Introduction
Oral cancer is characterized by high incidence and
mortality rates in developing countries, and is the 11th most
common cancer in the world (1).
Tongue squamous cell carcinoma (TSCC) is the most common oral
malignancy with different histopathological symptoms and
etiopathogenesis of tumorigenesis (2,3).
Epidemiological data have indicated alterations in the demographic
profile of patients suffering from TSCC (4). Although numerous advanced anticancer
treatments have been proposed for use in the clinic, TSCC has been
traditionally considered to be associated with frequent recurrence
and metastasis, and a poor prognosis, due to rapid migration and
invasion (5). Local migration to
parenchymal tissues and long distance metastasis to organs are the
principal reasons underlying the mortality rate of TSCC and the
poor survival rate among patients with TSCC (6). Therefore, understanding the potential
mechanisms of TSCC cell growth and aggressiveness is important to
inhibit tumor metastasis and improve survival for patients.
Obovatol is a phenolic compound extracted from the
bark of Magnolia obovata, which is renowned for its
pharmacodynamic functions of antioxidation, neuroprotection,
antithrombosis, anti-inflammation and antitumor. Previously,
obovatol has been observed to exhibit growth inhibitory effects on
tumor cells and tissues through the induction of apoptotic cell
death (7,8). A study demonstrated that obovatol
inhibited prostate and colon cancer cell growth by inducing
apoptosis and inhibition of the nuclear factor-κB signaling pathway
(9). Another report indicated that
obovatol inhibited colorectal cancer growth and aggressiveness by
suppressing tumor cell proliferation and inducing apoptosis, and
that it may therefore be a potent inducer of tumor cell apoptosis
and a potent antitumor agent (10). Additionally, obovatol may induce
apoptosis in non-small cell lung cancer cells via C/EBP homologous
protein activation, activated caspase 9/3 and apoptosis regulator
Bax, and that it attenuated the expression of cyclin D1 in A549 and
H460 non-small cell lung cancer cells (11).
The present study investigated the inhibitory
effects of obovatol on the growth and aggressiveness of SCC9 TSCC
cells. The present study examined obovatol-mediated pro-epidermal
growth factor (EGF)-mediated Janus kinase (JAK)-signal transducer
and activator of transcription (STAT) signaling in SCC9 TSCC cells.
The results demonstrated that obovatol may induce apoptosis in SCC9
TSCC cells by increasing caspase 9/3 and apoptotic protease
enhancing factor 1 (Apaf-1) expression levels mediated by the
EGF-induced JAK-STAT signaling pathway.
Materials and methods
Ethics statement
The present preclinical study was performed
according to the recommendations in the Guide for the Tianjin First
Center Hospital (Dental; Tianjin, China). All experimental
protocols and animals were approved by the Committee on the Ethics
of Animal Experiments in Defence Research of Tianjin First Center
Hospital (TFCH-023/0016; Tianjin, China). All surgery was performed
under intravenous sodium pentobarbital (37 mg/kg). The present
study was additionally approved by the Ethical Committee of Tianjin
Stomatological Hospital and Maxillofacial Surgery (Tianjin,
China).
Cells and reagents
SCC9 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA) and tumor cells were
cultured in minimum essential medium (MEM; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cells were cultured in an incubator at 37°C and 5%
CO2.
MTT assay
SCC9 cells (6×103 cells) were treated
with obovatol (5.00 mg/ml) in 96-well plates for 24 h in triplicate
for each condition, with PBS as a control. MTT (20 µl; 5 mg/ml) was
added to each well subsequent to incubation at 37°C. All plates
were further incubated for 4 h and the medium was subsequently
removed, and 100 µl dimethyl sulfoxide was added into the wells to
solubilize the crystals. The optical density was measured using a
ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at
wavelength of 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was isolated from SCC9 cells using an RNA
Easy Mini Extract kit (Sigma-Aldrich; Merck KGaA). RNA samples with
a A260/A280 ratio between 1.8 and 2.0 were used to synthesize cDNA
via RT using SuperScript III Reverse Transcriptase (Thermo Fisher
Scientific, Inc.). Reaction conditions for RT were: 65°C for 5 min,
50°C for 35 min and 85°C for 5 min. The expression of fibronectin
(FN), vimentin (VM), E-cadherin (ED), EGF and JAK in SCC9 cells was
detected using an SYBR® Green Real-Time PCR Master Mixes
(Thermo Fisher Scientific, Inc.), with β-actin expression as an
endogenous control. All procedures were performed according to the
manufacturer's protocol. qPCR reaction conditions were: 95°C for 45
sec (initial denaturation), followed by 40 cycles of 95°C for 15
sec (denaturation), 60°C for 45 sec (anealing/elongation) and 72°C
for 30 sec (final extension). All the primers were synthesized by
Invitrogen (Invitrogen; Thermo Fisher Scientific, Inc.). Primer
sequences were: FN forward, 5′-CCCACCGTCTCAACATGCTTAG-3′, reverse
5′-CTCGGCTTCCTCCATAACAAGTAC-3′; VM forward,
5′-TCTACGAGGAGGAGATGCGG-3′, reverse 5′-GGTCAAGACGTGCCAGAGAC-3′; ED
forward, 5′-GTCAGTTCAGACTCCAGCCC-3′, reverse
5′-AAATTCACTCTGCCCAGGACG-3′; EFG forward,
5′-GTGCAGCTTCAGGACCACAA-3′, reverse
5′-AAATGCATGTGTCGAATATCTTGAG-3′; JAK forward,
5′-AAGCTTTCTCACAAGCATTTGGTTT-3′, reverse
5′-AGAAAGGCATTAGAAAGCCTGTAGTT-3′ and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse 5′-AGTACTTGCGCTCAGGAGGA-3.
Relative mRNA expression levels were determined using
2−ΔΔCq (12). The final
results were presented in the n-fold manner compared with
β-actin.
Cells invasion and migration
assays
SCC9 cells were incubated with obovatol (5.00
mg/ml). SCC9 cells were suspended at a density of 1×106
in 500 µl serum-free MEM. Cells were seeded at the top of BD
BioCoat Matrigel Migration Chambers (BD Biosciences, Franklin
Lakes, NJ, USA), and MEM containing 20% fetal calf serum
(Sigma-Aldrich, Merck KGaA) was applied to the lower chamber. Cells
were incubated at 37°C for 24 h and 0.5% crystal violet
(Sigma-Aldrich; Merck KGaA) staining was performed at room
temperature for 20 min to analyze cell migration, according to the
manufacturer's protocol. For the migration assay, SCC9 cells were
seeded into a control insert (BD Biosciences) instead of a Matrigel
Migration Chamber. SCC9 cell migration and invasion was counted in
at least three randomly stained fields using a light microscope
(magnification, ×100) (Olympus Corporation, Tokyo, Japan) for every
membrane.
