Introduction
Alcoholic liver disease (ALD), which is mainly
caused by excessive alcohol drinking, endangers human health
worldwide (1). Individuals with
ALD that progress to advanced disease stages with liver fibrosis
and cirrhosis have increased risk of complications such as portal
hypertension, liver cancer and liver failure (2).
The liver contains diverse enzymatic systems to
metabolize ethanol associated with many important metabolic
functions (3). Under normal
circumstances, most of the ethanol in the body could be catalyzed
by alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase
(ALDH) (4). Therefore, the
bioactivity for dealing with ethanol can be reflected by the
activation rate of ADH and ALDH. Tumor necrosis factor α (TNF-α),
one of the most important proinflammatory factors, is produced and
secreted by hepatic parenchymal cells and Kupffer cells during the
process of alcoholic liver injury (5,6).
Moreover, interleukin 6 (IL-6) can induce significant injuries in
Kupffer cells mainly by reduction in ADH and ALDH activities owing
to the long-term excessive ethanol consumption, causing aggravating
damage to liver cells by ethanol and acetaldehyde (4). Furthermore, reduction in liver cell
function results in decreased expression of the intracellular
response factors, AMP-activated protein kinase (AMPK)-α2 and
peroxisome proliferator-activated receptor (PPAR)-α (7), and enhanced expression of the
inflammatory factors, TNF-α and IL-6, leading to liver cell
injury.
Edible mushrooms, which are rich in proteins as well
as trace minerals, and low in fat (8), have long been used in folk medicines
and health foods. Lentinus edodes, also named Xianggu in
Chinese and Shiitake in Japanese, is one of the most widely used
edible mushrooms in the global market because of its flavor and
nutritional profile (9). Research
on effective components of Lentinus edodes has revealed that
they have diverse beneficial effects, including immune regulation,
anti-tumor, anti-aging, liver protection and resistance to
respiratory infections (10–12).
Mycelia zinc polysaccharides of Lentinus edodes have been
found to upregulate the anti-aging activity of total antioxidant
capacity, GSH peroxide and superoxide dismutase (SOD), and
downregulate the malondialdehyde (MDA) content in vivo
(13). Other compounds such as
polyphenols and crude polysaccharides in Lentinus edodes
have also been reported to exhibit potent antioxidative
bioactivities (14–17). Taking advantages of the high
content of protein and balanced amino acid compositions, we
previously obtained enzymatic hydrolyzed peptides from Letinous
edodes umbrellas and feet, and found that Letinous
edodes foot peptides possessed strong activation abilities on
ADH and ALDH activities in vitro (18,19).
In the present study, we established a cell model of
ethanol-induced liver damage to evaluate the hepatoprotective
effect of Letinous edodes foot peptides in human L02
hepatocytes. The regulation on aminotransferases, alcohol metabolic
enzymes, antioxidation capacities and proinflammatory cytokines was
also investigated at the cellular and molecular level.
Materials and methods
Letinous edodes foot peptides
Letinous edodes foot peptides were obtained
from a preliminary experiment (19). The Letinous edodes foot
peptides were prepared by an alkali-solution and acid-isolation
method, assisted by ultra-high-pressure processing with a pressure
of 400 MPa and a processing time of 10 min. After ultrafiltration,
three molecular weights of peptides were obtained, of which 0–3 kDa
had the highest activity to activate ADH and ALDH by 70.79 and
71.35%, respectively. Therefore, we used Letinous edodes
foot peptides with molecular weight of 0–3 kDa as material in this
research to evaluate the protective effect.
Cells and chemicals
Human liver cells (L02) were bought from Shanghai Bo
research Biological Technology Co., Ltd. (Shanghai, China).
Ethanol, analytical reagent, was obtained from Tianjin Yongda
Chemical Reagent Co., Ltd. (Tianjin, China). Alanine
aminotransferase (ALT), aspartate aminotransferase (AST), MDA and
SOD test kits, and ADH and ALDH ELISA kits were obtained from the
Nanjing Institute of Biological Engineering. RPMI-1640 culture
medium, 0.25% pancreatin, penicillin-streptomycin and fetal bovine
serum (FBS) were obtained from Gibco; Thermo Fisher Scientific,
Inc., (Waltham, MA, USA). PMSF and RIPA cell lysis solutions were
obtained from Beyotime Institute of Biotechnology, Haimen, China.
The MTT was obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). DMSO and PBS were procured from Shanghai Jixing
Biological Science and Technology Co., Ltd. TRNzol reagent
(DP405-02) was obtained from Tian Gen Biochemical Technology Co.,
Ltd., (Beijing, China). PrimeScript™ RT reagent kit with gDNA
Eraser RR047B, SYBR®Premix Ex Taq™ II (Tli
RNaseH Plus), ROX plus RR82LR, and DL2,000 DNA Marker 3427Q were
obtained from Takara Bio, Inc., (Otsu, Japan). Primer synthesis was
performed by Invitrogen; Thermo Fisher Scientific, Inc.
Cell culture
L02 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS and 100 U/ml penicillin at 37°C in a 5%
CO2 humidified environment.
Determination of the damaging
concentration of ethanol in the experiment model
L02 cell viability after treatment with ethanol and
Letinous edodes foot peptides was measured by the MTT assay.
Certain concentration of Letinous edodes foot peptides and
ethanol were prepared in a serum-free medium, using
Puerariae as control. Puerariae is a famous traditional
Chinese medicine with the effect of anti-inebriation. It has been
reported that pretreatment with Puerariae extract significantly
prolonged the ethanol tolerance duration and shortened intoxication
duration, which was accompanied by decreased blood ethanol
concentration, elevated ADH and ALDH activities in the liver and
decreased ALT and AST activities in the serum (20). Cells were seeded in 96-well plates
at a cell density of 5×104 cells/well. After treatment
with 0, 25, 50, 75, 100, 200, 300, 400, 500 or 800 mmol/l ethanol,
10 µl of MTT (5 mg/ml) were added for 4 h. Then, the medium was
removed and 150 µl of DMSO were added for 10 min. The absorbance
was measured at 492 nm.
Determination of the optimal
concentration of Letinous edodes foot peptides for protection
against ethanol-induced damage
To determine the best concentration of Letinous
edodes foot peptides for protection against ethanol-induced
damage, 100 µl of Letinous edodes foot peptides were added
to a 96-well plate prior to ethanol (200 mmol/l). The final
concentrations were 0, 6.25, 12.5, 25, 50, 75, 100, 200, 400, 600
or 1,200 mg/l, which were proved to have nontoxic effect to the L02
cell in the preliminary experiment (data not shown).
Determination of the addition order of
Letinous edodes foot peptides and ethanol
To determine the best addition order of Letinous
edodes foot peptides to obtain the best results, we had three
experimental groups. In the first group Letinous edodes foot
peptides at different concentrations was added to the cells 24 h
prior to ethanol addition. In the second group ethanol was added to
the cells 24 h prior to Letinous edodes foot peptides. In
the last group Letinous edodes foot peptides and ethanol
were added simultaneously.
