Introduction
Infection with hepatitis B virus (HBV) is a public
health issue worldwide (1).
Epidemiological studies have estimated that 350 million people are
chronic carriers of HBV, with the potential to develop acute and
chronic hepatitis, which is a major risk factor for the development
of hepatocellular carcinoma (2,3).
Standard treatment regimens such as pegylated interferons (IFNs)
and nucleoside/nucleotide analogs are used to treat chronic
hepatitis B but are only partially successful (4–7).
Drug resistance and severe side effects limit current therapies for
chronic HBV infection (8).
The role of adaptive immune responses in the control
of HBV infection have been extensively investigated. Toll-like
receptors (TLRs) accept pathogen-associated molecule patterns, and
activate antiviral mechanisms including intracellular antiviral
pathways and the production of antiviral effectors like IFNs and
pro-inflammatory cytokines. Activation of the TLR3 signaling is
necessary for the induction of liver damage induced by Concanavalin
A in vivo; TLR3 regulates inflammation and the adaptive T
cell immune response in the absence of viral infection (9). A previous study indicated that the
ubiquitin proteasome pathway regulates the HBV life cycle through
TLR signaling pathway (8). The
ubiquitin proteasome system (UPS) is a conserved cellular signaling
pathway that controls several critical processes in the cell by
regulating expression of the proteins involved in cell cycle, DNA
repair, innate immunity, and other processes (2). The UPS mediates the majority of
protein degradation in eukaryotic cells preventing accumulation of
damaged, misfolded and mutant proteins via proteolysis (10,11).
The UPS serves an important role in the cellular response to
multiple stimulation signals. Although the primary function of
ubiquitin was associated with proteolysis, it is now considered as
a key regulator of functions such as signaling cascades,
transcription, apoptosis or oncogenesis. Malfunction of the UPS is
associated with the development of multiple pathological processes
including metabolic disorders, immune diseases, inflammation and
cancer (12).
Traditional Chinese medicine has served a role in
the prevention of hepatitis B in China. The characteristics of
Chinese medicine with its multi-links and multi-pathways are
increasingly demonstrating great therapeutic potential and
development prospects. Bushen recipe used in traditional Chinese
medicine and is mainly comprised of herba epimedii, cuscuta
chinensis lam and radix rehmanniae recen, is used to tonify
kidneys. However, many Chinese studies recently demonstrated that
Bushen recipe is effective for the treatment of numerous diseases
such as early stage diabetes, osteoporosis, osteosarcoma and
chronic liver disease and our research group previously
demonstrated another important efficacy on HBV (13–16).
Furthermore, our previous study indicated that not only the whole
prescription of Bushen Recipe, but also its disassembled
prescriptions may exhibit certain efficacy on liver injury to an
extent (Nie et al, unpublished data). It was suggested that
Bushen Recipe and its disassembled prescriptions have a
bi-directional regulatory effect on ubiquitin ligase, and the
ubiquitination of myeloid differentiation primary response (MyD)88
is affected by the ubiquitin proteasome pathway, which can inhibit
the production of inflammatory mediator and activate
TIR-domain-containing adapter-inducing interferon-β (TRIF) and
tumor necrosis factor (TNF) receptor-associated factor-binding
kinase (TBK)1. Ultimately the ubiquitin proteasome signaling
pathway could serve a dual effect as an anti-inflammatory and
inhibitor of replication of hepatitis B virus. In the present
study, through simulation of the liver injury induced by viral
hepatitis attacks by Concanavalin A treatment, it was verified that
Bushen prescription exerted an anti-inflammatory and protective
effect on the liver through the multi-link regulation of
immunity.
Materials and methods
Materials
A total of 60 normal Balb/c mice were purchased from
Shanghai Jiesijie Experimental Animals Co., Ltd. (Shanghai, China)
and 40 HBV transgenic mice were obtained from the Guangzhou 458th
Hospital of Chinese People's Liberation Army (Guangzhou, China).
Concanavalin A was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The primary antibodies used in the western
blot analysis were as follows: Anti-TLR4 (cat. no. NB100-56566),
anti-TLR3 (cat. no. NB100-56571) and anti-TLR9 (cat. no.
nbp2-24729), purchased from Novus Biologicals LLC (Littleton, CO,
USA); anti-TNF-associated factor (TRAF)6 (cat. no. ab33915),
anti-IFN regulatory factor (IRF)3 (cat. no. ab25950), anti-TBK1
(cat. no. ab109735), anti-TRAF-interacting protein (TIRP) (cat. no.
ab205100) purchased from Abcam (Cambridge, UK); anti-nuclear factor
(NF)-κB (cat. no. 8242), anti-MyD88 (cat. no. 4283) purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). The secondary
antibodies were obtained from R&D Systems, Inc. (Minneapolis,
MN, USA). ELISA kits to detect interleukin (IL)-6, IL-1, TNF-α and
IFN-γ were purchased from Wuhan Huamei Biotech Co., Ltd. (Wuhan,
China). Alanine aminotransferase Assay Kit (cat. no. C009-2) and
Cholinesterase assay kit (cat. no. A023-2) were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Animal experiments
The experimental mice were fed in Specific Pathogen
Free Animal Rooms of Animal Experimental Center of Shanghai
University of Traditional Chinese Medicine (Shanghai, China) with
free access to clean drinking water and food. All animal
experiments were carried out according to the regulations of and
approved by Laboratory Animal Administration of Shanghai University
of Traditional Chinese Medicine. All the mice were divided into two
groups: Group 1 and group 2. The group 1 were all normal and the
group 2 were all HBV transgenic mice: A replicative HBV transgenic
mouse produced by the Department of Genetics Engineering,
Infectious Disease Center, Guangzhou 458th Hospital of Chinese
People's Liberation Army (Guangzhou, China). The HBV genome was
transferred into the genotype of 1.3 times D genotype by
microinjection of fertilized eggs, serum HBs Ag >2,000 U/ml, HBV
DNA levels of 104−106 copies/ml, HBV
replication intermediates exist in the liver. All the experimental
mice were germ-free grade, 6–8 weeks old, weighing 20–22 g, and
purchased following testing for HBs Ag. Animals of both sexes
(males/females 1:1) were included. Mice in both groups were divided
into seven sub-groups: A, Control; B, Concanavalin A; C, Bushen
recipe (BS); D, Bushen-yang (BSY); E, Bushen-yin (BSC); F, QingHua
(QH); G, GanYanLing (GYL). Each sub-group in group 1 had 8 mice,
while 5 mice in each sub-group in group 2; the remaining mice were
not employed. Acute liver injury was induced using tail vein
injection of Concanavalin A in both normal and HBV transgenic mice
from B to G sub-groups and the mice in each sub-group were treated
with different traditional Chinese medicines (diluted in saline),
respectively: A, (Control): Normal Saline; B, (Concanavalin A):
with Normal Saline, 15 mg/kg; C, (BS): the Whole Bushen recipe; D,
(BSY): the disassembled prescriptions of Bushen recipe; E, (BSC):
the disassembled prescriptions of Bushen recipe; F, (QH): The
disassembled prescriptions of Bushen recipe; G, (GYL).
These traditional Chinese medicines were prepared in
our laboratory or were purchased from different companies and their
effective constituents and manufacturer were enumerated as
follows:
The Whole Bushen recipe (BS) was prepared according
to the formula of ‘Bushen Granule’ (Shanghai Pharmacy) which was
produced in our laboratory and is comprised of 15 g herba epimedii,
15 g cuscuta chinensis lam, 15 g cistanche deserticola ma, 15 g
fallopia multiflora, 15 g radix rehmanniae recen, 15 g lyceum
barbaruml, 15 g giant knotweed rhizome, 15 g radix scutellariae, 15
g radix sophorae flavescentis and 10 g pericarpium citri
reticulatae viride.
The Bushen-yang group (BSY) of Bushen recipe was
boil-free particles of Chinese medicine and comprised of 15 g herba
epimedii, 15 g cuscuta chinensis lam, 15 g cistanche deserticola ma
which was produced by Shenzhen Resources Sanjiu Modern Chinese
Medicine Co., Ltd. (Shenzhen, Guangdong, China).
