Introduction
Osteosarcoma (OS) is the most common and aggressive
bona tumor that usually occurs among adolescents and young people
(1). OS gives rise to many
cancer-related deaths worldwide every year (2). Surgery combined with chemotherapeutic
treatment is the major strategy for OS intervention (3). Despite extensive advances achieved on
OS therapy in recent years, the five-year survival rate of OS
patients is quite poor (4). Thus,
there is an urgent need to understand the underlying mechanism
involved in OS development and progression, which is crucial for
the development of effectively therapeutic targets.
Long noncoding RNAs (lncRNAs) is a subgroup of
noncoding RNAs that possess a length of more than 200 nucleotides
(5,6). In the past decade, lncRNAs have
become a very hot topic in the field of biology. More and more
evidences indicate that lncRNAs are involved in almost all
physiological processes, such as immune, development and
tumorigenesis (6–8). Due to the important functions of
lncRNAs on cell proliferation, migration and invasion (9), lncRNA aberrant expression is closely
correlated with the development of human cancers, including
cholangiocarcinoma (10),
pancreatic cancer (11), breast
cancer (12), gastric cancer
(13), hepatocellular carcinoma
(14) and OS (8). Furthermore, several reports indicate
that lncRNAs may serve as biomarkers for cancer diagnosis or
prognosis (15). Therefore,
understanding the functions and mechanism of lncRNAs is critical
for cancer therapy.
LncRNA AFAP1-AS1 has been demonstrated to promote
the development or progression of some cancers, such as thyroid
cancer (16) and tongue squamous
cell carcinoma (17). Whether
AFAP1-AS1 has a role in OS progression remains unclear. In the
present study, we found that AFAP1-AS1 expression was significantly
upregulated in OS tissues and cell lines. AFAP1-AS1 high expression
is linked to OS patients' poor prognosis. Moreover, we showed that
AFAP1-AS1 knockdown significantly suppressed the proliferation and
invasion of OS cells. Mechanistically, we found that AFAP1-AS1
sponged microRNA (miR)-4695-5p to upregulate the expression of
transcription factor (TCF)4, which is a pivot transcription factor
in Wnt/β-catenin pathway. Taken together, our results demonstrated
that AFAP1-AS1/miR-4695-5p/TCF4/β-catenin signaling cascade was
involved in OS progression.
Materials and methods
Clinical specimens
A total of 49 samples of OS tissues and adjacent
normal tissues were obtained from patients who underwent surgery at
The First Affiliated Hospital of Jiamusi University (Jiamusi,
China). Two pathologists independently evaluated the histological
diagnosis and differentiation of the tissue samples, according to
the World Health Organization classification system. All of the
tissue samples collected were immediately snap-frozen in liquid
nitrogen and stored at −80°C until required. The present study was
approved by the Research Ethics Committee of The First Affiliated
Hospital of Jiamusi University, and informed consent was obtained
from all patients.
Cell culture and transfection
Normal human osteoblasts, hFOB 1.19, and four human
OS cell lines, Saos-2, U2OS, MG-63 and 143B, were purchased from
American Type Culture Collection (Manassas, VA, USA). Cell lines
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 100 units/ml of
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified incubator containing 5%
CO2.
A siRNA against AFAP1-AS1 (named as si-AFAP1-AS1),
corresponding scramble negative control (NC) (named as scramble),
miR-4695-5p mimics, mimic NCs were designed and constructed by
Ribobio (Guangzhou, China). The target sequence was as followed:
si-AFAP1-AS1: 5′-AGGACACAGACUGCUUCAU-3′; and scramble:
5′-UUCUCCGAACGUGACGUTT-3′. AFAP1-AS1 and TCF4 overexpression
plasmids (pCDNA3.1-AFAP1-AS1 or pCDNA3.1-TCF4) were cloned into
pCDNA3.1 Vector. The sequences were determined by DNA sequencing.
These plasmids were transfected into OS cells in 6-well plates at a
final concentration of 100 ng using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Cell proliferation assay
For cell proliferation assays, the viable cells were
tested by Cell Counting Kit-8 (CCK-8) assay kit according to the
manufacturer's instructions. In brief, cells were grown in 96-well
plate with 1×104 per well and incubated in 37°C with 5%
CO2 until cell confluent rate reached 70%. After
transfected with plasmid for 48 h, cells were still incubated for
24, 48 and 72 h. 10 µl CCK-8 solution was seed into each well. The
absorbance at 450 nm was measured with SUNRISE Microplate Reader
(Tecan Group, Ltd., Mannedorf, Switzerland).
Transwell invasion assay
Cell invasion was assayed using a Transwell invasion
assay (invasion Transwell chambers; EMD Millipore, Billerica, MA,
USA) with Matrigel™ (BD Biosciences). The cells were seeded at a
density of 1×105 cells on the upper chamber with 200 µl
serum-free DMEM. Following transfection for 48 h, 600 µl DMEM
supplemented with 20% FBS, which served as a chemoattractant, was
added to the lower chamber. After 48 h incubation at 37°C, the
upper side of the membrane was wiped with cotton wool to remove
non-invasive cells; the membranes were then fixed with 4% methanol
at 4°C and stained with 0.1% crystal violet at room temperature.