Determination of enzyme activity
L02 cells in the logarithmic growth phase were
cultured in 96-well plates (1×106 cells/well) for 24 h.
The cells were divided into six groups: negative control (no
ethanol, no peptides), positive control (ethanol treated),
Puerariae group (ethanol treated with pretreatment of 10
mg/l Puerariae) and peptides groups (ethanol treated with
pretreatment of 25, 50,100 mg/l peptides). Six wells were set up
for each group. The experiment was performed in an incubator with
5% CO2 at 37°C. The interval between adding ethanol and
the peptides was 24, and 24 h between the last sampling and
testing.
After treatment, the cells were rinsed twice with
PBS (0.01 mol/l). PMSF (1 mmol/l, 100 µl) was added to each well
and the supernatant was removed after centrifugation (20,000 × g,
10 min). The indexes of oxidative damage in the cell lysis
solution, including MDA, SOD, ALT and AST, were examined with the
relevant kits.
ELISA assay
Cell treatment and grouping were as described above.
Serum-free cell culture medium was added to the negative control
group, 200 mmol/l ethanol solution to the positive control group,
and the peptides prior to 200 mmol/l ethanol solution to the
peptides groups for 24 h. After another 24 h, the supernatant was
collected to test ADH and ALDH activity.
The relative expression of mRNA
measured by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
RT-qPCR was used to detect expressions of target
genes. The primers listed in Table
I were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. Total RNA was extracted with TRNzol, and cDNA reverse
transcription was performed by the PrimeScript RT reagent kit with
gDNA Eraser in accordance with the manufacturer's protocol.
Amplification was carried out by SYBR Premix Ex Taq II & reg
(Tli RNaseH Plus; Takara Bio, Inc., Otsu, Japan), ROX plus
according to the product specifications, using cDNA templates
diluted 10 times. Amplification was performed at 95°C for 30 sec,
followed by 45 cycles of 95°C for 5 sec and 60°C for 40 sec. The
2−∆∆Cq method of quantification was employed (21).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer name | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| IL-6 upstream
primer |
ATGAGGAGACTTGCCTGGTGAAAAT | 104 |
| IL-6 downstream
primer |
TCTGGCTTGTTCCTCACTACT |
|
| TNF-α upstream
primer |
CTCCTCACCCACACCATCAGCCGCA | 135 |
| TNF-α downstream
primer |
ATAGATGGGCTCATACCAGGGCTTG |
|
| AMPK-α2 upstream
primer |
CGAAGTCAGAGCAAACCGTATG | 248 |
| AMPK-α2 downstream
primer |
GAACGCTGAGGTGTTGAGGAA |
|
| PPAR-α upstream
primer |
GATCTGAGAAAGCAAAACTGAAAGC | 293 |
| PPAR-α downstream
primer |
GCAGTGAAAGATGCGGACCTC |
|
| ADH upstream
primer |
TCCGACCTGGAGCTGAGACA | 152 |
| ADH downstream
primer |
GGCGACGGCAGGTAGTTCTC |
|
| ALDH2 upstream
primer |
AGTTTGTGGAGCGGAGCGT | 128 |
| ALDH2 downstream
primer |
CGTGTTGATGTAGCCGAGGA |
|
| ACTIN upstream
primer |
CTGAAGTACCCCATCGAGCAC | 223 |
| ACTIN downstream
primer |
ATAGCACAGCCTGGATAGCAAC |
|
Statistical analysis
All data are presented as means ± standard error of
three experiments. The total variation was estimated by analysis of
variance followed by a Duncan multiple comparison test with SPSS
ω20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Establishment of cell model of
ethanol-induced damage
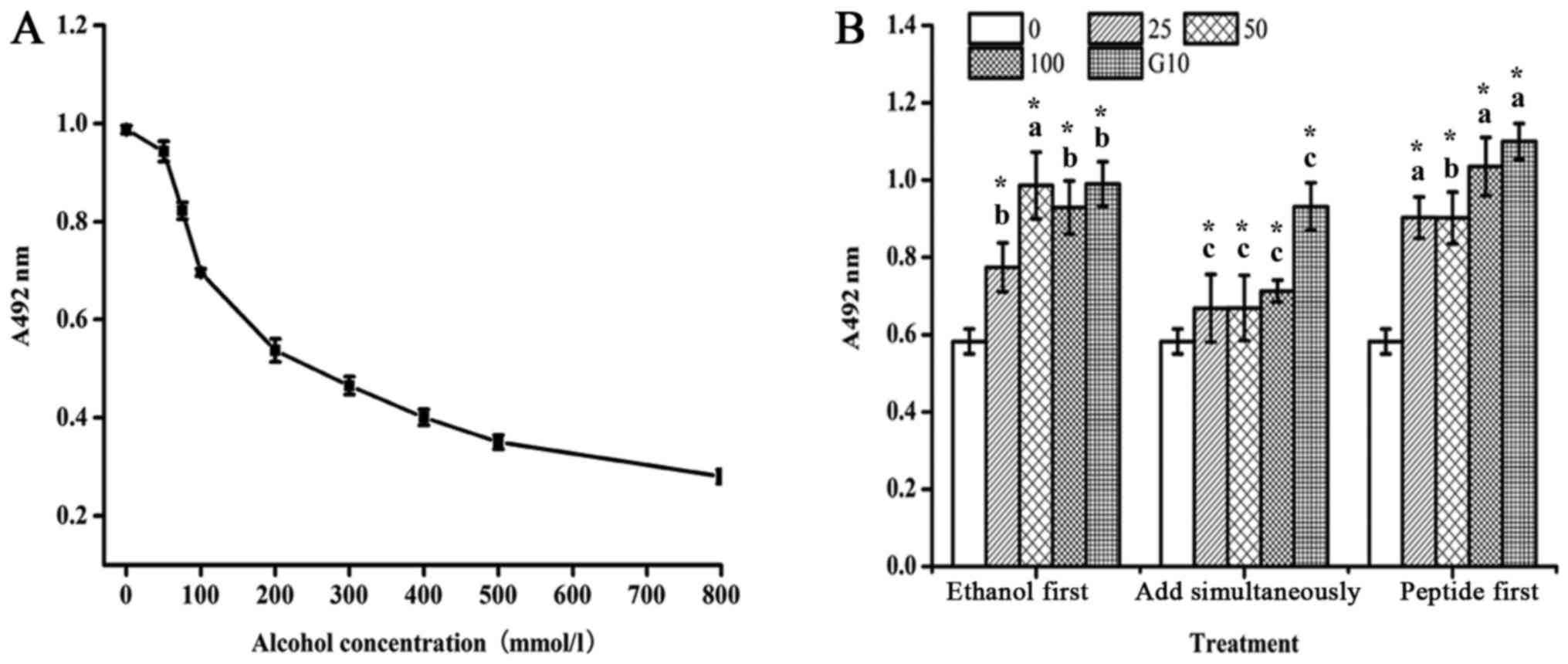
As shown in Fig.