The Bushen-yin group (BSC) of Bushen recipe was also
boil-free particles of Chinese medicine and comprised of 15 g
fallopia multiflora, 15 g radix rehmanniae, 15 g lyceum barbaruml
that was produced by Shenzhen Resources Sanjiu Modern Chinese
Medicine Co., Ltd.
The QingHua group (QH) of Bushen Recipe has 15 g
giant knotweed rhizome, 15 g radix scutellariae, 15 g radix
sophorae flavescentis and was produced by Shenzhen Resources Sanjiu
Modern Chinese Medicine Co., Ltd. These were all produced as
boil-free particles of Chinese medicine.
GYL was purchased from Changshu Lei Yun Shang
Pharmaceutical Co., Ltd (Changshu, China) and had 35 mg matrine in
2 ml solution of GYL.
The administration dose of these drugs was
calculated according to the human and mice dose conversion method
in the second edition of ‘Experimental Zoology’. The conversion
coefficient between human (~70 kg) and mice (~20 g) is 0.0026.
Assuming the dose for human (70 kg) is n, the dose for mice may be
calculated as: n mg/kg × 70 kg ×0.0026/20 g=9.1n mg/kg. Therefore,
the dosage which was used in this study was 9 times of the adult
daily dose. The mice in sub-groups A to F underwent intragastric
administration, while intraperitoneal injection occurred for
sub-group G. The drug was administered once daily for 7 days at a
dose of 18 mg/kg.
Microplate method to detect liver
function indexes
The mice were sacrificed by excessive ether
inhalation at 18 h after injection with Concanavalin A and
administration with Bushen recipe and its disassembled
prescriptions. Peripheral blood samples (0.5 ml) were collected
from hearts of mice and centrifuged at 375 × g for 30 min at 4°C to
obtain serum for the following experiments. Subsequently, serum was
seeded into 96-well plates and detection reagents were added
according to the manufacturer's protocol of Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Finally, these plates
were analyzed at a wavelength of 505 nm by a Thermo Scientific
Varioskan Flash (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
to detect the content of alanine aminotransferase (ALT) and
cholinesterase (ChE).
Hematoxylin and eosin staining
Mice were sacrificed at 18 h following injection
with Concanavalin A and administration with Bushen recipe and its
disassembled prescriptions, liver tissues of all mice were obtained
to inspect liver injury and antiviral effect of Bushen recipe and
its disassembled prescriptions, and they were fixed with 10%
paraformaldehyde. Subsequently, the paraffin-embedded sections were
prepared for histological analysis. The slices were cut in 5 µm
sections and stained in dye liquor of hematoxylin (3 min) and eosin
(15 sec) at room temperature for histological evaluation. Finally,
these slices were observed under an inverted microscope (ix73,
Olympus Corporation, Tokyo, Japan) and evaluated by a pathologist
blindly. A total of five random fields of hepatic lobule were
imaged at ×200 magnification.
Western blot analysis
Liver tissues of all mice were homogenized and cells
were suspended in PBS. The cell suspension was centrifuged with PBS
at 600 × g at 4°C and were lysed with radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Jiangsu,
China) for 30 min at 4°C. Subsequently, cell lysates were
centrifuged at 9,000 × g at 4°C for 15 min and a Takara BCA Protein
Assay Kit (cat. no. T9300A; Takara Bio, Inc., Otsu, China) was used
to detect the total protein concentrations. Subsequently, total
protein was mixed with an equal volume of loading buffer and boiled
for 5 min. Equal amounts of proteins (20 µg) in each lane were
separated by 10% SDS-polyacrylamide gel electrophoresis and then
transferred to Immobilon-polyvinylidene fluoride Transfer Membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5%
nonfat milk in TBS with Tween 20 (TBST) for 1 h at room temperature
and incubated with the indicated primary antibodies overnight at
4°C in a dilution of 1:1,000. Following washing 3 times with TBST,
these membranes were incubated with secondary antibodies, including
IgG(H+L) horseradish peroxidase (HRP)-labeled goat anti-mouse IgG
(cat. no. A0216) and IgG(H+L) HRP-labeled goat anti-rabbit IgG
(cat. no. A0208), purchased from Beyotime Institute of
Biotechnology, for 1 h at room temperature at a dilution of
1:10,000. GAPDH served as the loading control. Finally, membranes
were visualized using the enhanced chemiluminescence system (ECL;
PerkinElmer Inc., Waltham, MA, USA).
ELISA analysis
Mouse IL-6 ELISA kit (cat. no. EK0411) and mouse
TNF-α ELISA kit (cat. no. EK0527) were purchased from Boster
(Hubei, China). Mouse IFN-γ ELISA kit was purchased from R&D
Systems, Inc. (cat. no. MIF00). Mouse IL-1 ELISA kit was purchased
from Mlbio (cat. no. ml037875, Shanghai, China). ELISA analysis in
the present study was conducted according to the manufacturer's
protocol of Wuhan Sanying Bioengineering (Wuhan, China). In brief,
blank wells without any solution were set in a 96-well plate and
100 µl negative control (PBS with 0.05% Tween-20), positive control
or serum from rats of the aforementioned experimental groups per
well were added into the test wells. The plate was covered with the
adhesive strip and incubated in a humidified atmosphere of 5%
CO2 at 37°C for 1 h. Subsequently, 50 µl of
HRP-conjugated antibodies were added into each well (apart from the
blank well). Subsequently, plates were covered with another
adhesive strip and incubated for 30 min at 37°C again. Following
this, wells were washed with wash buffer (included in kit; 200 µl
per well) three times and were left to dry for 20 sec completely
removing the liquid. Following washing, any remaining wash buffer
was removed through inverting the plate on clean paper towels. In
addition, 50 µl substrate A and 50 µl substrate B were added to
each well and plates were incubated for 30 min at 37°C in the dark.
Finally, 50 µl of stop solution was added into each well, and
plates were gently tapped to mix the solution. Finally, optical
density (OD) of each well was determined within 10 min (25°C) using
a Thermo Scientific Varioskan Flash (Thermo Fisher Scientific,
Inc.) at a wavelength of 450 nm.
Statistical analysis
All experiments in the present study were repeated
at least three times. All data are presented as the mean ± standard
deviation. Data were analyzed by SPSS software, version 18.0 (SPSS,
Inc., Chicago, IL, USA). Two-way analysis of variance and
Student-Neuman-Keuls method were used to determine statistical
significance among experimental groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Bushen recipe and its disassembled
prescriptions inhibit liver injury by affecting liver function
indexes
ALT and ChE are both synthesized in liver and may
reflect the degree of liver injury. Following injection of
Concanavalin A, liver tissues of all mice presented liver injury
and the Bushen recipe and its disassembled prescriptions have
previously been demonstrated to decrease liver injury. Therefore,
the expression levels of ALT and ChE were investigated in
peripheral blood of normal and HBV transgenic mice following
treatment with Concanavalin A, Bushen Recipe and its disassembled
prescriptions. As presented in Fig.
1, the results demonstrated that the content of ALT was
increased immediately following administration of Concanavalin A,
whereas the ChE declined in the liver tissues of both normal and
HBV transgenic mice compared with the control. However, this
increase of ALT was suppressed following administration of Bushen
Recipe and its disassembled prescriptions (Fig. 1A), while the expression of ChE was
upregulated (Fig. 1B). These
results indicated that Bushen Recipe and its disassembled
prescriptions alleviated the liver injury induced by Concanavalin A
as observed within the Bushen Recipe and disassembled prescription
groups compared with in the Con A group.