Five visual fields with a magnification ×200 were randomly selected
from each membrane and the cell numbers were counted using a light
microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated using Trizol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufactures'
protocol. The first strand cDNA was compounded using a Tianscript
RT kit (Tiangen Biotech, Beijing, China). PCR amplification was
performed using TaqMan Human MicroRNA Assay (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and UltraSYBR Mixture (CW0957;
CWbio, Beijing, China) in LC 480 PCR System (Roche Diagnostics,
Indianapolis, IN, USA). U6 and 18S was employed as reference genes
to normalize the expression of miR-4695-5p or AFAP1-AS1 and TCF4.
The primers used were synthesized and purchased from Genecopoeia
(Guangzhou, China). Relative expression level was analyzed on the
basis of the 2−ΔΔCq method (18).
Tumor xenograft
4-6-weeks old female BALB/c nude mice were used for
all in vivo xenograft studies. All animal experiments were
approved by the Animal Care and Use Committee of The First
Affiliated Hospital of Jiamusi University. Cells were injected
subcutaneously into the flanks of nude mice (2×106 cells
per animal). All mice were sacrificed 35 days after seeding of
tumor cells, and the tumor weights measured.
Luciferase assays
Wild-type (WT) or mutant (Mut) fragment (801–1,660
bp) of AFAP1-AS1 containing the predicted miR-4695-5p binding sites
were designed, constructed and cloned by Sangon Biotech (Shanghai,
China) downstream of the firefly luciferase gene in the
pGL3-control vector (Promega Corporation, Madison, WI, USA) to form
pGL3-AFAP1-AS1-WT and pGL3-AFAP1-AS1-Mut, respectively.
For the luciferase reporter assays, OS cells were
seeded into 12-well plate cells and co-ransfected with 300 ng of
pGL3-AFAP1-AS1-WT or pGL3-AFAP1-AS1-Mut and 50 nM of miR-4695-5p or
NC using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). Cells were collected 48 h after transfection, and luciferase
activity was measured using a dual-luciferase reporter assay system
(Promega Corporation). The firefly luciferase activity of each
sample was normalized to the Renilla luciferase activity of each
sample.
Statistical analysis
Each experiment was repeated at least three times.
All data are expressed in terms of mean ± standard deviation. The
Kaplan-Meier method was used to calculate the survival curve, and
log-rank test to determine statistical significance. Student's
t-test and one-way ANOVA followed by Tukey's post hoc test were
used to analyze 2 or multiple groups, respectively, for statistical
significance. Pearson correlation coefficient analysis was used to
determine the correlations. P<0.05 was considered to indicate a
statistically significant difference.
Results
AFAP1-AS1 is highly expressed in OS
tissues
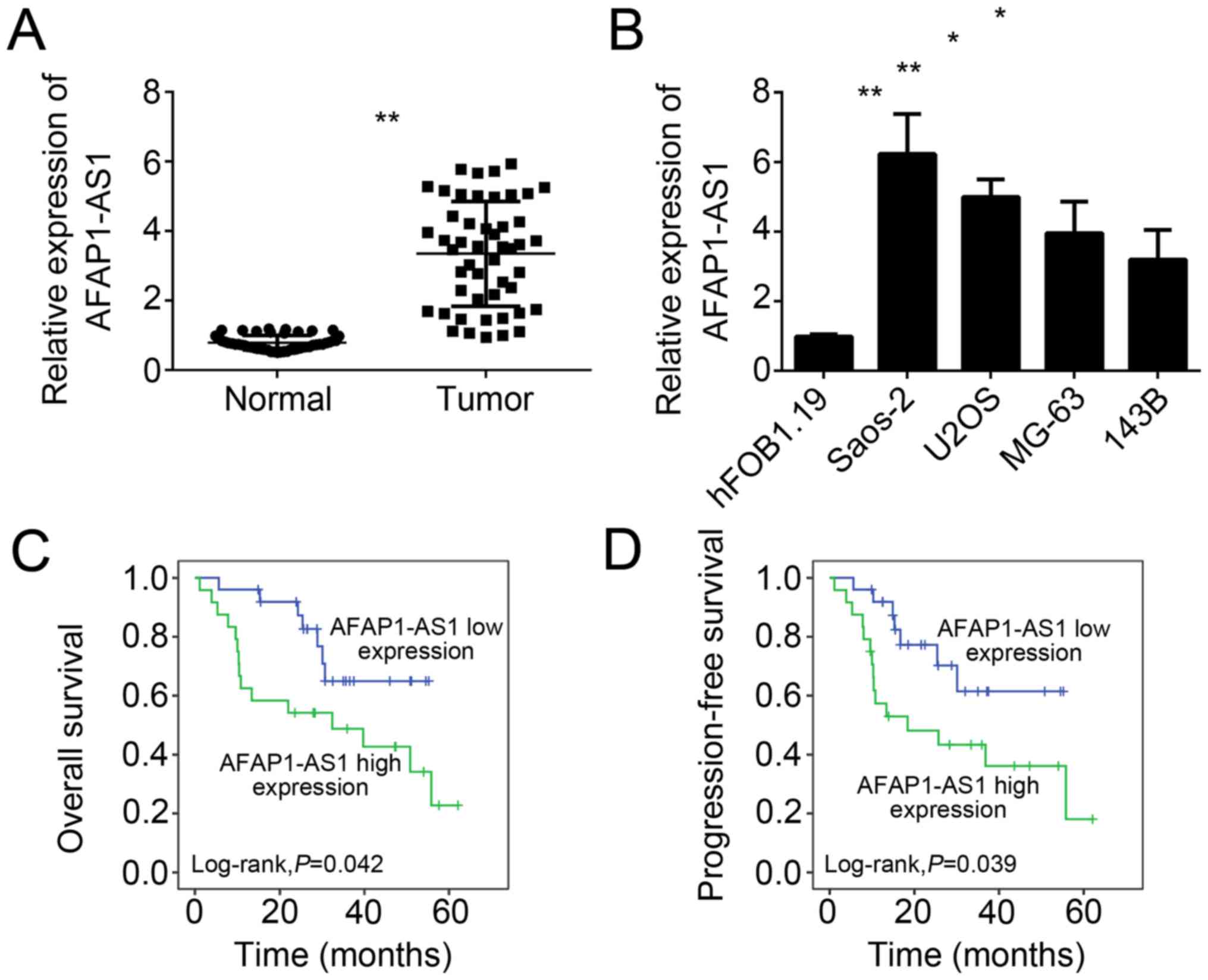
Firstly, we analyzed the expression patterns of
AFAP1-AS1 in OS tissues by RT-qPCR. We examined the levels of
AFAP1-AS1 in 49 OS tissues and paired adjacent normal tissues, and
found that the expression of AFAP1-AS1 was significantly
upregulated in OS tissues compared to adjacent normal tissues
(Fig. 1A). Consistently, the
expression of AFAP1-AS1 was also upregulated in OS cell lines,
including Saos-2, U2OS, MG-63 and 143B cells, compared with
hFOB1.19 cells (Fig. 1B). To
analyze whether AFAP1-AS1 could serve as a prognostic marker for OS
patients, we performed Kaplan-Meier survival curve analysis, and
found that higher expression of AFAP1-AS1 in OS patients showed
lower overall and progression-free survival rates (Fig. 1C and D).
