higher compared with in the C and CU groups 7 days
after MCAO (P<0.05); and the number of cells was also higher
compared with the EU group, however there was no statistically
significant difference. The E group demonstrated a significant
increase in the number of BrdU+/NeuN+ cells compared with all other
groups 14 and 21 days after MCAO surgery (P<0.05). The number of
cells in the C group was significantly higher than the CU group at
14 and 21 days after MCAO (P<0.05; Fig. 3).
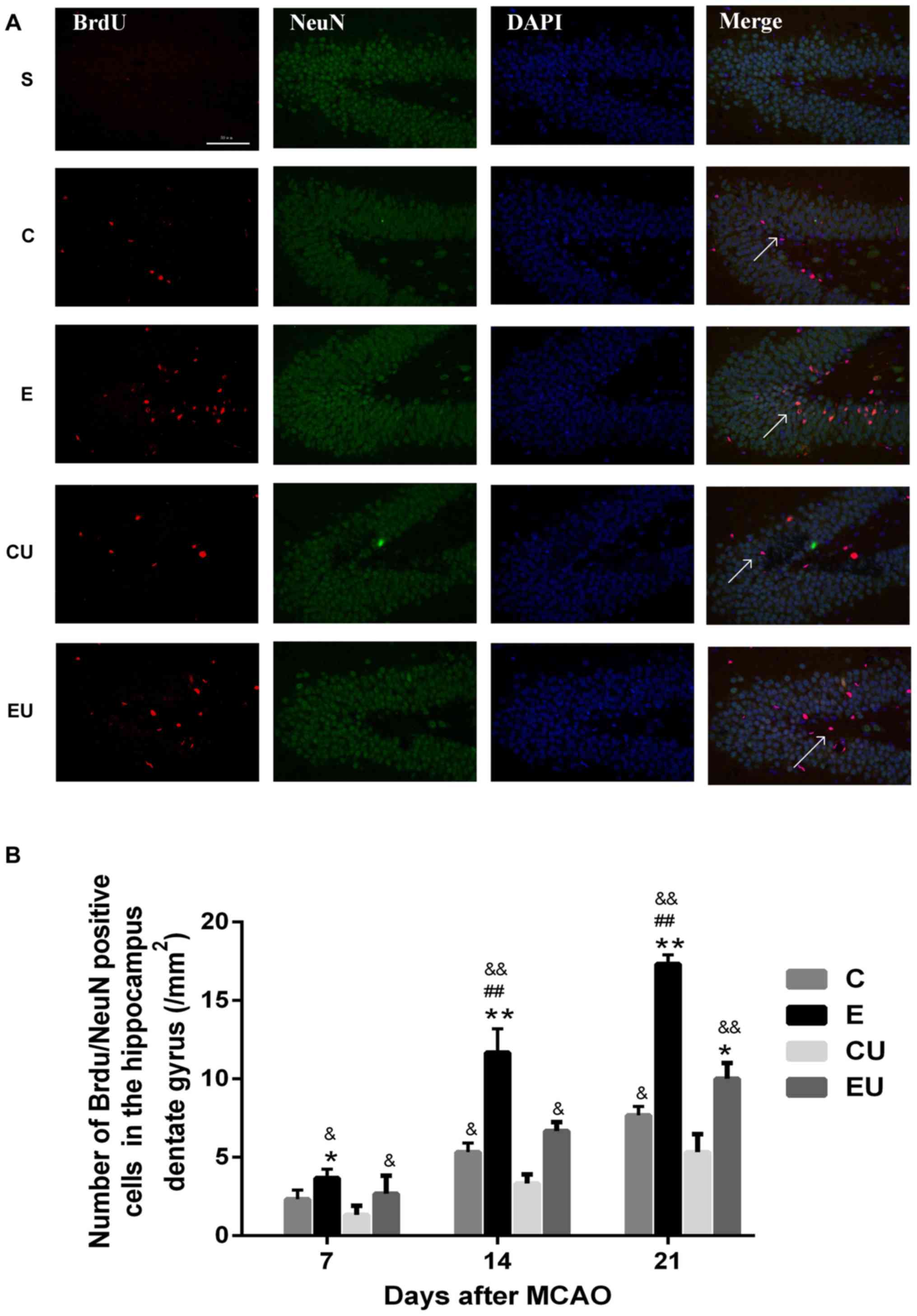 | Figure 3.Exercise enhances proliferation of
neurons in the hippocampal dentate gyrus. (A) Immunofluorescence
staining for BrdU (red) and NeuN (green) in rats 21 days after
MCAO. Scale bar=50 µm. (B) Quantification of
BrdU+/NeuN+ cells 7, 14 and 21 days after
MCAO. Data are presented as the means ± standard deviation. The
arrows in the images are indicating
BrdU+/NeuN+ cells. *P<0.05, **P<0.001
vs. C; ##P<0.001 vs. EU; &P<0.05,
&&P<0.001 vs. CU. BrdU, bromodeoxyuridine; C,
control group; CU, control group treated with U0126; E, physical
exercise group; EU, physical exercise group treated with U0126;
MCAO, middle cerebral artery occlusion; NeuN, neuronal nuclei. |
Expression of
BrdU+/GFAP+ cells in the hippocampal dentate
gyrus
Most cells in the S group were
BrdU+/GFAP+ with only a small number of
BrdU+/GFAP+ cells observed in some brain
slices. The E group had a significant increase in the number of
BrdU+/GFAP+ cells 7, 14, and 21 days after
MCAO (P<0.05), indicating proliferating astrocytes. The number
of cells in the C group was significantly higher compared with in
the CU group 14 and 21 days after MCAO (P<0.05; Fig. 4).
