Introduction
Wound healing includes two processes: Tissue
regeneration, which generally occurs in lower organisms and early
embryos in response to trauma, and scar healing, which typically
occurs in adult wound healing. Scars may be divided into
non-pathological scars (normal scars) that are not raised on the
skin and pathological scars (abnormal scars), which appear raised
on the skin and occur in the majority of wound healing processes;
pathological scars also include hypertrophic scars and keloids
(1–3).
Keloid scarring is a type of fibroproliferative
disease with pathological features that include excessive
proliferation of fibroblasts and collagen-based excessive
deposition of extracellular matrix components (1). Keloid scars are characterized by
persistent hyperplasia that is beyond the boundaries of original
lesion and gradually invade the surrounding normal skin tissue
(1). Keloid scarring severely
affects the appearance and function of skin and is one of the most
common clinical orthopedic diseases (1).
Tumors are neoplasms that arise from the abnormal
proliferation and differentiation of cells upon stimulation from
various tumor initiating and promoting factors. Once formed,
neoplasms grow uncontrollably and invade nearby organs and adjacent
normal tissues (2). Based on the
similarities between the features and biological behaviors of
keloid scars and tumors, the strategies applied to tumor therapy
may also be effective for keloid treatment. The primary treatment
strategy for keloid scarring is surgery with adjuvant physical
methods, including chemotherapy and photodynamic treatment
(2). However, the relapse rate of
keloid scarring is 45–100% via surgical resection only due to a
lack of efficacious treatment methods (2,3).
The principle of suicide gene therapy is the
transduction of cells with a gene that encodes an enzyme that can
convert an inactive prodrug into a cytotoxic metabolite (4). In addition to affecting cells
expressing the enzyme, adjacent untransduced cancer cells are
killed by the transfer of the phosphorylated nucleoside analog
between cells. This phenomenon, known as the ‘bystander effect’,
results in the killing of a larger portion of cells than is
transduced with the suicide gene (5–7).
Drosophila melanogaster multisubstrate deoxyribonucleoside
kinase (Dm-dNK) is a type of suicide gene that has been used for
the treatment of various cancers. Although four dNKs have been
identified in humans, the insect drosophila melanogaster has
only one known multisubstrate dNK. Dm-dNK has the ability to
phosphorylate purine and pyrimidine nucleosides, as well as
mediating DNA synthesis by integrating into the genome of cells
(5,6). Furthermore, Dm-dNK preferably
phosphorylates pyrimidines, which enhances cell sensitivity to
several cytotoxic nucleoside analogs, including
(E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU) and
1-β-D-arabinofuranosylthymine (araT) (8,9).
Zheng et al (10)
demonstrated that Dm-dNK may be expressed in human cells with
enzymatic activity.
Notably, the lentiviral vector has been identified
to facilitate the incorporation of target genes into the host
genome for efficient and stable expression of target genes in
dividing and non-dividing cells (11,12).
In the present study, a lentivirus vector was used to express the
Dm-dNK suicide gene in keloid fibroblasts (KF). The efficacy of
Dm-dNK combined with BVDU or araT as a type of keloid treatment was
investigated.
Materials and methods
Cell lines and culture
A total of 30 randomly selected patients (20 females
and 10 males) aged from 20 to 50 years old diagnosed as spontaneous
keloid from December 2014 to February 2015 in the Plastic Surgery
of Aoyang Hospital were included in the present study. All patients
underwent surgery and primary keloid fibroblasts were successfully
obtained from 13 patients (4 males, 9 females). The present study
was approved by the Ethics Committee of Aoyang Hospital
(Zhangjiagang, China) and written informed consent was provided by
all patients.
Keloid tissues were washed with PBS, cut into small
pieces. Finally, they were adhered and cultured on the bottom of
tissue culture flasks. After 3–5 d of culture, the keloid
fibroblasts were extracted from these tissues and cultured in
Dulbecco's Modified Eagle Medium (DMEM, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal
bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin in an atmosphere containing
5% CO2 at 37°C. After 5 days, 1 ml DMEM with 20% FBS
were added. On day 8, the medium was first replaced, then every 2–3
days until cells were cultured to 80% confluence and then passaged.
The 2–3 generation of cells with ~60% coverage rate were selected
for subsequent experiments.
Construction of the lentiviral plasmid
and virus production
Dm-dNK cDNA was amplified from PLXSN-dNK plasmid
(10) using the following primers:
Forward, 5′-CCGGAATTCACCATGGCGCAGGCA-3′ and reverse,
5′-CGCGGATCCTCATTATCTGGCGAC-3′, as previously described (10). The sequences of endonucleases
EcoRI and BamHI (New England Biolabs, Beverly, MA,
USA) were designed using the forward and reverse primers.