AFAP1-AS1 depletion suppresses the
proliferation and invasion of OS cells
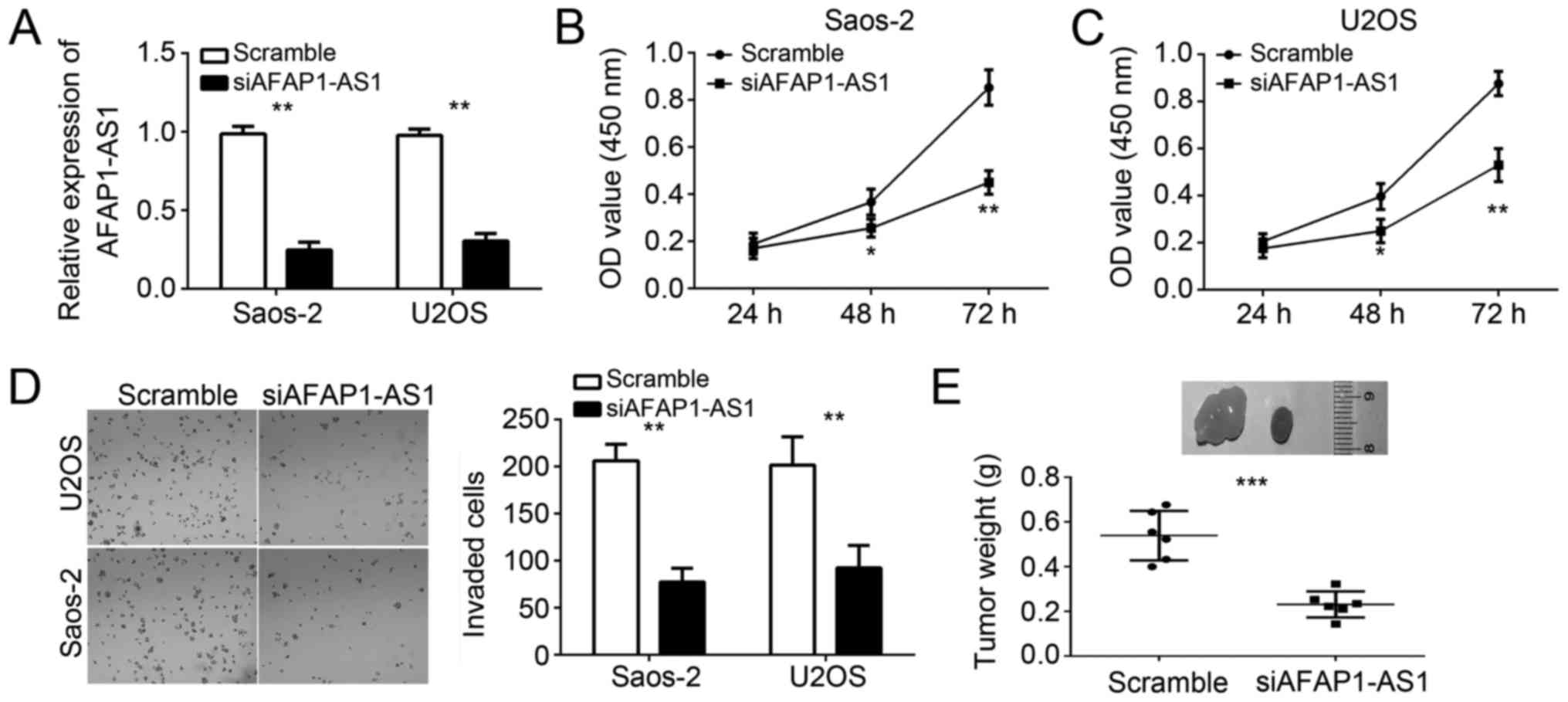
To explore the function of AFAP1-AS1 in OS cells, we
silenced AFAP1-AS1 in Saos-2 and U2OS cells. RT-qPCR analysis
indicated that AFAP1-AS1 expression was significantly downregulated
in Saos-2 and U2OS cells transfected with siAFAP1-AS1 (Fig. 2A). Then CCK-8 assays were used to
analyze the effect of AFAP1-AS1 knockdown on cell proliferation. As
shown, knockdown of AFAP1-AS1 significantly inhibited the
proliferation of Saos-2 and U2OS cells (Fig. 2B and C). Due to the correlation
between cancer cell metastasis and tumor malignance, we then
determined the effect of AFAP1-AS1 on tumor cell metastasis.