Small interfering RNA (siRNA)
transfections
SCC9 cells (5×105 cells in 100 µl MEM)
were cultured to 85% confluence and transfected with siRNA (40 nM;
5′-CAGCATCTGTCTAATCAAA-3′) targeting EGF (EGFKD) or scrambled siRNA
(5′-AATCAATCCATCCTT-3′) by incubating with
Lipofectamine™ RNAi MAX (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a CO2 incubator for 4 h
according to the manufacturer's protocols, followed by incubation
in MEM at 37°C for 48 h prior to harvest. siRNA targeting EGF and
scrambled siRNA were obtained from GenePharma (Shanghai,
China).
Western blotting
SCC9 cells were homogenized in
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing protease inhibitor and were centrifuged at 3,800 ×
g at 4°C for 10 min. The supernatant of the mixture was used for
the analysis of protein expression. Concentration of protein
samples was measured by Bicinchoninic Acid assay. Then, 10%
SDS-PAGE gel electrophoresis was performed using 30 µg protein from
each sample, followed by transfer to a polyvinylidene difluoride
membrane. For western blotting, primary antibodies (Abcam,
Cambridge, UK) were added following blocking with 5% skimmed milk
for 1 h at 37°C. Primary antibodies included: Rabbit anti-CDK1
(1:1,000; ab131450, Abcam), anti-CDK2 (1:1,000; ab64669, Abcam),
anti-FN (1:1,000; ab23750, Abcam), anti-VM (1:1,000; ab137321,
Abcam), anti-ED (1:1,000; ab1416, Abcam), anti-Caspase-3 (1:1,000;
ab13847, Abcam), anti-Caspase-9 (1:1,000; ab2324, Abcam),
anti-Apaf-1 (1:1,000; ab53152, Abcam), anti-p53 (1:1,000; ab131442,
Abcam), anti-Bcl-2 (1:1,000; ab32124, Abcam), anti-JAK (1:1,000;
ab125051, Abcam), anti-STAT (1:1,000; ab32520, Abcam),
anti-phosphorylated (p)JAK (1:1,000; ab138005, Abcam), anti-pSTAT
(1:1,000; ab76315, Abcam), anti-PDGF (1:1,000; ab124392, Abcam),
anti-VEGFR (1:1,000; ab32152, Abcam), anti-EFG (1:1,000; ab10409,
Abcam), and anti-β actin (1:1,000; ab6276, Abcam). The membrane was
incubated with all mentioned primary antibodies for overnight (8–10
h) at 37°C. Membranes were subsequently incubated with horseradish
peroxidase-conjugated anti-immunoglobulin G for 24 h at 4°C
(1:10,000; ab6721, Abcam). The results were visualized using a
chemiluminescence detection system (LumiGLO; Cell Signaling
Technology, Inc., Danvers, MA, USA). Relative expression levels of
each protein were normalized to endogenous control β-actin using
ImageJ v2 (National Institutes of Health, Bethesda, MD, USA).
Apoptosis assay
SCC9 cells were grown to 90% confluence. Apoptosis
was assessed by incubating the cells with obovatol (5.00 mg/ml) for
24 h. Following incubation, SCC9 cells were trypsinized and
collected. The cells were washed in PBS, adjusted to
1×106 cells/ml and labeled with annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (annexin V-FITC kit; BD
Biosciences). SCC9 cells were analyzed using a FACScan flow
cytometer (BD Biosciences). The percentage of labeled SCC9 cells
undergoing apoptosis in each group was determined and calculated
using BD Cell Quest pro software v5.1 (BD Biosciences).
Animal study
Male Balb/c (specific pathogen free) nude mice (age,
6–8 weeks, 20–25 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). Mice were raised in laminar
airflow cabinets under a 12 h light/dark cycle at a constant
temperature of 20–26°C and humidity of 30–70%. Animals were fed an
ad libitum diet of irradiated and sterilized dry food and
sterile drinking water. SCC9 cells (1×107) were mixed
with 100 µl PBS and injected subcutaneously into the flanks of
Balb/c mice (n =80). Xenografted mice were divided into two groups
(n=40 mice/group) and received treatment with obovatol (5.00 mg/kg)
once a day for 6 days following tumor implantation (diameter, 5–8
mm). The tumor volumes were calculated according to a previous
method (13). A fraction of the
mice (n=10 mice/group) were sacrificed and tumor sections were
obtained for further analysis on day 24. The remaining animals
(n=30 mice/group) were housed until day 120 to observe the survival
rate of experimental mice. Control mice were not treated.
Immunohistochemistry
TSCC tissues isolated from experimental mice were
fixed in formaldehyde (10%) for 24 h at room temperature, followed
by embedding in paraffin. Tumor tissues were sliced into tumor
sections with a thickness of 8 µm. Antigen retrieval was performed
by incubating with 10 mM citrate buffer (pH 6.0) at 95–100°C for 10
min. Slides were allowed to cool for 20 min, followed by two washes
with PBS, 5 min each and xylene was used to deparaffinize the
tissue, prior to rehydration by descending series of alcohol. Then
tissue sections were blocked using 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) in PBS at room
temperature for 1 h, followed by incubation with primary
antibodies, including rabbit anti-Caspase-3, anti-Caspase-9,
anti-Apaf-1, anti-EFG, anti-JAK and anti-STAT. Subsequently,
tissues were washed with PBS twice for 5 min per wash and incubated
with secondary antibody to Rabbit Immunoglobulin G-H&L (Biotin,
1:1,000, ab97049, Abcam) in a humidified chamber at room
temperature for 30 min. Following washing, color development was
performed by adding 3,3′-Diaminobenzidine (DAB; Sigma-Aldrich;
Merck KGaA). Signals were observed under a light microscope
(magnification, 20×, Olympus). A Ventana Benchmark automated
staining system (Ventana Medical Systems, Inc., Tucson, AZ, USA)
was used for observation of protein expression levels.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) to detect renal cell apoptosis
Paraffin-embedded sections (10% formaldehyde-fixed)
were subjected to antigen retrieval following dewaxing and
dehydration as aforementioned. The tissue sections were incubated
with proteinase K (20 µg/ml in Tris/HCl, pH 7.4–8.0 from Thermo
Fisher Scientific, Inc.) at room temperature for 15–30 min and
washed twice with PBS. Subsequently, the tissues were placed in
methanol solution containing 3% H2O2 at room
temperature for 5 min, followed by washing with PBS for 1 min. The
TUNEL reaction mixture (50 µl) was added and incubated at 37°C with
tissue in a wet box for 60 min, followed by 3 washes with PBS.
