Introduction
Morbidity of ovarian cancer ranks the third among
malignancies of the female genital tract. However, ovarian cancer
ranks first regarding mortality (1). Ovarian cancer has indistinct clinical
symptoms and hidden onset, and there is currently no reliable early
detection method (1). Thus, ~70%
of ovarian cancer patients are in advanced stages at initial
diagnosis. In addition, patients have frequently developed
extensive metastases. It is estimated that the 5-year survival rate
among these patients is only 30–45% (2). Epithelial ovarian cancer (EOC)
accounts for 80–90% of all ovarian cancer cases. It is the most
common pathological type of ovarian cancer. The standard treatment
regimen for patients with EOC at first diagnosis is maximum
cytoreductive surgery with postoperative adjuvant chemotherapy,
most commonly platinum-based chemotherapy (2).
A feature of microRNA (miR) expression is its tissue
specificity (3). Research has
demonstrated that miR expression abundance and function are
different in tumor tissues of various histological origins
(4). miR expression in various
types of tumor tissues and normal control tissues have been
analyzed (5). The results suggest
that tumors of different histological types have distinct miR
expression profiles (5).
Furthermore, different tumor types can be distinguished through the
differentially expressed miRs. Currently, research on miRs
generally concentrates on investigating tumorigenesis, development
and tumor-targeted therapy (6).
miRs have an important regulatory role in the genesis and
development of human tumors. This suggests that miRs may become a
novel direction for the diagnosis and treatment of human tumor
(4). Zhang et al (7) demonstrated that serum miR-195 is a
novel non-invasive biomarker for the detection of cervical
cancer.
Vascular endothelial growth factor (VEGF)
specifically binds the vascular endothelial growth factor receptor
(VEGFR) (8). Thus, it has a vital
role in tumor invasion and metastasis (3). Recent research has demonstrated that
ovarian cancer cells express VEGFR and VEGF. This indicates that
VEGF can directly act on ovarian cancer cells in an autocrine and
paracrine manner (9). Previous
studies have verified that VEGF is involved in the genesis,
development, invasion and metastasis of ovarian cancer (10).
Protein kinase B (AKT) is a serine/threonine protein
kinase. It is also the downstream target protein of
phosphoinositide 3-kinase (PI3K) (10). In addition, it is also the central
link of the PI3K/AKT signal transduction pathway. AKT is involved
in the genesis and development of multiple tumors (11). AKT regulates cell survival,
apoptosis, proliferation, malignant tumorigenesis and
chemoresistance (12). The
expression of AKT has been demonstrated to be upregulated in
ovarian cancer. In addition, AKT activation can inhibit ovarian
cancer cell apoptosis (11) and is
involved in the genesis, development, invasion and metastasis of
ovarian cancer (13). Sun et
al (14) indicate that miR-195
suppressed cell growth in renal cell carcinoma via PI3K/AKT
signaling pathways. Therefore, the present study aimed to
investigate the functional effects of miR-195 on ovarian cancer
cells and the underlying mechanism involved.
Materials and methods
Clinical cohorts
Peripheral blood samples from female patients with
ovarian cancer (n=8) and healthy female volunteers (n=8) were
collected and these patients were diagnosed at different stages at
the Department of Third Gynecological, Third Affiliated Hospital of
Qiqihar Medical College (Qiqihar, China) between December 2015 and
March 2016. Patient details are presented in Table I. A total of 5 ml peripheral blood
was obtained from patients with ovarian cancer and healthy
volunteers and following centrifugation at 2,000 × g for 10 min at
4°C, the obtained serum samples were preserved at −80°C.
 | Table I.Demographic and clinical features of
patients with ovarian cancer and healthy volunteers. |
Table I.
Demographic and clinical features of
patients with ovarian cancer and healthy volunteers.
| Characteristics | Ovarian cancer
(n=8) | Healthy volunteers
(n=8) |
|---|
| Age (years) | 48.92±8.35 | 47.44±7.34 |
| TNM stage |
|
|
| I | 1 |
|
| II | 2 |
|
|
III–IV | 5 |
|
| Type |
|
|
|
Carcinoma | 6 |
|
|
Adenocarcinoma | 2 |
|
Cell culture and transfections
Human OVCAR-3 ovarian cancer cells were purchased
from Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified incubator with 5% CO2.
miR-195 (5′-UAGCAGCACAGAAAUGGC-3′), miR-195 inhibitor
(5′-CAGUACUUUUGUGUAGUACAA-3′) and negative mimic
(5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). OVCAR-3 cells were transfected with
100 nM miR-195, miR-195 inhibitor and negative mimic using
Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
transfection for 4 h, AKT inhibitor (100 nM; LY294002) or VEGFR2
inhibitor (10 nM; vandetanib) was added post-transfection for 48 h
at 37°C. The control group was transfected with negative mimic.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and microarray samples
Total RNA was extracted from cell or serum sample
using a TRIzol™ reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA (200 ng) was converted into cDNA using
the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). qPCR was accomplished using FastStart Universal
SYBR Green Master mix (Rox; Invitrogen; Thermo Fisher Scientific,
Inc.) by the ABI PRISM® 7300 real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 94°C for 3 min; 40 cycles
of 94°C for 30 sec, 65°C for 40 sec and 72°C for 30 sec; final
extension at 72°C for 5 min. The primer sequences were as follows:
miR-195, forward 5′-ACACTCCAGCTGGGTAGCAGCACAGAAAT-3′ and reverse
5′-TGGTGTCGTGGAGTCG-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and
reverse 5′-AACGCTTCACGAATTTGCGT-3′. Relative expression level was
computed using 2−ΔΔCq method (15).
Total RNA (200 ng) was amplified by Low Input
Quick-Amp Labeling kit (Agilent Technologies, Inc., Santa Clara,
CA, USA) and labeled with Cy3 (Agilent Technologies, Inc.).
Cy3-labeled cRNAs were used for hybridization in TaqMan®
Array Standard Plates (cat. no. 4413266, Thermo Fisher Scientific,
Inc.). Cy3-signal scanned images were quantified using a Feature
Extraction 10.5.1.1 image analysis software (Agilent Technologies,
Inc.).
Cell proliferation assay
Following transfection for 24, 48 and 72 h, cell
viability was measured by the MTT assay. A total of 20 µl MTT (0.5
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added
into each well and incubated for 4 h at 37°C. Then, the medium was
removed and 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA)
were added to cell and incubated for 20 min at 37°C. The optical
density was detected with a Multiskan EX (Thermo Fisher Scientific,
Inc.) at 490 nm.