Through transwell invasion assays, we found that AFAP1-AS1
knockdown significantly reduced the numbers of invaded Saos-2 and
U2OS cells (Fig. 2D). To further
determine the effect of AFAP1-AS1 on tumor growth in vivo,
we conducted a xenograft experiment. We injected AFAP1-AS1-silenced
or control Saos-2 cells into nude recipient mice. After 5 weeks, we
sacrificed these mice and measured tumor weights. The results
indicated that knockdown of AFAP1-AS1 significantly decreased the
tumor size (Fig. 2E). Taken
together, our results demonstrated that AFAP1-AS1 serves as an
oncogene to regulate OS cell proliferation and invasion.
AFAP1-AS1 suppresses the expression of
miR-4695-5p in OS cells
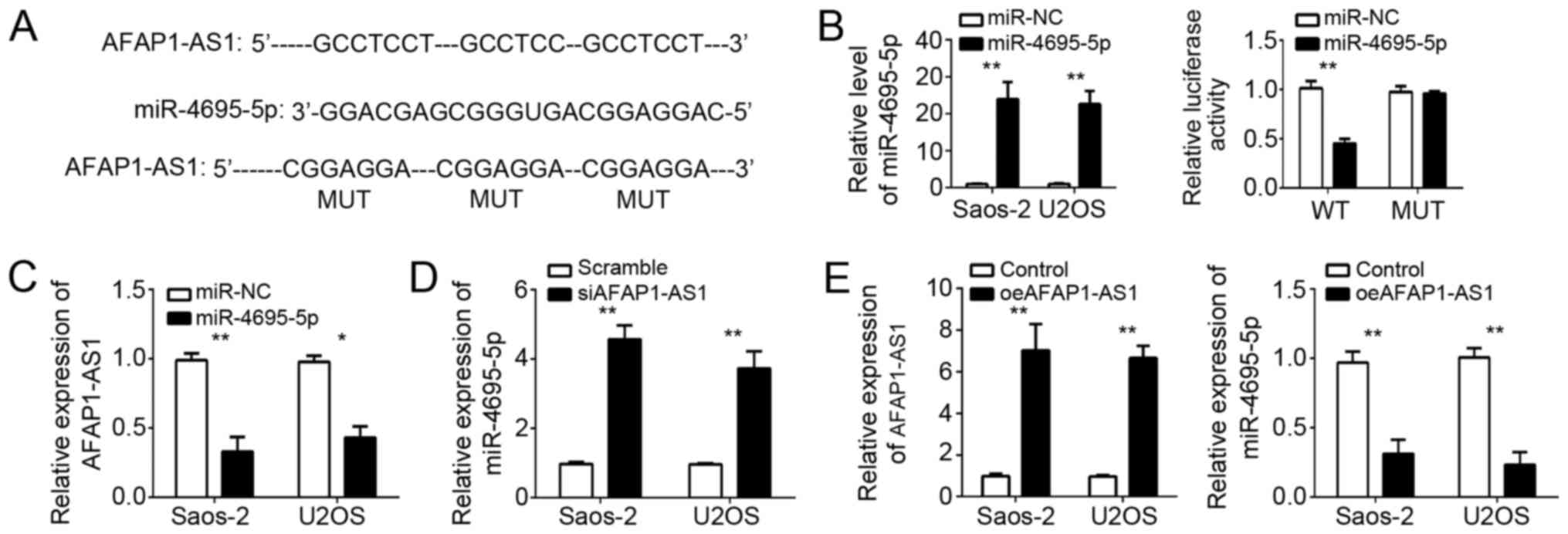
LncRNAs have been shown to sponge miRNAs and
regulate gene expression. In order to determine the mechanism of
AFAP1-AS1 in OS cells, we explored the target miRNAs. Through
bioinformatics analysis, we found that miR-4695-5p was the most
potential candidate. We found that there were three potential
binding sites of miR-4695-5p in AFAP1-AS1 (Fig. 3A). To verify their interaction, we
conducted luciferase reporter assays using WT or Mut AFAP1-AS1
reporter plasmid. We first confirmed the overexpression of
miR-4695-5p in Saos-2 and U2OS cells after transfection (Fig. 3B). Furthermore, overexpression of
miR-4695-5p significantly inhibited the luciferase activity in
Saos-2 cells transfected with WT-AFAP1-AS1, whereas mutation of
these binding sites abrogated this effect (Fig. 3B). Furthermore, RT-qPCR analysis
indicated that overexpression of miR-4695-5p markedly suppressed
the expression of AFAP1-AS1 in Saos-2 and U2OS cells (Fig. 3C). In addition, we found that
knockdown of AFAP1-AS1 also promoted the levels of miR-4695-5p in
Saos-2 and U2OS cells (Fig. 3D).
Then we overexpressed AFAP1-AS1 and overexpression of AFAP1-AS1
reduced the expression of miR-4695-5p in Saos-2 and U2OS cells
(Fig. 3E). Summarily, above data
indicated that AFAP1-AS1 sponged miR-4695-5p to regulate its
expression in OS cells.