Dm-dNK-3Flag was amplified by polymerase chain reaction (PCR) kit
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer's
protocols. Amplification was performed via quantitative (q)PCR with
the following primers: Upstream, 5′-CCGGAATTCACCATGGCGCAGGCA-3′ and
downstream, 5′-CGCGGATCCTCATTATCTGGCGAC-3′. The gene was amplified
by qPCR using following thermocycling conditions: 94°C for 4 min,
followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for
1.5 min. The PGC-FU plasmid (GeneChem, Inc., Shanghai, China)
consisted of a 5′-long terminal repeat (LTR), cytomegalovirus (CMV)
promoter, multiple clone sites, green fluorescent protein (GFP)
sequences and a 3′-LTR. The endonucleases AgeI and
EcoRI (New England Biolabs) were used to remove GFP of
PGC-FU plasmid and then co-cultured with Dm-dNK-3Flag in 293T cells
to generate the recombinant plasmid PGC-FU-dNK. Following the
manufacturer's protocol PGC-FU-dNK or PGC-FU plasmids together with
two packaging plasmids PHelper1.0 (with gag, pol and rev
components) and PHelper2.0 (with VSVG component) were
co-transfected into 293T cells using Lipofectamine™ 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
lentivirus-containing medium (aforementioned) was collected and
filtered. Lentiviruses were concentrated and stored at −80°C. The
titer of lentiviruses and multiplicity of infection (MOI) was
determined through dilution as described (13). Control viruses containing GFP were
used to determine the infection efficiency. For infection, keloid
fibroblasts were seeded in 6-well plates at a density of
4×105 cells/well. The cells were infected with Lenti-GFP
and Lenti-dNKflag viruses at 10 of MOI for 24 h at 37°C with 6
µg/ml polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany);
untransduced cells served as the control. Cells were viewed under
fluorescence microscopy at 488 nm, 24 h following transfection
(magnification, ×200).
Western blotting
Following 72 h of transfection, cells were harvested
and lysed in lysis buffer [20 mM Tris (pH 7.5), 150 mM sodium
chloride, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM
ethylenediaminetetraacetic acid, 1% sodium carbonate, 0.5 µg/ml
leupeptin, and 1 mM phenylmethanesulfonyl fluoride] with protease
inhibitors, The protein concentrations were determined via a
Bicinchoninic Acid protein assay and then boiled in a sample buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) at 100°C for 5 min.
Equal amounts of protein (~20 ug per lane) were separated using 10%
SDS-PAGE and then transferred to polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). Subsequently, samples were
blocked in 5% non-fat milk in Tris-buffered saline with Tween-20
(TBST) for 2 h at room temperature. Membranes were incubated with
antibodies against Flag (ab213519, 1:1,000, Abcam, Cambridge, MA,
USA) or β-actin (sc130300, 1:500, Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) at 4°C overnight. Subsequently, membranes were
washed in TBST five times each for 10 min and incubated with a
secondary horseradish peroxidase-conjugated antibody (sc-516180;
Santa Cruz Biotechnology, Inc.) at 1:5,000 dilution, at room
temperature for 2 h. Protein bands were detected using a
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.)
and autoradiography (BioMaxfilm, Kodak, Rochester, NY, USA).
β-actin served as an internal control.
Enzyme activity assay
Following 72 h of infection, cell proteins were
extracted as previously described (14). To detect the activity of Dm-dNK, a
35-ml reaction mixture containing 50 mM Tris-HCl (pH 7.6), 100 mM
KCl, 2 mM dithiothreitol, 15 mM NaF, 5 mM MgCl2, 5 mM
ATP, 0.5 mg/ml bovine serum albumin and 0.6 mg protein extract was
prepared. A total of 2.5 mM [methyl-3H] thymidine (dThd; Moravek,
Inc., Brea, CA, USA) was mixed with equal amounts of unlabeled
substrates. Aliquots of the samples were spotted on Whatman Grade
DE-81 filter paper. Following 10, 20 or 30 min incubation at 37°C,
samples were dried for 1 h and washed three times with 5 mM
ammonium formate. Subsequently, nucleoside monophosphates were
isolated using 0.5 M KCl. Radioactivity was sequentially quantified
through scintillation counting.
Cell viability assay
The cell viability and lethal bystander effects were
evaluated using the MTT assay. Briefly, 3×103 cells from
untransduced, Lenti-GFP and Lenti-Dm-dNK groups were cultured in
96-well plates overnight at 37°C. Following infection with
Lenti-GFP or Lenti-Dm-dNK for 3 days, cells were treated with
graded concentrations of BVDU or araT from 0.00001 to 100 mM for 4
days. The medium was replaced, as aforementioned and cells were
incubated with 20 µl MTT (Promega Corp., Madison, WI, USA; 5 mg/ml)
for 4 h at room temperature. Following this, the medium was
replaced with 200 µl dimethyl sulfoxide. The solubilized formazan
product was quantified according to the absorbance at the
wavelength of 570 nm. The assay was conducted in triplicate and
repeated in trilplicate.