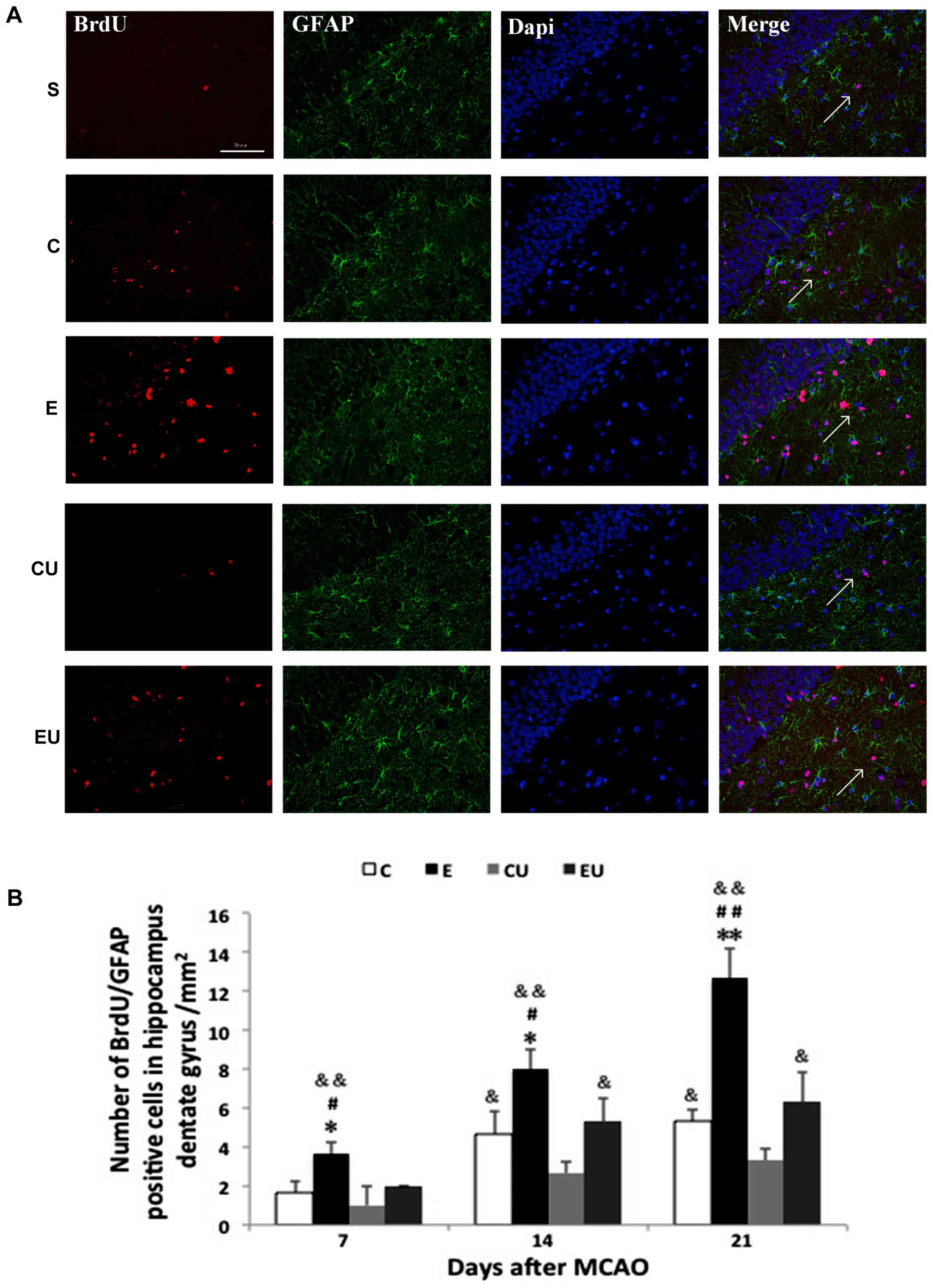 | Figure 4.Exercise enhances proliferation of
astrocytes in the hippocampal dentate gyrus. (A) Immunofluorescence
staining for BrdU (red) and GFAP (green) in rats 21 days after
MCAO. Scale bar=50 µm. (B) Quantification of
BrdU+/GFAP+ cells 7, 14 and 21 days after
MCAO. The arrows in the images are indicating
BrdU+/GFAP+ cells. Data are presented as the
means ± standard deviation. *P<0.05, **P<0.001 vs. C;
#P<0.05, ##P<0.001 vs. EU;
&P<0.05, &&P<0.001 vs. CU.