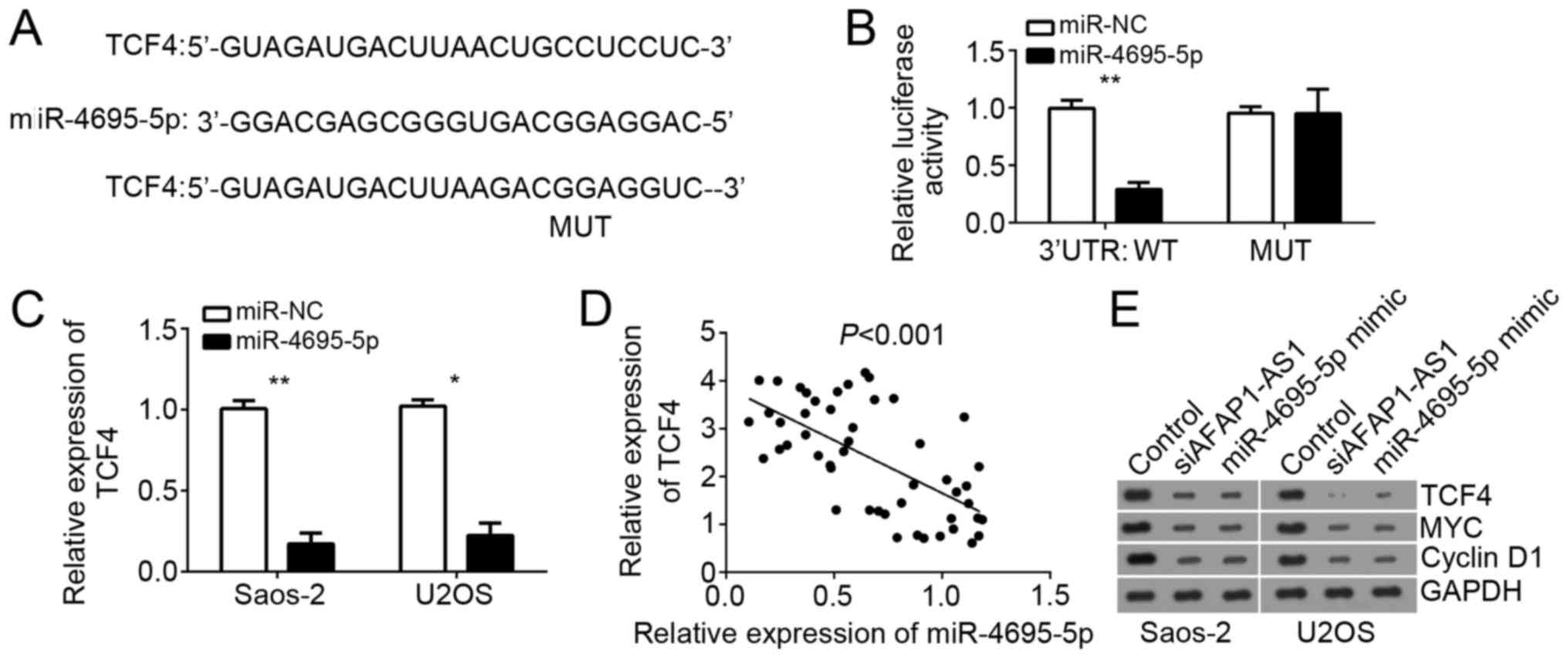
TCF4 is a direct target of miR-4695-5p
in OS cells
To further explore the downstream mechanism of
miR-4695-5p, we searched the target genes of miR-4695-5p. Using
TargetScan website, we found that TCF4 was one of potential target
genes of miR-4695-5p in OS cells. TCF4 is a pivot transcription
factor of Wnt/β-catenin pathway and involved in tumor progression
in various cancers. Therefore, we chose TCF4 for further
investigation. We also found that there was a potential binding
site of miR-4695-5p in the 3′-URT region of TCF4 mRNA (Fig. 4A). Luciferase reporter assays
indicated that overexpression of miR-4695-5p significantly
repressed the luciferase activity in Saos-2 cells transfected with
WT-TCF4-3′-UTR (Fig. 4B). Besides,
RT-qPCR showed that overexpression of miR-4695-5p inhibited the
mRNA levels of TCF4 in Saos-2 and U2OS cells (Fig. 4C). Moreover, there was a reversely
correlation between the expression of miR-4695-5p and TCF4 in OS
tissues (Fig. 4D). Then we
performed western blot to analyze the activation of Wnt/β-catenin
pathway. Cyclin D1 and MYC are two classical target genes of
Wnt/β-catenin pathway. Therefore, we chose them as markers for
evaluation of Wnt/β-catenin pathway activation. The results
indicated that both overexpression of miR-4695-5p and AFAP1-AS1
knockdown inhibited the protein levels of TCF4, Cyclin D1 and MYC
in Saos-2 and U2OS cells (Fig.
4E). Taken together, our results indicated that miR-4695-5p and
AFAP1-AS1 regulated TCF4 expression and Wnt/β-catenin pathway
activation in OS cells.