The assay for lethal and bystander effects was
performed as described (15). KF
cells expressing Dm-dNK were mixed at different ratios with their
respective parental cell lines. To promote cell contacts, the mixed
cells were plated in 24-well plates at 3×105 cells/well.
After 24-h incubation, cells were trypsinized and a 1:100 dilution
of the cells was distributed into 96-well plates in five
replicates. Cells were cultured subsequently in the presence of
BVDU or araT for 2 to 3 days until cells without prodrugs reached
confluency. The proliferation of the cells was measured by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay. We
calculated the inhibition of bystander cells proliferation using a
method described previously (16).
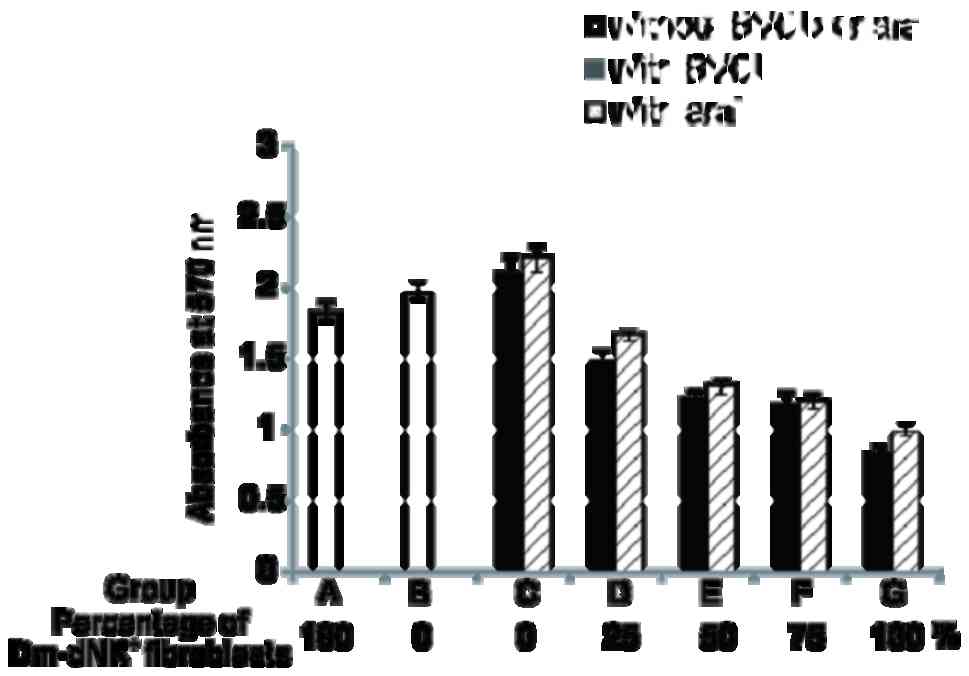
Cells were divided into seven groups: Groups A and B were controls
with 100% Dm-dNK+ transduced KF without drugs and
nontransduced KF cells, respectively whereas groups C-G were
experimental groups constituting Dm-dNK gene-transduced KF cells of
various percentages (0, 25, 50, 75 and 100% Dm-dNK gene-transduced
cells), incubated in the presence of the 1 mM BVDU or araT.
