Introduction
Ischemic heart disease, especially myocardial
infarction (MI), remains the leading cause of death in the world
(1). Sustained myocardial ischemia
causes multiple kinds of damage to cardiac tissue (2). It is of great importance to alleviate
detrimental damage by MI with pharmacological treatment.
Apoptosis plays a key role in the pathogenesis of
myocardial ischemia, indicating that inhibition of myocardial
apoptosis could decrease or alleviate the loss of terminally
differentiated myocardial cells (3,4).
Thus, an anti-apoptosis and anti-necrotic strategy serves as a
potential target for multiple therapies of myocardial ischemia
(5). The phosphoinositide 3-kinase
(PI3K)/AKT/glycogen synthase kinase-3β (GSK3β) pathway plays a key
role in the regulation of anti-apoptosis in multiple tissues,
including heart, brain, ovaries and kidney.
Herba leonuri, one kind of traditional Chinese
medicine, has been widely used to treat dysmenorrhea, menoxenia and
gynecological dysfunctions (6).
Several reports of pharmaceutical preparations based on H. leonuri
suggest that it may have protective effects against cardio-cerebral
vascular diseases (7,8). Leonurine, a specific component of H.
leonuri, has been reported to reveal cardio-protective effects
in vivo and in vitro (9,10).
Leonurine's effects also result in increased phosphorylation of AKT
and expression of HIF-1, Surviving and VEGF in rats with myocardial
ischemia. Leonurine also inhibited apoptosis in H9c2 cell under
hypoxia by increasing expression of the anti-apoptotic protein
Bcl-2 and decreasing expression of the pro-apoptotic protein Bax
(11). Although multiple studies
have been carried out to study the effect of Leonurine on
myocardial protection, the underlying mechanisms remain unclear.
Thus, in the present study, we investigated the mechanism of
cardio-protection by Leonurine in rats with MI.
Materials and methods
Our study included 45 male SD rats (200–250 g) from
Hunan Provincial Center for Disease Control and Prevention in
China. They were cared for under a room temperature of 22–25°C and
12:12-h light/dark cycle environment. Five rats were housed in each
cage with sufficient food and water. We designed experimental
procedures and protocols conformed to the Guide for the Care and
Use of Laboratory Animals from the US National Institutes of Health
and to the Institutional Animal Care Committee from Wuhan
University in China (wdrm-20140812).
Model of MI and in vivo leonurine
administration
A total of 45 rats were randomly divided into three
groups: i) sham-operated group (Sham); ii) MI with saline group
(NS); and iii) MI with Leonurine group (Leonurine). The model of MI
was operated as previous study, male SD rats were anesthetized
intra-peritoneally with 3% pentobarbital sodium (30 mg/kg), then
intubated and ventilated at a respiratory rate of 70 times/min. A
thoracotomy was implemented from the third intercostal space on the
left side of rats. After the epicardium was stripped, the left
coronary artery was ligated 2 mm below the left atrial appendage
with 6-to-0 polypropylene. The electrocardiogram (ECG) of rats was
recorded by ECG monitoring, and a successful MI model was
determined by ST-segment elevation in Leads II and III in ECG and
pallor appearance in the infarcted area. The rats in sham group
underwent thoracotomy without left anterior descending (LAD)
ligation and served as control.
The rats in the Leonurine group were treated with
Leonurine (24697-74-3; Shanghai Yanyi Biotechnology Co., Ltd.,
Shanghai, China) at 15 mg/kg/d by oral gavage after the onset of MI
for 28 days. The rats in sham group and NS group were administered
with equal volume of saline in the same manner. According to
previous study, Leonurine at both 15 and 30 mg/kg decreased
myocardial fibrosis and down regulated Nox4-ROS-NF-κB pathway,
while there was no significant difference in inhibiting cell
fibrosis between these two doses (12). And in Liu et al study, only
15 mg/kg/day of Leonurine significantly reduced neurological
deficits and brain infarct volume compared with 7.5 mg/kg/day of
leonurine or vehicle group (13).
Therefore the dose of leonurine in our present study is relatively
low but safe and effective without causing any side effects.
Echocardiography
On Day 1 and Day 28 after the MI surgery, all the
rats were anesthetized with their chest shaved. We used a
transthoracic echocardiography (Acuson, Mountain View, CA, USA)
which was equipped with a 3- to 7-MHz probe to investigate their
left ventricular (LV) dimension and heart function in the long-axis
and short-axis views in a blind manner. The animals with visual
infarct areas less than 20% in the short-axis view were excluded in
order to minimize the variation in infarct areas. LV end-systolic
diameter (LVESD), LV end-diastolic diameter (LVEDD), and ejection
fractional (EF) were assessed.
Histopathological examinations
All the rats were sacrificed using a pentobarbital
overdose (200 mg/kg) after the echocardiography assessment. The
heart was immediately removed into diastole with 10% KCl and the
ventricles parts were fixed in 10% (vol/vol) buffered formalin
solution. Masson's trichrome stain and immunohistochemistry were
conducted on five different transversal levels in the
peri-injection site subsequently after embedded. In all 3 animal
groups, MI area and collagen content were investigated in sections
obtained from the border zone (about 2 mm from the infarction zone
edge) via light microscopy. In each LV transverse section, 4 random
microscopic fields were chosen in a blind fashion and subsequently
examined. By computer image analysis software, the surface area of
infarct wall and the entire surface area of the left ventricle
(magnification, ×10) was automatically calculated, and the ratio of
the former to the latter was identified as the Infarct size (%).
The image analysis software was also used to calculate the Collagen
content (%), which was identified as the ratio of the area of
collagen to the area of the entire high-power field (magnification,
×200).