1A, at a low concentration (<50 mmol/l) ethanol did not
inhibit the growth of L02 hepatic cells, but it promoted cell
proliferation. The reason for this could be that at a low
concentration the cells used ethanol as an energy source, resulting
in accelerated cell proliferation. As the concentration increased,
ethanol inhibited L02 cell proliferation, with an IC50
of 222.8±14.7 mmol/l. Therefore, the final concentration chosen for
the model of ethanol-induced damage was 200 mmol/l.
Next, we incubated L02 cells with different
concentrations of Letinous edodes foot peptides for 24 h.
Different effects on the proliferation of normal hepatic L02 cells
were observed (Table II). When
the concentration of Letinous edodes foot peptides was low,
the proliferation rate was similar to the cells exposed to ethanol
only. However, as the concentration was increased, the L02 cell
proliferation rate increased. Furthermore, from 25, 50 and 100 mg/l
the positive effect on proliferation was significant. Thus, we
concluded that the peptides could promote the growth of normal
human liver L02 cells of ethanol-induced damaged.
 | Table II.Effect of Letinous edodes foot
peptides on the proliferation of ethanol-damaged L02 cells. |
Table II.
Effect of Letinous edodes foot
peptides on the proliferation of ethanol-damaged L02 cells.
|
|
A492nm |
|
|---|
|
|
|
|
|---|
| Reagent and
concentration | Repeat 1 | Repeat 2 | Repeat 3 | Average |
|---|
| No ethanol, no
peptides |
|
|
|
|
| 0 | 0.99 | 1.077 | 0.987 | 1.018±0.051 |
| Ethanol
(mmol/l) |
|
|
|
|
|
200 | 0.39 | 0.382 | 0.406 |
0.393±0.012a |
| Peptides
(mg/l) |
|
|
|
|
|
6.25 | 0.366 | 0.43 | 0.426 |
0.407±0.036a |
|
12.5 | 0.433 | 0.433 | 0.354 |
0.407±0.045a |
| 25 | 0.48 | 0.446 | 0.428 |
0.452±0.026b |
| 50 | 0.465 | 0.524 | 0.484 |
0.491±0.030b |
| 75 | 0.474 | 0.483 | 0.528 |
0.495±0.029b |
|
100 | 0.543 | 0.496 | 0.552 |
0.530±0.030b |
|
200 | 0.592 | 0.498 | 0.547 |
0.546±0.047b |
|
400 | 0.644 | 0.561 | 0.562 |
0.589±0.048b |
|
600 | 0.59 | 0.625 | 0.607 |
0.607±0.018b |
|
1,200 | 0.71 | 0.63 | 0.744 |
0.695±0.058b |
| Puerariae
(mg/l) |
|
|
|
|
| 10 | 0.665 | 0.694 | 0.626 |
0.662±0.034b |
| 25 | 1.015 | 0.938 | 0.947 |
0.967±0.042b |
The MTT assay was used to examine the cell viability
of L02 cells treated with different concentrations of Letinous
edodes foot peptides and 200 mmol/l ethanol, which were added
in three different sequences: Ethanol first, both substances
simultaneously, or peptides first (Fig. 1B). Similar to the 10 mg/l
Puerariae control, in the cells in which the Letinous
edodes foot peptides of 25, 100 mg/l was added 24 h before the
ethanol, the cell viability tended to be extremely significantly
higher than that of the other two groups (P<0.01). In a
comprehensive way, we added the Letinous edodes foot
peptides prior to the ethanol treatment in the following
experiments.
Measurement of aminotransferases'
activity
As shown in Fig.
2A, the AST enzyme activity in the positive control group
significantly increased compared with that in the negative control
group. However, a significant decrease was observed in the groups
pretreated with the Letinous edodes foot peptides. With the
increase in the Letinous edodes foot peptides concentration,
AST activity decreased gradually, though the Puerariae group
showed an even lower AST activity. The results suggest that
Letinous edodes foot peptides can effectively suppress the
liver cell damage-induced elevation in AST activity. Compared with
the negative control, the ALT activity in the positive control
group was significantly enhanced (Fig.
2B). Letinous edodes foot peptides at 25–100 mg/l
prominently suppressed the increase in ALT enzyme activity in L02
liver cells induced by ethanol. Thus, Letinous edodes foot
peptides have an obvious protective effect on ethanol-induced
damage in L02 liver cells.
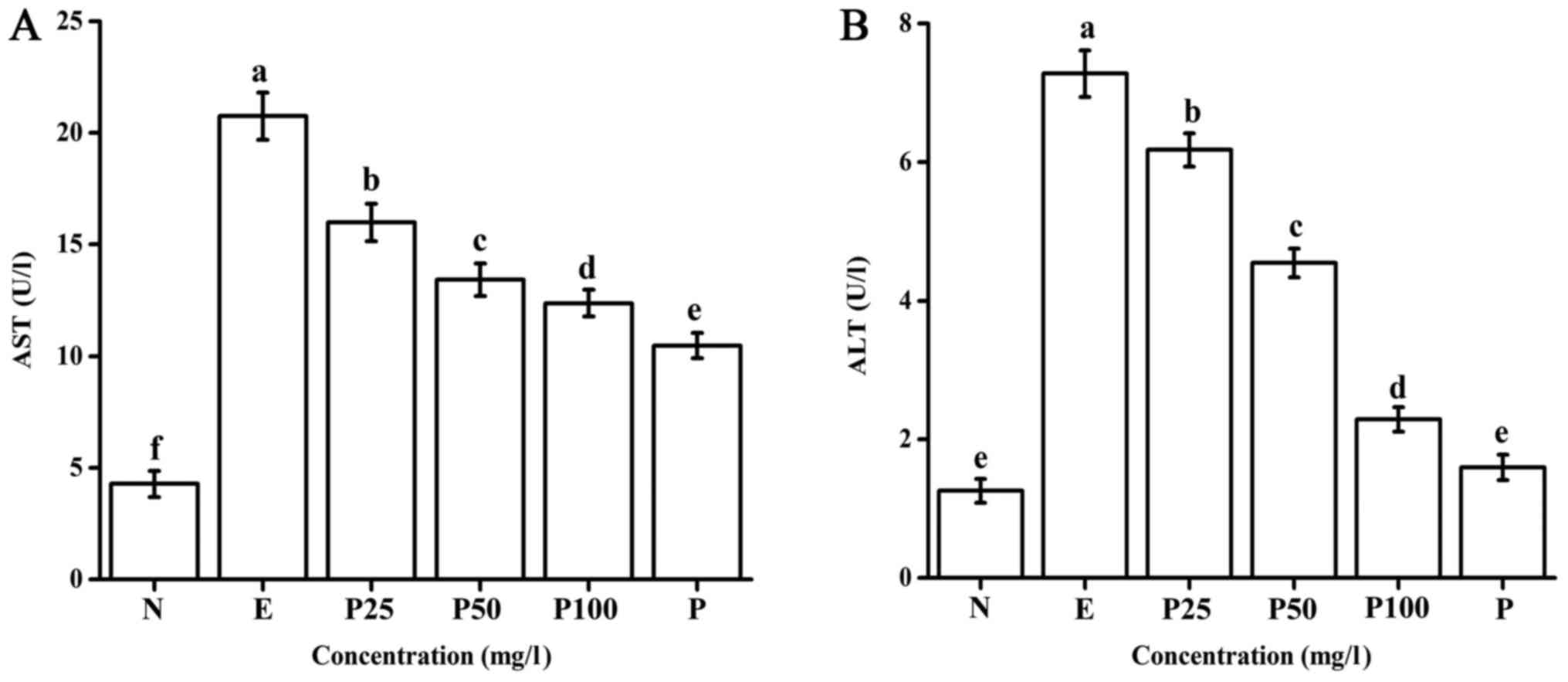 | Figure 2.Effect of Letinous edodes foot
peptides on (A) AST and (B) ALT activity. Samples were divided into
the following groups: N, negative control (no ethanol, no
peptides), E, positive control (ethanol treated), P25, peptides
group (ethanol treated with pretreatment of 25 mg/l peptides), P50,
peptides group (ethanol treated with pretreatment of 50 mg/l
peptides), P100, peptides group (ethanol treated with pretreatment
of 100 mg/l peptides), P, Puerariae group (ethanol treated with
pretreatment of 10 mg/l Puerariae). a-fP<0.05. AST,
aspartate aminotransferase; ALT, alanine aminotransferase. |
Measurement of antioxidant
indexes
As shown in Fig.