AFAP1-AS1 promotes OS cell
proliferation and invasion by regulating activation of
TCF4/β-catenin pathway
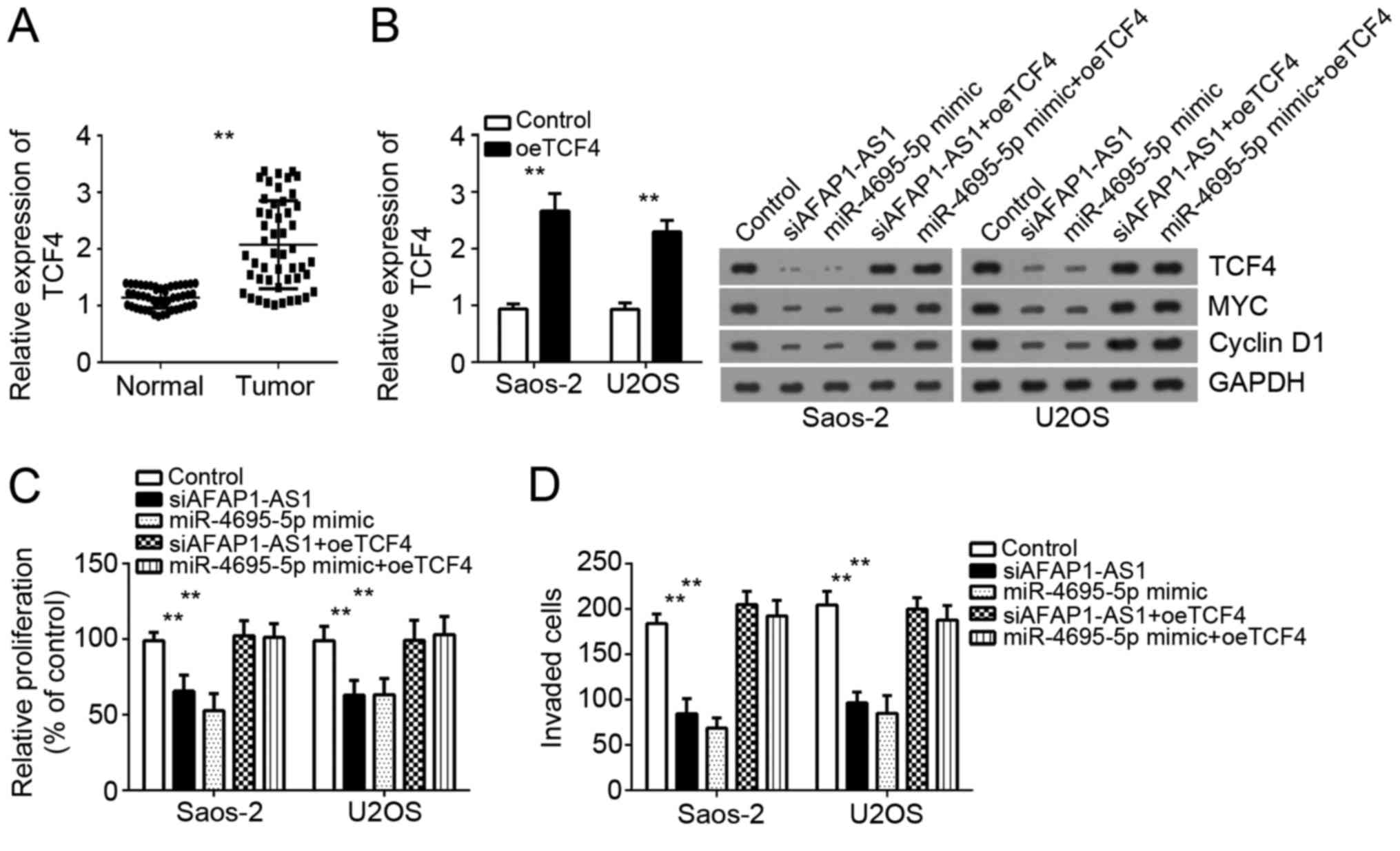
Then we analyzed the expression of TCF4 in OS
tissues and found that TCF4 expression was significantly
upregulated in OS tissues compared with adjacent normal tissues
(Fig. 5A). To further confirm that
TCF4 is indispensable for the role of AFAP1-AS1 in OS cells, we
restored the protein level of TCF4 in Saos2 and U2OS cells
transfected with siAFAP1-AS1 or miR-4695-5p mimics. RT-qPCR and
Western blot showed that the expression of TCF4 was significantly
upregulated in these two cells (Fig.
5B). Moreover, CCK-8 and transwell invasion assays indicated
that miR-4695-5p overexpression or AFAP1-AS1 knockdown inhibited
cell proliferation and invasion, while restoration of TCF4
significantly rescued the proliferation and invasion of Saos-2 and
U2OS cells (Fig. 5C and D). In
conclusion, these data indicated that AFAP1-AS1 promoted OS cell
proliferation and invasion by regulating miR-4695-5p/TCF4/β-catenin
signaling.
Discussion
LncRNA AFAP1-AS1 has been demonstrated to be
overexpressed and act as an oncogene in several cancers (16,17).
For instance, Han et al reported that AFAP1-AS1 is
overexpressed in colorectal cancer, and promotes tumor growth and
metastasis (19). Zhang et
al reported that upregulated AFAP1-AS1 expression promotes
hepatocellular carcinoma cell proliferation and invasion, and
predicts a poor prognosis (20).