Statistical analysis
All data were presented as means ± standard error or
standard deviation where appropriate, from three independent
experiments. SPSS software (version 10.1, SPSS, Inc., Chicago, IL,
USA) was used for all analyses. Comparisons amongst groups were
performed using one-way analysis of variance and the
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of Dm-dNK in keloid
fibroblasts
The expression of GFP (Lenti-GFP) and Dm-dNK
(Lenti-CMV-dNK) was driven by lentiviral plasmids. In PGC-FU-dNK
plasmid, the GFP fragment in the PGC-FU plasmid was replaced by
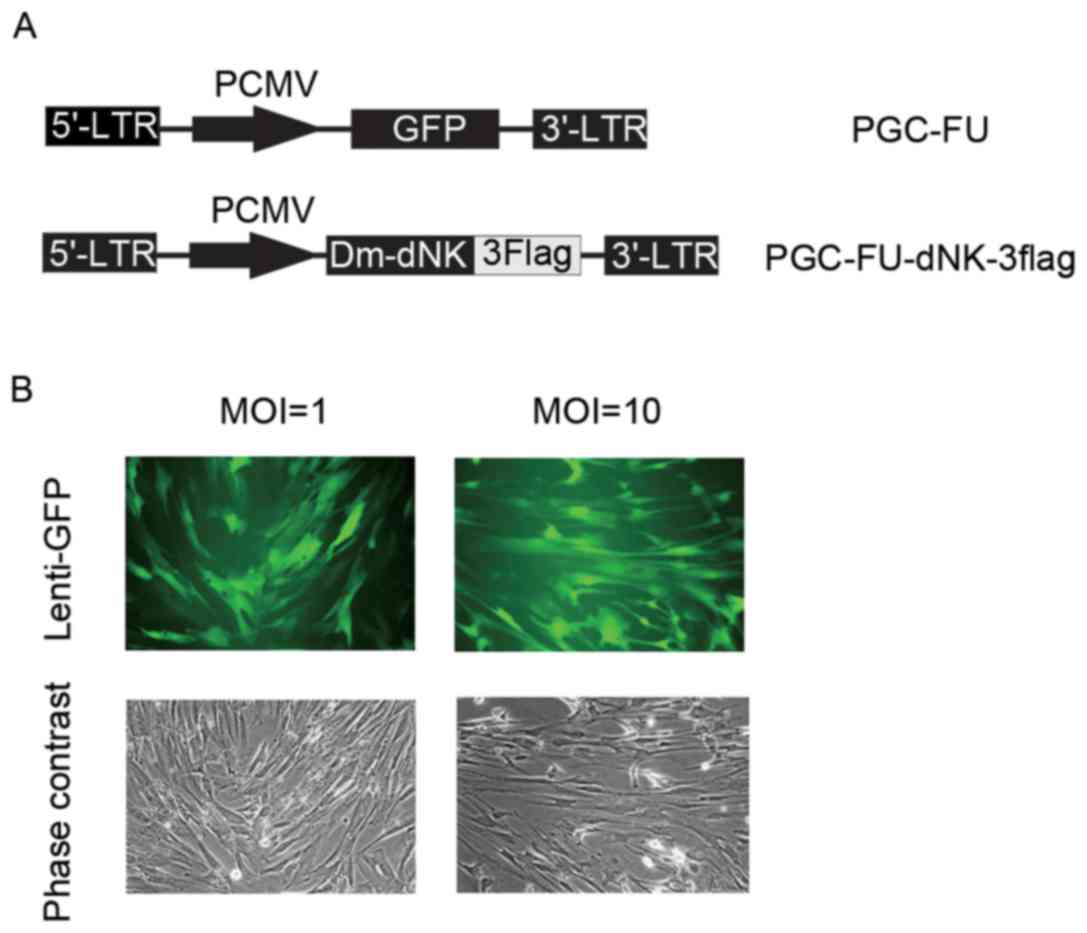
Dm-dNK-3flag (Fig. 1A). To examine
infection efficiency, lentiviruses with GFP at a MOI of 1 or 10
were used to infect cells. As indicated in Fig. 1B, the expression of GFP was
observed in both infections, however, the percentage of
GFP-positive cells infected with lentiviruses at a MOI of 10 was
not markedly different from that infected with lentiviruses at a
MOI of 1.
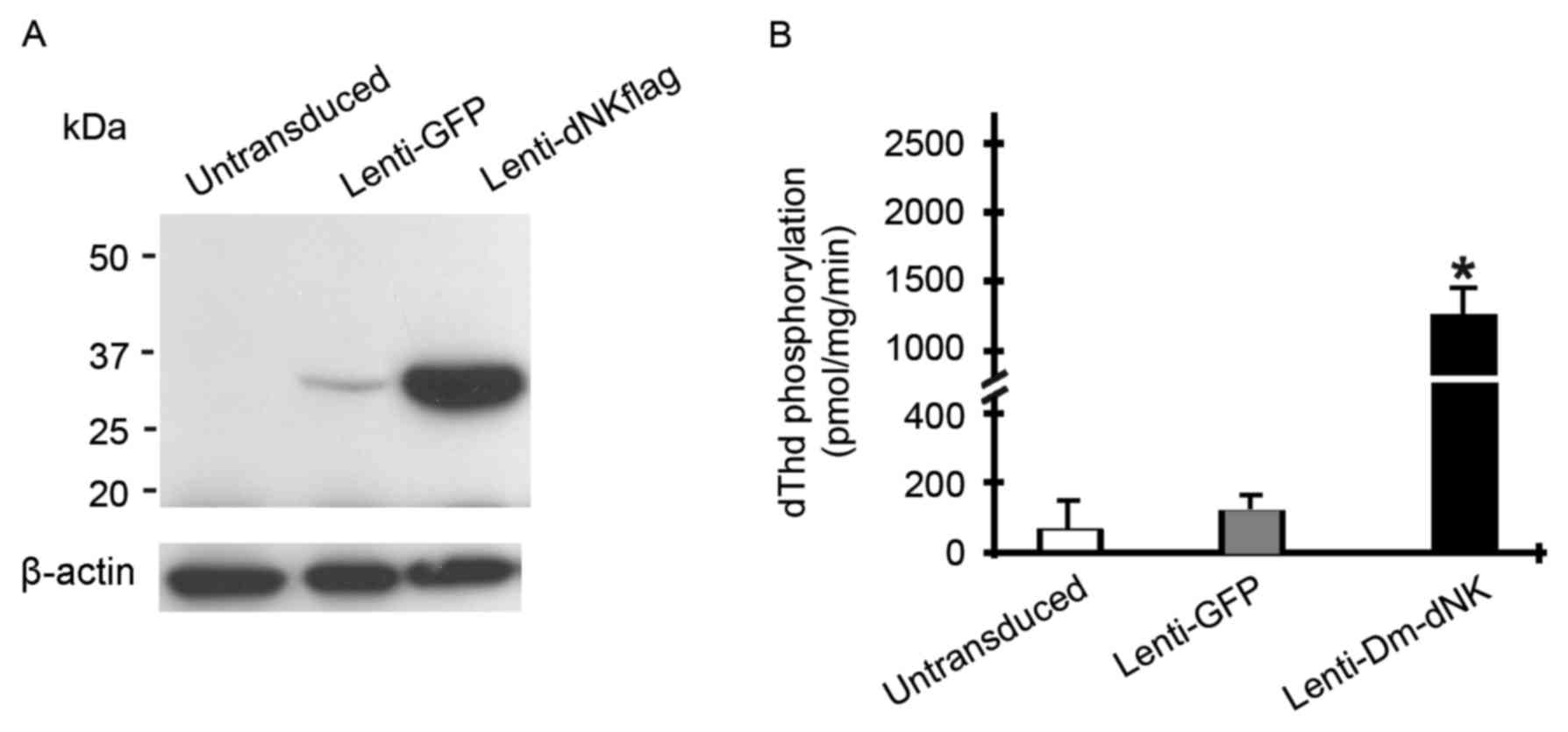
Keloid fibroblasts were initially infected with
lentivirus at a MOI of 10. The expression level of Dm-dNK protein
was assessed using western blotting. Dm-dNK protein expression was
observed in the keloid fibroblasts following Lenti-dNKflag
transfection, whereas the cells transfected with Lenti-GFP and
untransduced cells demonstrated low or no Dm-dNK protein
expression, respectively (Fig.
2A).
To evaluate enzymatic activity of transfected Dm-dNK
in human keloid fibroblasts, phosphorylation of dThd in cell
extracts was assessed. dThd kinase activity in keloid fibroblasts
with Lenti-Dm-dNK was significantly increased by 10–15-fold
following 72 h infection compared with untransduced cells or
following infection with Lenti-GFP (P<0.05; Fig. 2B).
In summary, these experiments revealed that human KF
cells transduced with the Lenti-CMV-dNK may express Dm-dNK protein,