3A, compared with the positive control, the Letinous
edodes foot peptides significantly decreased the MDA content in
liver cells injured by ethanol in a dose-dependent manner.
Furthermore, 100 mg/l peptides significantly decreased the MDA
level compared to that observed in the Puerariae group.
These results indicate that the Letinous edodes foot
peptides can decrease the content of MDA in ethanol-damaged liver
cells.
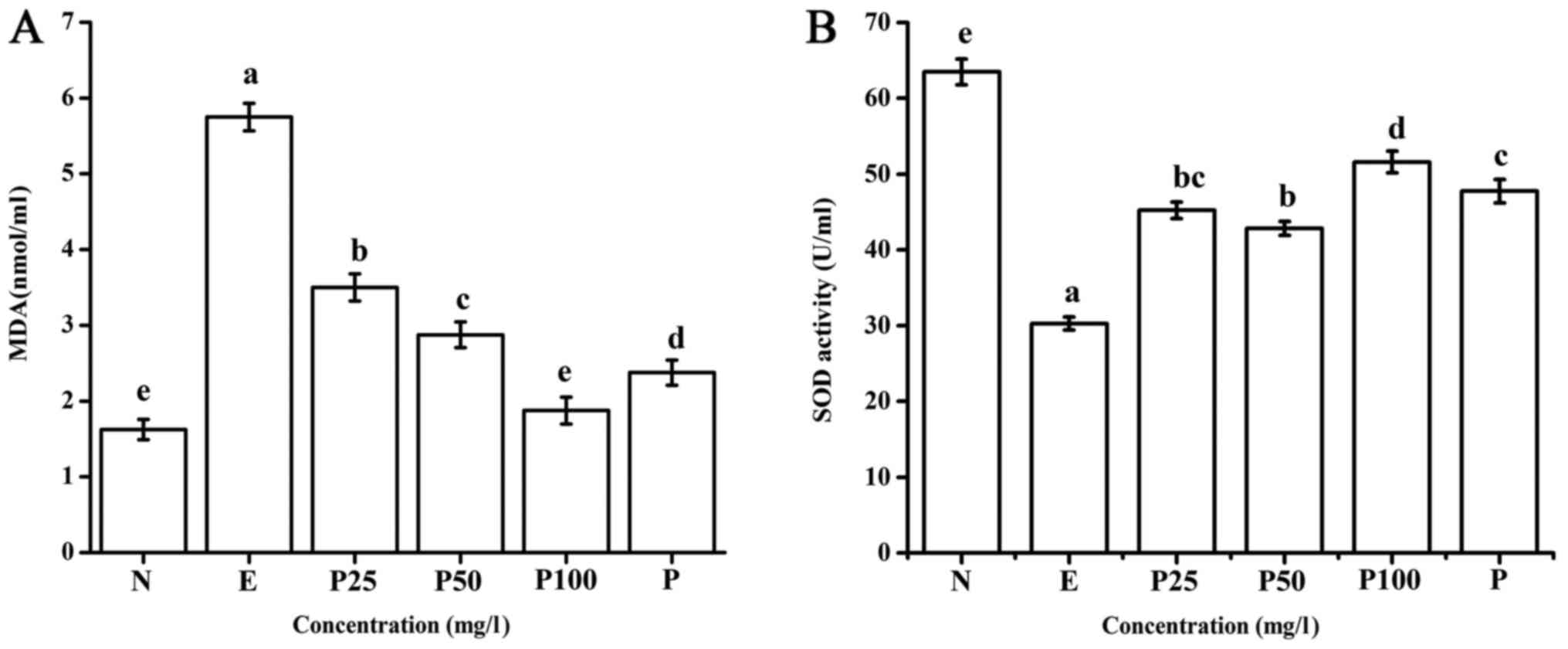 | Figure 3.Effect of Letinous edodes foot
peptides on the (A) MDA and (B) SOD content.
a-eP<0.05. SOD, superoxide dismutase; MDA,
malondialdehyde; N, negative control (no ethanol, no peptides), E,
positive control (ethanol treated), P25, peptides group (ethanol
treated with pretreatment of 25 mg/l peptides), P50, peptides group
(ethanol treated with pretreatment of 50 mg/l peptides), P100,
peptides group (ethanol treated with pretreatment of 100 mg/l
peptides), P, Puerariae group (ethanol treated with pretreatment of
10 mg/l Puerariae). |
Next, we examined the effect of Letinous
edodes foot peptides on SOD activity. Compared with the
negative control, ethanol significantly reduced SOD activity
(Fig. 3B). In contrast,
Letinous edodes foot peptides (25–100 mg/l) significantly
improved the SOD activity in ethanol-damaged L02 cells, and the
same effect was observed with 10 mg/l Puerariae.
Measurement of dehydrogenases by ELISA
and RT-qPCR
As shown in Fig. 4A and
B, the amount of ADH secretion increased after ethanol exposure
for 24 h compared with the negative control. This increase was
slightly suppressed by pretreating with Letinous edodes foot
peptides or Puerariae. ADH secretion in the Puerariae
group was slightly higher than that in the 50 mg/l Letinous
edodes foot peptides group, but lower than that in the 25 mg/l
group. Ethanol also increased ALDH secretion compared with the
negative control; however, Letinous edodes foot peptides
reversed this increase. The amount of ALDH secretion was the lowest
in the 25 mg/l peptides group and the highest in the
Puerariae group.