BrdU, bromodeoxyuridine; C, control group; CU, control group
treated with U0126; E, physical exercise group; EU, physical
exercise group treated with U0126; GFAP, glial fibrillary acidic
protein; MCAO, middle cerebral artery occlusion. |
Western blot analysis
The expression levels of Cyclin D1 in the E group
were significantly increased at all time points compared with in
the other groups (P<0.05). The expression levels of CDK4 and
p-Rb in the E group were significantly increased 14 and 21 days
compared with in the other groups (P<0.05). Although the
expression levels of CDK4 and p-Rb in the E group were increased at
7 days compared with in the C group, the difference was not
statistically significant. The expression levels of Cyclin D1, CDK4
and p-Rb in the C group were significantly higher compared with the
CU group 7, 14, and 21 days after MCAO (P<0.05). The expression
levels of P-16 in the E group were significantly lower at 14 days
compared with in the other groups (P<0.05), and the expression
levels of P-16 in the E group were lower at 7 and 21 days compared
with in the C group, but the difference was not statistically
significant. The expression levels of P-16 in the C group were
significantly lower than in the CU group 7 and 14 days after MCAO
(P<0.05; Fig. 5).
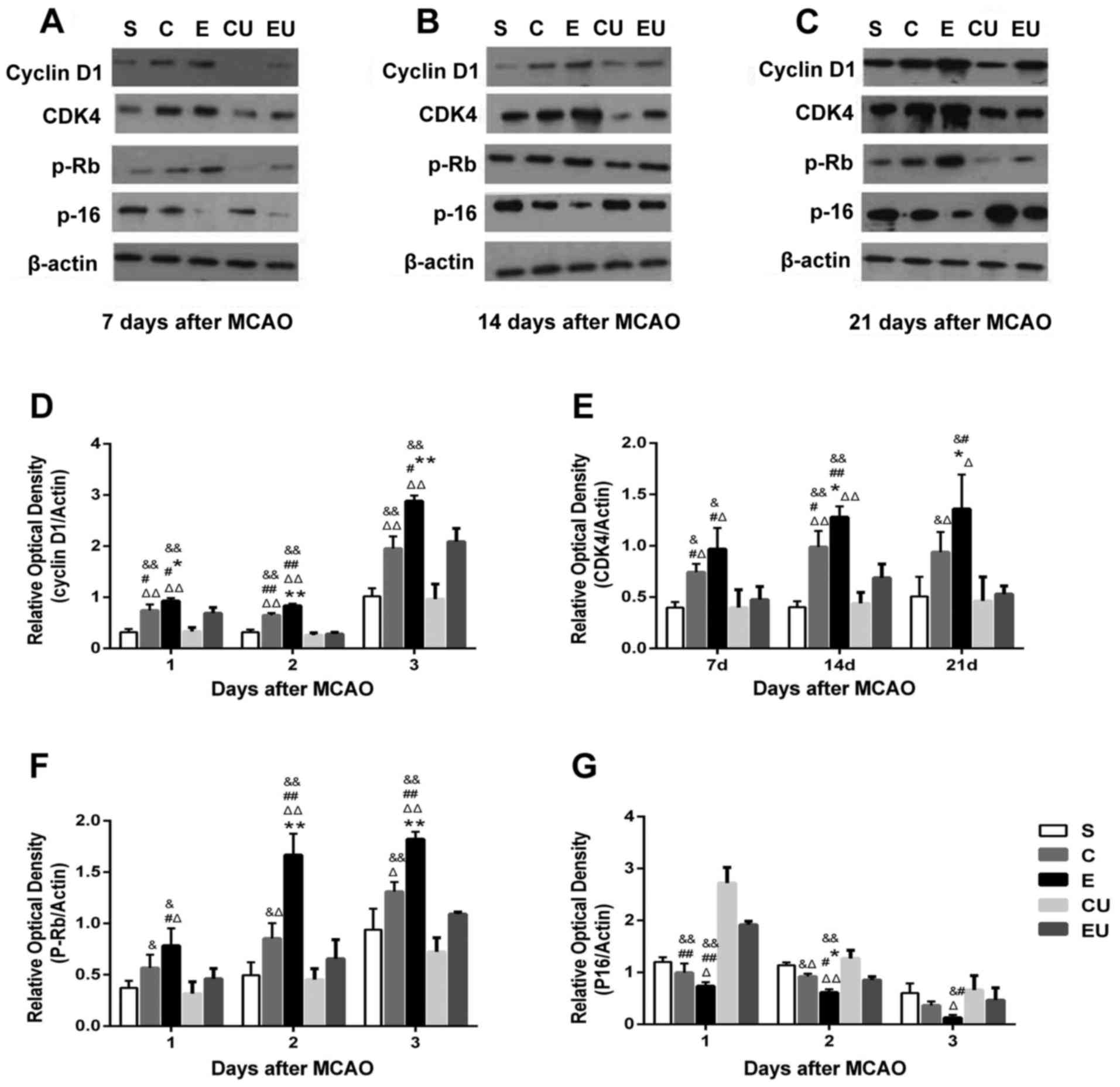 | Figure 5.Western blot analysis of Cyclin D1,
CDK4, p-Rb and P-16 in the hippocampal tissue of rats (A) 7, (B)
14, (C) 21 days after MCAO. Relative expression of (D) Cyclin D1,
(E) CDK4, (F) p-Rb and (G) P-16 7, 14 and 21 days after MCAO. Data
are presented as the means ± standard deviation. *P<0.05,
**P<0.001 vs. C; #P<0.05, ##P<0.001
vs. EU; &P<0.05, &&P<0.001
vs. CU; ΔP<0.05, ΔΔP<0.001 vs. S. C,
control group; CDK4, cyclin-dependent kinase 4; CU, control group
treated with U0126; E, physical exercise group; EU, physical
exercise group treated with U0126; MCAO, middle cerebral artery
occlusion; p-Rb, retinoblastoma protein; S, sham surgery group. |
The expression levels of p-ERK1/2 and c-Fos in the E
group were significantly increased at each time point compared with
in the other groups (P<0.05). The expression levels of c-Fos in
the C group were significantly higher compared with in the CU group
7, 14, and 21 days after MCAO (P<0.05). The expression levels of
p-ERK1/2 in the C group were significantly higher compared with in
the CU group 7 and 21 days after MCAO (P<0.05; Fig. 6).