In addition, Deng et al showed that AFAP1-AS1 overexpression
is associated with a poor prognosis in NSCLC patients (21). Nevertheless, the function of
AFAP1-AS1 in OS remains largely unknown. In this study, we found
that AFAP1-AS1 was significantly upregulated in OS tissues and cell
lines. Moreover, AFAP1-AS1 overexpression predicted a poor
prognosis in OS patients. Furthermore, functional experiments
indicated that loss of AFAP1-AS1 expression led to reduced
proliferation and invasion of OS cells in vitro. We also
showed that AFAP1-AS1 knockdown resulted in delayed tumor growth
in vivo. Taken together, our data indicated that AFAP1-AS1
functions as an oncogene and might be involved in OS development
and progression.
Accumulating evidences indicate that lncRNAs could
regulate gene expression through various mechanisms, including RNA
decay, chromatin remodeling and miRNA sponging (22,23).
It was reported that AFAP1-AS1 regulates PTEN/AKT pathway to
enhance gastric cell proliferation (23). Wang et al reported
Upregulation of AFAP1-AS1 promotes the proliferation, invasion and
survival of tongue squamous cell carcinoma cell by activation of
Wnt/β-catenin signaling pathway (17). However, how AFAP1-AS1 activates
these signaling pathway remains unclear. And the underlying
molecular mechanism of AFAP1-AS1 in cancer cells requires to be
investigated. In this study, our results indicated that AFAP1-AS1
could directly associate with miR-4695-5p to serve as a competing
endogenous RNA. Though luciferase reporter assays and RT-qPCR
analysis, we demonstrated their direct interaction. miRs have been
widely acknowledged as essential regulators in human cancers,
including OS (24). miRs could
bind to the complementary sequence in the 3′-UTR region of target
mRNAs and regulate gene expression (25). Until now, the function of
miR-4695-5p remains totally unclear. Therefore, to determine the
role of miR-4695-5p in OS and whether AFAP1-AS1 promoted OS cell
proliferation and invasion by sponging miR-4695-5p, we further
investigated the downstream mechanism of miR-4695-5p. Through
bioinformatics analysis and functional experiments, we demonstrated
TCF4 was a direct target of miR-4695-5p in OS cells, which
suggested that miR-4695-5p might act as a tumor suppressor via
targeting TCF4.
After initiation of Wnt/β-catenin signaling,
β-catenin translocates to the nucleus and binds to TCF4, which
leads to transcriptional activation of downstream oncogenic genes,
such as MYC and Cyclin D1, by β-catenin-TCF4 complex (26). Aberrant activation of this
signaling has been observed in many cancers, including colorectal
cancer (27), esophageal squamous
cell carcinoma (28), clear cell
renal cell carcinoma (29) and
lung cancer (30). The knowledge
about how TCF4 expression is regulated is limited. In our study, we
demonstrated AFAP1-AS1/miR-4695-5p axis regulated the expression of
TCF4 and consequently influenced the activation of Wnt/β-catenin
pathway in OS cells. Moreover, we showed that knockdown of
AFAP1-AS1 or miR-4695-5p overexpression suppresses OS cell
proliferation and invasion. However, restoration of TCF4 protein
levels significantly rescued the proliferation and invasion of OS
cells transfected with siAFAP1-AS1 or miR-4695-5p mimics. These
data suggested that upregulation of TCF4 by AFAP1-AS1/miR-4695-5p
axis promoted OS progression.
In conclusion, our findings demonstrate that
AFAP1-AS1 is overexpressed in OS tissues and associated with poor
prognosis of OS patients. AFAP1-AS1 promotes the proliferation and
invasion of OS cells through inhibition of miR-4695-5p and
activation of TCF4-β-catenin signaling. Our data elucidated a
potential mechanism underlying the tumor-oncogenic role of
AFAP1-AS1 in OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RL and SL initiated, designed this work, analyzed,
interpreted the results and wrote this manuscript. YL, QT, YX and
RZ performed certain experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for this
study was approved by the Institutional Ethics Committee of The
First Affiliated Hospital of Jiamusi University and all enrolled
patients signed a written informed consent document. In addition,
all procedures involving animals conformed to the national
guidelines of and were approved by the Animal Care Ethics Committee
of The First Affiliated Hospital of Jiamusi University.
Consent for publication
All patients within this study provide consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, Greenfield EM and Getty PJ: Osteosarcoma. J Am Acad Orthop
Surg. 17:515–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu W, Tang L, Lin F, Yao Y, Shen Z and
Zhou X: High-intensity focused ultrasound: noninvasive treatment
for local unresectable recurrence of osteosarcoma. Surg Oncol.
24:9–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bacci G, Ferrari S, Bertoni F, Ruggieri P,
Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M and
Campanacci M: Long-term outcome for patients with nonmetastatic
osteosarcoma of the extremity treated at the istituto ortopedico
rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2
protocol: An updated report. J Clin Oncol. 18:4016–4027. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye B, Liu B, Yang L, Zhu X, Zhang D, Wu W,
Zhu P, Wang Y, Wang S, Xia P, et al: LncKdm2b controls self-renewal
of embryonic stem cells via activating expression of transcription
factor Zbtb3. EMBO J. 37:pii: e97174. 2018. View Article : Google Scholar
|
|
7
|
Liu B, Ye B, Yang L, Zhu X, Huang G, Zhu
P, Du Y, Wu J, Qin X, Chen R, et al: Long noncoding RNA lncKdm2b is
required for ILC3 maintenance by initiation of Zfp292 expression.