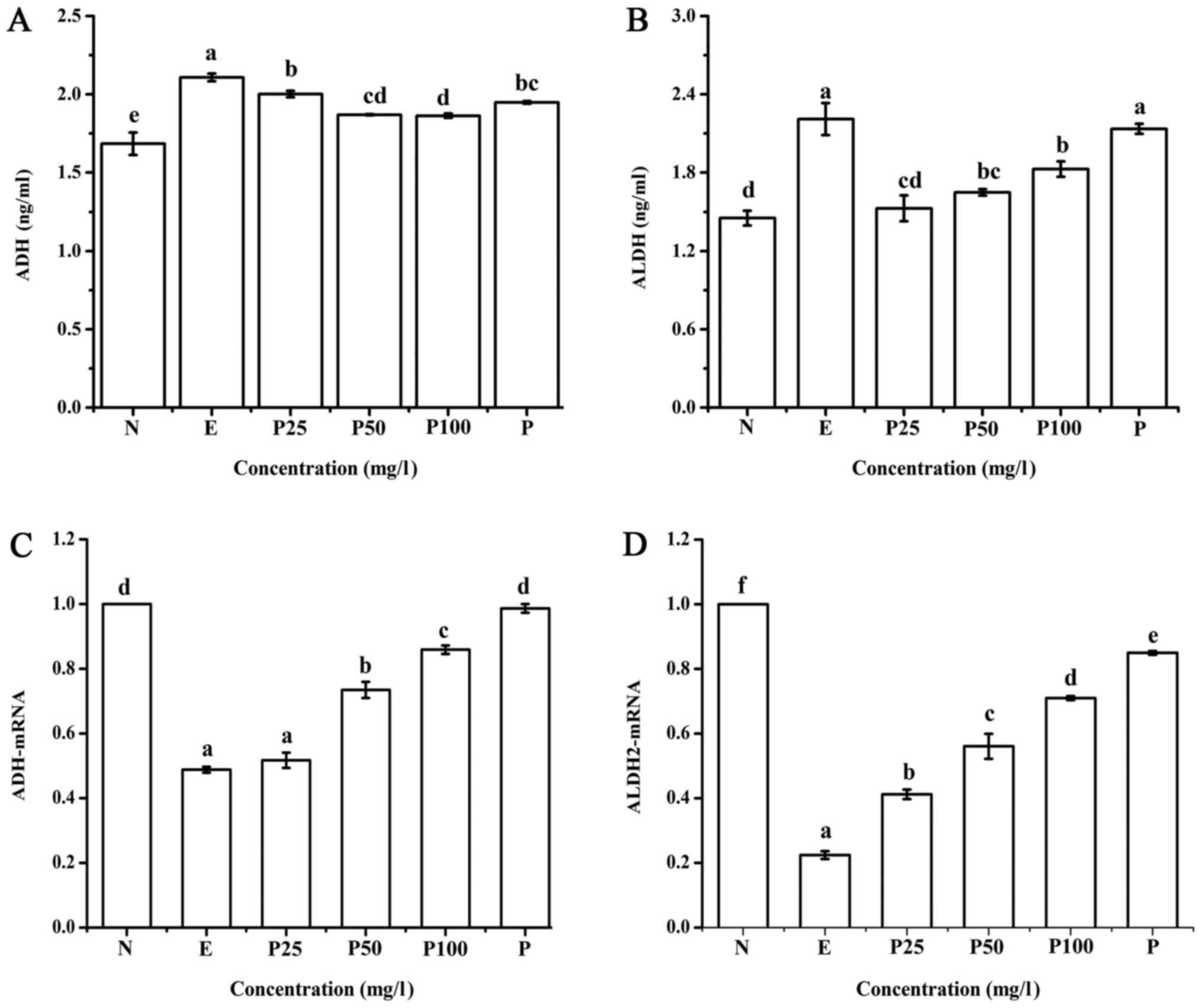 | Figure 4.Content of the dehydrogenases (A) ADH
and (B) ALDH was measured by ELISA assay. The mRNA expression of
(C) ADH and (D) ALDH2 was measured by reverse
transcription-quantitative polymerase chain reaction.
a-fP<0.05. ADH, alcohol dehydrogenase; ALDH,
acetaldehyde dehydrogenase; N, negative control (no ethanol, no
peptides), E, positive control (ethanol treated), P25, peptides
group (ethanol treated with pretreatment of 25 mg/l peptides), P50,
peptides group (ethanol treated with pretreatment of 50 mg/l
peptides), P100, peptides group (ethanol treated with pretreatment
of 100 mg/l peptides), P, Puerariae group (ethanol treated with
pretreatment of 10 mg/l Puerariae). |
As displayed in Fig. 4C
and D, compared with the negative control, the upregulation of
ADH and ALDH2 mRNA stimulated by ethanol in the positive control
group was significantly suppressed. This effect was reversed by
pretreatment with Puerariae and Letinous edodes foot
peptides in a dose-dependent manner. The ADH and ALDH2 mRNA
relative expression was in the following order: Negative control
> Puerariae group (10 mg/l)>peptides group (100
mg/l)>peptides group (50 mg/l)>peptides group (25
mg/l)>positive control, and the positive control group showed
significant differences compared with the other groups.
Expression of proinflammatory
cytokines
As shown in Fig. 5,
compared with the negative control, the expression of IL-6 and
TNF-α mRNA in the positive control group was significantly higher.
This upregulation was significantly suppressed by treatment with
Letinous edodes foot peptides in a dose-dependent manner,
even lower with 100 mg/l of peptides group compared with
Puerariae group. These results indicate that Letinous
edodes foot peptides can relieve liver injury.