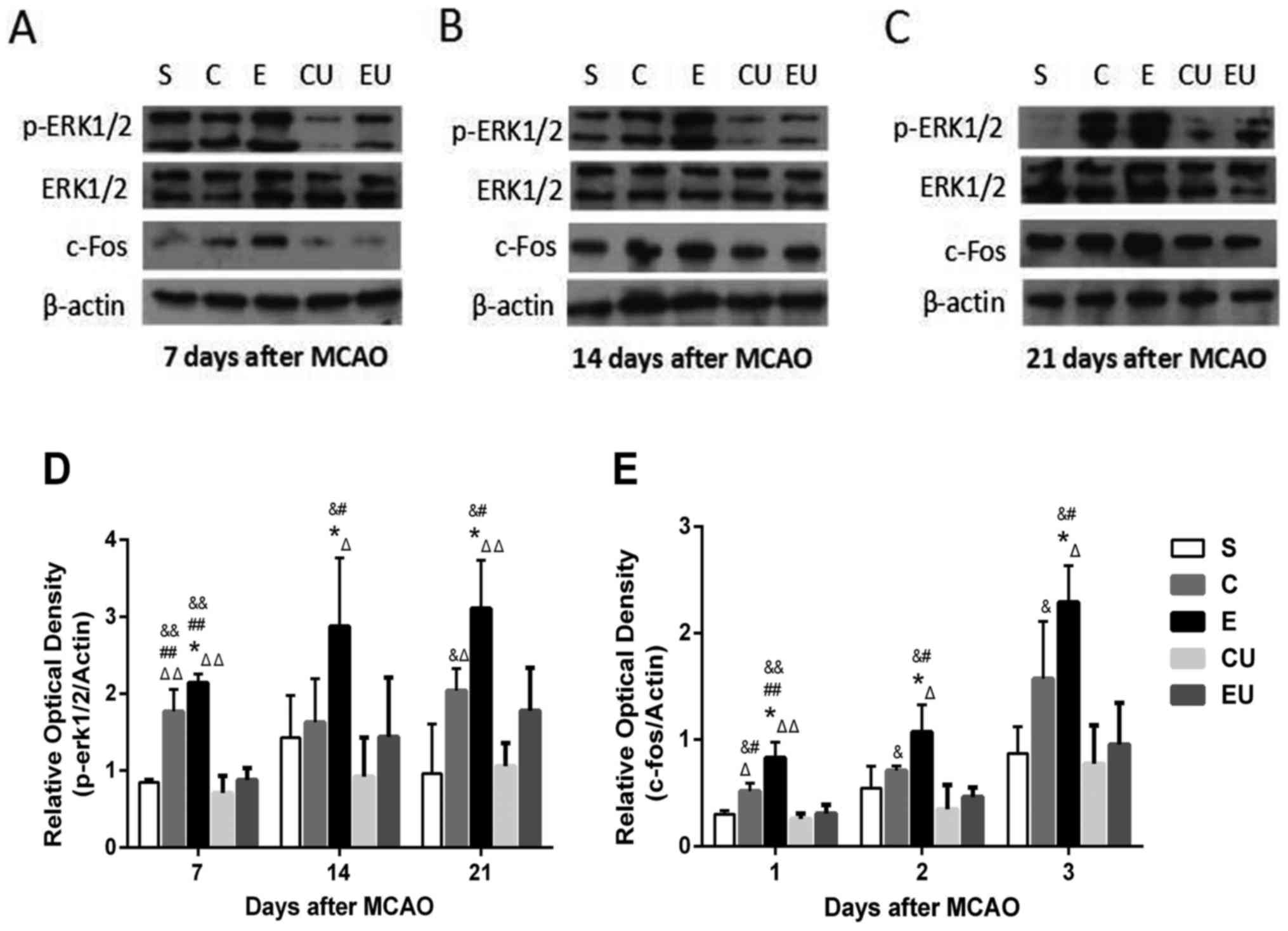 | Figure 6.Western blot analysis of p-ERK1/2,
ERK1/2 and c-Fos in the hippocampal tissue of rats (A) 7, (B) 14
and (C) 21 days after MCAO. Relative protein expression of (D)
p-ERK1/2 and (E) c-Fos 7, 14 and 21 days after MCAO. Data are
presented as the means ± standard deviation. *P<0.05 vs. C;
#P<0.05, ##P<0.001 vs. EU;
&P<0.05, &&P<0.001 vs. CU;
ΔP<0.05, ΔΔP<0.001 vs. S. C, control
group; CU, control group treated with U0126; E, physical exercise
group; ERK, extracellular signal-regulated kinase; EU, physical
exercise group treated with U0126; MCAO, middle cerebral artery
occlusion; p, phosphorylated; S, sham surgery group. |
Discussion
Studies have demonstrated that physical exercise
serves an important role in recovery of neurological function in
patients with cerebral infarction (14–16).