which resulted in an increase of nucleoside and nucleoside analog
phosphorylation.
In vitro cytotoxicity and lethal
bystander effects of Dm-dNK/prodrug system in keloid
fibroblasts
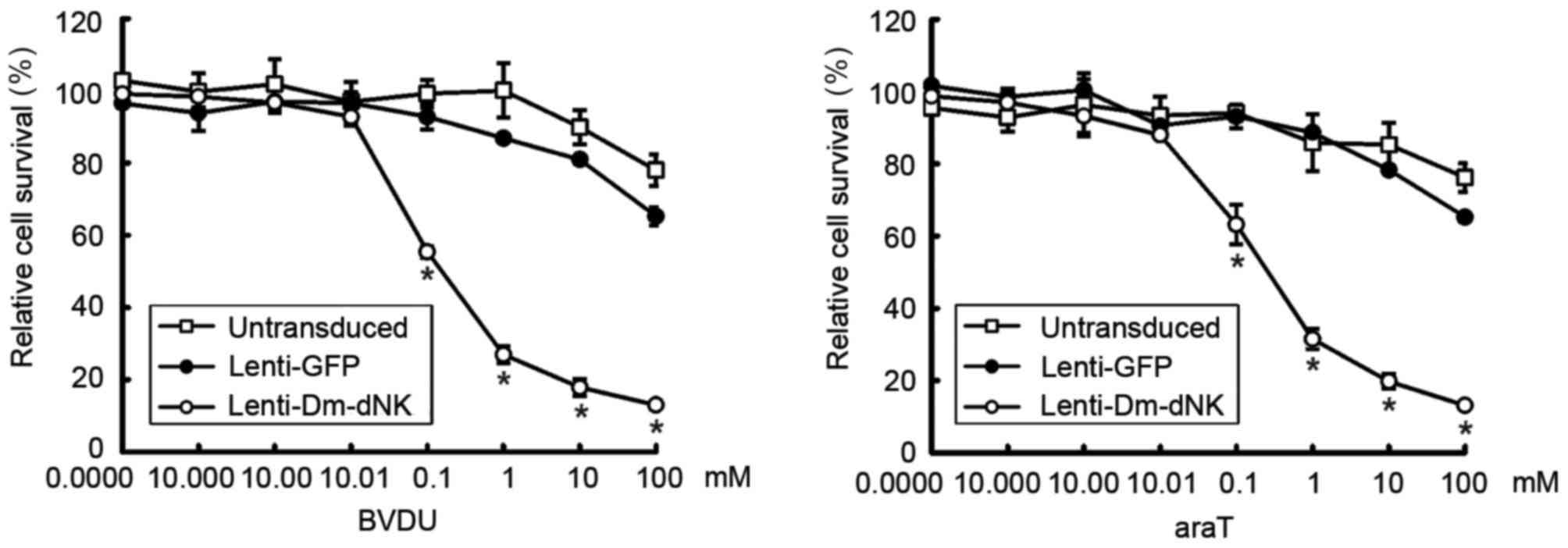
To evaluate the cytotoxicity of lentiviral vectors
with Dm-dNK in vitro, keloid fibroblasts were transfected
with lentivirus at a MOI of 10. Prodrug BVDU or araT was added at
concentrations of 0.00001–100 mM for 4 days and cytotoxicity was
examined using an MTT assay. Results indicated that the viability
of keloid fibroblasts was reduced by 70% following transfection
with Lenti-DM-dNK combined with 1 mM bromovinyldeoxyuridine (BVDU)
or araT (Fig. 3).
No significant difference in lethality (cell death)
lethal effect was observed among the three control groups (groups
A-C). However, the lethal effects in the experimental group (groups
D-G) were markedly higher compared with those in the control group
(groups A-C). Furthermore, 50% Dm-dNK-expressing KF cells (group E)
induced cell death to a degree similar to that obtained with 25%
Dm-dNK-expressing cells (group F; Fig.
4).
In summary, the results of the present study
demonstrated that Dm-dNK-expressing KF cells exhibited increased
sensitivity of cell death in combination with BVDU and araT and may
have also induced bystander cell death in KF cells.
Discussion
In past decades, several types of prodrugs that are
activated and sensitized by suicide genes have been reported to
serve roles in the treatment of cancers (17). For example, the herpes simplex
virus thymidine kinase gene and the Escherichia coli
cytosine deaminase gene may combine with ganciclovir and
5-fluorocytosine, respectively (18). Xu et al (19) demonstrated that the combination of
recombinant adenovirus-mediated-double suicide genes E. coli
cytosine deaminase and herpes simplex virus type 1 thymidine