Transformation pod/TUNEL pod (50 µl; Sigma-Aldrich; Merck KGaA) was
then added and incubated at 37°C with tissue in a wet box for 60
min, followed by 3 washes with PBS. Finally, DAB substrate solution
(50–100 µl) was added and incubated with tissue at room temperature
for 10 min, followed by 3 washes with PBS. Hematoxylin staining was
performed at 37°C for 15 min and the slides were sealed. The sealed
slides were observed under microscope.
Statistical analysis
All data are presented as the mean ± standard
deviation of triplicate dependent experiments, and were analyzed
using Student's t-tests or one-way analysis of variance (Tukey
honest significant difference post hoc test). All data were
analyzed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA) and
GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA), in addition to Microsoft Excel 2010 (Microsoft Corporation,
Redmond, WA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
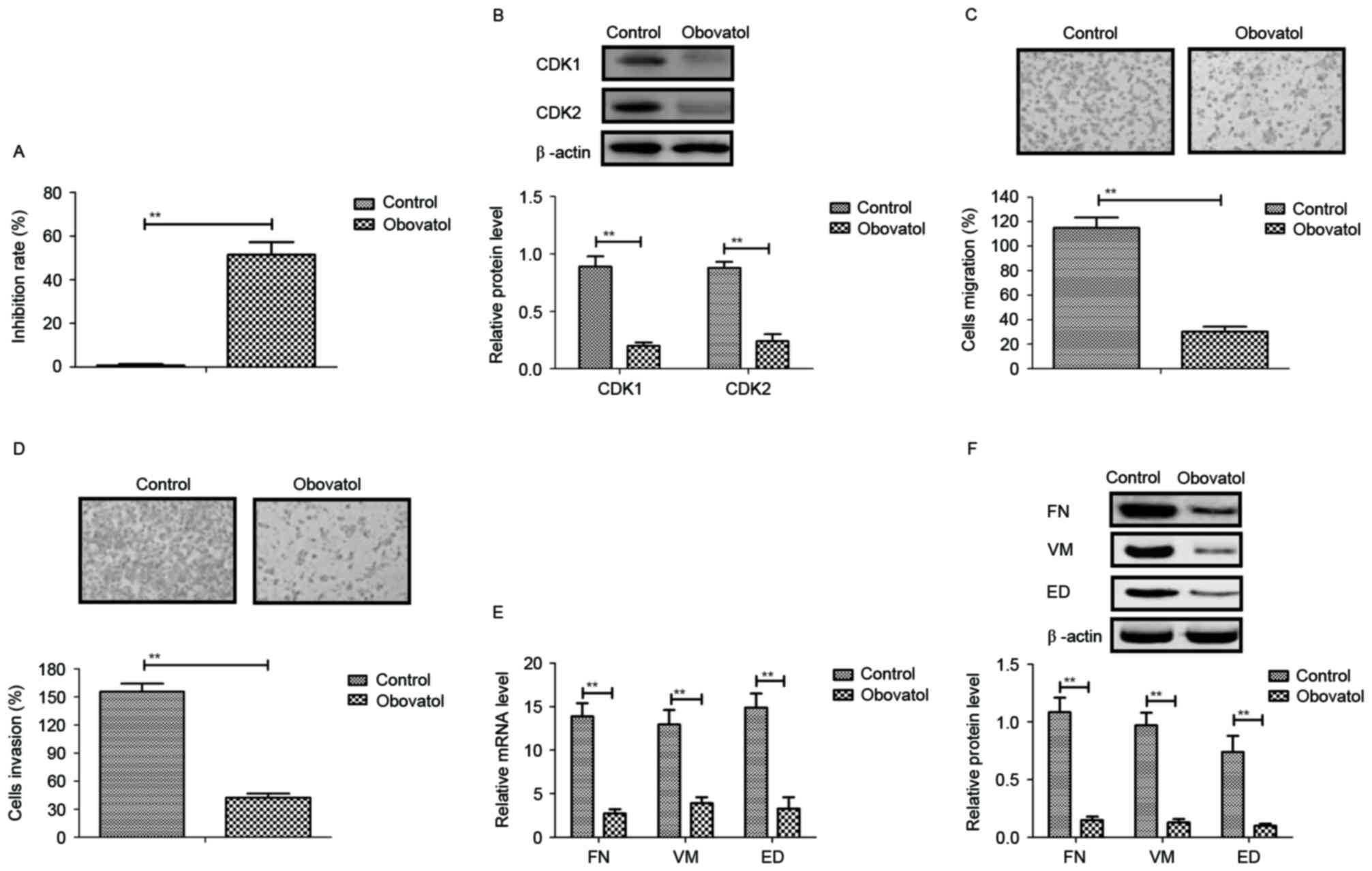
Obovatol inhibits TSCC cell growth and
aggressiveness through cell cycle arrest and downregulation of
metastasis-associated protein expression
The inhibitory effects of obovatol on the growth and
aggressiveness of TSCC cells were analyzed in the present study.
The results demonstrated that obovatol significantly inhibited SCC9
TSCC cell growth (Fig. 1A). The
expression levels of cyclin dependent kinase (CDK)1 and CDK2 were
downregulated in SCC9 TSCC cells following obovatol administration
(Fig. 1B). As presented in
Fig. 1C and D, migration and
invasion assays demonstrated that treatment with obovatol
suppressed the migration and invasion of SCC9 TSCC cells. It was
additionally observed that the expression levels of FN, VM and ED
were decreased by treatment with obovatol, as determined by RT-qPCR
and western blot analyses (Fig.
1E-F). The results of the present study indicated that obovatol
administration inhibited the growth and aggressiveness of SCC9 TSCC
cells.