Apoptosis rate
Following transfection for 48 h, OVCAR-3 cells were
harvested and stained with Annexin V-PE and propidium iodide using
an Apoptosis kit (BD Pharmingen; BD Biosciences, Franklin Lakes,
NJ, USA) according to the manufacturer's protocol. The rate of
apoptosis was quantified with a flow cytometer
(FACSCalibur™ system; BD Biosciences) and apoptosis rate
was analyzed using FlowJo software (version 7.6.1; FlowJo LLC,
Ashland, OR, USA).
Caspase-3/9 activity
Caspase-3/9 activity of cells was measured using
Caspase-3/9 activity kits (Beyotime Institute of Biotechnology,
Shanghai, China) following transfection for 48 h. The optical
density was detected with a Multiskan EX (Thermo Fisher Scientific,
Inc.) at 450 nm.
Western blotting
Following transfection for 48 h, cell proteins were
extracted using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology) and the protein concentration was
determined using a bicinchoninic acid kit (Beyotime Institute of
Biotechnology). Equal amount of proteins (30–50 µg) were separated
on 8–12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (Mai Bio Co., Ltd., Shanghai, China). The membrane was
blocked using 5% skimmed milk for 1 h at 37°C and incubated with
Bcl2 associated X apoptosis regulator (Bax; 1:1,000; cat no.
sc-6236; Santa Cruz Biotechnology), VEGFR2 (1:2,000; sc-6236; Santa
Cruz Biotechnology), phosphorylated (p)-AKT (1:1,000; cat no.
sc-7985-R; Santa Cruz Biotechnology) and GAPDH (1:5,000; cat no.
ab8245; Abcam, Cambridge, UK) at 4°C overnight. The membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat no. 7054; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 2 h at room temperature. Enhanced
chemiluminescence reagent (Beyotime Institute of Biotechnology) was