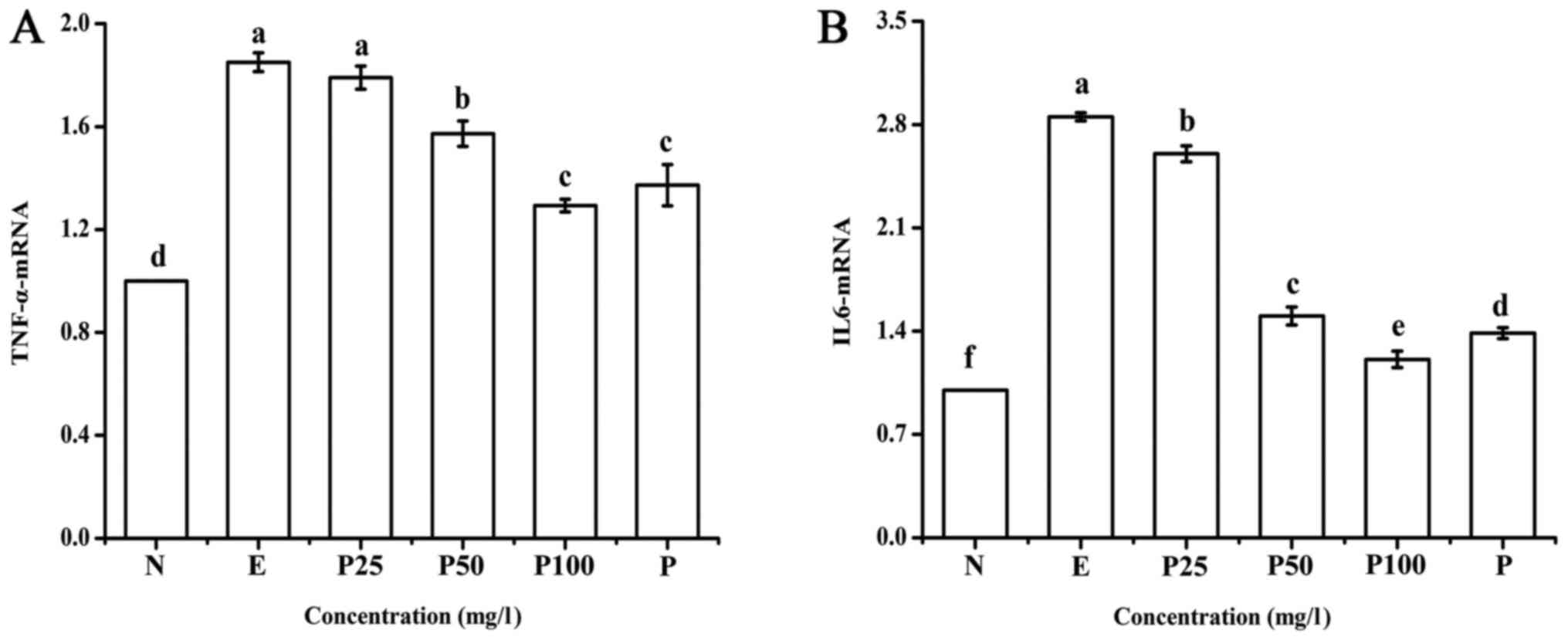 | Figure 5.Effect of Letinous edodes foot
peptides on the mRNA expression of (A) TNF-α and (B) IL-6.
a-fP<0.05. IL, interleukin; TNF, tumor necrosis
factor; N, negative control (no ethanol, no peptides), E, positive
control (ethanol treated), P25, peptides group (ethanol treated
with pretreatment of 25 mg/l peptides), P50, peptides group
(ethanol treated with pretreatment of 50 mg/l peptides), P100,
peptides group (ethanol treated with pretreatment of 100 mg/l
peptides), P, Puerariae group (ethanol treated with pretreatment of
10 mg/l Puerariae). |
Expression of metabolic regulation
factors
As shown in Fig. 6,
compared with the negative control, the relative expression of
AMPK-α2 and PPAR-α mRNA in the positive control group was lower.
This effect was reversed by Letinous edodes foot peptides
and Puerariae. The influence of the low concentration (25
mg/l) of Letinous edodes foot peptides on PPAR-α mRNA
relative expression was not significant, but that of the other
concentration groups (50, 100 mg/l) was significant compared with
the positive control group. PPAR-α expression in the
Puerariae group showed no difference compared with the
positive control group.
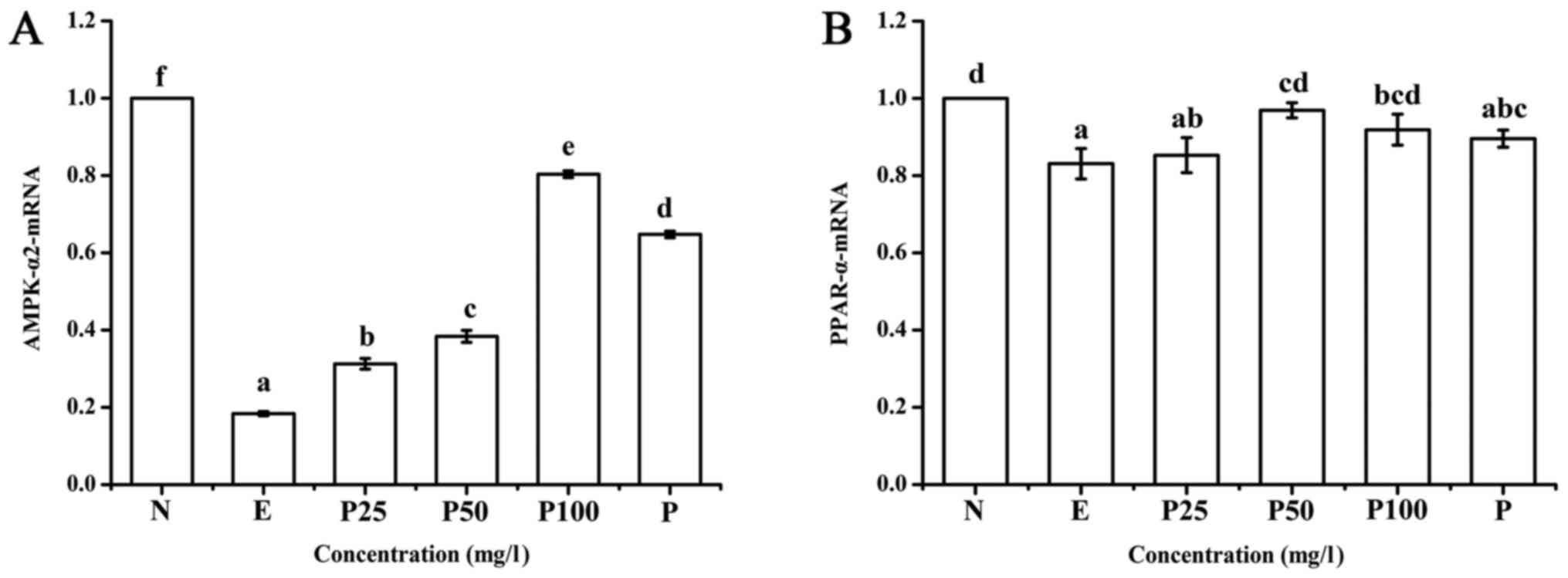 | Figure 6.Effect of Letinous edodes foot
peptides on the mRNA expression of (A) AMPK-α2 and (B) PPAR-α.