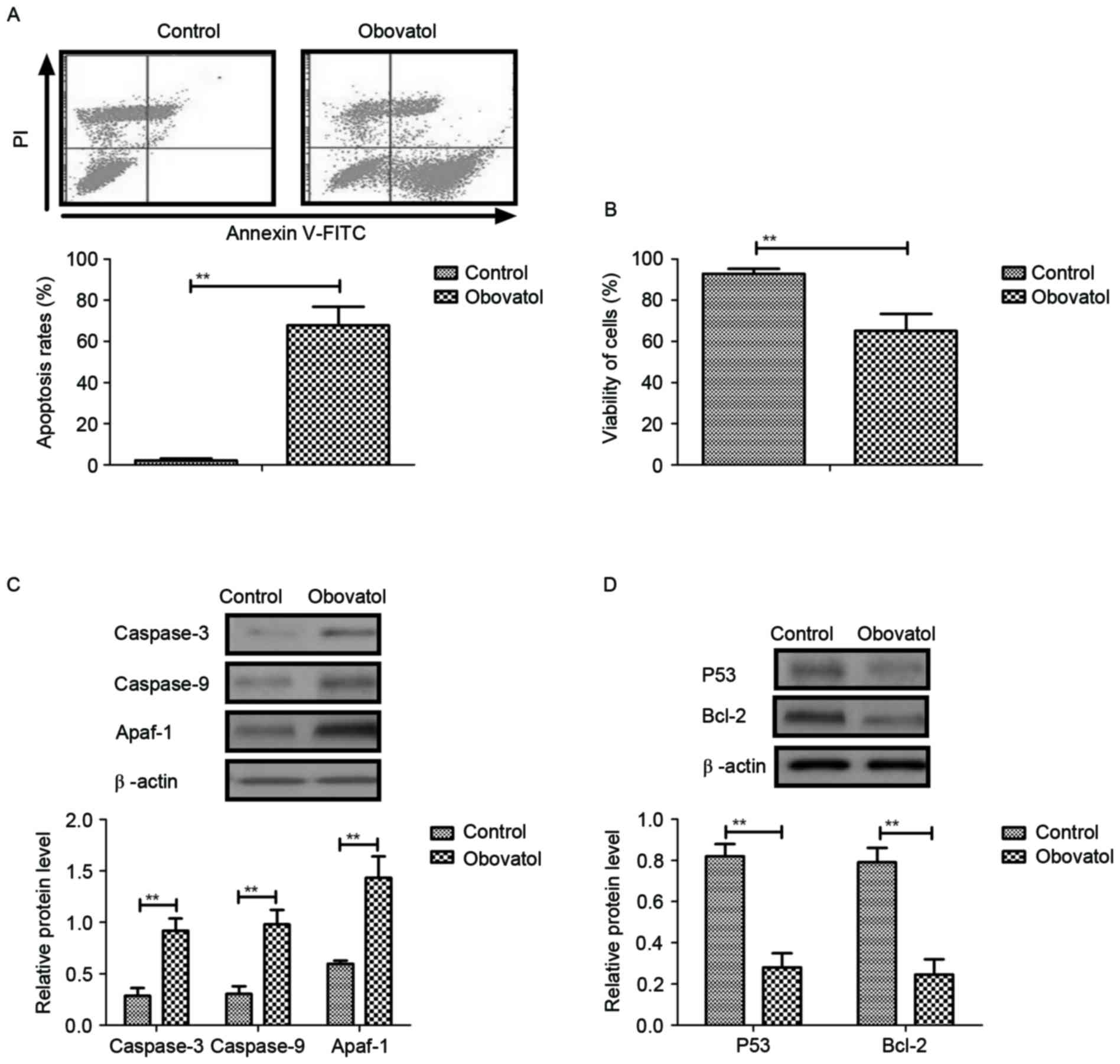
Obovatol induces apoptosis in TSCC
cells through regulation of apoptosis-associated gene
expression
The pro-apoptotic effects of obovatol in TSCC cells
were analyzed in the present study. The results demonstrated that
treatment with obovatol promoted apoptosis in SCC9 TSCC cells
(Fig. 2A). Cell viability analysis
demonstrated that obovatol enhanced the cellular atrophy of SCC9
TSCC cells (Fig. 2B). It was
observed that caspase-3, caspase-9 and Apaf-1 expression levels
were increased in SCC9 TSCC cells, as determined by western
blotting (Fig. 2C). The results
additionally demonstrated that the expression levels of p53 and
Bcl-2 were downregulated following obovatol administration in SCC9
TSCC cells (Fig. 2D). These
investigations suggested that treatment with obovatol promoted
apoptosis in SCC9 TSCC cells by increasing pro-apoptotic gene
expression and decreasing anti-apoptotic gene expression.
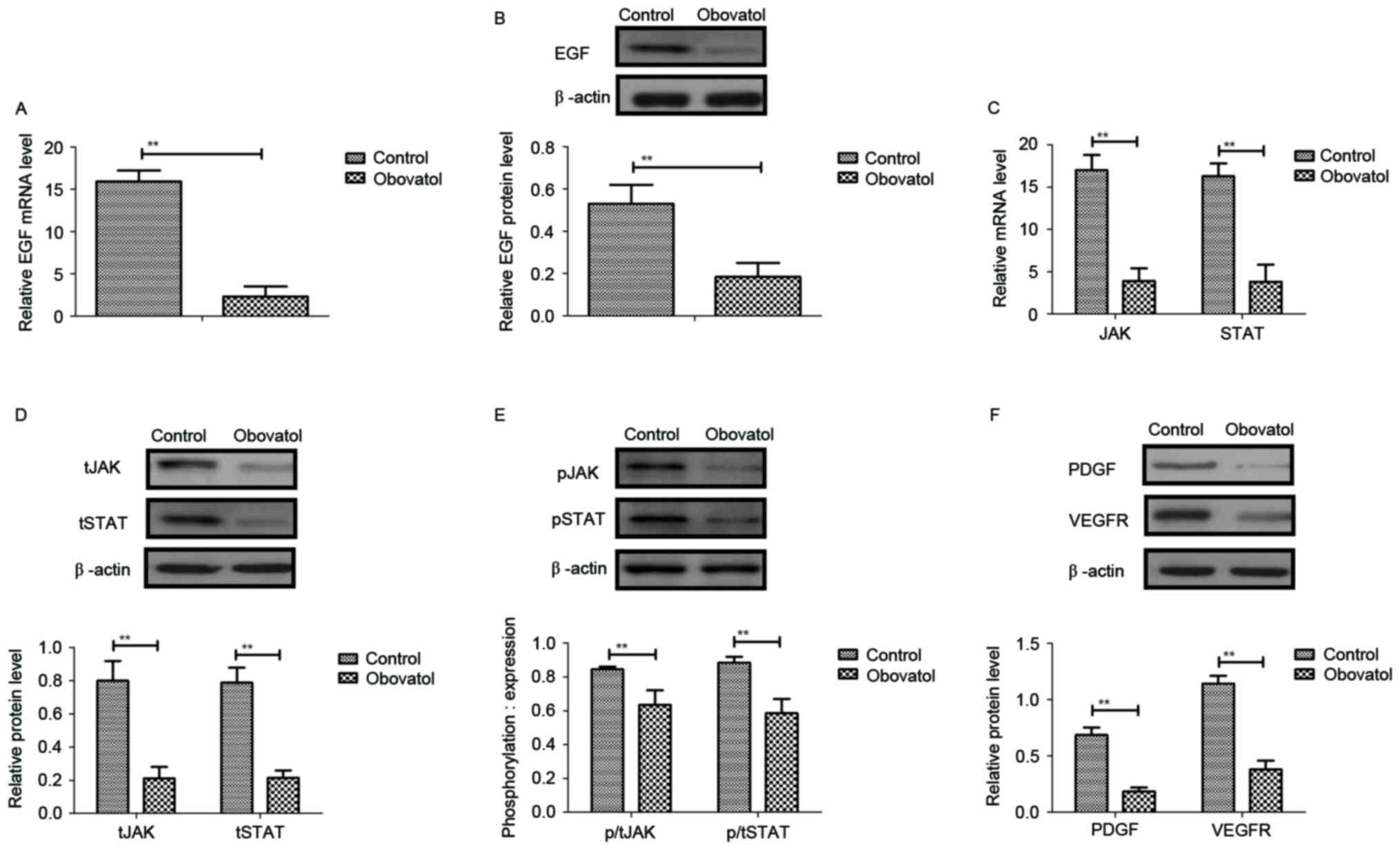
Obovatol inhibits the expression of
EGF and downregulates the JAK-STAT signaling pathway in TSCC
cells
Previous studies suggested that EGF expression
levels, and JAK-STAT transcriptional activation and mRNA
stabilization, may be associated with the proliferation and
metastasis of human carcinoma cells (14–16).