used to develop the protein and semi-quantified using Bio-Rad
Laboratories Quantity One software 3.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Data were represented as the mean ± standard
deviation of three independent experiments. All data were analyzed
with one-way analysis of variance followed by Tukey's post-hoc test
using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-195 in ovarian
cancer
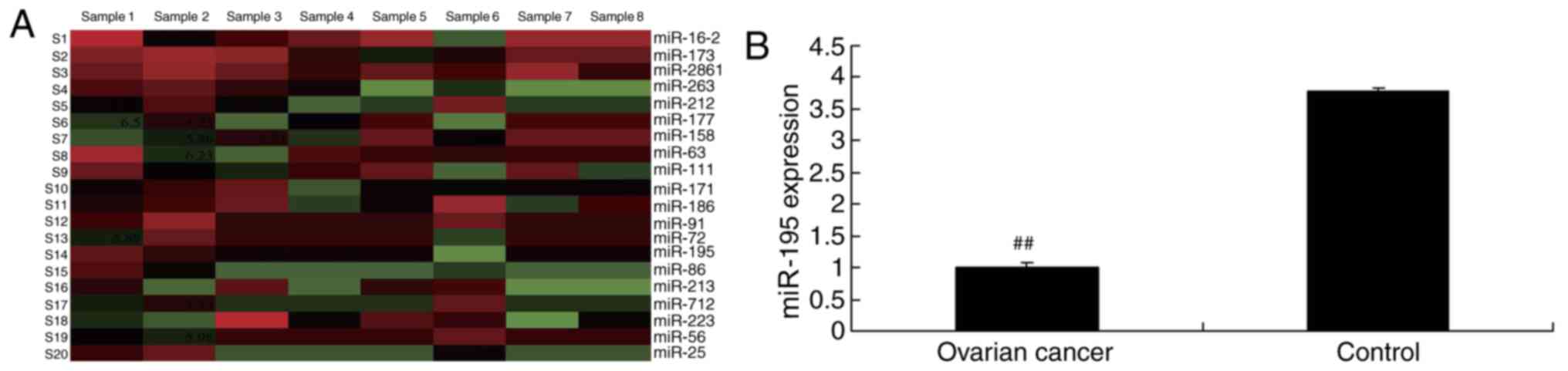
To observe the association between the expression of
miR-195 and human ovarian cancer, RT-qPCR was used to measure the
expression of miR-195 levels. As presented in Fig. 1, the expression of miR-195 was
significantly downregulated in ovarian cancer serum samples,
compared with the control normal group (P<0.01). These data
suggest that the expression of miR-195 may be implicated in the
pathogenesis of human ovarian cancer.
miR-195 suppresses cell proliferation
and induces apoptosis of ovarian cancer cells
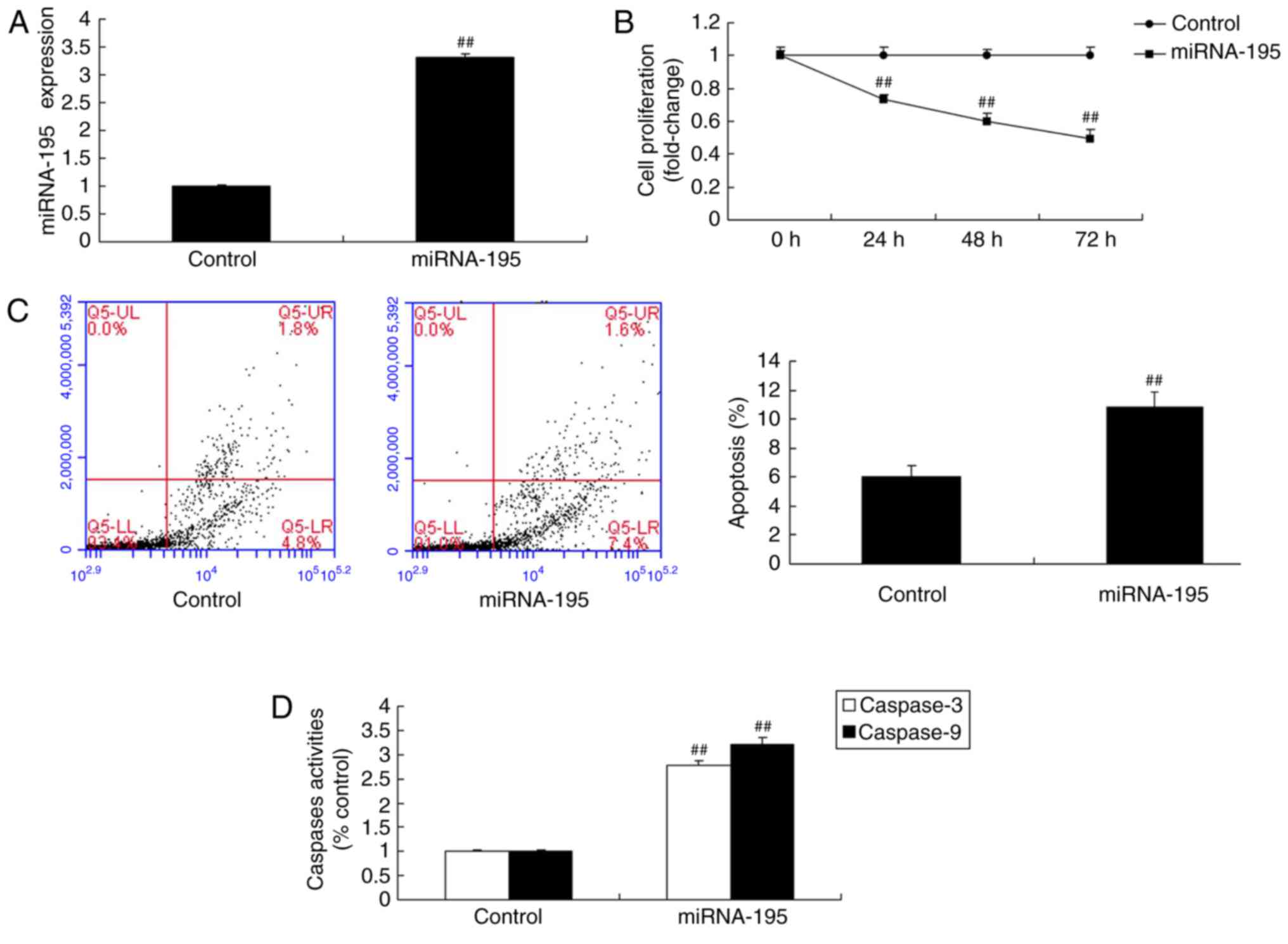
To validate the effects of miR-195 on the growth and
death of OVCAR-3 ovarian cancer cells, an miR-195 mimic was used to
significantly increase miR-195 expression (P<0.01; Fig. 2A). Fig. 2B-D demonstrated that miR-195
suppressed cell proliferation, and induced apoptosis and
caspase-3/9 activity in ovarian cancer cells, compared with the
control group.
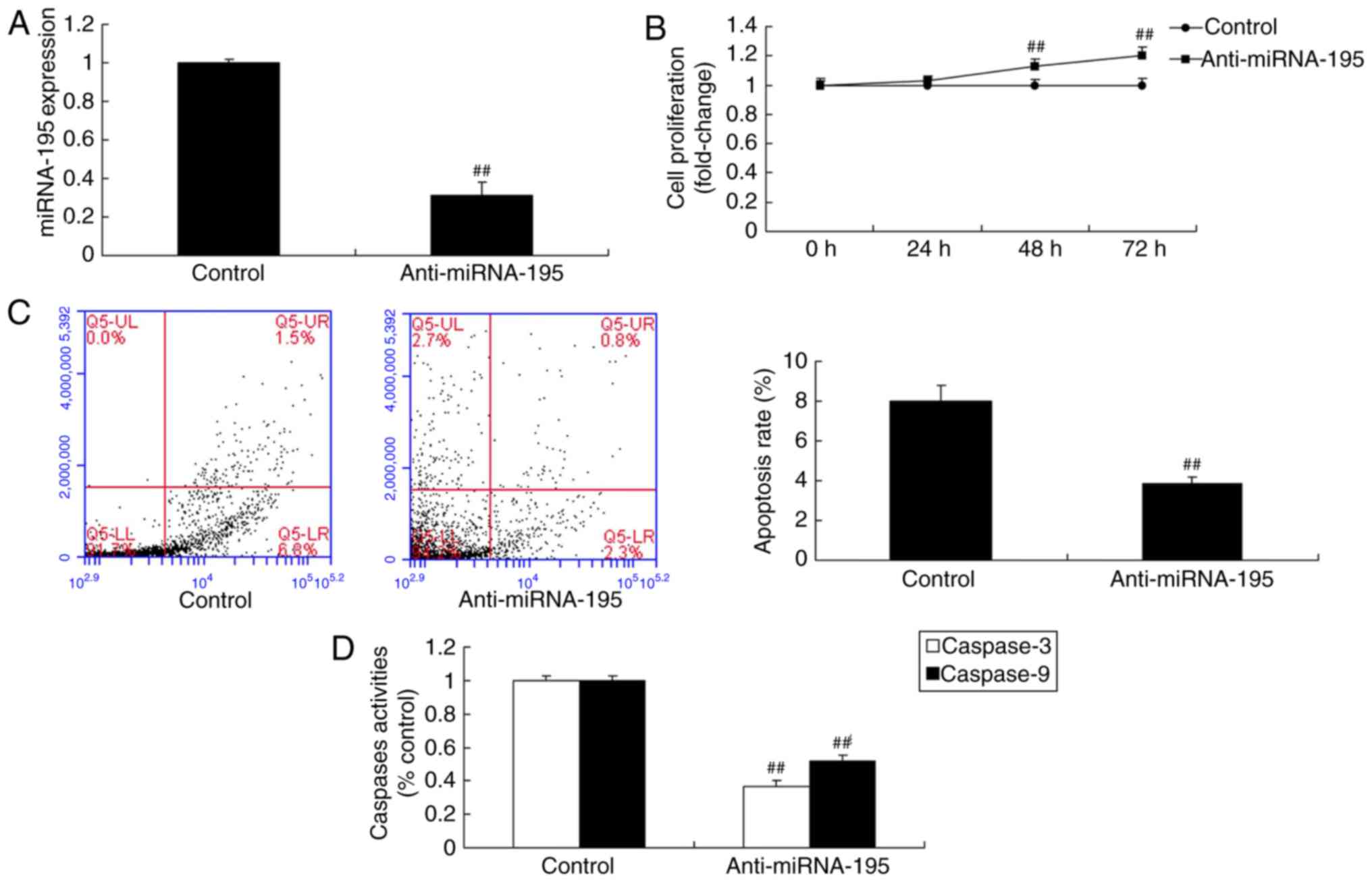
miR-195 inhibitor increases cell
proliferation and inhibits apoptosis of ovarian cancer cells
An miR-195 inhibitor mimic was used to significantly
reduce miR-195 expression, and the miR-195 inhibitor significantly
increased cell proliferation and inhibited apoptosis of ovarian
cancer cells (P<0.01; Fig.
3).