a-fP<0.05. AMPK, AMP-activated protein kinase; PPAR,
peroxisome proliferator-activated receptor; N, negative control (no
ethanol, no peptides), E, positive control (ethanol treated), P25,
peptides group (ethanol treated with pretreatment of 25 mg/l
peptides), P50, peptides group (ethanol treated with pretreatment
of 50 mg/l peptides), P100, peptides group (ethanol treated with
pretreatment of 100 mg/l peptides), P, Puerariae group (ethanol
treated with pretreatment of 10 mg/l Puerariae). |
Discussion
SOD is a scavenger of superoxide anion radicals,
transforming them into harmless oxygen and water molecules, thus
protecting cells from free radical damage. Acute ethanol excess can
cause a decrease in SOD activity and accumulation of a large amount
of free radicals, which raise the lipid peroxidation reaction with
polyunsaturated fatty acids in biological membranes, producing
peroxide lipids afterwards. This kind of substance, with poor
stability, will continue to produce MDA and other substances
(22,23). MDA can cause serious liver cell
damage which will change the cell membrane fluidity and
permeability. SOD and MDA are regularly measured together, as the
level of SOD activity is proportional to the free radical
scavenging ability of the organism, and the MDA content in the
cells reflects the degree of free radical attack. Combining SOD
with MDA analysis is helpful in studying the mechanism of
biological activity. Zhang et al (24) have reported that bamboo leaf
flavonoids significantly increased the SOD activity and reduced the
MDA content against ethanol-induced liver injury. She et al
(25) proved that corn peptides
protected liver cells from oxidative damage induced by ethanol
metabolism. Hong et al (26) confirmed that fermented Adlay
increased the activities of antioxidant enzymes such as SOD in the
liver. The results of this study showed that Letinous edodes
foot peptides significantly improved the SOD activity in the
ethanol-induced L02 cell model and reduced the MDA content,
demonstrating its hepatoprotective effect.
Under normal circumstances, the concentrations of
AST and ALT in liver cells are 1,000–5,000 times higher than in the
serum. If the liver tissue is injured, liver cell swelling and
necrosis, or increased membrane permeability of liver cells will
occur, followed by secretion of transaminase into the blood,
contributing to the increase in serum transaminase activity.
Because aminotransferase activity in the liver is much higher than
that in the blood under normal conditions, its change in serum is a
specific marker used to assess hepatocellular damage in clinic
(22,27). Zhang et al (3) investigated the protective effect of
anthocyanins from purple sweet potato on acute carbon
tetrachloride-induced liver injury in mice, which showed that the
anthocyanins could significantly reduce the carbon
tetrachloride-induced ALT and AST activity, demonstrating its
protective effect. The results of this study showed that the
Letinous edodes foot peptides reduced the damage index ALT
and AST enzyme activity in ethanol-induced liver cells, confirming
its protective effect on the liver.
It has been shown that when the body was stimulated
by ethanol, the liver cells produced ADH to metabolize ethanol,
which was oxidized to acetaldehyde, and then oxidized to acetic
acid by ALDH. Therefore, the release of ADH and ALDH gradually
increases with the increase in ethanol metabolism. ADH is a metal
enzyme with zinc atoms, which plays a major role in ethanol
metabolism. It is mainly expressed in the liver, intestines and
stomach; however, in the liver it is mainly located in the cell
fluid. The change in ADH activity has two sides. If the activity
increases, the oxidation of ethanol to acetaldehyde is accelerated,
providing the conditions for further metabolism of non-toxic
products. However, if the activity change is not timely for the
oxidation of acetaldehyde to acetic acid, the damage to the liver
cells will be more serious compared with ethanol. If ADH activity
is inhibited, the ethanol hazard for the body itself will increase
(28,29). ALDH is also an important enzyme in
ethanol metabolism, as it can transform toxic acetaldehyde
associated with alcoholic liver injury into acetic acid, which is a
nontoxic metabolic product. If the activities of ADH or ALDH are
weakened, the ethanol itself or the intermediate metabolite,
acetaldehyde, will produce toxic effects, which may lead to liver
cell mitochondria damage, affecting the energy metabolism of liver
cells, and inducing cell apoptosis and necrosis, thus aggravating
liver injury (30,31). At present, many studies have
indicated that apoptosis is involved in the occurrence of ALD,
which is an important part of the pathogenesis of ALD (32). The current study showed that the
Letinous edodes foot peptides increased the mRNA expression
of ADH and ALDH, which was obviously reduced by ethanol, confirming
its protective effect on the liver.
TNF-α, the most important cytokine in alcoholic
liver injury, is secreted by intrahepatic Kupffer cells, and its
level in vivo increases when ethanol activates the
extracellular receptor-activated kinase. Liver injury induced by
ethanol has also been associated with the level of AMPK and PPAR-α.
Besides, TNF-α promotes the release of other inflammatory cytokines
(such as IL-1 and IL-6), which promote liver cell damage caused by
inflammation, and even cause liver cell death. Ethanol can
influence inflammatory reactions by TNF-α, AMPK and PPAR-α, leading
to cell apoptosis and necrosis (33–36).
Vidyashankar et al (37)
have reported that ethanol in metabolic processes will consume
coenzyme Q10 in the liver, which can enhance TNF-α secretion, thus
producing substances harmful to HepG2 cells. Shearn et al
(38) have reported that AMPK
expression significantly decreased after ethanol intake in mice
with a high-fat diet. Studies in mouse and rat cultured liver cells
in vitro have demonstrated that ethanol suppressed the
expression of PPAR-α and the binding of PPAR-α/RXR to DNA, thus
promoting liver steatosis, inflammation, necrosis and fibrosis
(39). Park et al (34) found that Schisandra
chinensis has the ability to prevent ethanol-induced fatty
liver by a significant increase in AMPK and PPAR-α expression in
hepatic tissue of alcoholic rats. Our research revealed that the
Letinous edodes foot peptides have a role in protecting the
liver by inhibiting the mRNA expression of IL-6 and TNF-α and
enhanced the mRNA expression of the metabolic regulation factors
AMPK-α2 and PPAR-α, demonstrating the protective effect of
Letinous edodes foot peptides.