The rehabilitation mechanism of physical exercise is widely
believed to be associated with neural plasticity, and may be
related to the regulation of differentiation and proliferation of
endogenous NSCs; however, the molecular mechanisms still need to be
clarified.
BrdU is a thymine nucleotide analog that is
incorporated into DNA during the S phase of the cell cycle and is a
commonly used marker for cellular proliferation (17). Zhang et al (18) demonstrated that this is a common
method for detecting NSCs. Previous studies have demonstrated that
the presence of increased numbers of BrdU+ cells
identified the proliferation of NSCs (19,20).
In the present study, proliferating cells were visualized by
immunofluorescence double staining.
BrdU+/NeuN+ and
BrdU+/GFAP+ cells identified proliferating
neurons and proliferating astrocytes, respectively. Due to the
presence of increased numbers of BrdU+/NeuN+
and BrdU+/GFAP+ cells, and decreased mNSS
scores in the E group, it may be hypothesized that physical
exercise enhances the proliferation and differentiation of
endogenous NSCs to improve the neural function of rats with
cerebral infarction. Injection of U0126, an ERK signaling pathway
inhibitor, suppressed the proliferation of neural cells and
affected the mNSS score. The experimental results demonstrated that
physical exercise not only promoted differentiation of endogenous
NSCs into neurons, but also their differentiation into astrocytes.
It is thought that astrocytes develop a scar around the ischemic
region, thereby hindering the growth of new neurons; however,
growing evidence suggests that activated astrocytes can inactivate
excitatory glutamate and secrete nerve growth factor (NGF), which
supports neural cell survival and axon growth (21). Only live astrocytes can support
extension of axonal growth cones, thus potentially compensating for
injured nerve cells (22,23). According to the mNSS results,
astrocytes may have a role in endogenous neuroprotection and neural
recovery after cerebral ischemia, but the mechanisms need to be
further studied.
Clinical and animal studies have revealed that
physical exercise can promote nerve regeneration (24–27).
Ferreira et al (28)
demonstrated that short-term and moderate treadmill exercise in
rats with cerebral infarction improves hippocampal plasticity, and
Brandt et al (29)
identified that physical exercise promotes the proliferation of
NSCs and maturation of neurons in the dentate gyrus of rats, which
are in line with the results obtained in the present study. BrdU
labeling of proliferation is affected by dose, frequency and other
factors. In our previous experiment, BrdU was injected 1 day prior
to animal sacrifice (30), marking
fewer proliferative cells, whereas daily injection of BrdU may
exert potential toxicity in differentiating stem cells (7). Therefore, in the present study, BrdU
was injected 3 days prior to animal sacrifice, to mark cell
proliferation and avoid toxicity; however, whether this injection
scheme is the best remains to be determined.
The proliferation of endogenous NSCs is defined by
cells entering the next phase of the cell cycle from the resting
G0 phase. Cell cycle proteins, including cyclins and
CDKs, can control the progression of cells through the cell cycle
by forming a complex, thereby initiating activation. Conversely,
CDK inhibitors (CKIs) suppress CDK activity and act as negative
regulators (31). For example,
Cyclin D1 and CDK4 can form a complex known as Cyclin D1/CDK4,
which phosphorylates substrate protein p-Rb to drive cells through
the cell cycle G1/S checkpoint. The CKI, P-16, binds to
CDK4 and blocks its interaction with Cyclin D1, resulting in
inhibition of p-Rb phosphorylation, as well as inhibition of cell
proliferation (32). The results
presented in this study revealed that physical exercise upregulated
the expression of CDK4, Cyclin D1 and p-Rb, but downregulated the
expression of P-16. The use of U0126 inhibited the induction of
cell proliferation-associated proteins by physical exercise.
Therefore, it may be hypothesized that physical exercise promotes
progression of NSCs from the G0/G1 to S phase
by regulating cell proliferation-related protein expression leading
to proliferation, with the ERK signaling pathway potentially having
an important regulatory role.
ERK is a member of the mitogen-activated protein
kinase (MAPK) family. Hypoxia, ischemia, inflammatory cytokines,
growth factors, mechanical stress, and biochemical and physical
factors can activate the ERK signaling pathway, resulting in a
series of phosphorylation processes. The tyrosine kinase receptor
Raf-1 binds to Ras and activates the latter, which in turn
phosphorylates its downstream substrate MEK. Phosphorylation of MEK
activates the downstream phosphorylation of ERK1/2. p-ERK is the
active form of ERK that can not only phosphorylate cytosolic
proteins, but also transcription factors, including c-Fos, c-Jun,
Elk-1 and c-Myc (33). These
transcription factors participate in various processes, including
cell differentiation, proliferation, inflammation and oxidative
stress (34). In cerebral
ischemia, the expression and activation of ERK is caused by growth
factors, Ca2+ influx, oxygen free radicals and
excitatory amino acids. ERK is downstream of the NGF receptor and
through sequential activation of NGF receptor-Ras-Raf-MEK promotes
nerve regeneration (35). Studies
have demonstrated that activation of the ERK pathway inhibits
neuronal excitotoxicity and serves a neuroprotective role, whereas
blocking activation of the ERK pathway promotes cell death
(36). Poddar and Paul
(37) also observed that
inhibition of ERK phosphorylation reduces neuronal cell death.