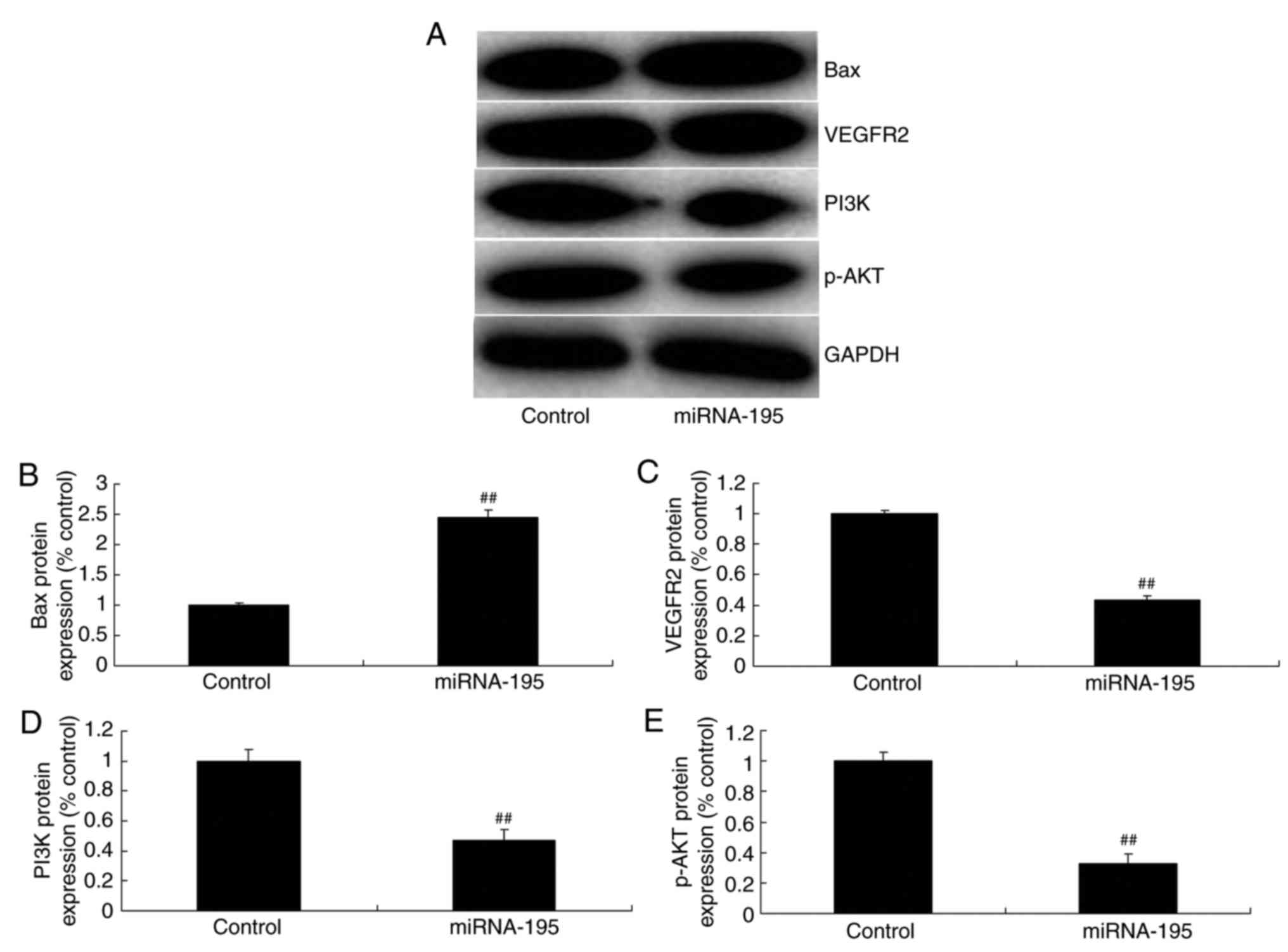
miR-195 induces Bax and suppresses
VEGFR2 and p-AKT protein expression in ovarian cancer cells
The effect of miR-195 on apoptosis of ovarian cancer
cells, was observed and the VEGFR2 and PI3K/AKT signaling pathway
was selected for investigation. As demonstrated in Fig. 4, miR-195 significantly induced Bax
and suppressed VEGFR2, PI3K and p-AKT expression in ovarian cancer
cells, compared with the control group (P<0.01; Fig. 4).
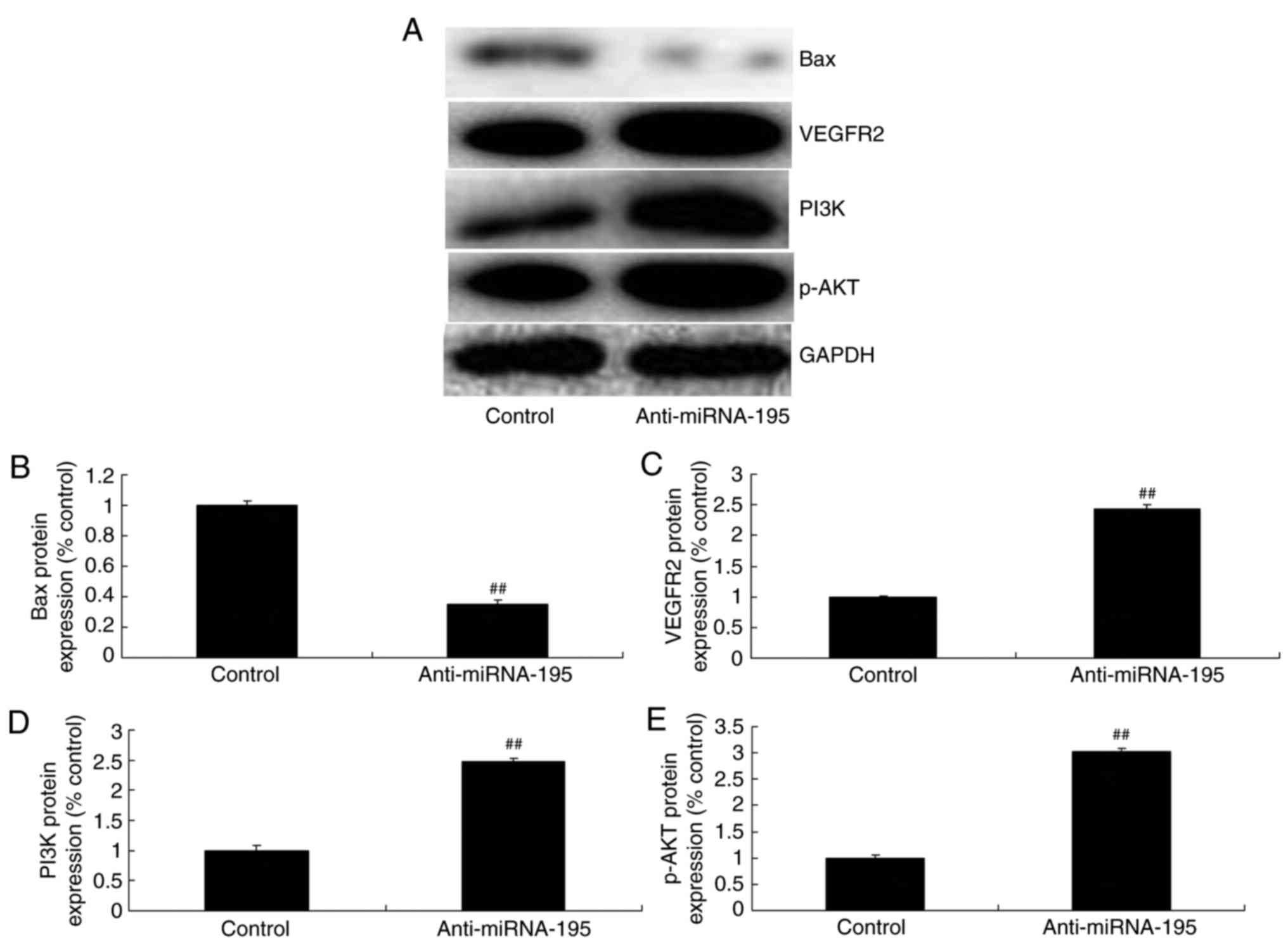
