Introduction
Tuberculosis (TB) is a contagious disease and
continues to be a major heath issue worldwide, especially in Asia
and Africa (1,2). Although the global incidence of
pulmonary TB has been reported to reduce over time, approximately
17% of the relapse and new cases of TB develop extra-pulmonary TB
(3). Among all types of emerging
extra-pulmonary TB, tuberculous pleural effusion (TPE) is the most
frequent manifestation, accounting for about 5% of all forms of TB,
and is the leading etiology of pleural effusion in many high TB
prevalence areas (4,5). Our understanding of the pathogenesis
of TPE has evolved. TPE once was thought to be an effusion
resulting from delayed hypersensitivity reaction. Recent evidences
suggest that it is a result of direct infection of Mycobacterium
tuberculosis (M. tuberculosis) in the pleura which leads to the
infiltration of inflammatory cells and chronic accumulation of
fluid in pleural space (6–8). The pathogenesis of TPE involves
intricate cellular and humoral immune responses, although the exact
underlying mechanisms are not completely understood.
B cell activating factor (BAFF) is a novel member of
the tumor necrosis factor family, a homotrimer expressed by T
cells, dendritic cells and macrophages (9–11).
BAFF is initially expressed on the cell surface and subsequently
released as a soluble form after enzymatic cleavage (12). In in vivo and in
vitro experiments, BAFF has been confirmed as a key cytokine in
B cell homeostasis. BAFF deficient mice lack a mature B cell
component (13). Recent evidence
has indicated that it's indispensable for peripheral B cell
survival (14), while excessive
BAFF stimulation in humans contributes to the development of a
variety of autoimmune diseases (15,16).
The level of BAFF has been reported to increase in human active
pulmonary TB (17). However, its
potential contribution to the modulation of B cell maturation in
patients with TPE remains elusive. We therefore detected the level
of BAFF and B cell compositions, and further investigated whether
such changes are linked to M. tuberculosis-induced immune
response.
Materials and methods
Study population and ethics
statement
A total of 45 cases of TPE were enrolled from
Shenzhen Third People's Hospital (Shenzhen, China). TPE was
diagnosed if i) acid fast bacilli (AFB) staining or M.
tuberculosis (MTB) cultures or MTB-DNA polymerase chain
reaction of pleural effusion or pleural biopsy specimens showed
positive; ii) or if parietal pleural biopsy specimens present
typical histopathology characterized with tuberculous granuloma or
caseous necrosis (18). A total of
40 cases of HC subjects who had received BCG vaccination at birth
and showed a negative tuberculin skin test (TST) were recruited.
All subjects were recruited from January 2016 to November 2016 in
Shenzhen Third People's Hospital. Subjects with HIV infection,
diabetes, cancer and autoimmune diseases were excluded from the
study. At the time of sample collection, all of the TPE patients
had not received any anti-TB therapy, corticosteroids or other
non-steroidal anti-inflammatory drugs. The characteristics of both
study cohorts are shown in Table
I, there was no significant differences in terms of age range
and gender ratio were noted between TPE patients (age range: 18–63
years; male/female: 1.0) and HCs (age range: 20–54; male/femal:
1.2). The study was approved by the Ethics Committee of Guangdong
Medical University and Shenzhen Third People's Hospital, and
written informed consent was obtained from all study subjects
before their participation.
 | Table I.Demographic characteristics of the
study groups. |
Table I.
Demographic characteristics of the
study groups.
|
Characteristics | Healthy control
group | Tuberculous pleural
effusion group | P-value |
|---|
| Patient number | 40 | 45 | – |
| Male/female, n
(ratio) | 22/18 (1.2:1) | 22/23 (1.0:1) | 0.57 |
| Age, years [medium
(range)] | 33 (20–54) | 36 (18–63) | 0.22 |
Isolation and preparation of
peripheral blood mononuclear cells (PBMCs) and pleural fluid
mononuclear cells (PFMCs)
PBMCs and PFMCs were isolated and prepared as
previously reported (19).
Briefly, approximately 10 ml pleural effusion collected from TPE
patients and 5 ml peripheral blood samples from TPE patients and
HCs were centrifuged at 2000 × g for 10 min at 4°C. The
supernatants were stored at −80°C for future analysis. Cell pellets
from pleural effusion were suspended in PBS and cellular components
of the blood samples were used for the PBMC isolation by standard
Ficoll-Hypaque (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
density gradient centrifugation (2000 × g for 20 min at 4°C). PBMCs
and PBFCs were then washed twice with pre-cooled PBS (pH 7.4; 4°C),
and then re-suspended in complete RPMI-1640 medium with 20%
heated-inactivation fatal calf serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). A cell viability of >95%
was seen in all experiments as determined by try-pan blue
exclusion.