Nat Immunol. 18:499–508. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang C, Wu K, Wang S and Wei G: Long
non-coding RNA XIST promotes osteosarcoma progression by targeting
YAP via miR-195-5p. J Cell Biochem. Jan 31–2018.(Epub ahead of
print). View Article : Google Scholar :
|
|
9
|
Dong X, Chen R, Lin H, Lin T and Pan S:
lncRNA BG981369 inhibits cell proliferation, migration, and
invasion, and promotes cell apoptosis by SRY-related high-mobility
group box 4 (SOX4) signaling pathway in human gastric cancer. Med
Sci Monit. 24:718–726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai JG, Tang RF, Shang JF, Qi S, Yu GD and
Sun C: Upregulation of long non-coding RNA CCAT2 indicates a poor
prognosis and promotes proliferation and metastasis in intrahepatic
cholangiocarcinoma. Mol Med Rep. 17:5328–5335. 2018.PubMed/NCBI
|
|
11
|
Cheng Y, Imanirad P, Jutooru I, Hedrick E,
Jin UH, Hoffman Rodrigues A, de Leal Araujo J, Morpurgo B, Golovko
A and Safe S: Role of metastasis-associated lung adenocarcinoma
transcript-1 (MALAT-1) in pancreatic cancer. PLoS One.
13:e01922642018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang P, Zhou H, Lu K, Lu Y, Wang Y and
Feng T: Exosome-mediated delivery of MALAT1 induces cell
proliferation in breast cancer. Onco Targets Ther. 11:291–299.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Chen Z, Yu S, Nie F, Yan S, Ma P,
Chen Q, Wei C, Fu H, Xu T, et al: Long noncoding RNA LINC01234
functions as a competing endogenous RNA to regulate CBFB expression
by sponging miR-204-5p in gastric cancer. Clin Cancer Res.
24:2002–2014. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu P, Wang Y, Wu J, Huang G, Liu B, Ye B,
Du Y, Gao G, Tian Y, He L and Fan Z: LncBRM initiates YAP1
signalling activation to drive self-renewal of liver cancer stem
cells. Nat Commun. 7:136082016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ
and Sun Q: Long noncoding RNA SNHG7 promotes the progression and
growth of glioblastoma via inhibition of miR-5095. Biochem Biophys
Res Commun. 496:712–718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai W, Tian Y, Jiang B and Chen W:
Down-regulation of long non-coding RNA AFAP1-AS1 inhibits tumor
growth, promotes apoptosis and decreases metastasis in thyroid
cancer. Biomed Pharmacother. 99:191–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZY, Hu M, Dai MH, Xiong J, Zhang S,
Wu HJ, Zhang SS and Gong ZJ: Upregulation of the long non-coding
RNA AFAP1-AS1 affects the proliferation, invasion and survival of
tongue squamous cell carcinoma via the Wnt/β-catenin signaling
pathway. Mol Cancer. 17:32018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han X, Wang L, Ning Y, Li S and Wang Z:
Long non-coding RNA AFAP1-AS1 facilitates tumor growth and promotes
metastasis in colorectal cancer. Biol Res. 49:362016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang JY, Weng MZ, Song FB, Xu YG, Liu Q,
Wu JY, Qin J, Jin T and Xu JM: Long noncoding RNA AFAP1-AS1
indicates a poor prognosis of hepatocellular carcinoma and promotes
cell proliferation and invasion via upregulation of the RhoA/Rac2
signaling. Int J Oncol. 48:1590–1598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng J, Liang Y, Liu C, He S and Wang S:
The up-regulation of long non-coding RNA AFAP1-AS1 is associated
with the poor prognosis of NSCLC patients. Biomed Pharmacother.
75:8–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu P, Wang Y, Huang G, Ye B, Liu B, Wu J,
Du Y, He L and Fan Z: lnc-β-Catm elicits EZH2-dependent β-catenin
stabilization and sustains liver CSC self-renewal. Nat Struct Mol
Biol. 23:631–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin T, Ma Q, Zhang Y, Zhang H, Yan J and
Gao C: MicroRNA-27a functions as an oncogene in human osteosarcoma
by targeting CCNG1. Oncol Lett. 15:1067–1071. 2018.PubMed/NCBI
|
|
25
|
Han C and Wang W: MicroRNA-129-5p
suppresses cell proliferation, migration and invasion via targeting
ROCK1 in osteosarcoma. Mol Med Rep. 17:4777–4784. 2018.PubMed/NCBI
|
|
26
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Xiao X, Chen X, Huo Y, Xi WJ, Lin
ZF, Zhang D, Li YF, Yang F, Wen WH, et al: Tumor-suppressive
miR-145 co-repressed by TCF4-β-catenin and PRC2 complexes forms
double-negative regulation loops with its negative regulators in
colorectal cancer. Int J Cancer. 142:308–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishiguro H, Wakasugi T, Terashita Y,
Sakamoto N, Tanaka T, Sagawa H, Okubo T and Takeyama H: Nuclear
expression of TCF4/TCF7L2 is correlated with poor prognosis in
patients with esophageal squamous cell carcinoma. Cell Mol Biol
Lett. 21:52016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao W, Zhou J, Deng Z, Gao Y and Cheng Y:
SPOP promotes tumor progression via activation of β-catenin/TCF4
complex in clear cell renal cell carcinoma. Int J Oncol.
49:1001–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Q, Gao M, Luo G, Han X, Bao W, Cheng
Y, Tian W, Yan M, Yang G and An J: Enhancement of radiation
sensitivity in lung cancer cells by a novel small molecule
inhibitor that targets the β-catenin/Tcf4 interaction. PLoS One.
11:e01524072016. View Article : Google Scholar : PubMed/NCBI
|