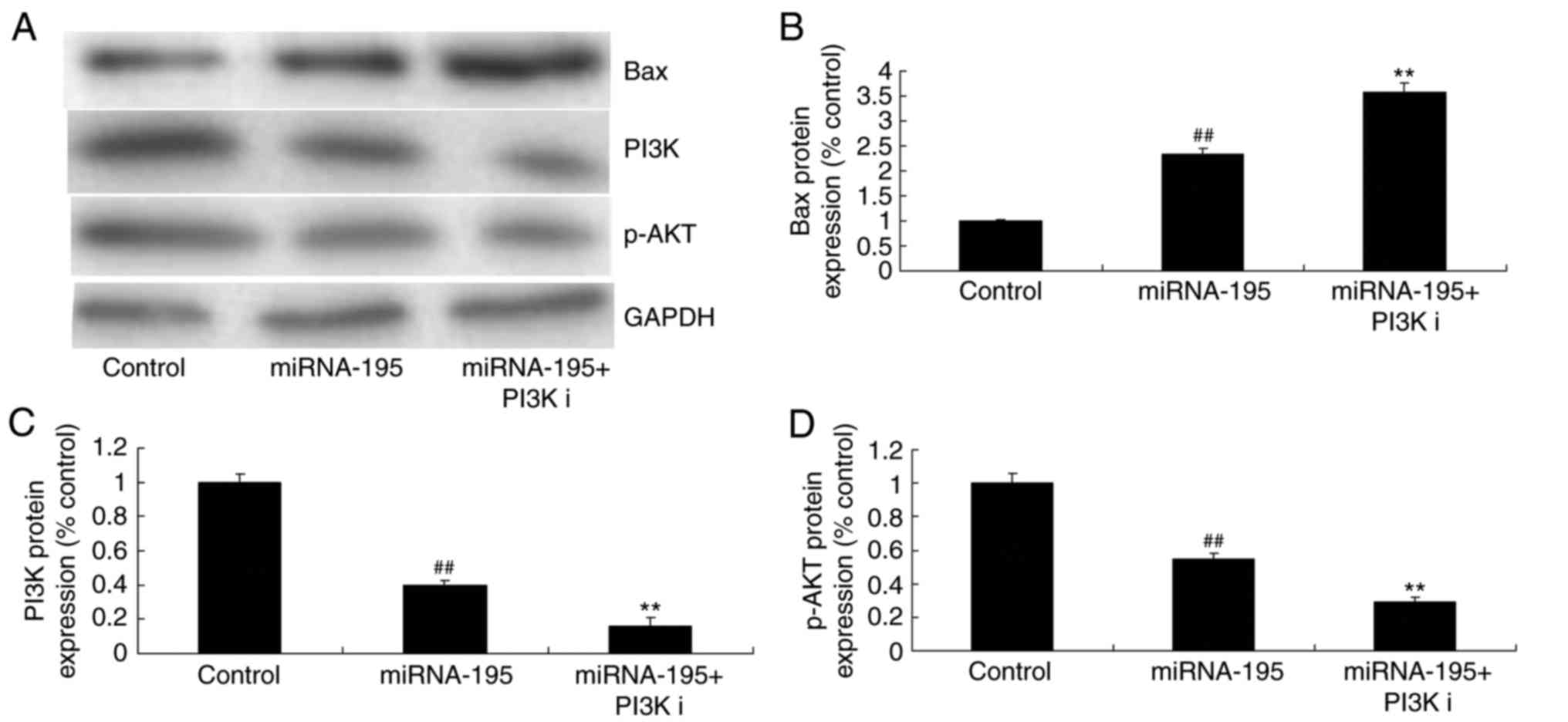
miR-195 inhibitor suppresses Bax and
induces VEGFR2 and p-AKT protein expression in ovarian cancer
cells
The anti-miR-195 significantly suppressed Bax
expression, and induced VEGFR2, PI3K and p-AKT expression in
ovarian cancer cells, compared with the control group (P<0.01;
Fig. 5). These results demonstrate
that the role of VEGFR2 and PI3K/AKT signaling pathways in miR-195
induced apoptosis regulation requires further investigation.