Therefore, the present study analyzed EGF expression and the
JAK-STAT signaling pathway in SCC9 TSCC cells. As presented in
Fig. 3A and B, the mRNA and
protein levels of EGF were decreased by treatment with obovatol in
SCC9 TSCC cells. The results additionally demonstrated that the
mRNA and protein expression levels of JAK and STAT were
downregulated in obovatol-treated SCC9 TSCC cells (Fig. 3C and D). Treatment with obovatol
decreased the phosphorylation levels of JAK and STAT in SCC9 TSCC
cells (Fig. 3E). It was
additionally observed that platelet-derived growth factor (PDGF)
and vascular endothelial growth factor receptor (VEGFR) protein
expression levels were downregulated by treatment with obovatol
(Fig. 3F). These data suggested
that obovatol may inhibit the expression of EGF and downregulate
the JAK-STAT signaling pathway in TSCC cells.
Obovatol inhibits TSCC cellular
apoptosis through the EGF-mediated JAK-STAT signaling pathway
In order to identify the mechanism through which the
EGF-mediated JAK-STAT signaling pathway may be inhibited by
obovatol, alterations in the expression and phosphorylation levels
of JAK and STAT were analyzed in EGF-knockdown SCC9 TSCC cells. As
presented in Fig. 4A and B, EGF
knockdown (EGFKD) inhibited the obovatol-mediated decreased
expression and phosphorylation levels of JAK and STAT in SCC9 TSCC
cells. Expression levels of caspase-3, caspase-9 and Apaf-1 were
increased in the EGFKD group compared with the EGFKD-OB group
(Fig. 4C). Notably, the
obovatol-induced increase in apoptosis was abolished in
EGF-knockdown SCC9 TSCC cells (Fig.
4D). These results indicated that treatment with obovatol
induced TSCC cellular apoptosis through the EGF-mediated JAK-STAT
signaling pathway.
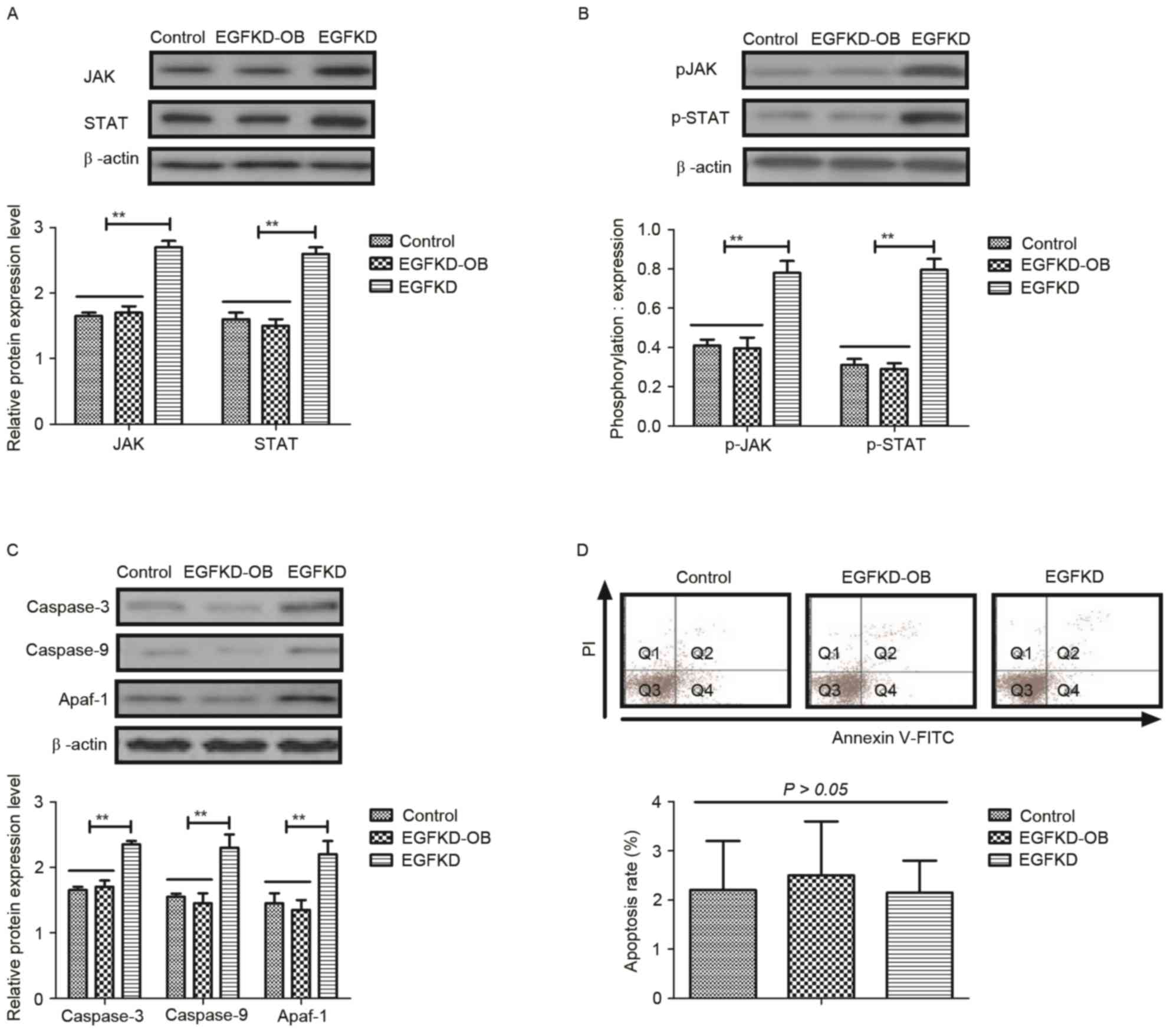 | Figure 4.Obovatol regulates SCC9 TSCC cellular
apoptosis through the EGF-mediated JAK-STAT signaling pathway. The
effects of EGF knockdown on the (A) expression and (B)
phosphorylation levels of JAK and STAT in SCC9 TSCC cells. (C) The
expression levels of caspase-3, caspase-9 and Apaf-1 in
EGF-knockdown SCC9 TSCC cells. (D) Obovatol-induced apoptosis was
inhibited by knockdown of EGF in SCC9 TSCC cells. All data are
presented as the mean ± standard error of the mean of triplicate
samples. **P<0.01: EGDKD vs. EGDKD-OB. TSCC, tongue squamous
cell carcinoma; EGF, pro-epidermal growth factor; JAK, Janus
kinase; STAT, signal transducer and activator of transcription;
Apaf-1, apoptotic protease enhancing factor 1; KD, knockdown; FITC,
fluorescein isothiocyanate; PI, propidium iodide; p,
phosphorylated; OB, treated with obovatol. |
In vivo anti-cancer effects of
obovatol in xenografted mice
The anti-tumor efficacy of treatment with obovatol
was studied in SCC9-bearing mice. The results demonstrated that
treatment with obovatol (12 mg/kg) significantly inhibited tumor
growth in a 24-day experiment (Fig.