TUNEL assay
Twenty-eight days post the MI surgery, the apoptotic
cells in the LV were examined using a TUNEL assay (Roche
Diagnostics GmbH, Mannheim, Germany). As instructed by the
manufacturer, we stained the nuclear DNA fragments in the apoptotic
cells using the transferase dUTP nick-end labeling technique and
the hematoxylin counterstaining. In a blind manner, we selected
three random fields (magnification, ×400) in each slide and
calculated the TUNEL-positive nuclei via a microscopy. The
apoptotic index (%) was termed as the ratio of the number of
TUNEL-positive nucleolus to the total number of nuclei.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
At 28 days post the MI surgery, we collected the
peri-infarct area of the hearts to do the total RNA isolation with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). According to the manufacturer's descriptions, we
reverse transcribed complementary DNA (cDNA) from total RNA with
oligo (dT) primers (Shanghai Sangon BET Company, Shanghai, China),
then amplified DNA fragments with a SYBR-Green-based assay kit
(Invitrogen; Thermo Fisher Scientific, Inc.). The RT-qPCR started
at 42°C for 1 h, 95°C for 5 min to make the cDNA; then followed the
condition including 1 cycle at 50°C for 2 min and 95°C for 10 min,
40 cycles at 95°C for 30 sec and 60°C for 30 sec. The RNA level of
Bax and Bcl-2 were calculated according to CT values between the
experimental group and the control group. β-actin was used as the
housekeeping gene. The specific primer sequence and amplicon size
of the selected genes are listed in Table I.
 | Table I.Specific primer sequence and amplicon
size of the selected genes rat β-actin, Bax and BCLcl-2. |
Table I.
Specific primer sequence and amplicon
size of the selected genes rat β-actin, Bax and BCLcl-2.
| Name | Primer | Sequence | Size |
|---|
| Rat β-actin | F |
5′-CACGATGGAGGGGCCGGACTCATC-3′ | 240 bp |
|
| R |
5′-TAAAGACCTCTATGCCAACACAGT-3′ |
|
| Rat Bax | F |
5′-CAGGCGAATTGGCGATGAAC-3′ | 134 bp |
|
| R |
5′-CCCAGTTGAAGTTGCCGTCT-3′ |
|
| Rat BCLcl-2 | F |
5′-CTGGCATCTTCTCCTTCCAG-3′ | 181 bp |
|
| R |
5′-CGGTAGCGACGAGAGAAGTC-3′ |
|
Western blot analysis
At 28 days post the MI surgery, western blotting was
conducted with the peri-infarct area of the hearts. The
Bicinchoninic Acid protein assay (Beyotime Institute of
Biotechnology, Haimen, China) was used to examine the protein
concentration. We added 50 µg of denatured protein in each hole of
the 10% SDS-polyacrylamide gels and transferred the protein to a
PVDF membrane (EMD Millipore, Billerica, MA, USA). Membranes were
probed with first antibodies against PI3K (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), p-PI3K (1:300; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), AKT (1:1,000; Cell Signaling
Technology, Inc.), p-AKT (1:800; Cell Signaling Technology, Inc.),
GSK3β (1:1,000; Abcam, Cambridge, UK), p-GSk3β (1:800, Abcam),
Caspase3 (1:300; Santa Cruz Biotechnology, Inc.), Cleaved-caspase3
(1:1,000; Cell Signaling Technology, Inc.), Bax (1:200; Santa Cruz
Biotechnology, Inc.), Bcl-2 (1:200; Santa Cruz Biotechnology,
Inc.), and second antibodies as HRP goat-anti-rat, HRP
goat-anti-rabbit, HRP rabbit-anti-goat. Finally the blots were
analyzed with the ImageJ software after been visualized by the
chemiluminescence methods. GAPDH was termed as an internal
control.
Statistical analysis
The data were showed as the mean ± standard
deviation. We used one-way ANOVA's test and the
Student-Newman-Keuls' test to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Totally 45 rats were initially used in this project.
According to original weight from low to high, 45 rats were
ear-marked with tags ranking number 1–45. Then, we transcribed
random numbers from the random number table from the first number
at the first row and copied down 45 numbers between 1 and 99. Each
number was divided by 3, and the remainder 1, 2 and 3 is on behalf
of the group sham, NS and Leonurine respectively. In case one or
more groups had more than 15 rats, for example the NS group had 16
rats, we continued to record 1 number between 1 and 99 from the 46
number and divided by 3, then this remainder stood for the
corresponding rat would be included into next group which was less
than 15 rats. Finally, the above three groups owned 15 rats,
respectively. A total of 3 rats (in sham group) were dead because
of hyper-anesthesia before surgery. A total of 5 rats (3 in NS
group and 2 in Leonurine group) died because of left heart failure
combined with wound infection within 1 week post MI experiments.
One rat (in Leonurine group) was excluded as infarct size was less
than 20%. None of the rest rats died with unexplained reasons or
suffered from any side effects such as vomiting, decreased body
weight, hair loss or physical weakness. At 28 days after the MI
surgery, all the rats were investigated on the heart functions with
Echocardiography before been sacrificed. And for each group, we as
well chose transcribed random numbers from the random number table
from the first number at different rows and copied down 12 numbers
between 1 and 99, and divided by 2, with the remainder 1 and 2 on
behalf of histological staining (6 rats) and biochemical testing (6
rats). Thus 6 rats from each group were chosen from random
selection and sacrificed to measure infarct size, collagen content
and apoptosis index, while another 6 rats from each group were
sacrificed to perform western blot and RT-PCR analyses.