kinase, via a polyglycine spacer, and prodrug therapy is effective
for the treatment of keloid fibroblasts. Additionally, our previous
studies suggested that the Dm-dNK/nucleoside analog system may be a
novel effective treatment for various types of cancer, including
breast cancer and gastric carcinoma, and may improve antitumor
effects (20,21). Furthermore, retrovirus-mediated
Dm-dNK may be used to treat osteosarcoma cells and pancreatic
adenocarcinoma cells (10). A
replicative adenovirus, ZD55-dNK, has been indicated to enhance
cancer-specific destruction through prodrug administration by
synergistically inducing apoptosis of human gastrocarcinoma cells
and suppressing replication of the adenovirus in vitro
(22). Similarly,
lentivirus-mediated Dm-dNK expression combined with gemcitabine
2′,2′-difluoro-deoxycytidine is an effective form of cancer therapy
(11). Notably, expressing suicide
genes via replication-defective adenoviral and lentiviral vectors
may be an effective way to treat human breast cancer (11,12).
A previous study demonstrated that the Dm-dNK/BVDU system may
provide a safe treatment for breast cancer with the aid of
lentiviral vector (12).
Considering the similarities between keloids and tumors, the
efficacy of Dm-dNK combined with prodrug BVDU or araT for the
treatment of keloids was investigated in the present study. The
results revealed that lentivirus-mediated Dm-dNK was successfully
expressed in keloid fibroblasts and presented with high enzymatic
activity. Furthermore, the Dm-dNK/nucleoside analog system had
significant efficacy in destroying keloid fibroblasts.
The bystander effect, which may result in the
destruction of more cells than those transduced with a suicide
gene, serves an important role in keloid fibroblast therapy
(23). There are two possible
mechanisms by which the bystander effect may be induced by a
suicide gene/prodrug system (23).