5A). Treatment with obovatol promoted cellular apoptosis in
tumors, as determined by TUNEL assay (Fig. 5B). Immunohistochemistry
demonstrated that caspase-3, caspase-9 and Apaf-1 expression levels
were upregulated in obovatol-treated tumor tissues compared with
controls (Fig. 5C). The results
demonstrated that treatment with obovatol decreased the expression
levels of EGF, JAK and STAT in tumor tissues (Fig. 5D). The results additionally
demonstrated that PDGF and VEGFR protein expression levels were
downregulated by treatment with obovatol in tumor tissues (Fig. 5E). Notably, it was observed that
treatment with obovatol prolonged the survival of tumor-bearing
mice in a 120-day observation (Fig.
5F). These outcomes suggested that obovatol may exhibit in
vivo anti-cancer effects and prolong survival in tumor-bearing
mice.
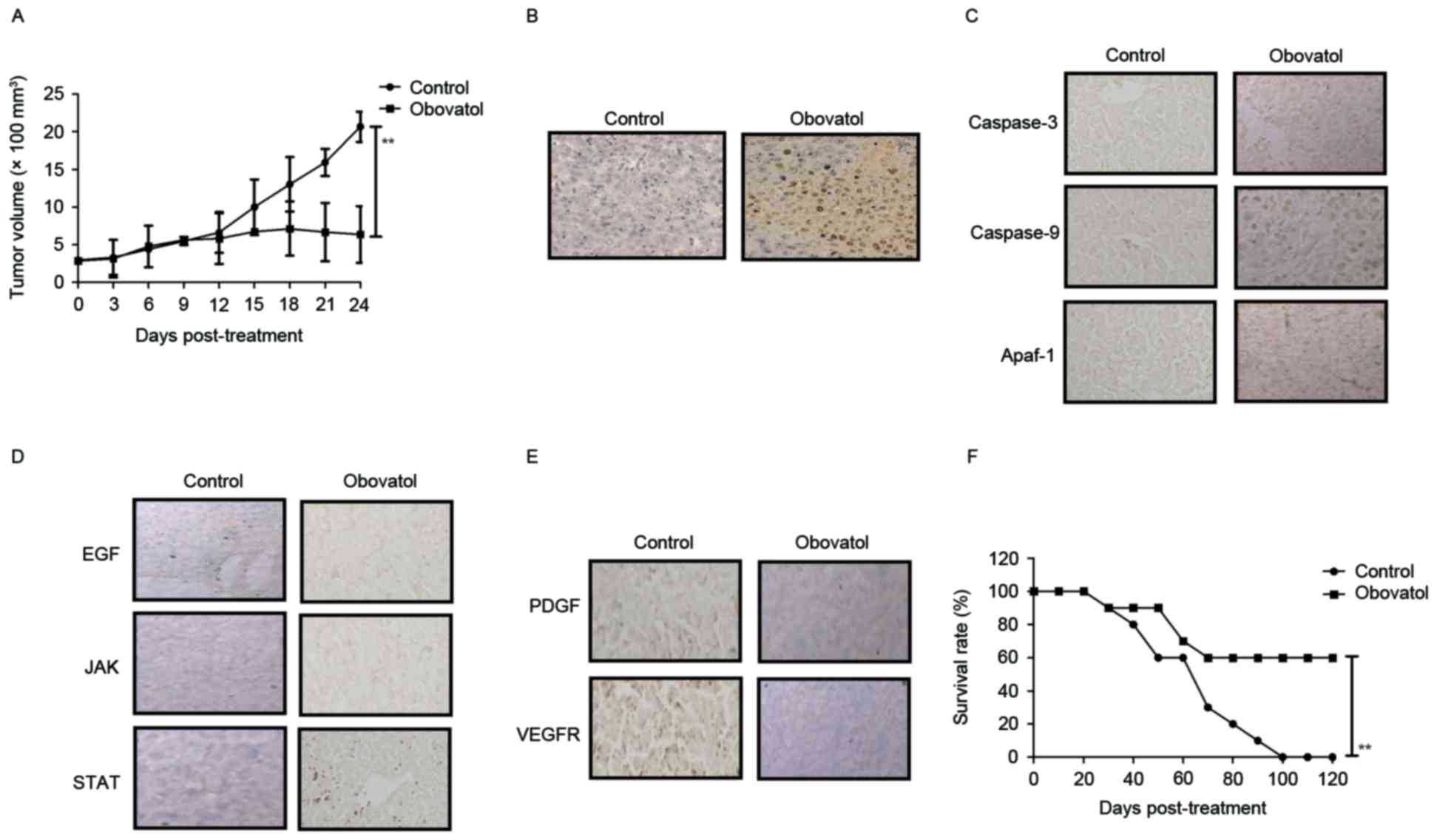 | Figure 5.In vivo anti-cancer efficacy of
treatment with obovatol in TSCC cell-bearing mice. (A) Treatment
with obovatol inhibited tumor growth in 24-day experiments. (B)
Treatment with obovatol promoted cellular apoptosis in tumor
tissues (magnification, ×20). (C) Immunohistochemistry was used to
analyze the effects of obovatol on caspase-3, caspase-9 and Apaf-1
expression levels in tumors tissues (magnification, ×20). (D)
Effects of treatment with obovatol on EGF, JAK and STAT in tumor
tissues (magnification, ×20). (E) Effects of treatment with
obovatol on PDGF and VEGFR protein expression levels in tumor
tissues (magnification, ×20). (F) Treatment with obovatol prolonged
the survival of SCC9-bearing mice in a 120-day experiment. n=8
animals/group. All data are presented as the mean ± standard error.