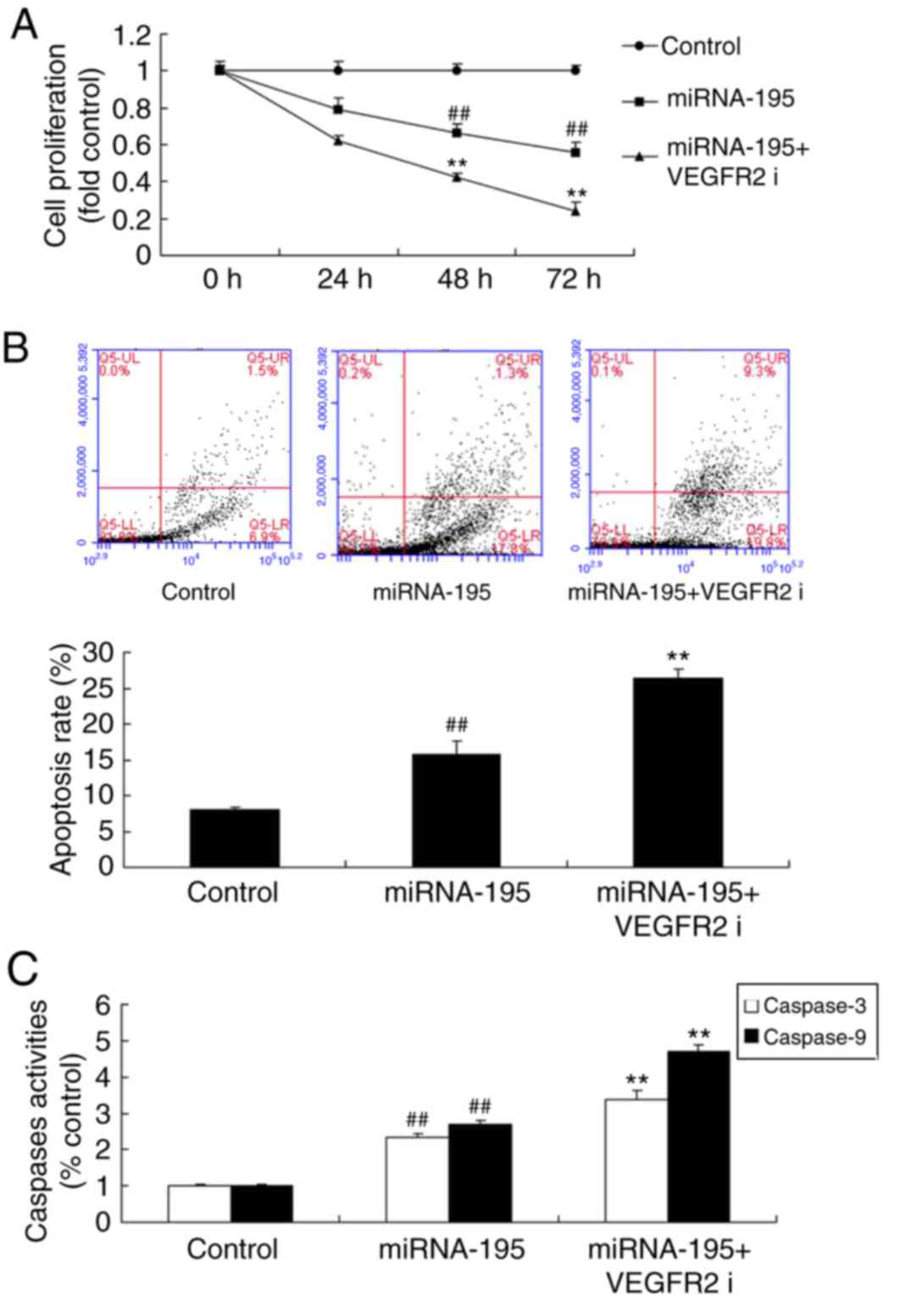
Inhibition of VEGFR2 increases the
functional effects of miR-195 on apoptosis of ovarian cancer
cells
To investigate the role of miR195 in regulating
VEGFR2 in ovarian cancer cells, a VEGFR2 inhibitor, vandetanib (10
nM) was added to cells following transfection with miR-195 mimics.
Vandetanib significantly increased the functional effects of
miR-195 on the inhibition of cell proliferation, and the promotion
apoptosis and caspase-3/9 activity of ovarian cancer cells,
compared with miR-195 group (Fig.
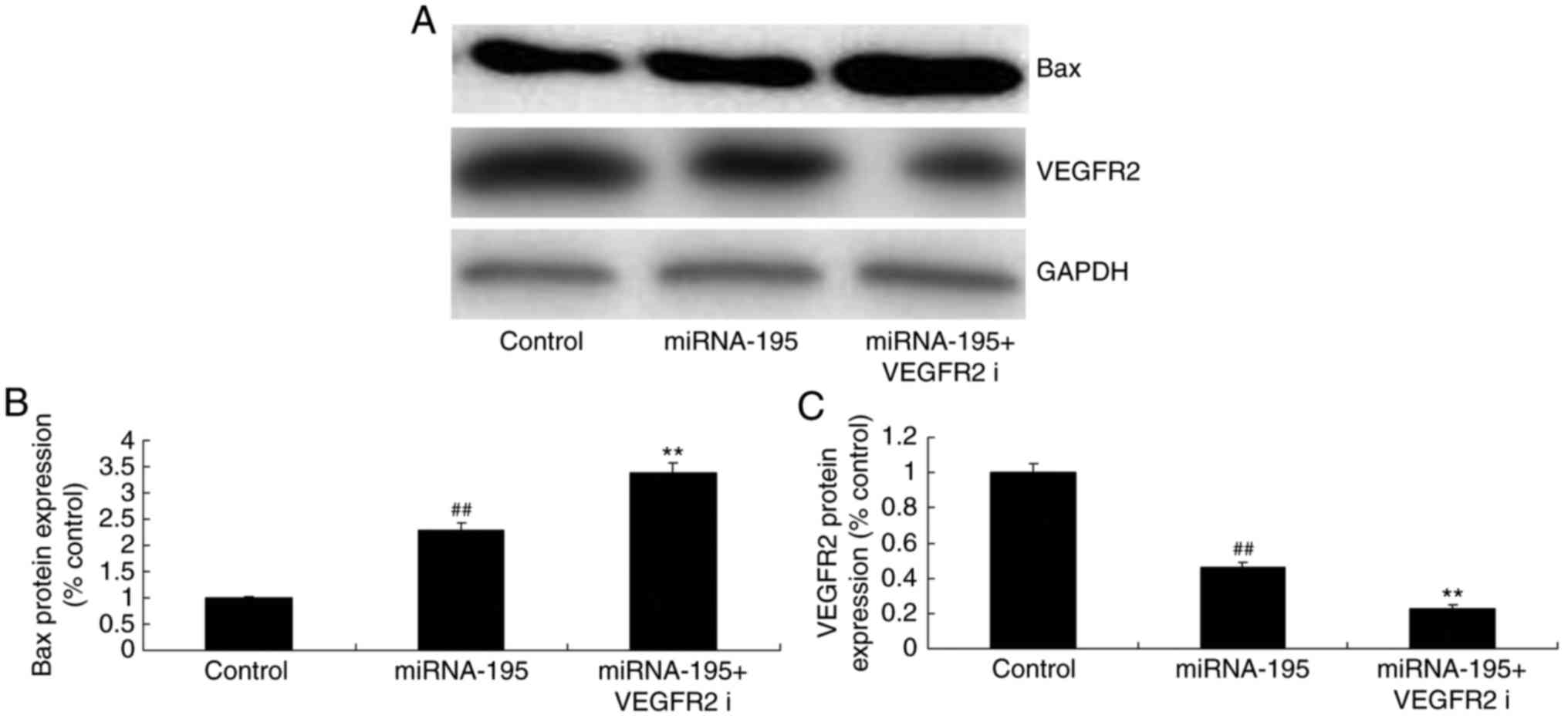
6). Furthermore, the inhibition of VEGFR2 significantly
suppressed VEGFR2 and increased Bax protein expression (P<0.01;
Fig. 7).