Notably, the bystander effect may be caused by the transfer of
phosphorylated prodrug through intercellular gap junctions and the
phagocytosis of apoptotic vesicles containing the prodrug
metabolites from suicide gene-expressing cells (7,15,24–26).
In the present study, the lethal effect was observed when 25% of
fibroblasts were transduced with Lenti-CMV-dNK in the presence of
BVDU or araT; however, no marked difference was observed in the
lethal effect among the groups with 50, 75 or 100%
Dm-dNK+ fibroblasts. Furthermore, when only 25% of
fibroblasts were Dm-dNK+, the bystander effect was
notable. Thus, the lethal bystander effects may effectively enhance
the inhibition the growth of keloid fibroblasts and might destroy
more keloid fibroblasts cells.
To date, various treatment methods, including
assisted-physical treatment, chemotherapy and photodynamic
treatment for keloid fibroblasts have emerged (27). Arno et al (27) indicated that some members of the
transforming growth factor (TGF)-β superfamily, including Smads,
Ski, SnoN, Fussels, endoglin, DS-Sily, Cav-1p, AZX100 and
thymosin-β4 and other associated molecules, may be targets for
preventing and treating keloid and hypertrophic scars. Fan et
al (2) suggested that
oxymatrine (OMT) is associated with the TGF-β/Smad signaling
pathway and inhibits collagen synthesis. This indicates that OMT
may prevent keloid and other fibrotic diseases. Furthermore, a
previous study reported that Wharton's jelly-derived mesenchymal
stem cells from human umbilical cords exhibited antifibrotic
properties through paracrine signaling (3). Differently, the principle of suicide
gene therapy is the transduction of cells with a gene that encodes
an enzyme that can convert an inactive prodrug into a cytotoxic
metabolite, regardless of pathogenic mechanism, and this strategy
was always used for cancer treatment. The present study
demonstrated that Dm-dNK can be expressed in human KF cell; the
enzyme retained its enzymatic activity and the cells expressing
Dm-dNK exhibited increased sensitivity to some cytotoxic nucleoside
analogs, including BVDU and araT. Additionally, the bystander
effect may enhance KF cell death. The findings of the present study
indicated that Dm-dNK/prodrugs may exhibit a therapeutic potential
to treat keloids and may become a novel treatment method of
clinical keloids; however, in vivo experimental models of
this suicide gene strategy require further evaluation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81172199
and 81272920).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS analyzed and interpreted the patient data and
performed parts of experiments. HJ was a major contributor in
writing the manuscript and performed parts of experiments. MG
performed majority of experiments. XZ designed the whole project
and revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Aoyang Hospital (Zhangjiagang, China) and written
informed consent was provided by all patients.
Consent for publication
The patient, or parent, guardian or next of kin (in
case of deceased patients) provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mogili NS, Krishnaswamy VR, Jayaraman M,
Rajaram R, Venkatraman A and Korrapati PS: Altered angiogenic
balance in keloids: A key to therapeutic intervention. Transl Res.
159:182–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan DL, Zhao WJ, Wang YX, Han SY and Guo
S: Oxymatrine inhibits collagen synthesis in keloid fibroblasts via
inhibition of transforming growth factor-β1/Smad signaling pathway.
Int J Dermatol. 51:463–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arno AI, Amini-Nik S, Blit PH, Al-Shehab
M, Belo C, Herer E and Jeschke MG: Effect of human wharton's jelly
mesenchymal stem cell paracrine signaling on keloid fibroblasts.
Stem Cells Transl Med. 3:299–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malekshah OM, Chen X, Nomani A, Sarkar S
and Hatefi A: Enzyme/prodrug systems for cancer gene therapy. Curr
Pharmacol Rep. 2:299–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johansson M, van Rompay AR, Degrève B,
Balzarini J and Karlsson A: Cloning and characterization of the
multisubstrate deoxyribonucleoside kinase of Drosophila
melanogaster. J Biol Chem. 274:23814–23819. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Springer CJ and Niculescu-Duvaz I:
Prodrug-activating systems in suicide gene therapy. J Clin Invest.
105:1161–1167. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freeman SM, Abboud CN, Whartenby KA,
Packman CH, Koeplin DS, Moolten FL and Abraham GN: The ‘bystander
effect’: Tumor regression when a fraction of the tumor mass is
genetically modified. Cancer Res. 53:5274–5283. 1993.PubMed/NCBI
|
|
8
|
Munch-Petersen B, Knecht W, Lenz C,
Søndergaard L and Piskur J: Functional expression of a
multisubstrate deoxyribonucleoside kinase from Drosophila
melanogaster and its C-terminal deletion mutants. J Biol Chem.
275:6673–6679. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munch-Petersen B, Piskur J and Sondergaard
L: Four deoxynucleoside kinase activities from Drosophila
melanogaster are contained within a single monomeric enzyme, a
new multifunctional deoxynucleoside kinase. J Biol Chem.