Therefore, ERK activation appears to have an important dual role in
brain protection and injury.
In rats with spinal cord injury, it has been
established that physical exercise activates the ERK signaling
pathway leading to regeneration of axons and improved motor
function (38). Studies have also
demonstrated that electric treadmill training can improve
neurological function in rats after cerebral ischemia, which may be
related to activation of the ERK signaling pathway; however, the
activation pathway remains largely unknown (39). In the present study, it was
identified that the protein expression levels of p-ERK1/2 and c-Fos
in the E group were higher compared with in the other experimental
groups 7, 14 and 21 days after MCAO. When the MEK1/2 inhibitor
U0126 was applied, the expression levels of p-ERK1/2 and the
downstream protein c-Fos were diminished. These results indicated
that physical exercise upregulated the ERK signaling pathway and
U0126 blocked the phosphorylation of ERK1/2 caused by physical
exercise. This suggested that physical exercise has a positive
regulatory role in promoting the proliferation and differentiation
of endogenous NSCs via the ERK signaling pathway. Notably, Li et
al (11) demonstrated that
pre-exercise training in mice may upregulate the exercise-induced
hormone irisin, which activates the ERK signaling pathway and
improves neurological function. The present study identified the
potential molecular regulatory mechanisms of physical exercise in
improving neurological function after cerebral ischemia in rats,
but whether the timing of exercise can also impact activation of
the ERK signaling pathway remains to be elucidated in future
experiments.
c-Fos is a proto-oncogene, and the rapid and
transient expression of the c-Fos protein can be induced by
ischemia, hypoxia, injury and drug action. c-Fos is a transcription
factor that regulates the expression of downstream target genes. It
is involved in an array of important biological processes,
including cell proliferation, differentiation, survival and
regulation of axon elongation (40,41).
Studies have demonstrated that low frequency electrical stimulation
can induce the expression of c-Fos to improve neurological function
(42). In addition, it has been
reported that high levels of c-Fos serve an important role in the
differentiation and maturation of cerebellar granule neurons
(43). c-Fos can also promote the
expression of NGF to enhance neuronal repair (44), accelerate the reconstruction of
neural networks and promote neuritis (45). Certain studies have identified that
physical exercise promotes the expression of c-Fos in the
hippocampus of rats, and may be associated with age and exercise
intensity (46,47). However, c-Fos has been linked to
apoptosis (48); therefore, there
has been some controversy over whether c-Fos is protective or
damaging in cerebral ischemia. The results of the present study
revealed that physical exercise increased the expression of c-Fos
and improved neurological function in rats, and that treatment with
U0126 inhibited c-Fos expression. These results indicated that
c-Fos may be involved in regulating the proliferation and
differentiation of NSCs, and that physical exercise can upregulate
the expression of c-Fos to protect brain cells.
There are some limitations to the present study.
Firstly, the experiments only utilized one exercise regime, and
whether or not exercise type, intensity, duration and time of
intervention could have different effects on activation of the ERK
signaling pathway remains to be determined. Secondly, the
experiments revealed that U0126 did not completely inhibit
proliferation and differentiation of endogenous NSCs, suggesting
that other signaling pathways may be involved. These questions will
be explored in future experiments.
In conclusion, the present study demonstrated that
physical exercise increased the number of
BrdU+/NeuN+ and
BrdU+/GFAP+ cells in the hippocampus of rats
following cerebral infarction. Physical exercise promoted
proliferation and differentiation of endogenous NSCs via the ERK
signaling pathway by upregulating the expression of CDK4, Cyclin D1
and p-Rb, but downregulating the expression of P-16, to improve
neurological function. These results will aid further understanding
of the mechanisms underlying the beneficial neurological effects of
exercise and may provide a theoretical basis for clinical
rehabilitation.
Acknowledgements
The authors would like to thank Professor Wei Hao
from the Central Laboratory at Zhujiang Hospital, Southern Medical
University (Guangzhou, China) for the generous help.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, GL and WW conceived and designed the
experiments; WL, GL, JC and YZ performed the experiments; WL, JC
and YS analyzed the data; WL and WW analyzed and interpreted the
data, performed statistical analysis, and drafted the manuscript.
All authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Procedures involving animals and their care were
conducted inconformity with NIH guidelines (NIH Pub. No. 85-23,
revised 1996) and was approved by Animal Care and Use Committee of
the Southern Medical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shinozuka K, Dailey T, Tajiri N, Ishikawa
H, Kim DW, Pabon M, Acosta S, Kaneko Y and Borlongan CV: Stem cells
for neurovascular repair in stroke. J Stem Cell Res Ther.
4:129122013.PubMed/NCBI
|
|
2
|
Ambroginia P, Lattanzi D, Ciuffoli S,
Betti M, Fanelli M and Cuppini R: Exercise and environment
exploration affect synaptogenesis in adult-generated neurons in the
rat dentate gyrus: Possible role of BDNF. Brain Res. 1534:1–12.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai MJ, Tsai SK, Huang MC, Liou DY, Huang
SL, Hsieh WH, Huang WC, Huang SS and Cheng H: Acidic FGF promotes
neurite outgrowth of cortical neurons and improves neuroprotective
effect in a cerebral ischemic rat model. Neuroscience. 305:238–247.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang YR, Chang HC, Wang PS and Wang RY:
Motor performance improved by exercises in cerebral ischemic rats.