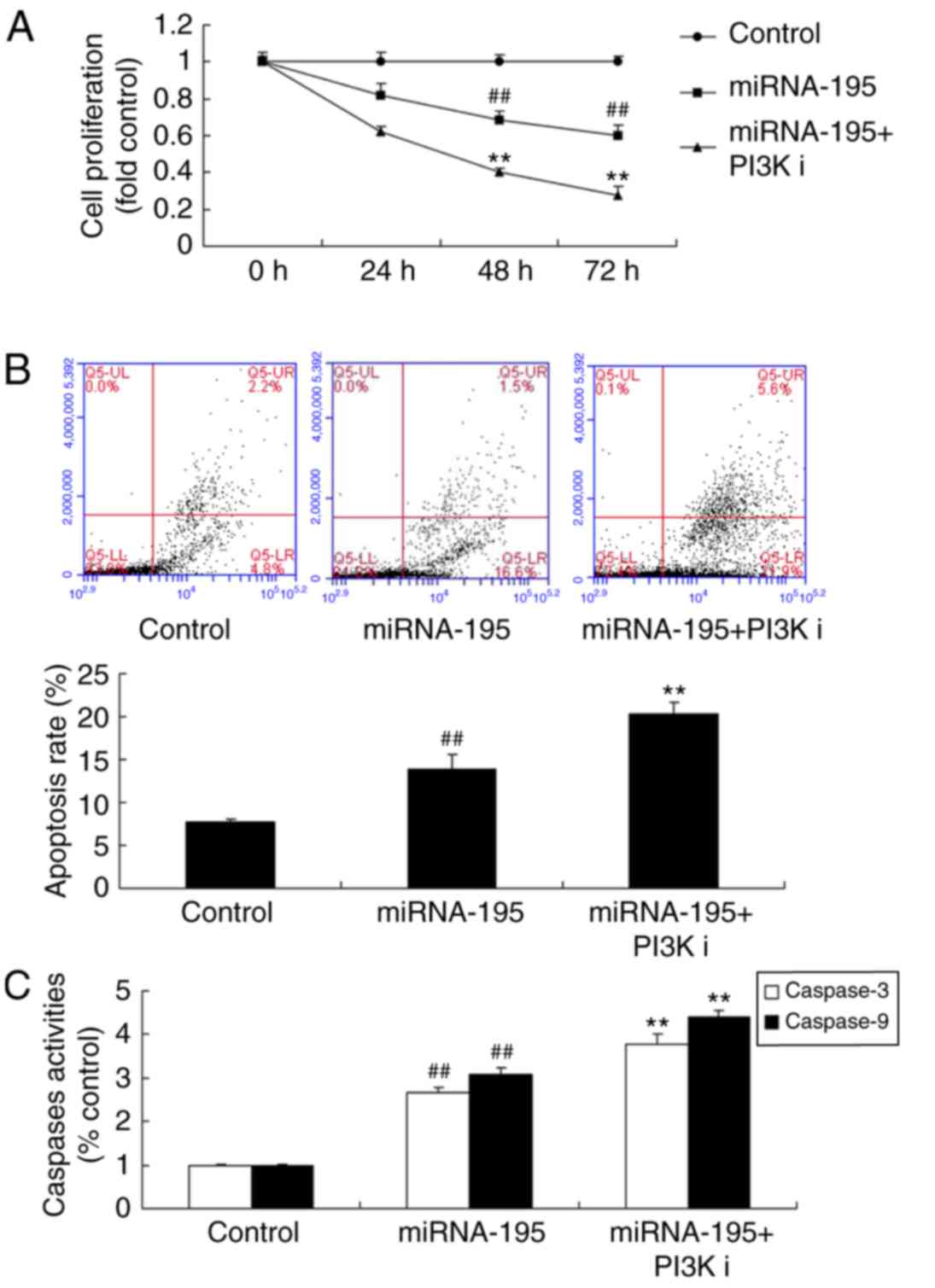
Inhibition of AKT increases the
functional effects of miR-195 on apoptosis of ovarian cancer
cells
The role of AKT on the functional effects of miR-195
on apoptosis of ovarian cancer cells was investigated. An AKT
inhibitor LY294002 (100 nM), increased the functional effects of
miR-195 on apoptosis of ovarian cancer cells (Fig. 8), and increased Bax, and suppressed
PI3K and p-AKT expression, compared with the miR-195 group
(Fig. 9).
Discussion
Ovarian cancer is a common malignancy of the female
genital tract. Epithelial ovarian cancer is the most common
histopathological type of ovarian cancer (1). The mortality rate for ovarian cancer
is the highest of all gynecological malignancies (16). Ovarian cancer has silent onset and
~2/3 patients are in the advanced stage at the onset of symptoms
(16). In the current study, the
expression of miR-195 was downregulated in ovarian cancer serum
samples, compared with the normal control group.
miRs can directly regulate the genesis and
development of ovarian cancer (17). The known mechanisms include miR
gene deletion and epigenetic modification (17). In addition, abnormal expression of
key enzymes involved in miR synthesis is also involved in the
development of ovarian cancer. Further studies on the
differentially expressed miRs in tissue and serum of patients with
ovarian cancer should be performed. This is of great important for
the diagnosis and treatment of ovarian cancer (18). The results of the present study
demonstrated that miR-195 suppressed cell proliferation and induced
apoptosis of ovarian cancer cells. Only the OVCAR-3 cell line was
used, which is a deficiency in the present study and other cell
lines will be used in future studies.
VEGFA in the VEGF protein family is crucial in
angiogenesis (19). The biological
functions of VEGF are achieved through binding with receptors on
the target cell surface (19).
VEGFR2 is the major receptor through which VEGF family proteins
exert their biological functions (8). There are autocrine or paracrine loops
of VEGFA and VEGFR2 in tumor cells and vascular endothelial cells,
which stimulate angiogenesis (17). Notably, the present study
demonstrated that miR-195 suppressed VEGFR2 protein expression in
ovarian cancer cells. Sun et al (14) demonstrated that miR-195 suppresses
angiogenesis and metastasis of hepatocellular carcinoma through
VEGF expression. Wang et al (20) reported that miR-195-5p inhibits
tumorigenesis of gastric cancer.