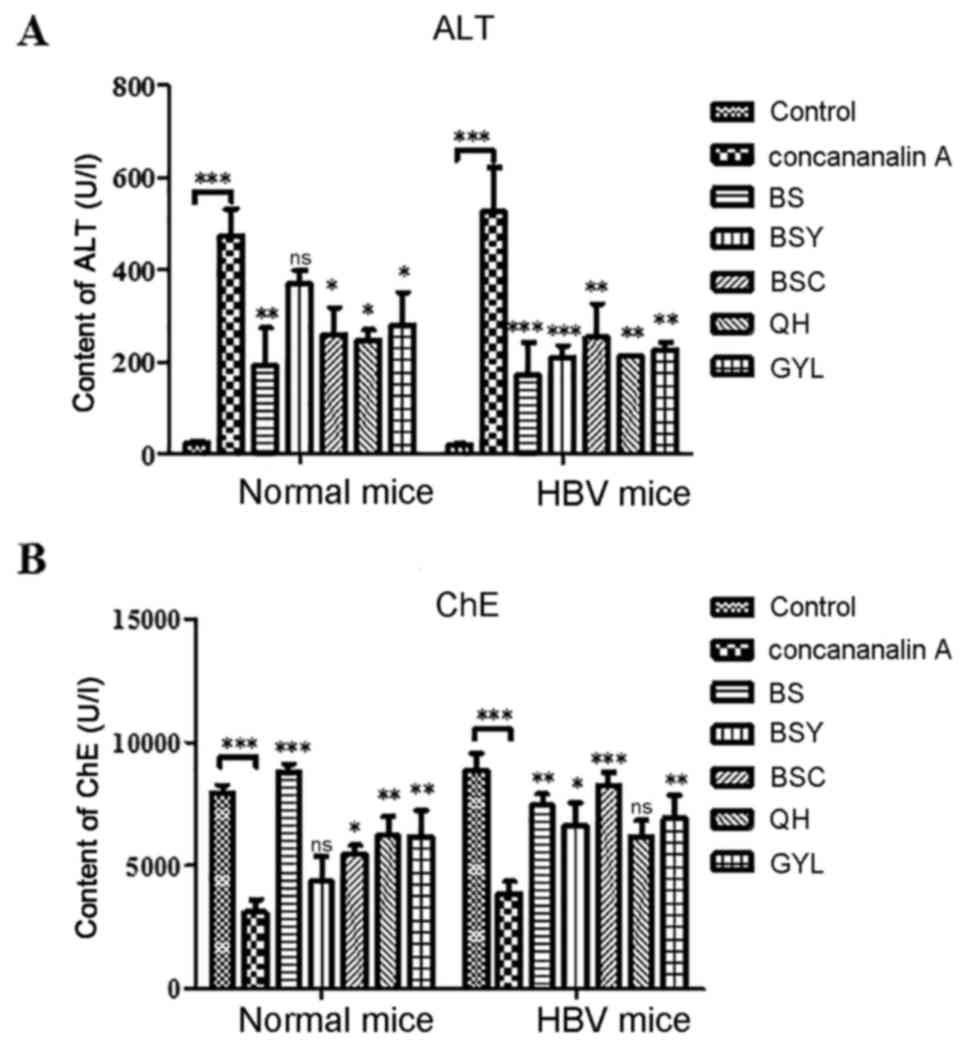 | Figure 1.Results of liver function indexes.
Mice liver function indexes (A) ALT and (B) ChE in serum were
determined. ns indicates no statistical significance, *P<0.05,
**P<0.01, ***P<0.001, vs. the Concanavalin A group or.
control group. ALT, Alanine aminotransferase; BS, Bushen recipe;
BSC, Bushen-yin; BSY, Bushen-yang; ChE, Cholinesterase; GYL,
GanYanLing; HBV, hepatitis B virus; IFN, interferon; IL,
interleukin; QH, QingHua; NS, not significant. |
Bushen recipe and its disassembled
prescriptions repair liver injury induced by Concanavalin A
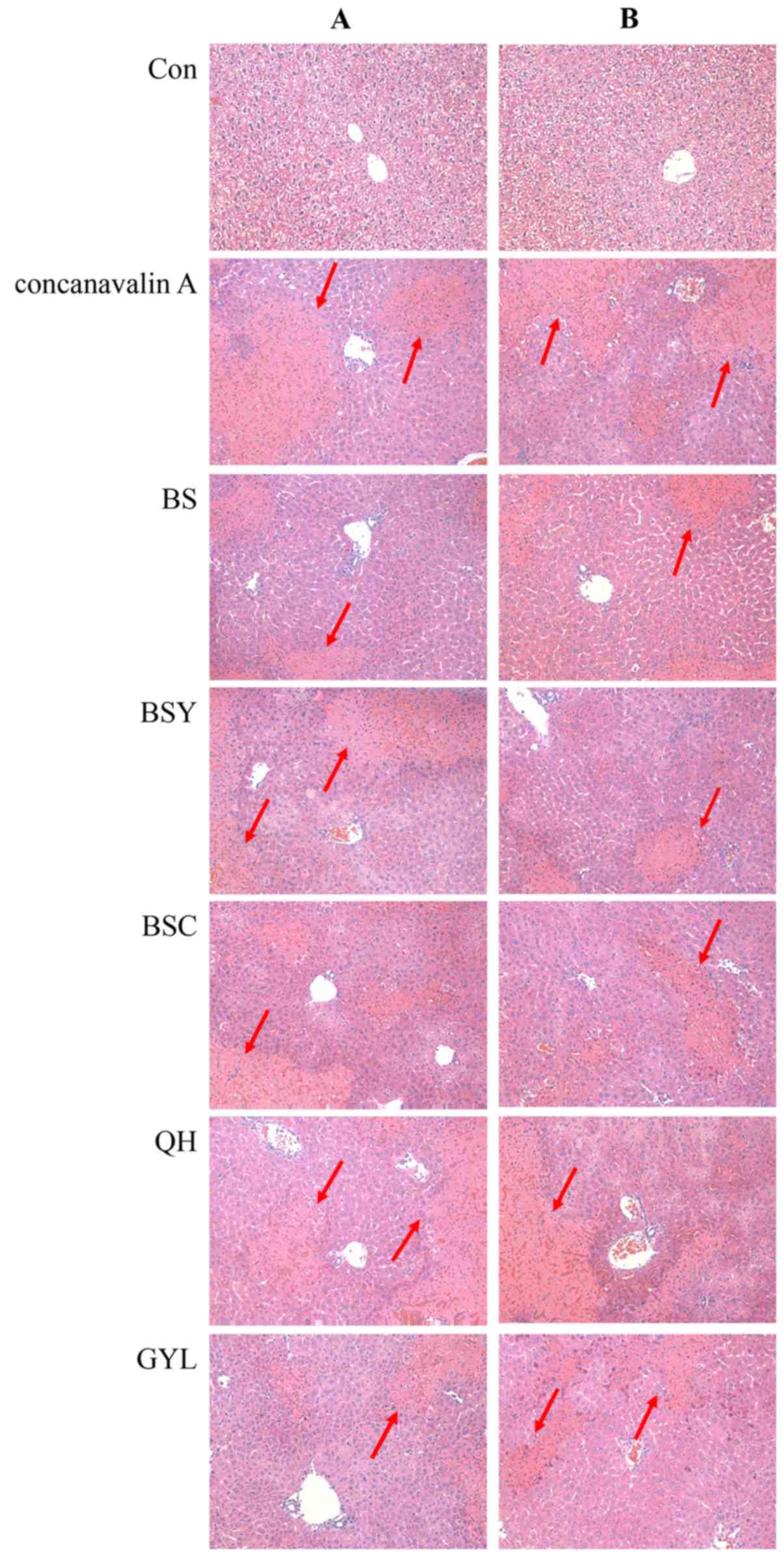
Livers of mice demonstrated acute immune hepatitis
following injection of Concanavalin A. The results obtained in this
experiment are presented in Fig.
2. Liver cells of normal mice were well-formed and the
structure of hepatic cord was clear and intact. The hepatic lobule
had no necrosis (which is a form of cell injury resulting in the
premature death of cells in living tissue by autolysis), bleeding,
and infiltration of inflammatory cells. However, the opposite
phenomenon was observed in the sections of mice injected with
Concanavalin A, whereas Bushen recipe and its disassembled
prescriptions relieved the pathological features in the HBV
transgenic mice, for example, the hepatic necrotic area was
significantly decreased in the Bushen recipe and its disassembled
prescriptions treated groups. The results indicated that Bushen
recipe and its disassembled prescriptions relieved the liver injury
induced by Concanavalin A.