**P<0.01: Control vs. Obovatol. TSCC, tongue squamous cell
carcinoma; EGF, pro-epidermal growth factor; JAK, Janus kinase;
STAT, signal transducer and activator of transcription; Apaf-1,
apoptotic protease enhancing factor 1; PDGF, platelet-derived
growth factor; VEGFR, vascular endothelial growth factor
receptor. |
Discussion
Epidemiology has indicated that the morbidity and
mortality rate of TSCC is increasing in young people (16). The current management and treatment
strategy for the majority of patients with TSCC is partial surgical
glossectomy, followed by radiotherapy and chemotherapy in the
clinic (17–19). However, the rapid growth of TSCC
cells, local migration towards adjacent tissues and long-distance
metastasis to the neck, lymphatic system and other organs, shortens
the five-year survival period (20,21).
A previous study indicated that inhibiting a number of signaling
pathways in TSCC cells may be regarded as a potential biomarker and
therapeutic target in mobile TSCC (22). Evidence has demonstrated that
obovatol may be regarded as an anti-tumor agent, with the potential
to inhibit tumor cell growth by inducing apoptosis and arresting
the cell cycle in tumor cells (9).
In the present study, the inhibitory effects of treatment with
obovatol in TSCC cells and tissues were analyzed in vitro
and in vivo. The present study additionally investigated the
potential mechanism underlying obovatol-mediated apoptosis
stimulation and growth inhibition in SCC9 TSCC cells. The results
indicated that treatment with obovatol inhibited growth and
aggressiveness, and additionally promoted apoptosis in TSCC cells
through the EGF-mediated JAK-STAT signaling pathway. In vivo
assays demonstrated that treatment with obovatol significantly
suppressed tumor growth and prolonged survival, suggesting that
obovatol may be a promising anti-cancer agent for TSCC therapy.
At present, reports have indicated that obovatol may
efficiently inhibit the growth of various human cancer cells
through regulation of cellular signaling pathways (10,11).
Lee et al (23) reported
that obovatol enhanced docetaxel-induced prostate and colon cancer
cell death through inactivation of nuclear transcription factor-κB,
and suggested that obovatol may possess therapeutic potential in
combination with other antineoplastic chemotherapeutics. Arora
et al (24) indicated that
the role of obovatol in arresting the cell cycle may potentiate the
cytotoxic effects and induce apoptosis in human pancreatic cancer
cells. An additional study demonstrated that treatment with
obovatol inhibited cell proliferation and induced cell death by
decreasing the phosphorylation of RAC-α serine/threonine-protein
kinase and serine/threonine-protein kinase mTOR, which further
inhibited γ-secretase activity by downregulating the expression of
γ-secretase complex proteins, particularly γ-secretase subunit
aph-1, in malignant melanoma cancer cells (24). Although these reports have
presented the inhibitory effects of obovatol in human cancer, the
underlying anti-tumor mechanism is not well understood in TSCC
cells.
Studies have demonstrated that the inhibition of EGF
expression contributes to the inhibition of tumor growth and
further inhibits tumor metastasis (25,26).
Al-Hazzaa et al (27)
reported that EGF receptor tyrosine kinase inhibitor ZD1839
promoted cisplatin-induced apoptosis in SCC-15 cells. In addition,
it has been reported that targeting of the JAK-STAT signaling
pathway may induce tumor cell apoptosis and inhibit tumor cell
proliferation in tumor bearing mice treated with mBIIB036 (28). Furthermore, Hernandez et al
(29) demonstrated that tumor
growth and aggressiveness was able to be mediated through
JAK-STAT-interferon signaling pathways in HCT116 cells. In the
present study, the results indicated that obovatol downregulated
p53 and Bcl-2 expression levels in TSCC cells, which led to
increased apoptosis in SCC9 TSCC cells (30,31).
It was additionally observed that obovatol suppressed EGF
expression, and the expression and phosphorylation levels of JAK
and STAT in SCC9 TSCC cells. Obovatol treatment abolished the
upregulation of caspase-3, caspase-9 and Apaf-1 expression levels
in SCC9 TSCCs induced by EG knockdown. The present study design
identified that treatment with obovatol induced TSCC cellular
apoptosis through the EGF-mediated JAK-STAT signaling pathway.
In conclusion, the present study indicated that
treatment with obovatol efficiently inhibited TSCC cell growth
through downregulation of the expression levels of CDK1 and CDK2.
The aggressiveness of TSCC cells was suppressed by obovatol
administration via decreased expression of FN, VM and ED. The
findings additionally indicated that obovatol administration
induced apoptosis in TSCC cells through inhibition of the
EGF-mediated JAK-STAT signaling pathway, which further contributed
to the inhibition of tumor growth in vivo and prolonged the
survival of tumor-bearing mice. Overall, the results of the present
study suggested that obovatol administration may be a potential
anti-cancer agent for the treatment of TSCC via blockade of the
EGF-mediated JAK-STAT signal pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD, JZ and XD made substantial contributions to the
concept and design of the present study. GR, MD and YDZ made
substantial contributions to the conception of the present study
and conducted data analysis. YZ, SS and JZ performed statistical
analysis. Also, XD and JZ drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee on
the Ethics of Animal Experiments in Defence Research of Tianjin
First Center Hospital (TFCH-023/0016).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dik EA, Willems SM, Ipenburg NA, Rosenberg
AJ, Van Cann EM and van Es RJ: Watchful waiting of the neck in
early stage oral cancer is unfavourable for patients with occult
nodal disease. Int J Oral Maxillofac Surg. 45:945–950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen G, Cai X, Ren JG, Jia J and Zhao YF:
Unexpected development of tongue squamous cell carcinoma after
sclerotherapy for the venous malformation: A unique case report and
literature review. Diagn Pathol. 8:1822013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kradin RL, Sheldon TA, Nielsen P, Selig M
and Hunt J: Malacoplakia of the tongue complicating the site of
irradiation for squamous cell carcinoma with review of the
literature. Ann Diagn Pathol. 16:214–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy J, Berman DR, Edwards SP,
Prisciandaro J, Eisbruch A and Ward BB: Squamous cell carcinoma of
the tongue during pregnancy: A case report and review of the
literature. J Oral Maxillofac Surg. 74:2557–2566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martínez C, Hernández M, Martínez B and
Adorno D: Frequency of oral squamous cell carcinoma and oral
epithelial dysplasia in oral and oropharyngeal mucosa in Chile. Rev
Med Chil. 144:169–174. 2016.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shrestha A, Marla V, Shrestha S and
Agrawal D: Awareness of undergraduate dental and medical students
towards oral cancer. J Cancer Educ. 32:778–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim Y, Kwon JS, Kim DW, Lee SH, Park RK,
Lee JJ, Hong JT, Yoo HS, Kwon BM and Yun YP: Obovatol from Magnolia
obovata inhibits vascular smooth muscle cell proliferation and
intimal hyperplasia by inducing p21Cip1. Atherosclerosis.