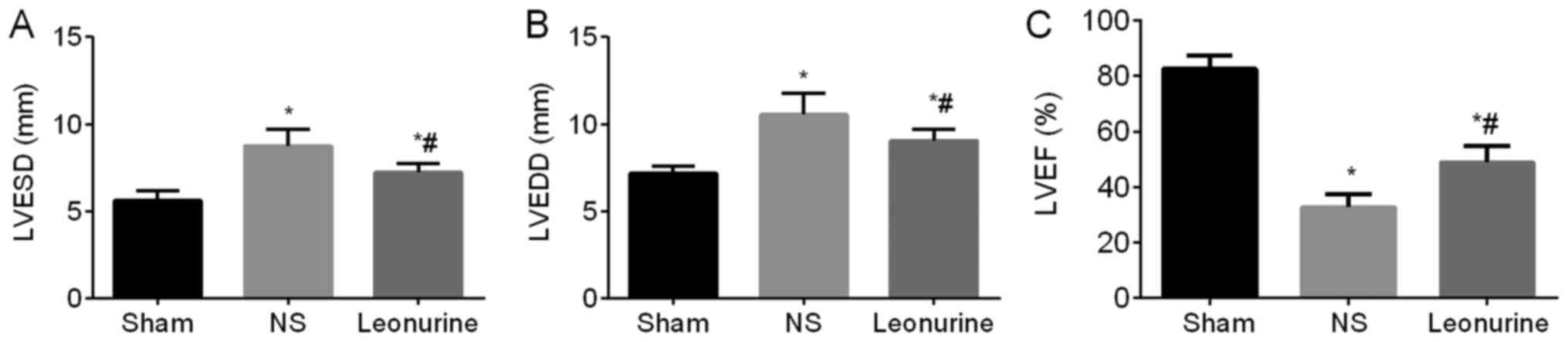
LV diameter and LVEF
The first day after MI, the NS and the Leonurine
group showed an obvious impaired heart function (decreased LVEF and
increased LV diameters) compared with the sham group, while the NS
group and the Leonurine group showed similar heart function between
each other (data not shown). At 28 days after MI, the MI groups
still showed an impaired heart function (significant increase in
the LVESD and LVEDD and an obvious decrease in the LVEF) compared
to the sham group (LVESD, 5.58±0.15 mm; LVEDD, 7.17±0.13 mm; LVEF,
82.62±1.41%). However, the Leonurine group (LVESD, 7.15±0.16 mm;
LVEDD, 9.07±0.18 mm; LVEF, 48.93±1.73%) showed not only a
remarkable reduction in LV diameters (P<0.05; Fig. 1A and B) but also an increase in
LVEF (P<0.05; Fig. 1C) compared
with the NS group (LVESD, 8.73±0.27 mm; LVEDD, 10.53±0.36 mm; LVEF,
32.73±1.38%).
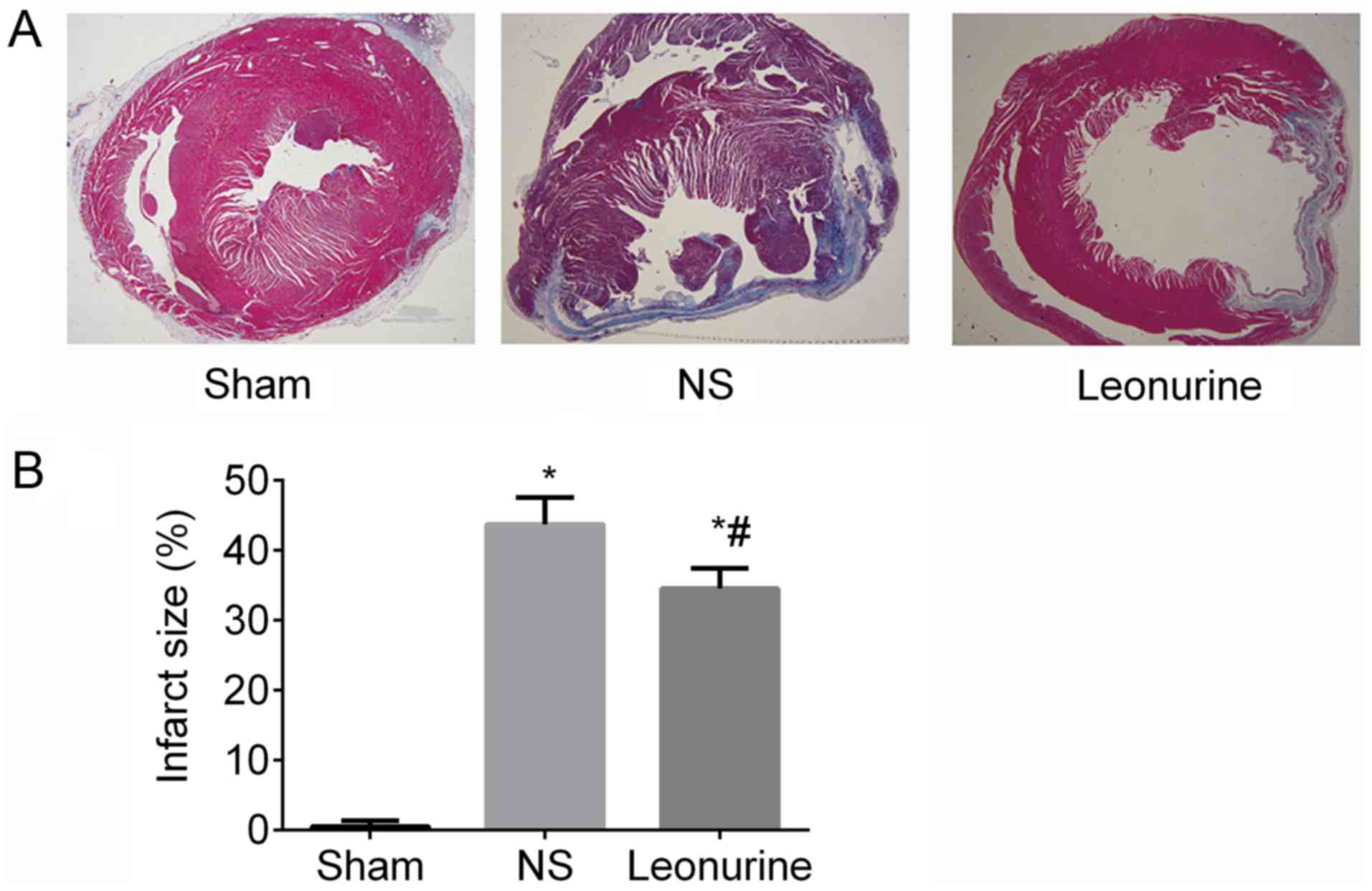
Infarct size
At 28 days after MI, the Leonurine group
(34.49±1.19%) showed a significant reduction in infarct size
compared with the NS (43.66±1.60%) group (P<0.05; Fig. 2).