Bushen recipe and its disassembled
prescriptions repair liver injury through inhibition of TLR3/9
signaling pathway
Protein expression levels of MyD88, TBK1, TRAF6,
IRF3, TLR3, TLR9, TLR4, NF-κB and TIRP that are members of TLR3/9
signaling pathway in all mice were detected by western blot
analysis. In Figs. 3–5, the protein expression levels of MyD88,
TBK1, IRF3, TLR4, TLR3, TRAF6 and NF-κB were increased in normal
mice following administration of Concanavalin A compared with the
mice in control group without any treatment. However, these
expression levels did not increase in HBV transgenic mice following
administration of Concanavalin A (Figs. 6–8). The increased expression of MyD88,
IRF3, TBK1 and NF-κB due to immunological hepatitis has been
reported previously, which is in agreement with the present
findings (17). Furthermore,
following administration with Bushen recipe and its disassembled
prescriptions, these proteins had no upregulated trend in either
normal or HBV groups. NF-κB further decreased in HBV groups
following treatment with Bushen recipe and its disassembled
prescriptions (Figs. 6–8). The expression of TRAF6 in QH group
and GYL group decreased more notably upon interaction of
corresponding treatment. Additionally, compared with the control
group, the expression levels of MyD88, TBK1, TRAF6, IRF3, TLR3,
TLR9 and TIRP were decreased to varying extents, indicating the
pharmaceutical effect of Bushen recipe and all its disassembled
prescriptions. In conclusion, these results indicated that the
TLR3/9 signaling pathway may be inhibited by Bushen Recipe and its
disassembled prescriptions to repair liver injury induced by
Concanavalin A.
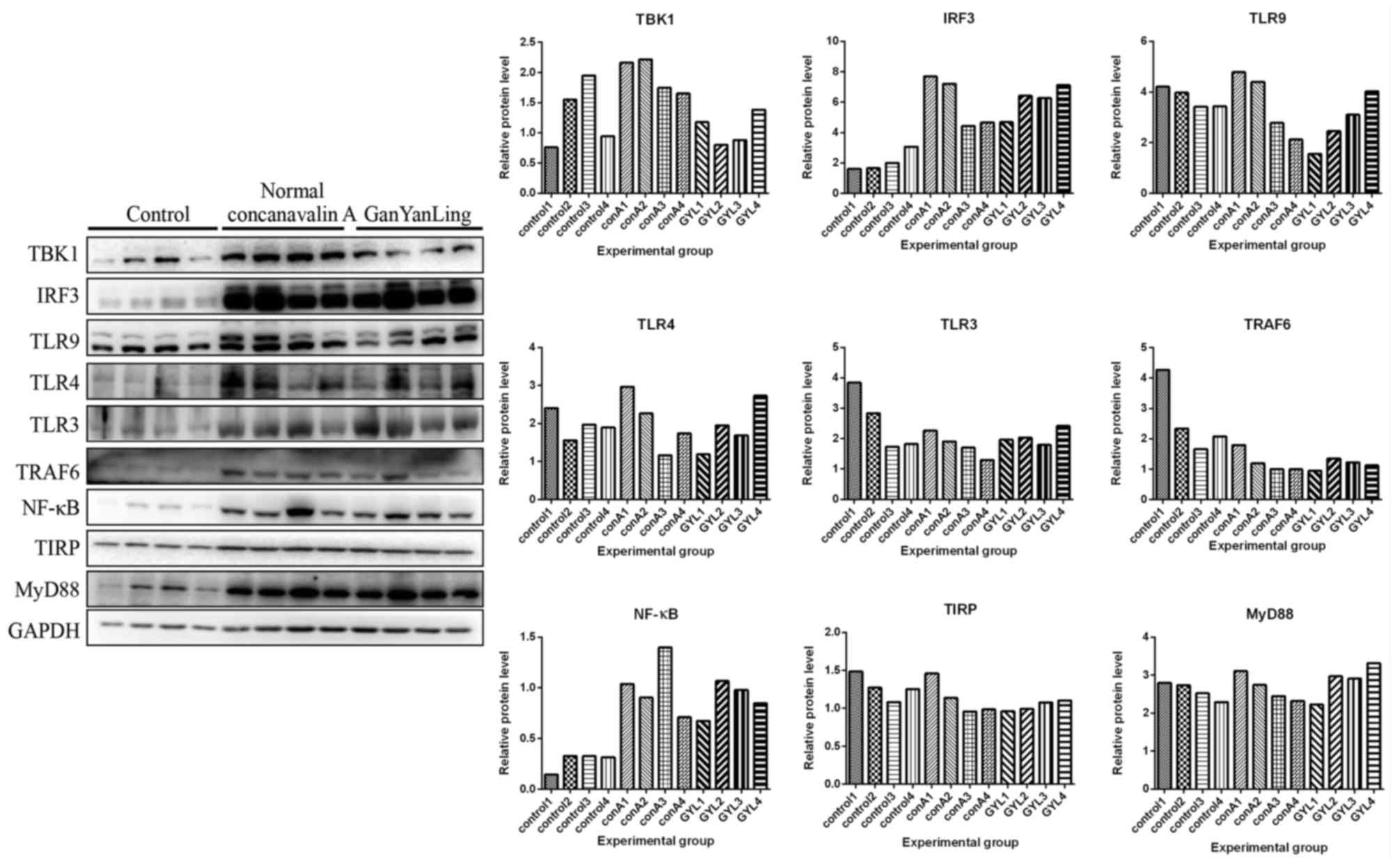 | Figure 3.Liver injury in normal mice was
induced by Concanavalin A, which were then treated with normal
saline (control) and GYL. Liver tissues were obtained to detect
protein expression levels of MyD88 (33 kDa), TBK1 (84 kDa), TRAF6
(60 kDa), IRF3 (55 kDa), TLR3 (104 kDa), TLR9 (120 kDa), TLR4
(97–116 kDa), NF-κB (65 kDa) and TIRP (26 kDa) by using western
blot analysis. GAPDH was used to confirm the equal amount of
protein loaded in each lane. The quantification of the western blot
analysis through gray analysis was also performed. conA1-4, mice
1–4 treated with Concanavalin A in the same condition; GYL,
GanYanLing; IRF3, interferon regulatory factor 3; MyD88, myeloid
differentiation primary response 88; NF-κB, necrosis factor-κB;
TBK1, tumor necrosis factor receptor-associated factor-binding
kinase; TRAF6, tumor necrosis-associated factor 6; TLR, Toll-like
receptor; TIRP, TRAF-interacting protein. |
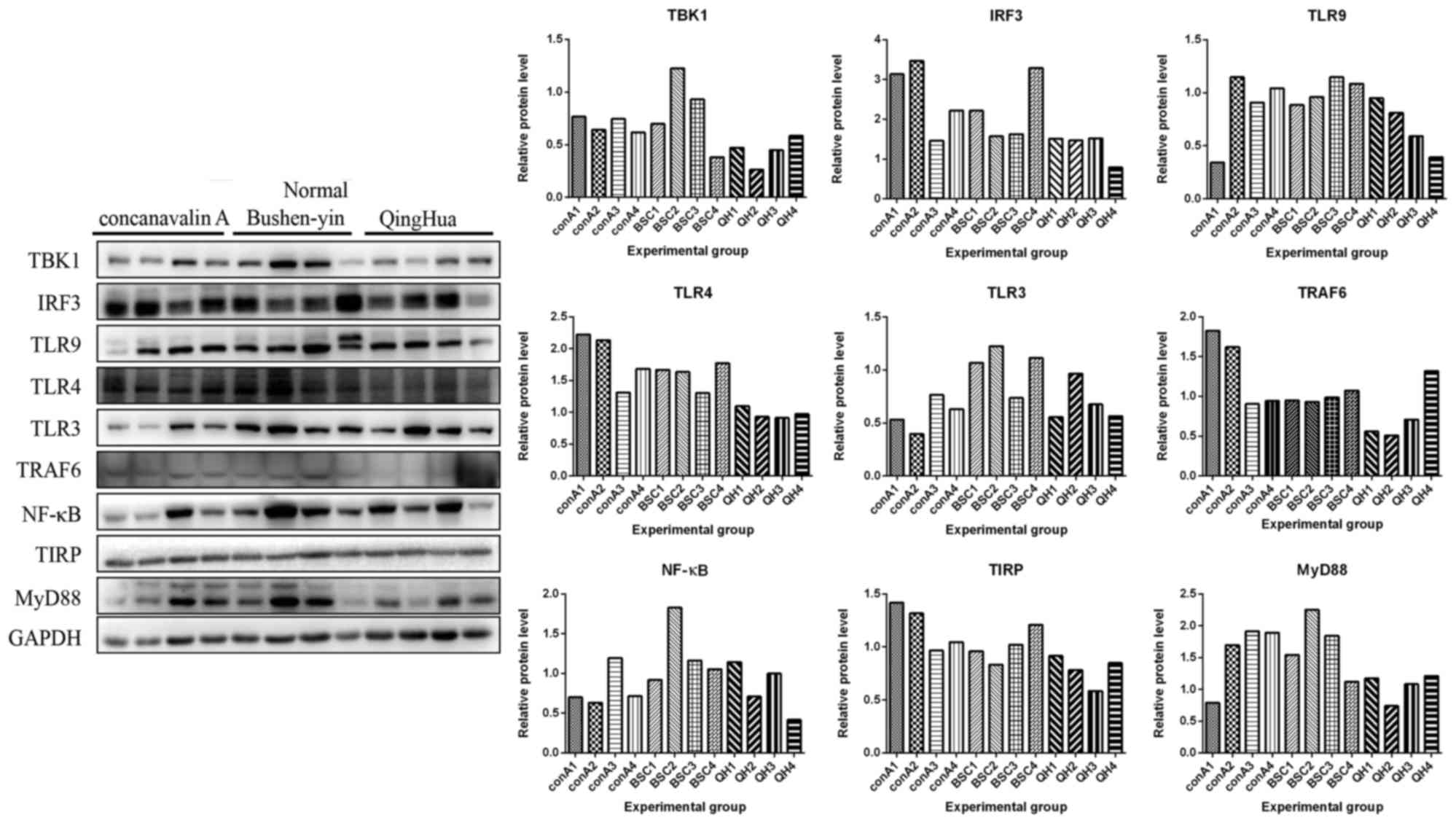 | Figure 5.Liver injury in normal mice was
induced by Concanavalin A, which were then treated with BSC and QH.