ELISA
For quantitative ELISA assay, BAFF in plasma and
supernatants of pleural effusion was tested using the Human BAFF
Quantikine ELISA Kit (SBLYS0B; R&D Systems, Inc., Minneapolis,
MN, USA) following the manufacturer's protocol.
Flow cytometry analysis
The freshly isolated PBMCs and PFMCs were washed
with PBS (4% FBS) and suspended at a concentration of
1×107/200 µl, followed by staining with CD19-APC,
IgD-FITC, CD27-PE-Cy7, CD38-APC-Cy7 (Biolegend, San Diego, CA, USA)
for 30 min at 4°C in the dark. Cells were then washed twice and
re-suspended in 200 µl PBS (4% FBS). Within 2 h, the samples were
acquired on a modified BD Canto II™ flow cytometer (BD Biosciences,
San Jose, CA, USA). Data analysis was performed using FlowJo
software (Tree Star, Inc., Ashland OR, USA).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA).
Differences in sex ratio of the two study cohorts were compared by
Pearson's χ2 test. Differences in age between the two
study cohorts were evaluated by Student's t-test. Differences in
BAFF level and the proportion of each B cell subset were evaluated
by analysis of variance with Tukey's post hoc test for multiple
comparisons. Correlations between two variables were analyzed by
Spearman's analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
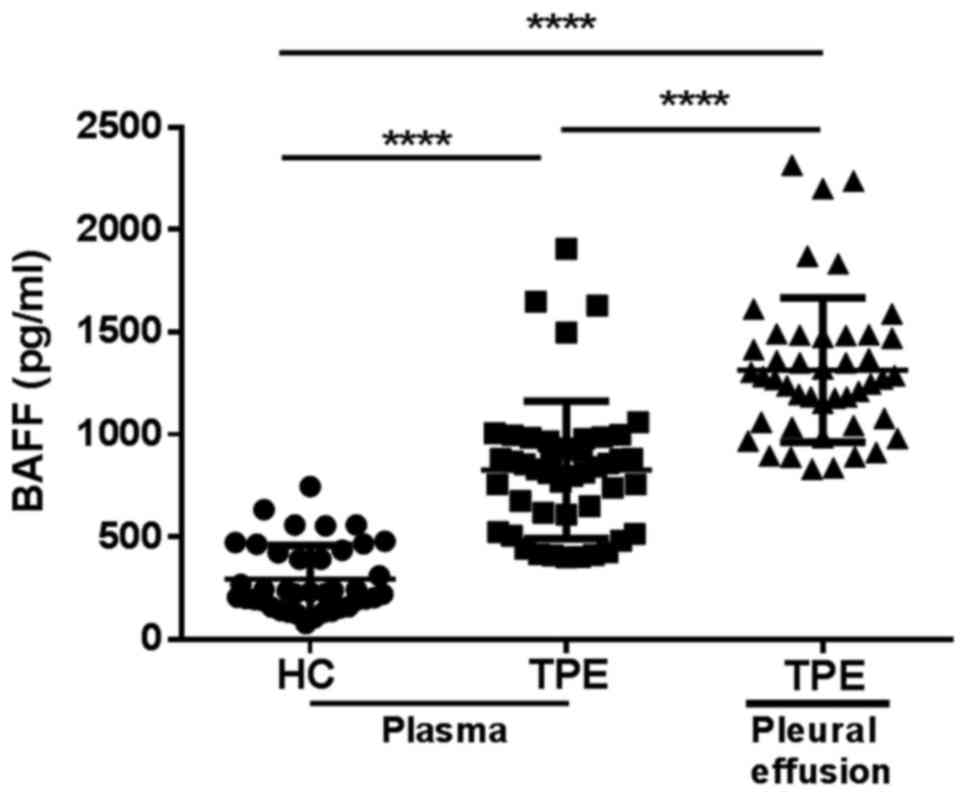
BAFF is increased in the plasma and
pleural effusion of patients with TPE
BAFF were previously reported to increase in the
development of human active pulmonary TB (17). It's uncertain whether BAFF is
similarly increased in the patients with TPE. In this study, we
detected the level of BAFF in plasma from 40 cases HCs and 45 cases
TPE patients using a sandwich ELISA kit. We found that the level of
plasma BAFF in TPE patients was 2.8-fold higher than that in HCs
(Fig. 1). Concomitantly, we
investigated the levels of BAFF in pleural effusion of these TPE
patients, and BAFF level was higher in pleural effusion compared to
that in plasma (Fig. 1).