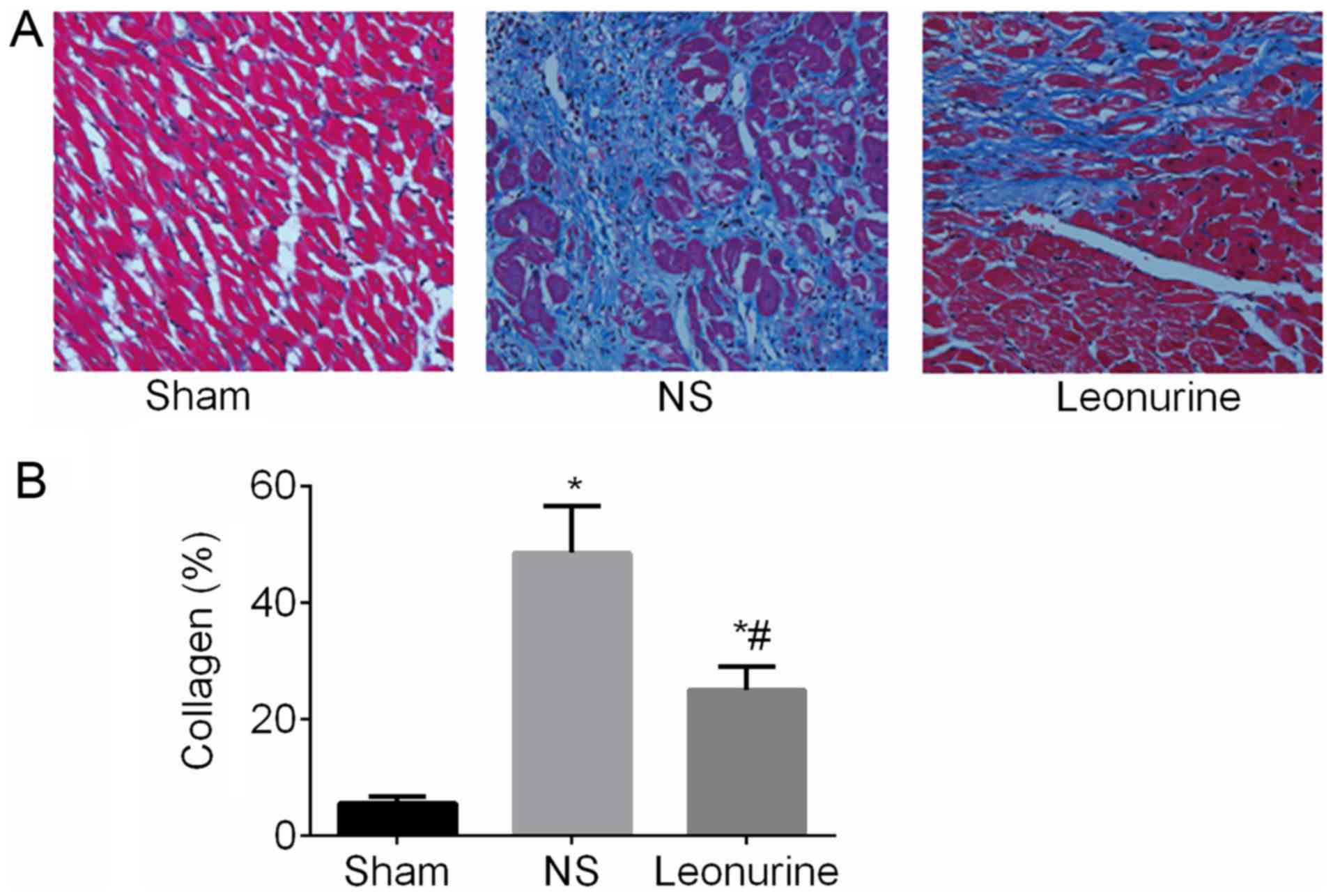
Collagen content
At 28 days after MI, collagen content was remarkably
increased (P<0.05; Fig. 3) in
the MI group compared with the sham (5.52±0.46%) group. After
Leonurine (24.99±1.63%) treatment, the collagen content was
remarkably decreased compared with the results of the NS group
(48.55±3.24%).
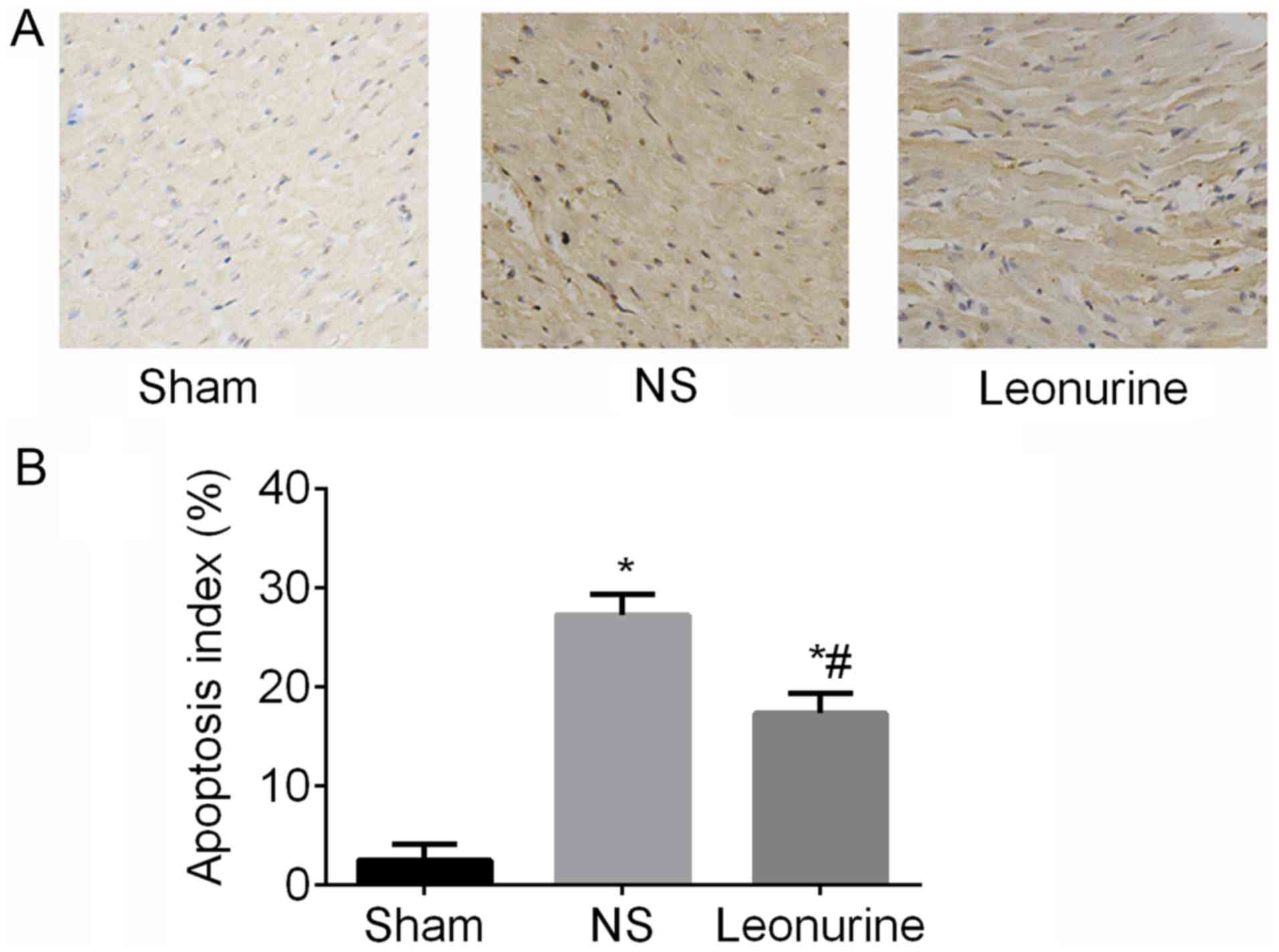
Apoptosis
At 28 days after MI, the NS (27.21±0.84%) and
Leonurine group (17.28±0.82%) exhibited an obvious increase
(P<0.05; Fig. 4) of the
apoptosis index compared with the sham group (2.5±0.66%), while the
Leonurine group showed a smaller apoptosis index (P<0.05;
Fig. 4) compared with the NS group
(Fig. 4).