210:372–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi DY, Lee YJ, Lee SY, Lee YM, Lee HH,
Choi IS, Oh KW, Han SB, Nam SY and Hong JT: Attenuation of
scopolamine-induced cognitive dysfunction by obovatol. Arch Pharm
Res. 35:1279–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SY, Yuk DY, Song HS, Yoon DY, Jung JK,
Moon DC, Lee BS and Hong JT: Growth inhibitory effects of obovatol
through induction of apoptotic cell death in prostate and colon
cancer by blocking of NF-kappaB. Eur J Pharmacol. 582:17–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SK, Kim HN, Kang YR, Lee CW, Kim HM,
Han DC, Shin J, Bae K and Kwon BM: Obovatol inhibits colorectal
cancer growth by inhibiting tumor cell proliferation and inducing
apoptosis. Bioorg Med Chem. 16:8397–8402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim H, Shin EA, Kim CG, Lee DY, Kim B,
Baek NI and Kim SH: Obovatol induces apoptosis in non-small cell
lung cancer cells via C/EBP homologous protein activation.
Phytother Res. 30:1841–1847. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai FL, Yu YH, Tian H, Ren GP, Wang H,
Zhou B, Han XH, Yu QZ and Li DS: Genetically engineered Newcastle
disease virus expressing interleukin-2 and TNF-related
apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther.
15:1226–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao YS, Zhou J, Fan J, Sun QM, Zhao Y,
Sun RX, Liu YK and Tang ZY: Interferon-alpha upregulates thymidine
phosphorylase expression via JAK-STAT transcriptional activation
and mRNA stabilization in human hepatocellular carcinoma SMMC-7721
cells. Zhonghua Zhong Liu Za Zhi. 30:444–447. 2008.(In Chinese).
PubMed/NCBI
|
|
15
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalinowski FC, Giles KM, Candy PA, Ali A,
Ganda C, Epis MR, Webster RJ and Leedman PJ: Regulation of
epidermal growth factor receptor signaling and erlotinib
sensitivity in head and neck cancer cells by miR-7. PloS one.
7:e470672012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong WM, Parvathaneni U, Jewell PD,
Martins RG, Futran ND, Laramore GE and Liao JJ: Squamous cell
carcinoma of the oral tongue in a patient with Fanconi anemia
treated with radiotherapy and concurrent cetuximab: A case report
and review of the literature. Head Neck. 35:E292–E298.
2013.PubMed/NCBI
|
|
19
|
Iyengar NM, Ghossein RA, Morris LG, Zhou
XK, Kochhar A, Morris PG, Pfister DG, Patel SG, Boyle JO, Hudis CA
and Dannenberg AJ: White adipose tissue inflammation and
cancer-specific survival in patients with squamous cell carcinoma
of the oral tongue. Cancer. 122:3794–3802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goepfert RP, Kezirian EJ and Wang SJ: Oral
tongue squamous cell carcinoma in young women: A matched
comparison-do outcomes justify treatment intensity? ISRN
Otolaryngology. 2014:5293952014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seppälä M, Pohjola K, Laranne J,
Rautiainen M, Huhtala H, Renkonen R, Lemström K, Paavonen T and
Toppila-Salmi S: High relative density of lymphatic vessels
predicts poor survival in tongue squamous cell carcinoma. Eur Arch
Otorhinolaryngol. 273:4515–4524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Theocharis S, Giaginis C, Dana E, Thymara
I, Rodriguez J, Patsouris E and Klijanienko J: Phosphorylated
epidermal growth factor receptor expression is associated with
clinicopathologic parameters and patient survival in mobile tongue
squamous cell carcinoma. J Oral Maxillofac Surg. 75:632–640. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SY, Cho JS, Yuk DY, Moon DC, Jung JK,
Yoo HS, Lee YM, Han SB, Oh KW and Hong JT: Obovatol enhances
docetaxel-induced prostate and colon cancer cell death through
inactivation of nuclear transcription factor-kappaB. J Pharmacol
Sci. 111:124–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arora S, Bhardwaj A, Srivastava SK, Singh
S, McClellan S, Wang B and Singh AP: Honokiol arrests cell cycle,
induces apoptosis, and potentiates the cytotoxic effect of
gemcitabine in human pancreatic cancer cells. PloS one.
6:e215732011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gotz R and Sendtner M: Cooperation of
tyrosine kinase receptor TrkB and epidermal growth factor receptor
signaling enhances migration and dispersal of lung tumor cells.
PloS One. 9:e1009442014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andersson N, Anttonen M, Färkkilä A,
Pihlajoki M, Bützow R, Unkila-Kallio L and Heikinheimo M:
Sensitivity of human granulosa cell tumor cells to epidermal growth
factor receptor inhibition. J Mol Endocrinol. 52:223–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Hazzaa A, Bowen ID, Randerson P and
Birchall MA: The effect of ZD1839 (Iressa), an epidermal growth
factor receptor tyrosine kinase inhibitor, in combination with
cisplatin, on apoptosis in SCC-15 cells. Cell Prolif. 38:77–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chapman MS, Wu L, Amatucci A, Ho SN and
Michaelson JS: TWEAK signals through JAK-STAT to induce tumor cell
apoptosis. Cytokine. 61:210–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hernandez JM, Elahi A, Clark W, Humphries
LA, Wang J, Achille A, Seto E and Shibata D: The tumor suppressive
effects of HPP1 are mediated through JAK-STAT-interferon signaling
pathways. DNA Cell Biol. 34:541–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fourati A, El May MV, Ben Abdallah M,
Gamoudi A, Mokni N, Goucha A, Boussen H, Ladgham A and El May A:
Prognostic evaluation of p53, heat shock protein 70, Ki67, and CD34
expression in cancer of the tongue in Tunisia. J Otolaryngol Head
Neck Surg. 38:191–196. 2009.PubMed/NCBI
|
|
31
|
Lin YT, Yang JS, Lin HJ, Tan TW, Tang NY,
Chaing JH, Chang YH, Lu HF and Chung JG: Baicalein induces
apoptosis in SCC-4 human tongue cancer cells via a Ca2+-dependent
mitochondrial pathway. In Vivo. 21:1053–1058. 2007.PubMed/NCBI
|