Alteration of B subsets in PBMCs and
PFMCs of patients with TPE
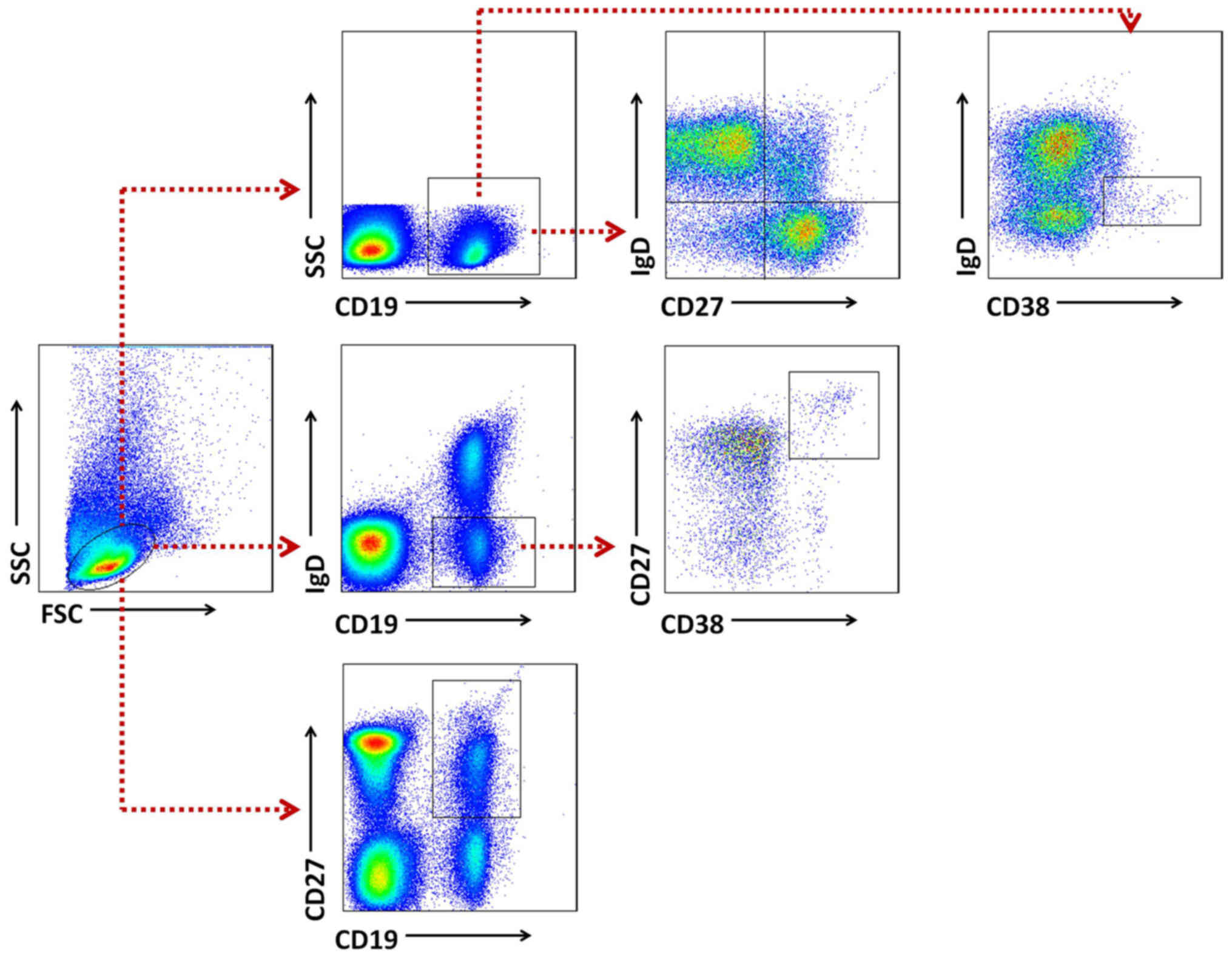
Gating strategies were set to evaluate B cell
subsets (Fig. 2). Naïve B cells
were classified as CD19+IgD+CD27−,
while total memory B cells were defined as
CD19+CD27+, including an unswitched
IgD+ population and a switched IgD−
population. Plasma cells were identified as
CD19+IgD−CD38+CD27+and
transitional B cell as CD19+IgDdim
CD38+. Definitions of B cell subsets are also listed in
Table II (18,20).
 | Table II.Definitions of B cell subsets. |
Table II.