J Mot Behav. 44:97–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuda F, Sakakima H and Yoshida Y: The
effects of early exercise on brain damage and recovery after focal
cerebral infarction in rats. Acta Physiol (Oxf). 201:275–287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo J, Hu X, Zhang L, Li L, Zheng H, Li M
and Zhang Q: Physical exercise regulates neural stem cells
proliferation and migration via SDF-1α/CXCR4 pathway in rats after
ischemic stroke. Neurosci Lett. 578:203–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Q, Ye T, Zhu LW, Wu XJ, Li HY and
Tian Y: Effects of acupuncture-rehabilitation therapy on
neurological function and extracellular signal-regulated Ki-nase
1/2 signaling pathway after focal cerebral ischemia in rats. Chin J
Rehabil Theory Pract. 23:27–31. 2017.
|
|
9
|
Weeber EJ and Swear JD: Molecular
neurobiology of human cognition. Neuron. 33:845–848. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kilic U, Yilmaz B, Reiter RJ, Yüksel A and
Kilic E: Effects of memantine and melatonin on signal transduction
pathways vascular leakage and brain injury after focal cerebral
ischemia in mice. Neuroscience. 237:268–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li DJ, Li YH, Yuan HB, Qu LF and Wang P:
The novel exercise-induced hormone irisin protects against neuronal
injury via activation of the Akt and ERK1/2 signaling pathways and
contributes to the neuroprotection of physical exercise in cerebral
ischemia. Metabolism. 68:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniotomy in rat. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin J, Gong G, Sun S, Qi J, Zhang H, Wang
Y, Wang N, Wang QM, Ji Y, Gao Y, et al: Functional recovery after
transplantation of induced pluripotent stem cells in a rat
hemorrhagic stroke model. Neurosci Lett. 554:70–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dragert K and Zehr EP: High-intensity
unilateral dorsiflexor resistance training results in bilateral
neuromuscular plasticity after stroke. Exp Brain Res. 225:93–104.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimodozono M, Noma T, Nomoto Y, Hisamatsu
N, Kamada K, Miyata R, Matsumoto S, Ogata A, Etoh S, Basford JR and
Kawahira K: Benefits of a repetitive facilitative exercise program
for the upper paretic extremity after subacute stroke: A randomized
controlled trial. Neurorehabil Neural Repair. 27:296–305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoller O, de Bruin ED, Knols RH and Hunt
KJ: Effects of cardiovascular exercise early after stroke:
Systematic review and meta-analysis. BMC Neurol. 12:452012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kee N, Sivalingam S, Boonstra R and
Wojtowicz JM: The utility of Ki-67 and BrdU as proliferative
markers of adult neurogenesis. J Neurosci Methods. 115:97–105.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang R, Zhang Z, Wang L, Wang Y, Gousev
A, Zhang L, Ho KL, Morshead C and Chopp M: Activated neural stem
cells contribute to stroke-induced neurogenesis and neuroblast
migration toward the infarct boundary in adult rats. J Cereb Blood
Flow Metab. 24:441–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato K, Iwai M, Nagano I, Shoji M and Abe
K: Temporal and spacial changes of BrdU immunoreactivity in
amygdala kindling development. Neurol Res. 24:593–596. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang M, Luo J, Bai Y and Liu MF: Neural
stem cell proliferation and differentiation in a neonatal rat model
of hypoxia/ischemia injury Acupuncture at Ren, Du and urinary
bladder meridians. Neural Regenerat Res. 5:267–272. 2010.
|
|
21
|
Bani-Yaghoub M, Underhill TM and Naus CC:
Gap junction blockage interferes with neuronal and astroglial
differentiation of mouse P19 embryonl carcinoma cells. Dev Genet.
24:69–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y and Swanson RA: Astrocytes and
brain injury. J Cereb Blood Flow Metab. 23:137–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayakawa K, Esposito E, Wang X, Terasaki
Y, Liu Y, Xing C, Ji X and Lo EH: Transfer of mitochondria from
astrocytes to neurons after stroke. Nature. 535:551–555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macaluso F and Myburgh KH: Current
evidence that exercise can increase the number of adult stem cells.
J Muscle Res Cell Motil. 33:187–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niwa A, Nishibori M, Hamasaki S, Kobori T,
Liu K, Wake H, Mori S, Yoshino T and Takahashi H: Voluntary
exercise induces neurogenesis in the hypothalamus and ependymal
lining of the third ventricle. Brain Struct Funct. 221:1653–1666.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DiFeo G and Shors TJ: Mental and physical
skill training increases neurogenesis via cell survival in the
adolescent hippocampus. Brain Res. 1654:95–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu
Y, Zhao Y, Tu F, Liu C and Chen X: Role of caveolin-1/vascular
endothelial growth factor pathway in basic fibroblast growth
factor-induced angiogenesis and neurogenesis after treadmill
training following focal cerebral ischemia in rats. Brain Res.