Expression of PI3K/AKT/GSK3β signaling
pathway and apoptosis-related proteins
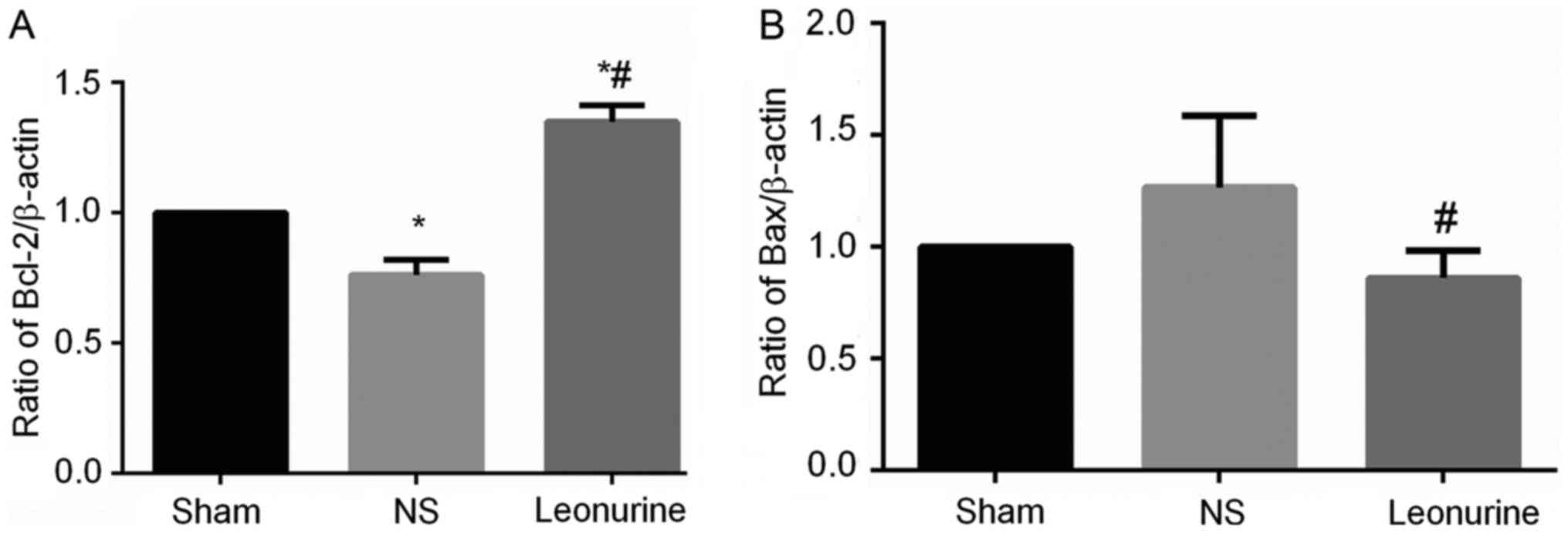
The gene expression of Bax in NS group 28 days after
MI is higher than the expression in the sham group, though not to a
statistically significant extent (Fig.