Definitions of B cell subsets.
| Subset | Parameter |
|---|
| Naïve B cell |
CD19+IgD+CD27− |
| Unswitched B
cell |
CD19+IgD+CD27+ |
| Switched B
cell |
CD19+IgD−CD27+ |
| Total memory B
cell |
CD19+CD27+ |
| Plasma B cell |
CD19+IgD−CD38+CD27+ |
| Transitional B
cell |
CD19+IgDdimCD38+ |
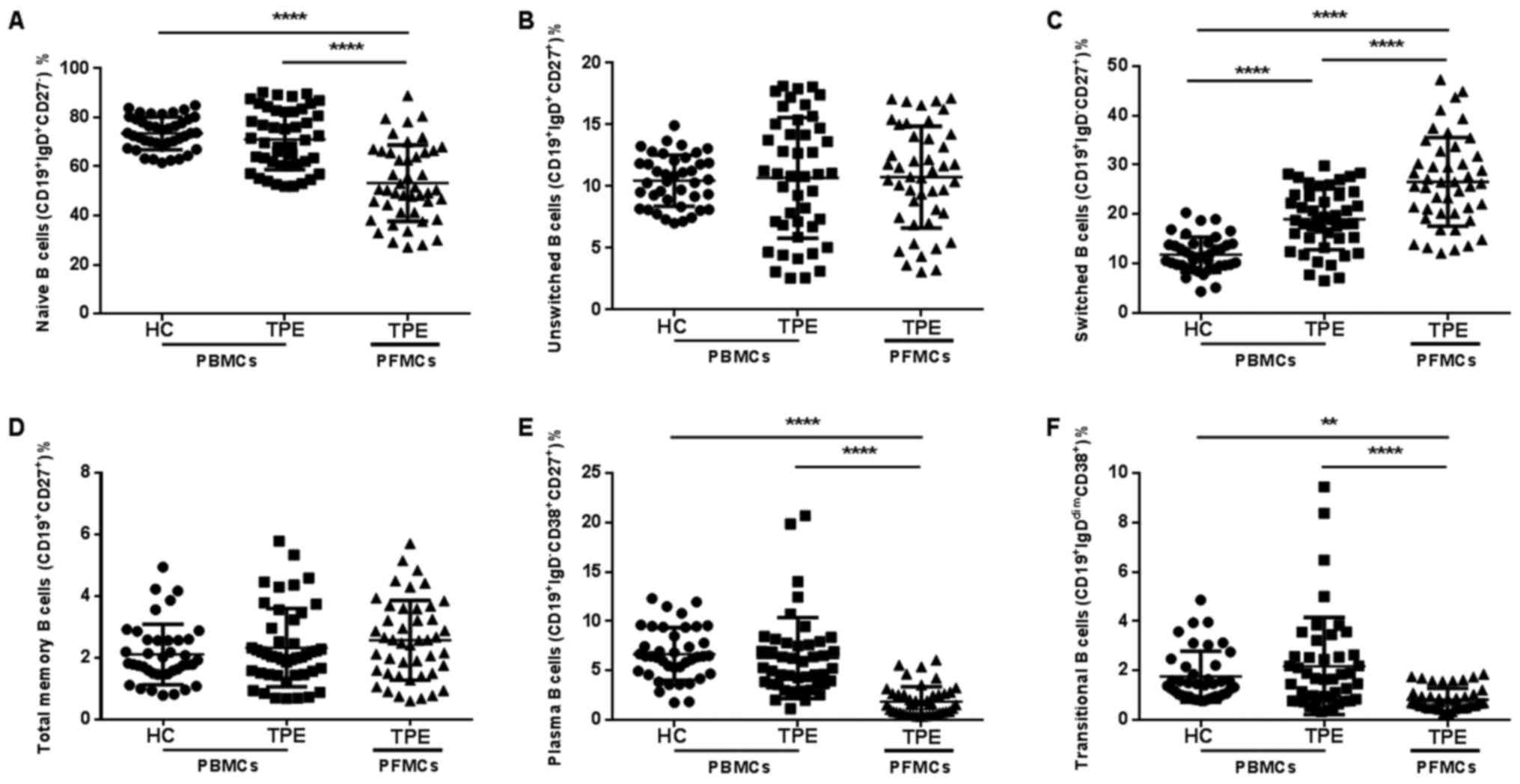
We analyzed B cell profile to evaluate whether B
cell subsets in peripheral blood or pleural effusion were altered
among the study groups. In PBMCs, the proportions of naïve B cells,
total memory B cell, unswitched B cell, plasma B cell and
transitional B cell were all similar between the two study groups
(Fig. 3). Compared to PBMCs in TPE
patients, the proportions of total memory B cell and unswitched B
cell were similar in PFMCs of TPE patients, but the proportions of
naïve B cells, plasma B cell and transitional B cell were much
lower in PFMCs of TPE patients. It is noteworthy that the
proportion of switched B cell was increased in PBMCs of patients
with TPE, and higher switched B cell proportion in PFMCs than that
in PBMC was also seen in these patients (Fig. 3C). Thus, these results suggest that