AKT is the downstream target of PI3K. The
phosphorylated form of AKT is its active form. The activated
phosphorylated AKT detaches from the cell membrane (12). Subsequently, it moves through the
cytoplasm or to the cell nucleus. It can therefore activate or
inhibit downstream protein molecules (21). The downstream protein molecules
include mammalian target of rapamycin, Bad, caspase-9, tuberin,
glycogen synthase kinase-3β and the forkhead transcription factor
family. Additionally, it can also mediate cell apoptosis,
proliferation and angiogenesis (22). In the present study, it was
demonstrated that miR-195 suppressed the p-AKT level in ovarian
cancer cell. Sun et al (14) indicated that miR-195 suppressed
cell growth in renal cell carcinoma via PI3K/AKT signaling
pathways. However, only p-AKT expression was measured in the
present study, not total AKT expression. Thus, the exact effect of
miR-195-5p on AKT cannot be determined and requires further
study.
In summary, miR-195 suppressed cell proliferation
and induced the apoptosis of ovarian cancer cells through
suppression of VEGFR2 and AKT signaling pathway proteins. These
findings indicate that miR-195 may be a novel noninvasive
biomarker, which would provide considerable diagnostic value in
screening for ovarian cancer.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JC designed the experiment, performed the
experiment, analyzed the data and wrote the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committee of the Third Affiliated Hospital of Qiqihar Medical
College (Qiqihar, China). Written informed consent was provided by
all participants.
Consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5:e14792014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Jin B, Chen L, Zhuo D, Zhang Z, Gong
K and Mao Z: MiR-30d induces apoptosis and is regulated by the
Akt/FOXO pathway in renal cell carcinoma. Cell Signal.
25:1212–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xin Q, Li J, Dang J, Bian X, Shan S, Yuan
J, Qian Y, Liu Z, Liu G, Yuan Q, et al: miR-155 deficiency
ameliorates autoimmune inflammation of systemic lupus erythematosus
by targeting S1pr1 in Faslpr/lpr mice. J Immunol. 194:5437–5445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mi L, Chen Y, Zheng X, Li Y, Zhang Q, Mo D
and Yang G: MicroRNA-139-5p suppresses 3T3-L1 preadipocyte
differentiation through notch and IRS1/PI3K/Akt insulin signaling
pathways. J Cell Biochem. 116:1195–1204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishnan K, Steptoe AL, Martin HC,
Pattabiraman DR, Nones K, Waddell N, Mariasegaram M, Simpson PT,
Lakhani SR, Vlassov A, et al: miR-139-5p is a regulator of
metastatic pathways in breast cancer. RNA. 19:1767–1780. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maoa R, Zou F, Yang L, Lin S, Li Y, Ma M,
Yin P, Liang X and Liu J: The loss of MiR-139-5p promotes
colitis-associated tumorigenesis by mediating PI3K/AKT/Wnt
signaling. Int J Biochem Cell Biol. 69:153–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Zhang D, Wang F, Xu D, Guo Y and
Cui W: Serum miRNAs panel (miR-16-2*, miR-195, miR-2861, miR-497)
as novel non-invasive biomarkers for detection of cervical cancer.
Sci Rep. 5:179422015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alevizos I and Illei GG: MicroRNAs in
Sjögren's syndrome as a prototypic autoimmune disease. Autoimmun
Rev. 9:618–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mameli G, Arru G, Caggiu E, Niegowska M,
Leoni S, Madeddu G, Babudieri S, Sechi GP and Sechi LA: Natalizumab
therapy modulates miR-155, miR-26a and proinflammatory cytokine
expression in MS patients. PLoS One. 11:e01571532016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Xiao X, Shen Y, Chen L, Xu C, Zhao
H, Wu Y, Zhang Q, Zhong J, Tang Z, et al: MicroRNA-32 promotes
calcification in vascular smooth muscle cells: Implications as a
novel marker for coronary artery calcification. PLoS One.
12:e01741382017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li M and Zhang J: Circulating MicroRNAs:
Potential and emerging biomarkers for diagnosis of cardiovascular
and cerebrovascular diseases. Biomed Res Int.
2015:7305352015.PubMed/NCBI
|
|
12
|
Ke ZP, Xu P, Shi Y and Gao AM: MicroRNA-93
inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by
targeting PTEN. Oncotarget. 7:28796–28805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schulte C, Karakas M and Zeller T:
microRNAs in cardiovascular disease-clinical application. Clin Chem
Lab Med. 55:687–704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun P, Wang L, Lu Y, Liu Y, Li L, Yin L,
Zhang C, Zhao W, Shen B and Xu W: MicroRNA-195 targets VEGFR2 and
has a tumor suppressive role in ACHN cells via PI3K/Akt and
Raf/MEK/ERK signaling pathways. Int J Oncol. 49:1155–1163. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JQ, Papp G, Póliska S, Szabó K, Tarr
T, Bálint BL, Szodoray P and Zeher M: MicroRNA expression profiles
identify disease-specific alterations in systemic lupus
erythematosus and primary Sjögren's syndrome. PLoS One.
12:e01745852017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jimenez SA and Piera-Velazquez S:
Potential role of human-specific genes, human-specific microRNAs
and human-specific non-coding regulatory RNAs in the pathogenesis
of systemic sclerosis and Sjogren's syndrome. Autoimmun Rev.
12:1046–1051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams AE, Choi K, Chan AL, Lee YJ,
Reeves WH, Bubb MR, Stewart CM and Cha S: Sjögren's
syndrome-associated microRNAs in CD14(+) monocytes unveils targeted
TGFβ signaling. Arthritis Res Ther. 18:952016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tandon M, Gallo A, Jang SI, Illei GG and
Alevizos I: Deep sequencing of short RNAs reveals novel microRNAs
in minor salivary glands of patients with Sjögren's syndrome. Oral
Dis. 18:127–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Li L, Jiang M and Li Y:
MicroRNA-195 inhibits human gastric cancer by directly targeting
basic fibroblast growth factor. Clin Transl Oncol. 19:1320–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ustun N, Aras M, Ozgur T, Bayraktar HS,
Sefil F, Ozden R and Yagiz AE: Thymoquinone attenuates trauma
induced spinal cord damage in an animal model. Ulus Travma Acil
Cerrahi Derg. 20:328–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohta K, Hoshino H, Wang J, Ono S, Iida Y,
Hata K, Huang SK, Colquhoun S and Hoon DS: MicroRNA-93 activates
c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by
directly inhibiting PTEN and CDKN1A. Oncotarget. 6:3211–3224. 2015.
View Article : Google Scholar : PubMed/NCBI
|