5). However, the Leonurine group had significantly decreased
the gene expression of Bax while increased the gene expression of
Bcl-2 compared with the result in the NS group (Fig. 5A). Thus, the ratio of Bcl-2/Bax was
significantly increased in the Leonurine group compared with that
of NS group. The NS group showed a smaller ratio of Bcl-2/Bax than
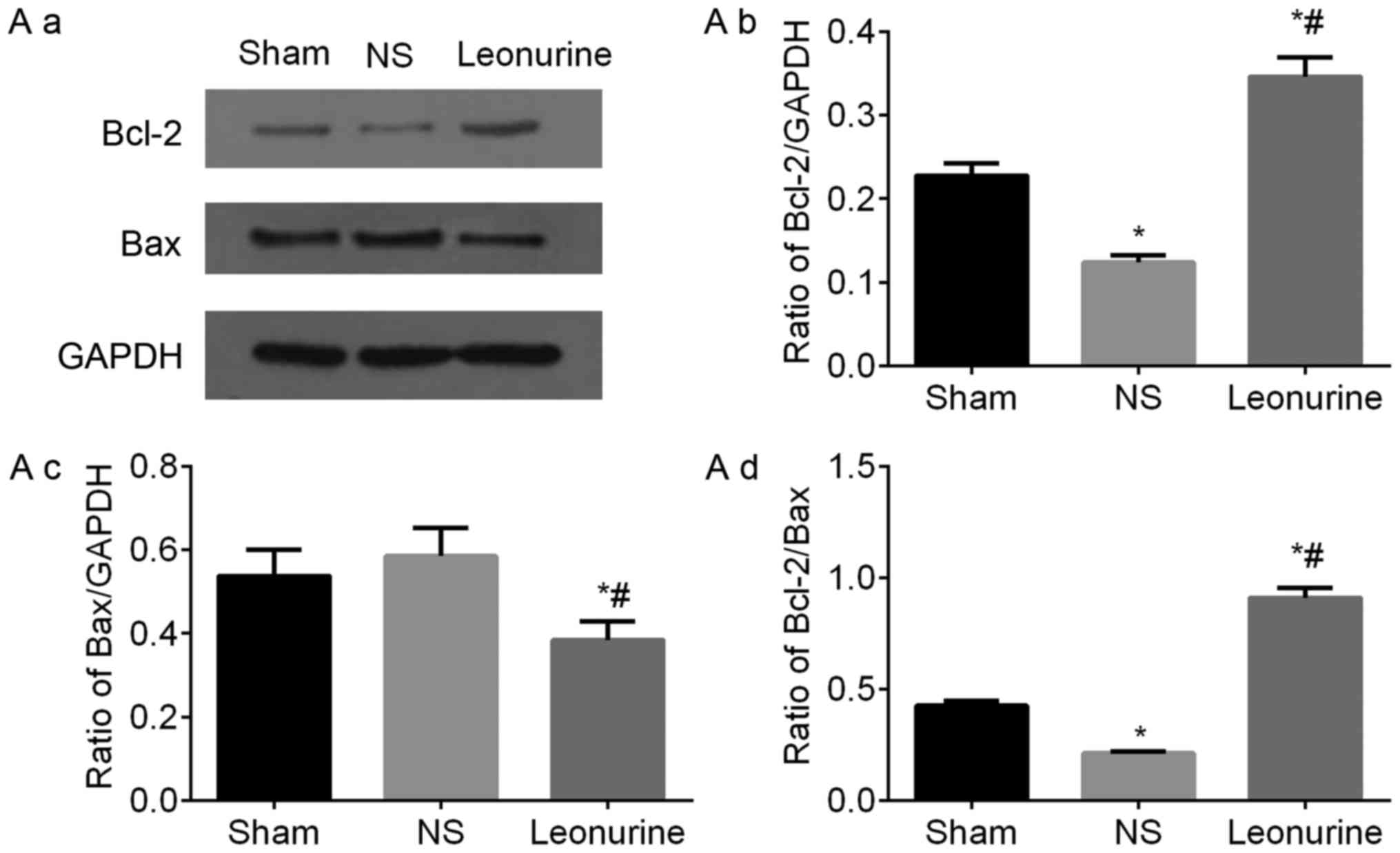
the sham group. The protein expression of Bax and Bcl-2 had the
same development trend in genetic levels (Fig. 6A).
The protein level of PI3K in all the groups had no
significant difference, but MI significantly decreased the level of
p-PI3K. Treatment with Leonurine significantly up-regulated the
phosphorylation of PI3K, accompanied with a significantly increased
ratio of p-PI3K/PI3K (Fig.
6B).
The protein level of AKT was significantly decreased
in the Leonurine group compared with the NS and sham group. The
protein level of p-AKT was decreased significantly in the NS group
compared with the sham group. After treatment with Leonurine, p-AKT
was significantly up-regulated, so as to significantly increase the
ratio of p-AKT/AKT in the Leonurine group (Fig. 6C).
The total protein level of GSK3β in the three groups
had no obvious difference. However the p-GSK3β was significantly
down-regulated in the NS group and significantly up-regulated in
response to Leonurine treatment. Moreover, the ratio of
p-GSK3β/GSK3β was significantly increased in the Leonurine group
compared to that of the NS group (Fig.
6D).
Caspase3 was significantly decreased in the NS group
and Leonurine group compared with the sham group. The
cleaved-caspase3 had been activated and cleaved in the NS group.
However treatment with Leonurine significantly reduced the
cleaved-caspase3 expression. Thus the ratio of
cleaved-caspase3/caspase3 is significantly decreased in Leonurine
group compared with that in NS group and sham group (Fig. 6E).
Discussion
The present study demonstrated that Leonurine
significantly decreased the stretch of the infarct zone, alleviated
the deposition of collagen, inhibited the apoptosis of
cardiomyocytes, prevented LV dilation and improved cardiac
function. These effects were due to that the Leonurine activated
PI3K/AKT/GSK3β signaling pathway and decreased the expression of
pro-apoptosis protein Bax and the activation of apoptosis marker
caspase3, and increased the expression of anti-apoptosis protein
Bcl-2.
With the feature that myocardium is terminally
differentiated tissue, it is critical to preserve cardiomyocyte
viability against ischemia diseases. The wide occurrences of cell
apoptosis in the ischemic heart, especially in the peri-infarct
size, largely hinder the recovery of cardiac function. Therefore,
activation of the anti-apoptosis signaling pathway and expression
of specialized anti-apoptotic proteins is crucial in preserving
cardiac function during ischemia.
The PI3K/AKT/GSK3β signaling pathway has been well
documented in mediating growth, differentiation, proliferation and
cell survival. In Yu-Shengyou' study, Dex induced anti-apoptotic
effects through activating PI3K/AKT and the downstream target GSK3β
against PAN-induced apoptosis in cultured podocytes (14).
PI3K, named phosphatidylinositol 3-kinase,
specifically catalyzes the phosphorylation of the hydroxyl group on
the 3 position of phosphatidylinositol (PI), forming 3,
4-diphosphate phosphatidylinositol and 3, 4, 5-triphosphate
phosphatidylinositol which are downstream messengers to activate
AKT (15). AKT is a member of
serine/threonine-protein kinases that contain SH2 (Src homology
2-like) domains and is commonly referred to as PKB.
PI3K/AKT is a critical signaling pathway mediating
survival, growth and apoptosis (16). In Hirokazu Ohashi' study, PI3K/AKT
induces the expression of surviving and decreases the activity of
Caspase-3 to inhibit the apoptotic effects induced by Angiotensin
II in microvascular endothelial cells (17). Moreover, EPO reduced myocardial
apoptosis and enhanced cardiac-protection under hypoxia and
ischemia conditions by activating the PI3K/AKT signaling pathway
(18,19). It is likely that in the Yizhun Zhu'
study, PI3K/AKT plays an important role in ischemia heart diseases
(2,11).