the B cell compartment were different in the peripheral blood of
TPE patients, especially in pleural effusion of TPE. The increased
switched B cell may play a major role in acquired immunity against
M. tuberculosis.
BAFF level was corrected with the
increased proportion of switched B cell in both blood and pleural
effusion of patients with TPE
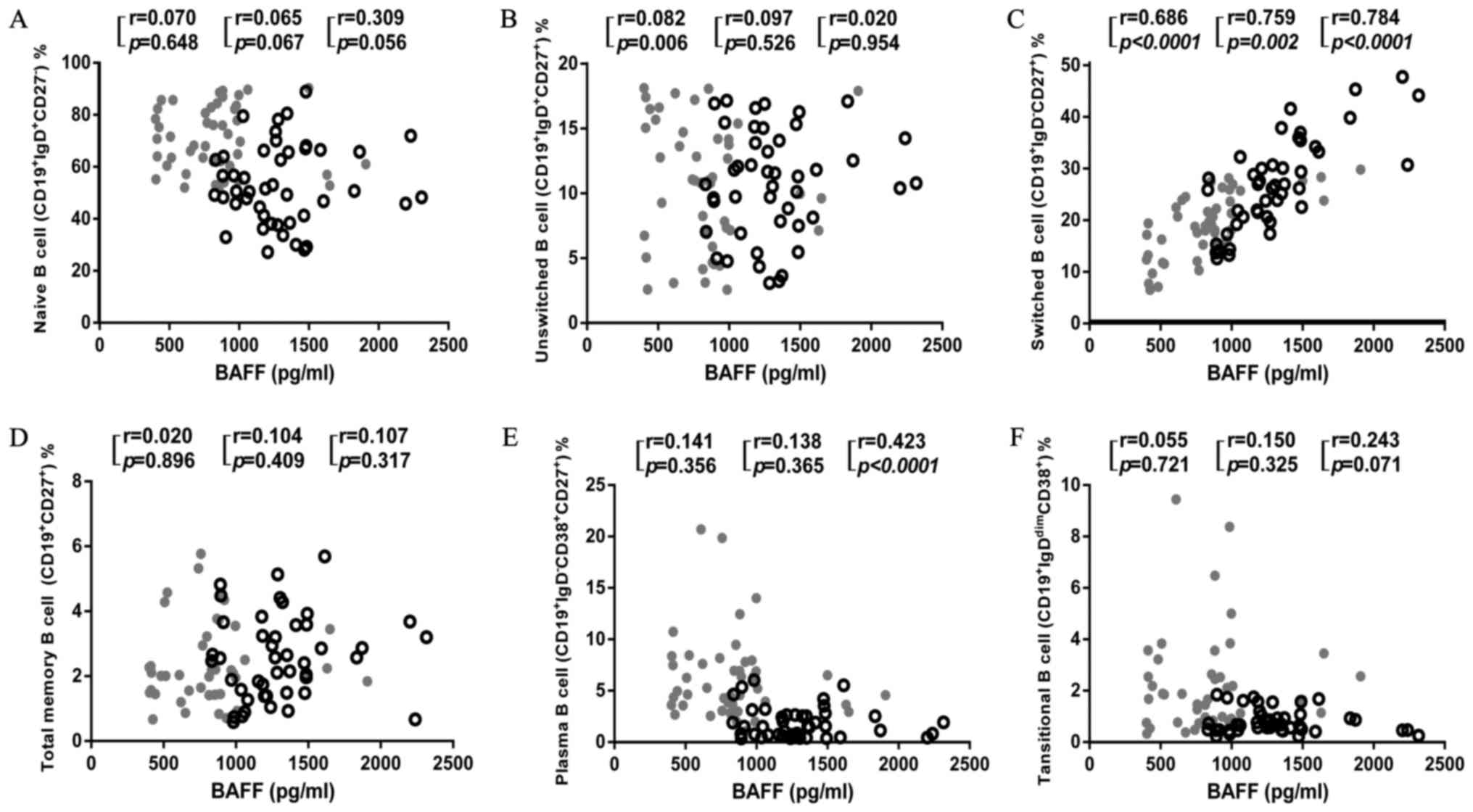
BAFF is a fundamental survival factor for the
maturation and differentiation of B cell (13,14,21).