1663:9–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferreira AF, Real CC, Rodrigues AC, Alves
AS and Britto LR: Short-term, moderate exercise is capable of
inducing structural, BDNF-independent hippocampal plasticity. Brain
Res. 1425:111–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandt MD, Maass A, Kempermann G and
Storch A: Physical exercise increases Notch activity, proliferation
and cell cycle exit of type-3 progenitor cells in adult hippocampal
neurogenesis. Eur J Neurosci. 32:1256–1264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi HS, Xu JL, Lin GY and Wu W: Effect of
11 mHz ultra-low frequency transcraniai magnetic stimulation on
expressions of nestin and Brdu in the hippocampus of rats with
focal cerebral ischemia and reperfusion. Chin J Neuromed.
13:1117–1122. 2014.
|
|
31
|
Kim MS, Kim KH, Lee EH, Lee YM, Lee SH,
Kim HD and Kim YZ: Results of immunohistochemical staining for cell
cycle regulators predict the recurrence of atypical meningiomas. J
Neurosurg. 121:1189–1200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim S and Kaldis P: Loss of Cdk2 and Cdk4
induces a switch from proliferation to differentiation in neural
stem cells. Stem Cells. 30:1509–1520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brunet A, Roux D, Lenormand P, Dowd S,
Keyse S and Pouysségur J: Nuclear translocation of p42/p44
mitogen-activated protein kinase is required for growth
factor-induced gene expression and cell cycle entry. EMBO J.
18:664–674. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Avruch J: MAP kinase pathways: The first
twenty years. Biochim Biophys Acta. 1773:1150–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang L and Karin M: Mammalian MAP kinase
signaling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schrader LA, Birnbaum SG, Nadin BM, Ren Y,
Bui D, Anderson AE and Sweatt JD: ERK/MAPK regulates the Kv4.2
potassium channel by direct phosphorylation of the pore forming
subunit. Am J Physiol Cell Physiol. 290:C852–C861. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Poddar R and Paul S: Homocysteine-NMDA
receptor-mediated activation of extracellular signal-regulated
kinase leads to neuronal cell death. J Neurochem. 110:1095–1106.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oh MJ, Seo TB, Kwon KB, Yoon SJ, Elzi DJ,
Kim BG and Namgung U: Axonal outgrowth and Erk1/2 activation by
training after spinal cord injury in rats. J Neurotrauma.
26:2071–2082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang, Qiang YE Tao, ZHU Lu-Wen, WU
Xiao-Jun, LI Hong-Yu and Tian Yuan: Effects of
acupuncture-rehabilitation therapy on neurological function and
extracellular signal-regulated kinase 1/2 signaling pathway after
focal cerebral ischemia in rats. Chin J Rehabil Theory Pract.
23:27–31. 2017.
|
|
40
|
Güller M, Toualbi-Abed K, Legrand A,
Michel L, Mauviel A, Bernuau D and Daniel F: c-Fos overexpression
increases the proliferation of human hepatocytes by stabilizing
nuclear Cyclin D1. World J Gastroenterol. 14:6339–6346. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eriksson M and Leppä S: Mitogen-activated
protein kinases and activator protein 1 are required for
proliferation and cardiomyocyte differentiation of P19 embryonal
carcinoma cells. J Biol Chem. 277:15992–16001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian JB and Bishop GA: Stimulus-dependent
activation of c-Fos in neurons and glia in the rat cerebellum. J
Chem Neuroanat. 23:157–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eriksson M, Taskinen M and Leppä S:
Mitogen activated protein kinase-dependent activation of c-Jun and
c-Fos is required for neuronal differentiation but not for growth
and stress response in PC I2 cells. J Cell Physiol. 210:538–548.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aubert N, Morel Falluel A, Vaudry D, Xifro
X, Rodriguez-Alvarez J, Fisch C, de Jouffrey S, Lebigot JF,
Fournier A, Vaudry H and Gonzalez BJ: PACAP and C2-cerarnide
generate different AP-1 complexes through a MAP-kinase-dependent
pathway: Involvement of c-Fos in PACAP-induced Bcl-2 expression. J
Neurochem. 99:1237–1250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Willson ML, McElnea C, Mariani J, Lohof AM
and Sherrard RM: BDNF increases homotypic olivocerebellar
reinnervation and associated fine motor and cognitive skill. Brain.
131:1099–1l12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim SH, Kim H, Kim SS, Shin MS, Chang HK,
Lee TH, Jang MH, Shin MC, Lee HH, Kim YP and Kim CJ: The influence
of age on the treadmill exercise-induced c-Fos expression in the
hippocampus of rats. Neurosci Res Communicat. 35:41–50. 2004.
View Article : Google Scholar
|
|
47
|
Lee TH, Jang MH, Shin MC, Lim BV, Kim YP,
Kim H, Choi HH, Lee KS, Kim EH and Kim CJ: Dependence of rat
hippocampal c-Fos expression on intensity and duration of exercise.
Life Sci. 72:1421–1436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vyas S, Biguet NF, Michel PP, Monaco L,
Foulkes NS, Evan GI, Sassone-Corsi P and Agid Y: Molecular
mechanisms of neuronal cell death: Implications for nuclear factors
responding to cAMP and phorbol esters. Mol Cell Neurosci. 21:1–14.
2002. View Article : Google Scholar : PubMed/NCBI
|