Liver tissues were obtained to detect protein expression levels of
MyD88 (33 kDa), TBK1 (84 kDa), TRAF6 (60 kDa), IRF3 (55 kDa), TLR3
(104 kDa), TLR9 (120 kDa), TLR4 (97–116 kDa), NF-κB (65 kDa) and
TIRP (26 kDa) by using western blot analysis. GAPDH was used to
confirm the equal amount of protein loaded in each lane. The
quantification of the western blot analysis through gray analysis
was also performed. BSC, Bushen-yin; conA1-4, mice 1–4 treated with
Concanavalin A in the same condition; IRF3, interferon regulatory
factor 3; MyD88, myeloid differentiation primary response 88;
NF-κB, necrosis factor-κB; QH, QingHua; TBK1, tumor necrosis factor
receptor-associated factor-binding kinase; TRAF6, tumor
necrosis-associated factor 6; TLR, Toll-like receptor; TIRP,
TRAF-interacting protein. |
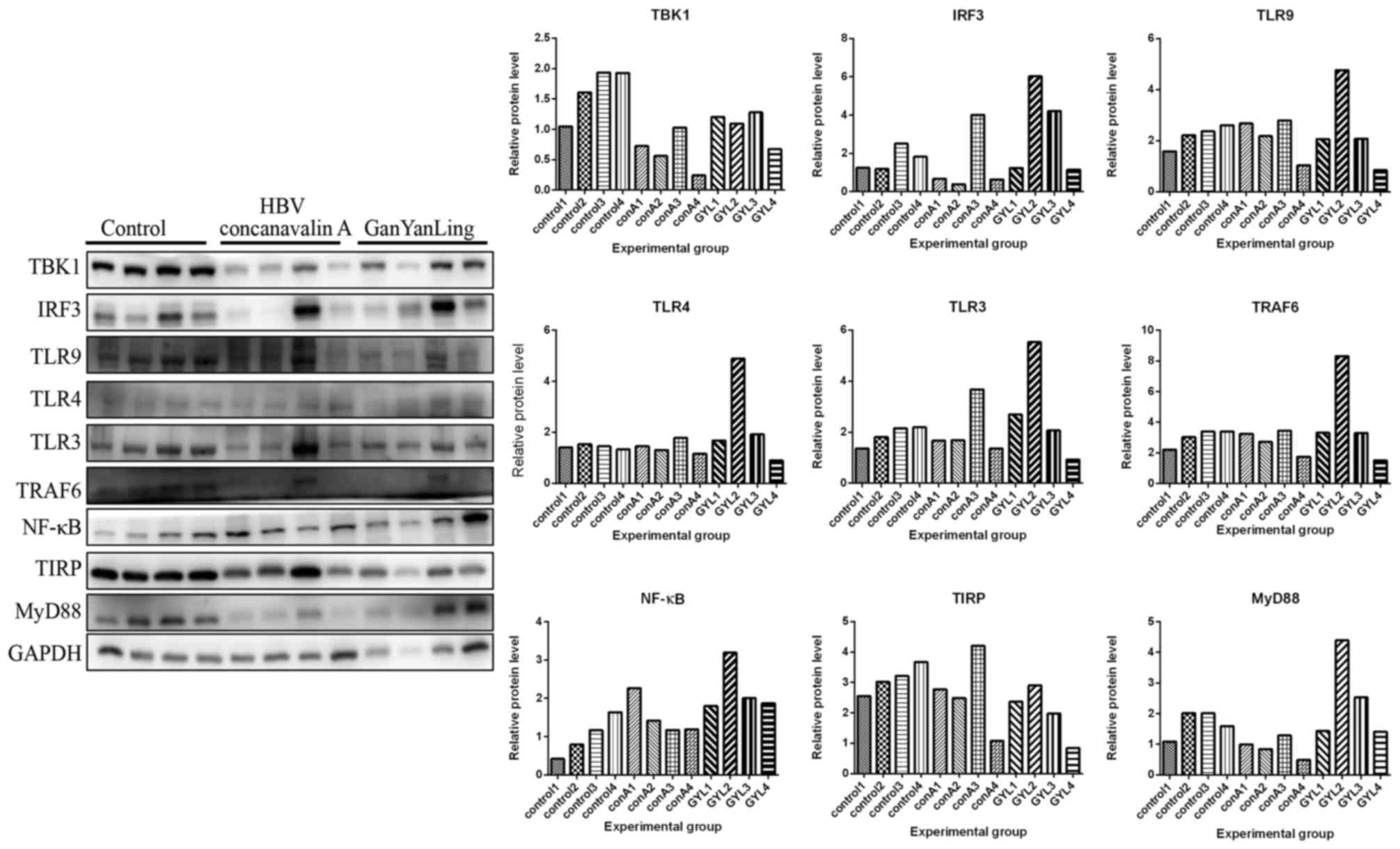 | Figure 6.Liver injury in HBV transgenic mice
was induced by Concanavalin A, which were then treated with normal
saline (control) and GYL. Liver tissues were obtained to detect
protein expression levels of MyD88 (33 kDa), TBK1 (84 kda), TRAF6
(60 kDa), IRF3 (55 kDa), TLR3 (104 kDa), TLR9 (120 kDa), TLR4
(97–116 kDa), NF-κB (65 kDa) and TIRP (26 kDa) by using western
blot analysis. GAPDH was used to confirm the equal amount of
protein loaded in each lane. The quantification of the western blot
analysis through gray analysis was also performed. conA1-4, mice
1–4 treated with Concanavalin A in the same condition; GYL,
GanYanLing; HBV, hepatitis B virus; IRF3, interferon regulatory
factor 3; MyD88, myeloid differentiation primary response 88;
NF-κB, necrosis factor-κB; TBK1, tumor necrosis factor
receptor-associated factor-binding kinase; TRAF6, tumor
necrosis-associated factor 6; TLR, Toll-like receptor; TIRP,
TRAF-interacting protein. |
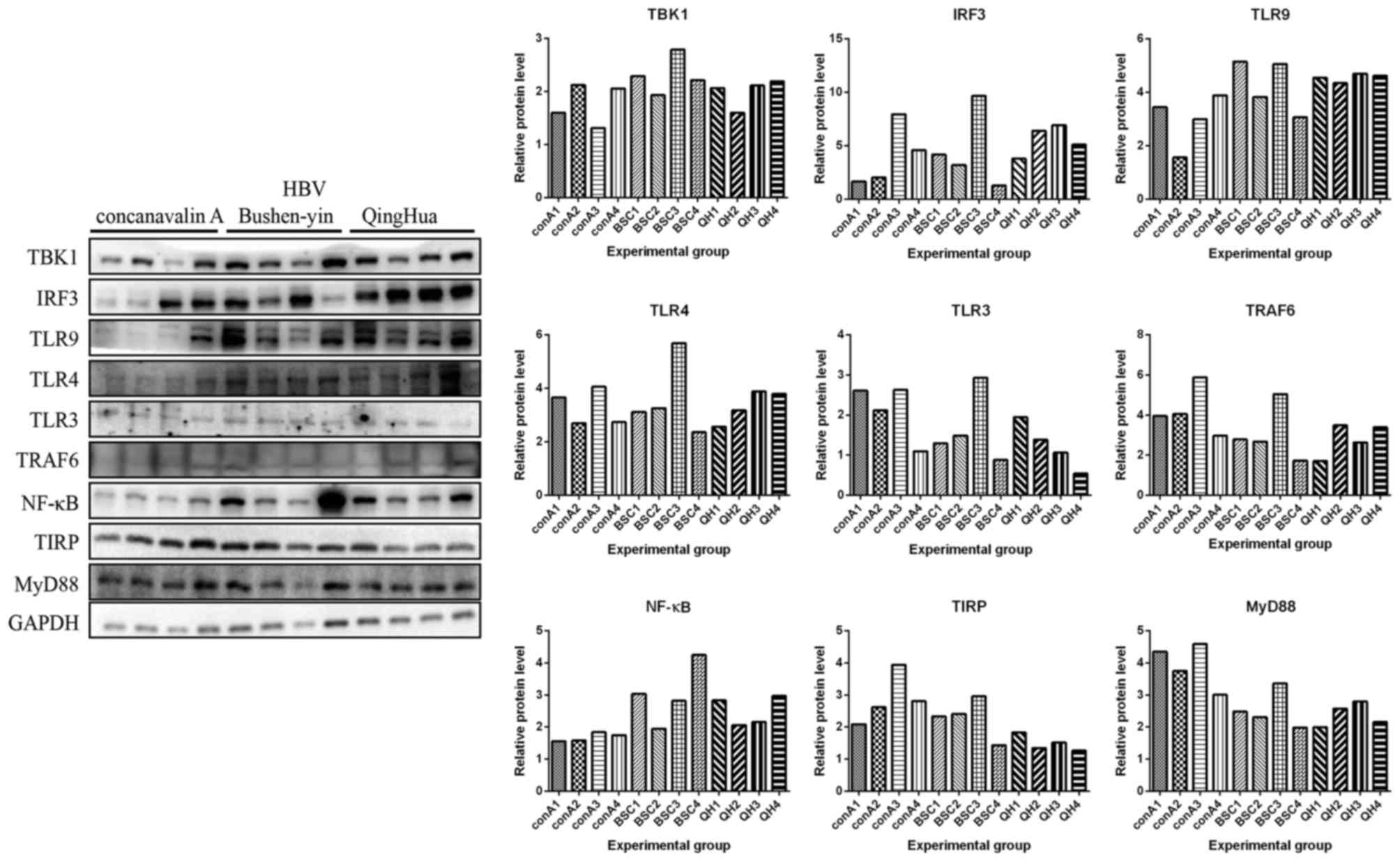 | Figure 8.Liver injury in HBV transgenic mice
was induced by Concanavalin A, which were then treated with BSC and
QH. Liver tissues were obtained to detect protein expression levels
of MyD88 (33 kDa), TBK1 (84 kDa), TRAF6 (60 kDa), IRF3 (55 kDa),
TLR3 (104 kDa), TLR9 (120 kDa), TLR4 (97–116 kDa), NF-κB (65 kDa)
and TIRP (26 kDa) by using western blot analysis. GAPDH was used to