To investigate how increased BAFF affects B cell survival in
patients with TPE, we further analyzed the correlation of the BAFF
level with the proportions of each B cell subset in PBMCs, PFMCs
and both together (Fig. 4). We
found that BAFF level had no correlation with naïve B cells, total
memory B cell, unswitched B cell and transitional B cell in both
PBMCs and PFMCs. Interestingly, BAFF level negatively correlated
with plasma B cell when combining PBMCs and PFMCs, despite there
was no significant correlation with either proportion alone
(Fig. 4E). Moreover, the BAFF
level had a high degree of correlation with the proportions of
switched B cell both in PBMCs and PFMCs (Fig. 4C). These findings suggest an
important role of BAFF in facilitating switched B cell
proliferation and redistribution, and potentially inhibiting plasma
B cell differentiation as a consequence of M.
tuberculosis-induced immune activation in patients with
TPE.
Discussion
Early researches on BAFF focused more on autoimmune
diseases. It has been reported that up-regulated BAFF is involved
in autoimmune disorders such as rheumatoid arthritis, systemic
lupus erythematosus, and autoimmune encephalomyelitis (15,16,22).
In human active pulmonary TB, the levels of BAFF and a
proliferation-inducing ligand (APRIL) were markedly increased. The
elevation of BAFF was closely related to the Th1 immune response
(17). When co-infected with
Strongyloides stercoralis, BAFF and APRIL level
significantly diminished in comparison to these patients with
latent TB (23). In our study, we
found that BAFF levels were dramatic increased in TPE patients,
particularly in the pleural effusion of these patients.
M. tuberculosis infection is well-known to
influence T cell responses, whether such infection also modulates
the maturation, differentiation and redistribution of B cell is
worth being revealed. Li et al scanned the profiling B cell
immune responses in TB patients, and they found the percentage of
tissue-like memory B cells
(CD19+CD10−CD27−CD21−CD20+)
was lower in the TB group than that in the HC group (24). Active TB has also been reported to
be directly associated with high frequencies of Bregs
(CD19+CD1d+CD5+), which
selectively inhibit Th17 activation by direct cell contact
(25). In this study, we
classified B cell subsets based on the expressions of surface cell
markers, including CD19, IgD, CD27 and CD38. Compared with PBMCs
from HC, we only found the proportion of switched B cell was
significantly different, higher in patients with TPE. These
apparent discrepancies reported across studies are most likely due
to the use of an imperfect panel of markers to characterize the B
cell subsets. Our study included previously unreported components
of B cell in the pleural effusion of TPE patients. We found the
proportion of switched B cell was significantly increased, while
naïve B cells, plasma B cell and transitional B cell decreased in
pleural effusion in comparison to peripheral blood of TPE patients.
The different B cell compartments may be affected by selective
activation and proliferation of B cell subsets during M.
tuberculosis infection or redistributions of individual
circulating B cell subsets between blood and pleural space.
The survival function of BAFF on B cells has been
well documented, but which B cell subsets are benefited from the
survival effect of BAFF is not clearly described. In the spleen of
BAFF−/− mice, B cells fail to proceed from naïve B cells
to the transitional type B cells (26,27),
consistent with the idea that transitional B cells are exquisitely
dependent on the activity of BAFF in vitro (28). Jaime and his colleagues reported
for the first time that BAFF could considerably attenuate plasma B
cell (CD27+CD38+ B cell) differentiation in
response to T cell-independent activation (29). Currently, we found BAFF level was
negatively correlated with plasma B cell when combining PBMCs and
PFMCs, which may validate Jaime's stand point to a certain extent.
Furthermore, BAFF level presented a high degree of correlation with
the proportions of switched B cell in both PBMCs and PFMCs. It
suggests that BAFF may facilitate switched B cell proliferation in
patients with TPE.