GSK3 belongs to the serine/threonine-protein kinase
family and consists of GSK3α and GSK3β (20,21).
GSK3β, a main downstream substrate of AKT, participates in the
PI3K/AKT pathway regulating glycogen and protein synthesis, cell
growth and anti-apoptosis (22,23).
Activated AKT can inhibit activation of GSK3β by phosphorylating
GSK3β at the Ser residue, and decrease the expression of Caspase3
as they induce anti-apoptosis effects (24,25).
AKT further facilitates the phosphorylation of GSK3β at the Ser
residue and p-GSK3β induces β-catenin phosphorylation to promote
cell survival (26). Wagner C
found that post-treatment rats model in vivo would obviously
decrease the infarct size through mediating PI3K/AKT to increase
the expression of p-GSk3β (Ser9) (27).
In this study, we observed a small decrease of PI3K
with a significant increase of p-PI3K in the Leonurine group
compared to the sham and NS group. Thus there was a significant
increase of the ratio of p-PI3K/PI3K after the treatment with
Leonurine for 28 days after MI.
To test whether AKT expression relevantly changed,
we investigated the level of AKT and p-AKT in the three groups. We
found that both p-AKT and the ratio of p-AKT/AKT were significantly
increased in the Leonurine group compared to the sham and NS group.
We also observed a similar change in p-GSK3β. There was increased
p-GSK3β expression and a higher ratio of p-GSK3β/GSK3β in the
Leonurine group compared to the sham and NS group. GSK3β further
activated the Bax family and Caspase family of apoptosis mediating
kinases (26,28).
Thus, we suggest that treatment with Leonurine
induces phosphorylation of PI3K, then the activated p-PI3K
facilitates downstream molecule AKT to be phosphorylated.
Subsequently, the p-AKT inhibits its downstream target protein
GSK3β. Ultimately the activated PI3K/AKT/GSK3β signaling axis
induces anti-apoptotic effect with the treatment of Leonurine in
rats 28 days after MI.
The Bcl-2 family is a potent regulator of apoptosis
pathways and it includes both anti-apoptotic proteins and
pro-apoptotic proteins. The ratio of Bcl-2 and Bax has been used as
a marker representing the apoptosis effect. In the present study,
we investigated the expression of Bcl-2 and Bax in both RNA level
and protein level. We found that Bcl-2/Bax at both levels in the
Leonurine group were significantly increased compared with that in
the NS group. This indicates Leonurine induced anti-apoptosis
effects by increasing the anti-apoptosis protein Bcl-2 and
decreasing the pro-apoptosis protein Bax.
Besides, caspase3 is a potential member of caspase
kinase family mediating cell apoptosis. Cleaved-caspase3 is able to
be induced by Bax and leads to apoptosis. As a result, it serves as
a marker for the extent of pro-apoptosis. In our study we also
exhibited that MI surgery caused significant apoptosis effect in NS
group with decreased caspase3 and largely increased
cleaved-caspase3 expression. However treatment with Leonurine was
able to reverse this phenomenon and significantly decreased the
expression of the activated form of caspase3 and decreased the
ration of cleaved caspase3/caspsase3 at the protein level. This
phenomenon was useful to alleviate the activation of the apoptosis
pathway and provide protective effects to myocardial myocytes.
Thus decreased infarct size and improved cardiac
function of rats are largely due to the activation of
PI3K/AKT/GSK3β signaling pathway and subsequent up-regulation of
anti-apoptosis proteins Bcl-2, and down-regulation of pro-apoptosis
protein Bax and cleaved-caspase3 caused by treatment of Leonurine.
Besides, several reports exhibited that Leonurine shows the effect
of anti-oxidation (29),
anti-inflammation (30),
anti-myocardial fibrotic response (12) and enhancing mitochondrial function
(31), which probably participate
in the cardio-protection in our study as well.
A limitation of the present study was that no
inhibitor of PI3K/AKT/GSK3β was set up to further validate the
signaling pathway mechanism because of insufficient Leonurine. The
lab will design relative experiments to further illustrate this
issue.
In conclusion, Leonurine induced cardiac protection
effects in vivo in MI by activating the PI3K/AKT/GSK3β
signaling pathway, which promoted the expression of anti-apoptosis
proteins, decreased the expression of pro-apoptosis proteins and
inhibits the activity of cleaved-caspase3. It indicates that
Leonurine represents a promising drug in treating ischemic heart
diseases.
Acknowledgements
Not applicable.
Funding
The experimental performance is supported by
National Nature Science Foundation of China; contract grant no.
81100130; Specialized Research Fund for the Doctoral Program of
Higher Education; contract grant no. 20120141110013; National Key
Basic Research Program of China; contract grant no. 81370283;
2005CB623903.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX, HLZ and XJJ designed the experiments. HLZ and FW
performed the experiments. LX and HLZ analyzed the data. LX, HLZ
and XJJ wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Renmin Hospital of Wuhan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PI3K
|
phosphoinositide 3-kinase
|
|
GSK3β
|
glycogen synthase kinase-3β
|
References
|
1
|
Zhu YZ, Chong CL, Chuah SC, Huang SH, Nai
HS, Tong HT, Whiteman M and Moore PK: Cardioprotective effects of
nitroparacetamol and paracetamol in acute phase of myocardial
infarction in experimental rats. Am J Physiol Heart Circ Physiol.
290:H517–H524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu C, Guo W, Maerz S, Gu X and Zhu Y:
3,5-Dimethoxy-4-(3-(2-carbonyl-ethyldisulfanyl)-propionyl)-benzoic
acid 4-guanidino-butyl ester: A novel twin drug that prevents
primary cardiac myocytes from hypoxia-induced apoptosis. Eur J
Pharmacol. 700:118–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallogly MM, Shelton MD, Qanungo S, Pai
HV, Starke DW, Hoppel CL, Lesnefsky EJ and Mieyal JJ: Glutaredoxin
regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2
and Bcl-xL: Implications for cardiac aging. Antioxid Redox Signal.