confirm the equal amount of protein loaded in each lane. The
quantification of the western blot analysis through gray analysis
was also performed. BSC, Bushen-yin; conA1-4, mice 1–4 treated with
Concanavalin A in the same condition; QH, QingHua; IRF3, interferon
regulatory factor 3; MyD88, myeloid differentiation primary
response 88; NF-κB, necrosis factor-κB; TBK1, tumor necrosis factor
receptor-associated factor-binding kinase; TRAF6, tumor
necrosis-associated factor 6; TLR, Toll-like receptor; TIRP,
TRAF-interacting protein. |
Bushen recipe and its disassembled
prescriptions decrease the Concanavalin A-induced increase of
various inflammatory factors expression
IL-6, TNF-α and IL-1 are primary inflammatory
factors that affect the downstream pathway of NF-κB, and IFN-γ
affects the downstream pathway of IRF3 (18,19).
These inflammatory factors were detected by ELISA as demonstrated
in Fig. 9. The expression levels
of IL-6, IL-1, TNF-α and IFN-γ were significantly increased in
normal and HBV mice following injection with Concanavalin A,
compared with in the control group. However, following treatment
with Bushen recipe and its disassembled prescriptions, the
Concanavalin A-induced upregulated expression levels of IL-6, IL-1
and TNF-α decreased significantly in the BS group, while only IL-1
in BSY group decreased significantly and only IL-6 in the BSC and
QH group decreased significantly. These results are consistent with
activation state of NF-κB in western blot analysis, and the
downstream pathway of NF-κB requires further investigation and
validation. Furthermore, the increase of IFN-γ expression was
evident following Concanavalin A injection in normal and HBV mice,
while the content of IFN-γ in the mice treated with the Bushen
recipe and its disassembled prescriptions decreased only slightly
in BSC and BSY groups. Therefore, the results of the present study
suggested that the Bushen recipe and its disassembled prescriptions
(BSC and BSY in particular) may repair liver injury induced by
Concanavalin A by inhibiting the expression of inflammatory
factors. Variations in the effects exhibited by Bushen recipe and
its disassembled prescription may be due to the differing compunds
present; however further research is required.