Our study had several limitations. First, a marked
limitation in the current study is the fact that we just found the
correction of increased BAFF level and the proportions of switched
B cell in PBMCs and PFMCs. The derivation that BAFF promote witched
B cell proliferation in TPE patients were not proved in vivo
or in vitro. Second, not all the B cell subset was covered
in our research, such as the regulatory B cell, a subset has been
reported to increase in active TB (24). Third, those pleural effusions with
the other aetiologies should also been enrolled in the sample
groups.
In conclusion, our study, for the first time,
demonstrate a clear alteration of B cell composition in pleural
effusion of TPE patients and that BAFF may activate switched B cell
to enhance the humoral immune response to M. tuberculosis
infection. The BAFF-switched B cell axis may be helpful to reveal
the pathogenesis and provide a potential immunotherapy for TPE. But
how BAFF affects switched B cell proliferation still need to be
elucidated.
Acknowledgements
The authors would like to thank the Department of
Respiration at Dongguan 6th Hospital (Guangdong, China) who
assisted with the recruitment of study subjects and the collection
of clinical data. The authors would also like to thank Dr. Jixin
Zhong (Cardiovascular Research Institute, Case Western Reserve
University, Cleveland, OH, USA) for assisting with the medical
English writing.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81570009 and
81273237) and The Natural Science Foundation of Guangdong Province
(grant nos. 2015A030313513 and 2017A030310666).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, JFX, KDL, JAZ and GBL conceived and designed the
experiments. XW, KDL, ZC, CC, ZGZ, YQL, HLL, RXL and BYZ performed
the experiments, and XW and JFX analysed the data. XW wrote the
paper, and XW and JFX critically reviewed the manuscript for
intellectual content.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangdong Medical University and Shenzhen Third
People's Hospital, and written informed consent was obtained from
all study subjects prior to their participation.
Consent for publication
Written informed consent was obtained from all study
subjects for the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BAFF
|
B cell activating factor
|
|
TPE
|
tuberculous pleural effusion
|
|
HC
|
health control
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PFMC
|
pleural fluid mononuclear cell
|
|
TB
|
tuberculosis
|
|
MTB
|
Mycobacterium tuberculosis
|
|
TST
|
tuberculin skin test
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Murray CJ, Ortblad KF, Guinovart C, Lim
SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown
JC, et al: Global, regional, and national incidence and mortality
for HIV, tuberculosis, and malaria during 1990–2013: A systematic
analysis for the global burden of disease study 2013. Lancet.
384:1005–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paulson T: Epidemiology: A mortal foe.
Nature. 502:S2–S3. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization (WHO): Global
tuberculosis report 2016. WHO; Geneva: 2016, http://apps.who.int/medicinedocs/documents/s23098en/s23098en.pdf
|
|
4
|
Vorster MJ, Allwood BW, Diacon AH and
Koegelenberg CF: Tuberculous pleural effusions: Advances and
controversies. J Thorac Dis. 7:981–991. 2015.PubMed/NCBI
|
|
5
|
Ruan SY, Chuang YC, Wang JY, Lin JW, Chien
JY, Huang CT, Kuo YW, Lee LN and Yu CJ: Revisiting tuberculous
pleurisy: Pleural fluid characteristics and diagnostic yield of
mycobacterial culture in an endemic area. Thorax. 67:822–827. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leibowitz S, Kennedy L and Lessof MH: The
tuberculin reaction in the pleural cavity and its suppression by
antilymphocyte serum. Br J Exp Pathol. 54:152–162. 1973.PubMed/NCBI
|
|
7
|
Seibert AF, Haynes J Jr, Middleton R and
Bass JB Jr: Tuberculous pleural effusion. Twenty-year experience.
Chest. 99:883–886. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Porcel JM: Advances in the diagnosis of
tuberculous pleuritis. Ann Transl Med. 4:2822016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackay F and Browning JL: BAFF: A
fundamental survival factor for B cells. Nat Rev Immunol.
2:465–475. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider P, MacKay F, Steiner V, Hofmann
K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea
H, et al: BAFF, a novel ligand of the tumor necrosis factor family,
stimulates B cell growth. J Exp Med. 189:1747–1756. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bornacelly A, Mercado D, Acevedo N and
Caraballo L: The strength of the antibody response to the nematode
Ascaris lumbricoides inversely correlates with levels of B-cell
activating factor (BAFF). BMC Immunol. 15:222014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakai J and Akkoyunlu M: The role of BAFF
system molecules in host response to pathogens. Clin Microbiol Rev.