12:1339–1353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee Y and Gustafsson AB: Role of apoptosis
in cardiovascular disease. Apoptosis. 14:536–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haunstetter A and Izumo S: Toward
antiapoptosis as a new treatment modality. Circ Res. 86:371–376.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XH, Xin H and Zhu YZ: More than a
‘mother-benefiting’ herb: Cardioprotective effect of Herba leonuri.
Sheng Li Xue Bao. 59:578–584. 2007.PubMed/NCBI
|
|
7
|
Kuang PG, Zhou XF, Zhang FY and Lang SY:
Motherwort and cerebral ischemia. J Tradit Chin Med. 8:37–40.
1988.PubMed/NCBI
|
|
8
|
Wang ZS, Li DW, Xia WJ, Qiu HQ and Zhu LY:
The therapeutic effect of herba leonuri in the treatment of
coronary myocardial ischemia. J Tradit Chin Med. 8:103–106.
1988.PubMed/NCBI
|
|
9
|
Liu XH, Xin H, Hou AJ and Zhu YZ:
Protective effects of leonurine in neonatal rat hypoxic
cardiomyocytes and rat infarcted heart. Clin Exp Pharmacol Physiol.
36:696–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XH, Chen PF, Pan LL, Silva RD and Zhu
YZ: 4-Guanidino-n-butyl syringate (Leonurine, SCM 198) protects
H9c2 rat ventricular cells from hypoxia-induced apoptosis. J
Cardiovasc Pharmacol. 54:437–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Pan L, Gong Q and Zhu Y: Leonurine
(SCM-198) improves cardiac recovery in rat during chronic
infarction. Eur J Pharmacol. 649:236–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu XH, Pan LL, Deng HY, Xiong QH, Wu D,
Huang GY, Gong QH and Zhu YZ: Leonurine (SCM-198) attenuates
myocardial fibrotic response via inhibition of NADPH oxidase 4.
Free Radic Biol Med. 54:93–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Zhang X, Du Y, Ji H, Li S, Li L,
Xing Y, Zhang X, Dong L, Wang C, et al: Leonurine protects brain
injury by increased activities of UCP4, SOD, CAT and Bcl-2,
decreased levels of MDA and Bax, and ameliorated ultrastructure of
mitochondria in experimental stroke. Brain Res. 1474:73–81. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu-Shengyou and LI Y: Dexamethasone
inhibits podocyte apoptosis by stabilizing the PI3K/Akt signal
pathway. Biomed Res Int. 2013:3269862013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vara Fresno JA, Casado E, De Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohashi H, Takagi H, Oh H, Suzuma K, Suzuma
I, Miyamoto N, Uemura A, Watanabe D, Murakami T, Sugaya T, et al:
Phosphatidylinositol 3-kinase/Akt regulates angiotensin II-induced
inhibition of apoptosis in microvascular endothelial cells by
governing survivin expression and suppression of caspase-3
activity. Circ Res. 94:785–793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tramontano AF, Muniyappar R, Black AD,
Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV and El-Sherif N:
Erythropoietin protects cardiac myocytes from hypoxia-induced
apoptosis through an Akt-dependent pathway. Biochem Biophys Res
Commun. 308:990–994. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai Z and Semenza GL:
Phosphatidylinositol-3-kinase signaling is required for
erythropoietin-mediated acute protection against myocardial
ischemia/reperfusion injury. Circulation. 109:2050–2053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rayasam GV, Tulasi VK, Sodhi R, Davis JA
and Ray A: Glycogen synthase kinase 3: More than a namesake. Br J
Pharmacol. 156:885–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maixner DW and Weng HR: The role of
glycogen synthase kinase 3 beta in neuroinflammation and pain. J
Pharm Pharmacol (Los Angel). 1:0012013.PubMed/NCBI
|
|
22
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Descamps S, Pawlowski V, Révillion F,
Hornez L, Hebbar M, Boilly B, Hondermarck H and Peyrat JP:
Expression of nerve growth factor receptors and their prognostic
value in human breast cancer. Cancer Res. 61:4337–4340.
2001.PubMed/NCBI
|
|
24
|
Morisco C, Zebrowski D, Condorelli G,
Tsichlis P, Vatner SF and Sadoshima J: The Akt-glycogen synthase
kinase 3beta pathway regulates transcription of atrial natriuretic
factor induced by beta-adrenergic receptor stimulation in cardiac
myocytes. J Biol Chem. 275:14466–14475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Badorff C, Ruetten H, Mueller S, Stahmer
M, Gehring D, Jung F, Ihling C, Zeiher AM and Dimmeler S: Fas
receptor signaling inhibits glycogen synthase kinase 3 beta and
induces cardiac hypertrophy following pressure overload. J Clin
Invest. 109:373–381. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong R, Rifai A and Dworkin LD: Activation
of PI3K-Akt-GSK3beta pathway mediates hepatocyte growth factor
inhibition of RANTES expression in renal tubular epithelial cells.
Biochem Biophys Res Commun. 330:27–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagner C, Tillack D, Simonis G, Strasser
RH and Weinbrenner C: Ischemic post-conditioning reduces infarct
size of the in vivo rat heart: Role of PI3-K, mTOR, GSK-3beta and
apoptosis. Mol Cell Biochem. 339:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Somervaille TC, Linch DC and Khwaja A:
Growth factor withdrawal from primary human erythroid progenitors
induces apoptosis through a pathway involving glycogen synthase
kinase-3 and Bax. Blood. 98:1374–1381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J, Huang SH, Zhu YC, Whiteman M, Wang
MJ, Tan BK and Zhu YZ: Anti-oxidative stress effects of Herba
leonuri on ischemic rat hearts. Life Sci. 76:3043–3056. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Pan L, Wang X, Gong Q and Zhu YZ:
Leonurine protects against tumor necrosis factor-α-mediated
inflammation in human umbilical vein endothelial cells.
Atherosclerosis. 222:34–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loh KP, Qi J, Tan BK, Liu XH, Wei BG and
Zhu YZ: Leonurine protects middle cerebral artery occluded rats
through antioxidant effect and regulation of mitochondrial
function. Stroke. 41:2661–2668. 2010. View Article : Google Scholar : PubMed/NCBI
|