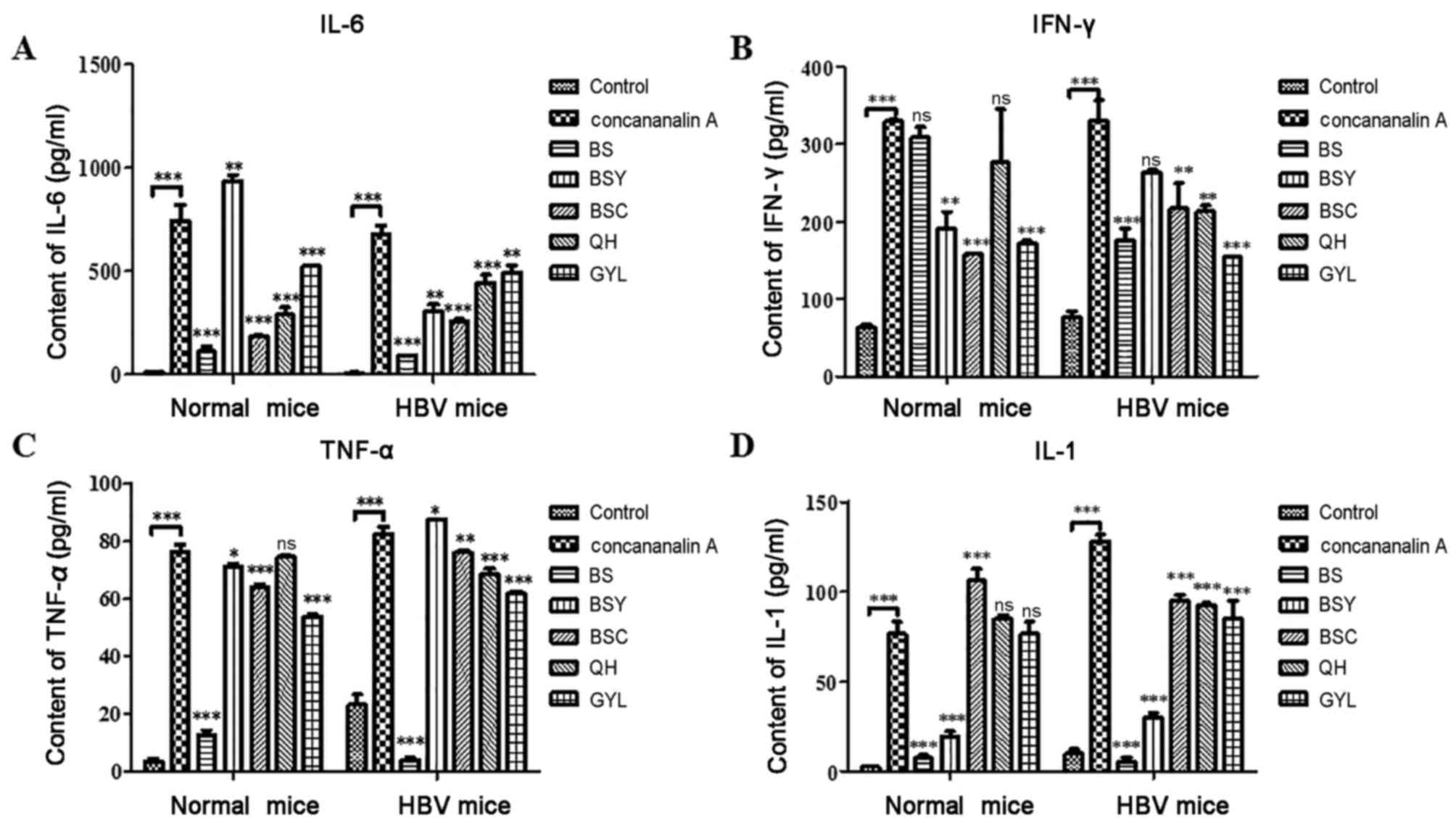 | Figure 9.Expression levels of inflammatory
factors in all mice treated with Concanavalin A, BS and its
disassembled prescriptions. Expression levels of (A) IL-6, (B)
IFN-γ and (C) TNF-α and (D) IL-1 as measured by ELISA. ns indicates
no statistical significance, *P<0.05, **P<0.01,
***P<0.001, vs. the Concanavalin A group or control group. BS,
Bushen recipe; BSC, Bushen-yin; BSY, Bushen-yang; GYL, GanYanLing;
HBV, hepatitis B virus; IFN, interferon; IL, interleukin; QH,
QingHuang; TNF, tumor necrosis factor; NS, not significant. |
Discussion
Hepatitis B has become one of the most prevalent and
serious infectious diseases in the world and persistently infects
350 million people worldwide with the added risk of development of
other chronic liver diseases (20–22).
It not only seriously affects human health, but it also brings a
vast economic burden to patients and society.
During acute HBV infection, viral clearance could be
achieved in a non-cytolytic manner. This process is mediated by
inflammatory cytokines such as IFN-α, IFN-β, IFN-γ and TNF-α which
are produced by activated T lymphocytes, natural killer cells or
antigen nonspecific macrophages (23–25).
IFN-γ inhibits HBV replication by disturbing the formation of viral
RNA-containing capsids or promoting their degradation; both of
these processes require proteasome activity (26–28).
The mechanism of the TNF-α-induced antiviral response remains
unclear. It has been reported that TNF-α blocks the formation or
stability of cytoplasmic viral capsids via activation of NF-κB,
however the antiviral effector genes remain unknown (29,30).
Therefore, herein, HBV mice were used to initiate liver injury
associated with Con A in order to study the effects of Con A on
liver injury.
Current treatment methods including vaccines,
immuno-modulators, interferons and nucleoside analogs are not
satisfactory. Traditional Chinese Medicines (TCMs) with various
components and multiple targets may have advantages in treating HBV
infection (31). The effect of
TCMs on serum HBs Ag of patients with chronic hepatitis B has been
systematically studied (32–34).
Notably, the Bushen recipe used in this study was prepared
according to the formula of ‘Bushen Granule’ (Shanghai Pharmacy),
which means that the recipe is unique. An additional Bushen recipe
with different ingredients, which is also produced by our hospital,
Shuguang Hospital (Shanghai, China), was reported to reduce both
the number of Th1/Th2 cells infiltrating the liver and serological
factors associated with liver function, alleviating pathological
alterations of the liver (35).
It is reported that the production of inflammatory
factors is associated with the TLR3/9 signaling pathway (36,37).
A particular subgroup within the TLR family including TLR3, TLR7,
TLR8, and TLR9 is localized in endosomes and recognizes nucleic
acids such as viral DNA or RNA. The other subgroup of
surface-expressed TLR1, TLR2, TLR4, TLR5, and TLR6 recognizes
components of the extracellular bacterial and fungal cell wall, as
well as some viral proteins (38,39).
Binding of TLR agonists to their receptors initiates the activation
of complex networks of intracellular signaling transduction
pathways to coordinate the inflammatory response. Conformational
changes and dimerization of TLRs occur upon binding with ligands.
The important components in these signaling networks are the
adaptor proteins and several protein kinases including
extracellular signal-regulated kinase, c-Jun N-terminal kinase,
p38, mitogen-activated protein kinase, and phosphoinositide
3-kinase, and the transcription factors IRF3/5/7, NF-κB, and
activator protein-1. The activation of these transcription factors
leads to the induction of type I IFNs, pro-inflammatory cytokines,
or co-stimulatory molecules, which are involved in antiviral
responses (40,41). The crucial adaptor proteins, such
as MyD88 are employed by almost all TLRs except TLR3; TIRAP is used
by TLR2 and TLR4, TRIF is used by TLR3 and TLR4, and TRAM is
employed only by TLR4 (42). The
results of the present study revealed that Bushen recipe and its
disassembled prescriptions may inhibit the activation of TLR3/9
signaling pathway, particularly NF-κB downstream. Combined with
ELISA results, it was observed that Bushen recipe and its
disassembled prescriptions decreased the expression of NF-κB and
its downstream inflammatory factors, including TNF-α, IL-6 and
IL-1; however future studies are required to confirm these
results.
In conclusion, in the present study, the combination
of the measurement of ALT and ChE, which are both synthesized in
liver and could be used to represent the degree of liver injury,
and the microscopic observation of Hematoxylin and Eosin Stained
liver tissues displayed that Bushen recipe and its disassembled
prescriptions could repair liver injury caused by Concanavalin A.
Furthermore, investigations verified that the capability of Bushen
recipe and its disassembled prescriptions results from the
inhibition of TLR3/9 signaling pathway and associated expression of
inflammatory factors. Notably, the present study may provide a
novel strategy for the development of anti-HBV drugs in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81373618).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HN and YG made substantial contributions to the
concept and design of the present study. HN, ZM and BZ conducted
the experiments. JC, LW and RW conducted data analysis. HN, LW and
RW produced the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were carried out according to
the regulations of and approved by Laboratory Animal Administration
of Shanghai University of Traditional Chinese Medicine.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
References
|
1
|
Tiollais P, Pourcel C and Dejean A: The
hepatitis B virus. Nature. 317:489–495. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minor MM and Slagle BL: Hepatitis B virus
HBx protein interactions with the ubiquitin proteasome system.
Viruses. 6:4683–4702. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Franchis R, Hadengue A, Lau G, Lavanchy
D, Lok A, McIntyre N, Mele A, Paumgartner G, Pietrangelo A, Rodés
J, et al: EASL International Consensus Conference on Hepatitis B.