30:991–1014. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mariño E, Walters SN, Villanueva JE,
Richards JL, Mackay CR and Grey ST: BAFF regulates activation of
self-reactive T cells through B-cell dependent mechanisms and
mediates protection in NOD mice. Eur J Immunol. 44:983–993. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mackay F, Woodcock SA, Lawton P, Ambrose
C, Baetscher M, Schneider P, Tschopp J and Browning JL: Mice
transgenic for BAFF develop lymphocytic disorders along with
autoimmune manifestations. J Exp Med. 190:1697–1710. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakayama Y, Kosek J, Capone L, Hur EM,
Schafer PH and Ringheim GE: Aiolos overexpression in systemic lupus
erythematosus B cell subtypes and BAFF-induced memory B cell
differentiation are reduced by CC-220 modulation of cereblon
activity. J Immunol. 199:2388–2407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becerra E, De La Torre I, Leandro MJ and
Cambridge G: B cell phenotypes in patients with rheumatoid
arthritis relapsing after rituximab: Expression of B
cell-activating factor-binding receptors on B cell subsets. Clin
Exp Immunol. 190:372–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu K, Zhang Y, Hu S, Yu Y, Yang Q, Jin D,
Chen X, Jin Q and Liu H: Increased levels of BAFF and APRIL related
to human active pulmonary tuberculosis. PLoS One. 7:e384292012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mensah F, Bansal A, Berkovitz S, Sharma A,
Reddy V, Leandro MJ and Cambridge G: Extended B cell phenotype in
patients with myalgic encephalomyelitis/chronic fatigue syndrome: A
cross-sectional study. Clin Exp Immunol. 184:237–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JA, Liu GB, Zheng BY, Lu YB, Gao YC,
Cai XZ, Dai YC, Yu SY, Jia Y, Chen C, et al:
Tuberculosis-sensitized monocytes sustain immune response of
interleukin-37. Mol Immunol. 79:14–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pozdzik A, Beukinga I, Gu-Trantien C,
Willard-Gallo K, Nortier J and Pradier O: Circulating
(CD3(−)CD19(+)CD20(−)IgD(−)CD27(high)CD38(high)) Plasmablasts: A
promising cellular biomarker for immune activity for anti-PLA2R1
related membranous nephropathy? Mediators Inflamm.
2016:76510242016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naradikian MS, Perate AR and Cancro MP:
BAFF receptors and ligands create independent homeostatic niches
for B cell subsets. Curr Opin Immunol. 34:126–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
von Budingen HC, Palanichamy A,
Lehmann-Horn K, Michel BA and Zamvil SS: Update on the autoimmune
pathology of multiple sclerosis: B-cells as disease-drivers and
therapeutic targets. Eur Neurol. 73:238–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anuradha R, Munisankar S, Bhootra Y, Dolla
C, Kumaran P, Nutman TB and Babu S: Modulation of Mycobacterium
tuberculosis-specific humoral immune responses is associated with
Strongyloides stercoralis co-infection. PLoS Negl Trop Dis.
11:e00055692017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XX, Chen JX, Wang LX, Sun J, Chen SH,
Chen JH, Zhang XY and Zhou XN: Profiling B and T cell immune
responses to co-infection of Mycobacterium tuberculosis and
hookworm in humans. Infect Dis Poverty. 4:202015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang M, Zheng X, Zhang J, Zhu Y, Zhu X,
Liu H, Zeng M, Graner MW, Zhou B and Chen X: CD19(+)CD1d(+)CD5(+) B
cell frequencies are increased in patients with tuberculosis and
suppress Th17 responses. Cell Immunol. 274:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schiemann B, Gommerman JL, Vora K, Cachero
TG, Shulga-Morskaya S, Dobles M, Frew E and Scott ML: An essential
role for BAFF in the normal development of B cells through a
BCMA-independent pathway. Science. 293:2111–2114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gross JA, Dillon SR, Mudri S, Johnston J,
Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, et al:
TACI-Ig neutralizes molecules critical for B cell development and
autoimmune disease. Impaired B cell maturation in mice lacking
BLyS. Immunity. 15:289–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Batten M, Groom J, Cachero TG, Qian F,
Schneider P, Tschopp J, Browning JL and Mackay F: BAFF mediates
survival of peripheral immature B lymphocytes. J Exp Med.
192:1453–1466. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darce JR, Arendt BK, Chang SK and Jelinek
DF: Divergent effects of BAFF on human memory B cell
differentiation into Ig-secreting cells. J Immunol. 178:5612–5622.
2007. View Article : Google Scholar : PubMed/NCBI
|