273:3926–3931. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng X, Johansson M and Karlsson A:
Retroviral transduction of cancer cell lines with the gene encoding
Drosophila melanogaster multisubstrate deoxyribonucleoside
kinase. J Biol Chem. 275:39125–39129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang N, Zhao L, Ma S, Gu M and Zheng X:
Lentivirus-mediated expression of Drosophila melanogaster
deoxyribonucleoside kinase driven by the hTERT promoter combined
with gemcitabine: A potential strategy for cancer therapy. Int J
Mol Med. 30:659–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang N, Dong X, Sun Y, Cai X, Zheng C, He
A, Xu K and Zheng X: Cytotoxic effects of adenovirus- and
lentivirus-mediated expression of Drosophila melanogaster
deoxyribonucleoside kinase on Bcap37 breast cancer cells. Oncol
Rep. 29:960–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma S, Qu W, Mao L, Zhu Z, Jia L, Zhao L
and Zheng X: Antitumor effects of oncolytic adenovirus armed with
Drosophila melanogaster deoxyribonucleoside kinase in
colorectal cancer. Oncol Rep. 27:1443–1450. 2012.PubMed/NCBI
|
|
14
|
Sandrini MP, Clausen AR, On SL, Aarestrup
FM, Munch-Petersen B and Piskur J: Nucleoside analogues are
activated by bacterial deoxyribonucleoside kinases in a
species-specific manner. J Antimicrob Chemother. 60:510–520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng X, Johansson M and Karlsson A:
Bystander effects of cancer cell lines transduced with the
multisubstrate deoxyribonucleoside kinase of Drosophila
melanogaster and synergistic enhancement by hydroxyurea. Mol
Pharmacol. 60:262–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao J, Black ME and Caruso M: Enhanced
ganciclovir killing and bystander effect of human tumor cells
transduced with a retroviral vector carrying a herpes simplex virus
thymidine kinase gene mutant. Hum Gene Ther. 11:1569–1576. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beltinger C, Uckert W and Debatin KM:
Suicide gene therapy for pediatric tumors. J Mol Med (Berl).
78:598–612. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hackman T, Doubrovin M, Balatoni J,
Beresten T, Ponomarev V, Beattie B, Finn R, Bornmann W, Blasberg R
and Tjuvajev JG: Imaging expression of cytosine deaminase-herpes
virus thymidine kinase fusion gene (CD/TK) expression with
[124I]FIAU and PET. Mol Imaging. 1:36–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu B, Liu ZZ, Zhu GY, Yang JF, Zhao JP,
Wang JC and Cai JL: Efficacy of recombinant adenovirus-mediated
double suicide gene therapy in human keloid fibroblasts. Clin Exp
Dermatol. 33:322–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma S, Zhao L, Zhu Z, Liu Q, Xu H,
Johansson M, Karlsson A and Zheng X: The multisubstrate
deoxyribonucleoside kinase of Drosophila melanogaster as a
therapeutic suicide gene of breast cancer cells. J Gene Med.
13:305–311. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Z, Zhao L, He A, Huang B, Karlsson A,
Xu H and Zheng X: Retrovirus-mediated Drosophila
melanogaster multisubstrate deoxyribonucleoside kinase gene
therapy of gastric cancer cells in vitro and in vivo. Anticancer
Res. 30:2641–2649. 2010.PubMed/NCBI
|
|
22
|
Zhu Z, Mao L, Zhao L, Sun Z, Wang Z, Xu H
and Zheng X: Synergistic therapeutic effect in gastric cancer cells
produced by oncolytic adenovirus encoding Drosophila
melanogaster deoxyribonucleoside kinase. Cancer Biol Ther.
11:874–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aghi M, Hochberg F and Breakefield XO:
Prodrug activation enzymes in cancer gene therapy. J Gene Med.
2:148–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bi WL, Parysek LM, Warnick R and Stambrook
PJ: In vitro evidence that metabolic cooperation is responsible for
the bystander effect observed with HSV tk retroviral gene therapy.
Hum Gene Ther. 4:725–731. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seachrist L: Successful gene therapy has
researchers looking for the bystander effect. J Natl Cancer Inst.
86:82–83. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fick J, Barker FG II, Dazin P, Westphale
EM, Beyer EC and Israel MA: The extent of heterocellular
communication mediated by gap junctions is predictive of bystander
tumor cytotoxicity in vitro. Proc Natl Acad Sci USA.
92:11071–11075. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arno AI, Gauglitz GG, Barret JP and
Jeschke MG: New molecular medicine-based scar management
strategies. Burns. 40:539–551. 2014. View Article : Google Scholar : PubMed/NCBI
|