13–14 September, 2002 Geneva, Switzerland. Consensus statement
(long version). J Hepatol. 39 Suppl 1:S3–S25. 2003.PubMed/NCBI
|
|
4
|
Inchauspé G and Michel ML: Vaccines and
immunotherapies against hepatitis B and hepatitis C viruses. J
Viral Hepat. 14 Suppl 1:S97–S103. 2007. View Article : Google Scholar
|
|
5
|
Lu M, Menne S, Yang D, Xu Y and Roggendorf
M: Immunomodulation as an option for the treatment of chronic
hepatitis B virus infection: Preclinical studies in the woodchuck
model. Expert Opin Invest Drugs. 16:787–801. 2007. View Article : Google Scholar
|
|
6
|
Michel ML and Tiollais P: Hepatitis B
vaccines: Protective efficacy and therapeutic potential. Pathol
Biol (Paris). 58:288–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roggendorf M and Lu M: Woodchuck Hepatitis
Virus. 2007. View Article : Google Scholar
|
|
8
|
Garcia ML: Regulation of hepatitis B virus
replication by the ubiquitin-proteasome pathway (unpublished PhD
thesis)Yale University School of Medicine. New Haven, CT: 2010
|
|
9
|
Xiao X, Zhao P, Rodriguez-Pinto D, Qi D,
Henegariu O, Alexopoulou L, Flavell RA, Wong FS and Wen L:
Inflammatory regulation by TLR3 in acute hepatitis. J Immunol.
183:3712–3719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keil JM, Shen Z, Briggs SP and Patrick GN:
Regulation of STIM1 and SOCE by the ubiquitin-proteasome system
(UPS). PLoS One. 5:e134652010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cacciapuoti F: Role of
ubiquitin-proteasome system (UPS) in left ventricular hypertrophy
(LVH). Am J Cardiovasc Dis. 4:1–5. 2014.PubMed/NCBI
|
|
12
|
Mata-Cantero L, Lobato-Gil S, Aillet F,
Lang V and Rodriguez MS: The Ubiquitin-Proteasome System (UPS) as a
cancer drug target: Emerging mechanisms and therapeuticsStress
Response Pathways in Cancer: From Molecular Targets to Novel
Therapeutics. Wondrak GT: Springer Netherlands; Dordrecht: pp.
225–264. 2015
|
|
13
|
Gao YQ, Yao Y and Li M: Effect of bushen
recipe on the immune effector molecules of natural killer cells in
patients with chronic hepatitis B. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 30:710–713. 2010.(In Chinese). PubMed/NCBI
|
|
14
|
Chen XF, Chen SH, Gui-Yuan L, Xiao LW and
Lou ZH: Research and discussion on mechanism of treating
osteoporosis by Bushen Huoxue Recipe. Chin Tradit Pat Med.
33:1130–1134. 2011.
|
|
15
|
Tan Y, Tan L, Huang S, Lu J and Yu L:
Content determination of active component in Huangqi Yinyanghuo
group and its effects on hTERT and Bcl-2 protein in osteosarcoma. J
Anal Methods Chem. 2014:7693502014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo HY: Clinical research of using Bushen
Huoxue prescription in the treatment of early diabetic nephropathy.
Sci Technol Eng. 8:2176–2179. 2008.
|
|
17
|
Zhang E and Lu M: Toll-like receptor
(TLR)-mediated innate immune responses in the control of hepatitis
B virus (HBV) infection. Med Microbiol Immunol. 204:11–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cortez M, Carmo LS, Rogero MM, Borelli P
and Fock RA: A high-fat diet increases IL-1, IL-6, and TNF-α
production by increasing NF-κB and attenuating PPAR-γ expression in
bone marrow mesenchymal stem cells. Inflammation. 36:379–386. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guinn Z, Brown DM and Petro TM: Activation
of IRF3 contributes to IFN-γ and ISG54 expression during the immune
responses to B16F10 tumor growth. Int Immunopharmacol. 50:121–129.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coluccio C, Begini P, Marzano A,
Pellicelli A, Imperatrice B, Anania G, Delle Fave G and Marignani
M: Hepatitis B in patients with hematological diseases: An update.
World J Hepatol. 9:1043–1053. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stanley M: Tumour virus vaccines:
Hepatitis B virus and human papillomavirus. Philos Trans R Soc Lond
B Biol Sci. 372:201602682017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stasi C, Silvestri C and Voller F:
Emerging trends in epidemiology of hepatitis B virus infection. J
Clin Transl Hepatol. 5:272–276. 2017.PubMed/NCBI
|
|
23
|
Guidotti LG, Ishikawa T, Hobbs MV, Matzke
B, Schreiber R and Chisari FV: Intracellular inactivation of the
hepatitis B virus by cytotoxic T lymphocytes. Immunity. 4:25–36.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guidotti LG, Rochford R, Chung J, Shapiro
M, Purcell R and Chisari FV: Viral clearance without destruction of
infected cells during acute HBV infection. Science. 284:825–829.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guidotti LG and Chisari FV: Noncytolytic
control of viral infections by the innate and adaptive immune
response. Annu Rev Immunol. 19:65–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robek MD, Wieland SF and Chisari FV:
Inhibition of hepatitis B virus replication by interferon requires
proteasome activity. J Virol. 76:3570–3574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wieland SF, Eustaquio A, Whitten-Bauer C,
Boyd B and Chisari FV: Interferon prevents formation of
replication-competent hepatitis B virus RNA-containing
nucleocapsids. Proc Natl Acad Sci USA. 102:9913–9917. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu C, Guo H, Pan XB, Mao R, Yu W, Xu X,
Wei L, Chang J, Block TM and Guo JT: Interferons accelerate decay
of replication-competent nucleocapsids of hepatitis B virus. J
Virol. 84:9332–9340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biermer M, Puro R and Schneider RJ: Tumor
necrosis factor alpha inhibition of hepatitis B virus replication
involves disruption of capsid Integrity through activation of
NF-kappaB. J Virol. 77:4033–4042. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puro R and Schneider RJ: Tumor necrosis
factor activates a conserved innate antiviral response to hepatitis
B virus that destabilizes nucleocapsids and reduces nuclear viral
DNA. J Virol. 81:7351–7362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen JJ: The Anti-HBV constituents from
traditional chinese medicine of swertia mileensis. Planta Med.
79:OP232013. View Article : Google Scholar
|
|
32
|
Dai XQ, Cai WT, Wu X, Chen Y and Han FM:
Protocatechuic acid inhibits hepatitis B virus replication by
activating ERK1/2 pathway and down-regulating HNF4α and HNF1α in
vitro. Life Sci. 180:68–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Jiang HY, Shi Y, He JL, Su S and
Chen Z: Chinese herbal medicine for carriers of the hepatitis B
virus: An updated systematic review and meta-analysis. Pharmazie.
69:723–730. 2014.PubMed/NCBI
|
|
34
|
Dai JJ, Tao HM, Min QX and Zhu QH:
Anti-hepatitis B virus activities of friedelolactones from Viola
diffusa Ging. Phytomedicine. 22:724–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiao B, Zhou ZH, Man LI, Sun XH, Jin SG,
Zhu XJ, Huang LY, Zhang X and Gao YQ: Experimental study of Bushen
Recipe on Th1/Th2 in liver tissue of chronic hepatic injury mice
model induced by ConA. China J Tradit Chin Med Pharm. 32:841–844.
2017.(In Chinese).
|
|
36
|
Xue D, Ma Y, Li M, Li Y, Luo H, Liu X and
Wang Y: Mycoplasma ovipneumoniae induces inflammatory response in
sheep airway epithelial cells via a MyD88-dependent TLR signaling
pathway. Vet Immunol Immunopathol. 163:57–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Wang S, Zhu R, Li H, Han Q and Zhao
RC: Lung tumor exosomes induce a pro-inflammatory phenotype in
mesenchymal stem cells via NFkB-TLR signaling pathway. J Hematol
Oncol. 9:422016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gay NJ and Gangloff M: Structure and
function of Toll receptors and their ligands. Annu Rev Biochem.
76:141–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schwabe RF, Seki E and Brenner DA:
Toll-like receptor signaling in the liver. Gastroenterology.
130:1886–1900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seki E and Brenner DA: Toll-like receptors
and adaptor molecules in liver disease: Update. Hepatology.
48:322–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee MS and Kim YJ: Signaling pathways
downstream of pattern-recognition receptors and their cross talk.
Annu Rev Biochem. 76:447–480. 2007. View Article : Google Scholar : PubMed/NCBI
|