In conclusion, this research, using a cell model
in vitro, demonstrated the effect of ethanol-induced damage
on cell proliferation and the protection effect on Letinous
edodes foot peptides using the MTT assay. Furthermore, we
studied the protective effect of Letinous edodes foot
peptides on ethanol-damaged liver cells at the cellular and
molecular levels by measuring the mRNA expression of
proinflammatory factors, metabolic regulation factors and sober
enzyme factors using ELISA and RT-qPCR. According to our results,
Letinous edodes foot peptides significantly improved the SOD
activity and the mRNA expression of ADH and ALDH, which were
obviously reduced by ethanol. It also reduced the intracellular MDA
content, and the AST and ALT activity. The ethanol-stimulated
activation of the proinflammatory cytokines, IL-6 and TNF-α, in L02
cells was significantly blocked, and the metabolic regulation
factors, AMPK-α2 and PPAR-α, and the sober enzyme factors, ADH and
ALDH2, were induced by Letinous edodes foot peptides. Hence,
we concluded that Letinous edodes foot peptides have a
protective effect on normal human liver L02 cells, preliminarily
determined that the peptides have the effect of sobering up. More
deep study of the pathway factors including protein expression, as
well as the exact extent of the effect in human should be
strengthened in further researches. Whether the peptides can be
used to treat ethanol-induced liver injury needs later animal and
clinical trials.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 31671963)
and the Major State Research Development Program of China (grant
no. 2016YFD0400604).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM, DFR and JL conceived, designed and supervised
the research. LM and CYH performed the experiments. CYH, XYZ and
CQQ conducted the data analysis. LM and JL wrote the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palipoch S, Koomhin P, Punsawad C, Na-Ek
P, Sattayakhom A and Suwannalert P: Heme oxygenase-1 alleviates
alcoholic liver steatosis: Histopathological study. J Toxicol
Pathol. 29:7–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coombes JD and Syn WK: Chapter
5-pathogenic mechanisms in alcoholic liver disease (ALD): Emerging
role of osteopontin. Mol Asp Alcohol Nut. 63–70. 2016. View Article : Google Scholar
|
|
3
|
Zhang M, Pan LJ, Jiang ST and Mo YW:
Protective effects of anthocyanins from purple sweet potato on
acute carbon tetrachloride-induced oxidative hepatotoxicity
fibrosis in mice. Food Agr Immunol. 27:157–170. 2016. View Article : Google Scholar
|
|
4
|
DeNucci SM, Tong M, Longato L, Lawton M,
Setshedi M, Carlson RI, Wands JR and de la Monte SM: Rat strain
differences in susceptibility to alcohol-induced chronic liver
injury and hepatic insulin resistance. Gastroent Res Pract.
2010:162010.
|
|
5
|
Liu WH, Liu TC and Yin MC: Beneficial
effects of histidine and carnosine on ethanol-induced chronic liver
injury. Food Chem Toxicol. 46:1503–1509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller AM, Wang H, Park O, Noriguchi N,
Lafdil F, Mukhopadhyay P, Moh A, Fu XY, Kunos G, Pacher P and Gao
B: Anti-inflammatory and anti-apoptotic roles of endothelial cell
STAT3 in alcoholic liver injury. Alcoholism Clin Exp Res.
34:719–725. 2010. View Article : Google Scholar
|
|
7
|
Larter CZ, Yeh MM, Van Rooyen DM, Brooling
J, Ghatora K and Farrel GC: Peroxisome proliferator-activated
receptor-α agonist, Wy 14,643, improves metabolic indices,
steatosis and ballooning in diabetic mice with non-alcoholic
steatohepatitis. J Gastroenterol Hepatol. 27:341–350. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gebrelibanos M, Megersa N and Taddesse AM:
Levels of essential and non-essential metals in edible mushrooms
cultivated in Haramaya, Ethiopia. Int J Food Contaminat. 3:22016.
View Article : Google Scholar
|
|
9
|
Zhao YM, Wang J, Wu ZG, Yang JM, Li W and
Shen LX: Extraction, purification and anti-proliferative activities
of polysaccharides from Lentinus edodes. Int J Biol Macromol.
93:136–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubost NJ, Ou B and Beelman RB:
Quantification of polyphenols and ergothioneine in cultivated
mushrooms and correlation to total antioxidant capacity. Food Chem.
105:727–735. 2007. View Article : Google Scholar
|
|
11
|
Finimundy TC, Dillon AJP, Henriques JAP
and Ely MR: A review on general nutritional compounds and
pharmacological properties of the Lentinula edodes mushroom. Food
Nut Sci. 5:1095–1105. 2014.
|
|
12
|
Bak WC, Park JH, Park YA and Ka KH:
Determination of glucan contents in the fruiting bodies and mycelia
of Lentinula edodes cultivars. Mycobiology. 42:301–304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Wang C, Gao X, Xu N, Lin L, Zhao
H, Jia S and Jia L: Purification, characterization and anti-aging
capacity of mycelia zinc polysaccharide by Lentinus edodes SD-08.
BMC Complement Altern Med. 15:1112015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grotto D, Bueno DC, Ramos GK, da Costa SR,
Spim SR and Gerenutti M: Assessment of the safety of the Shiitake
culinary-medicinal mushroom, Lentinus edodes (agaricomycetes), in
rats: Biochemical, hematological, and antioxidative parameters. Int
J Med Mushrooms. 18:861–870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palacios I, Lozano M, Moro C, D'Arrigo M,
Rostagno MA, Martínez JA, García-Lafuente A, Guillamón E and
Villares A: Antioxidant properties of phenolic compounds occurring
in edible mushrooms. Food Chem. 128:674–678. 2011. View Article : Google Scholar
|
|
16
|
Thetsrimuang C, Khammuang S and Sarnthima
R: Antioxidant activity of crude polysaccharides from edible fresh
and dry mushroom fruiting bodies of Lentinus sp. Strain RJ-2. Int J
Pharmacol. 7:58–65. 2011. View Article : Google Scholar
|
|
17
|
He JZ, Ru QM, Dong DD and Sun PL: Chemical
characteristics and antioxidant properties of crude water soluble
polysaccharides from four common edible mushrooms. Molecules.
17:4373–4387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng Z, Yang YD, Kuang QR, Sun BJ, Ren DF
and Lu J: Extraction optimization of Letinous edodes peptide and
its antioxidant antialcoholism activity in vitro. J Chin Inst Food
Sci Tech. 15:93–102. 2015.
|
|
19
|
Zhao RJ, Huo CY, Qian Y, Ren DF and Lu J:
Ultra-high-pressure processing improves proteolysis and release of
bioactive peptides with activation activities on alcohol metabolic
enzymes in vitro from mushroom foot protein. Food Chem.
231:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Cai F, Guo S, Ding F, He Y, Wu J
and Liu C: Protective effect of Flos Puerariae extract following
acute ethanol intoxication in mice. Alcohol Clin Exp Res.
38:1839–1846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhao Q, Wang L, Zhao M and Zhao
B: Protective effect of polysaccharide from maca (Lepidium meyenii)
on Hep-G2 cells and alcoholic liver oxidative injury in mice. Int J
Biol Macromol. 99:63–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Jiang Y, Ni Y, Zhang T, Duan C,
Huang C, Zhao Y, Gao L and Li S: Protective effects of
Lactobacillus plantarum C88 on chronic ethanol-induced liver injury
in mice. J Funct Foods. 35:97–104. 2017. View Article : Google Scholar
|
|
24
|
Zhang S, Chen J, Sun AD and Zhao LY:
Protective effects and antioxidant mechanism of bamboo leaf
flavonoids on hepatocytes injured by CCl4. Food Agr
Immunol. 25:386–396. 2014. View Article : Google Scholar
|
|
25
|
She XX, Wang F, Ma J, Chen X, Ren DF and
Lu J: In vitro antioxidant and protective effects of corn peptides
on ethanol-induced damage in HepG2 cells. Food Agr Immunol.
27:99–110. 2016. View Article : Google Scholar
|
|
26
|
Hong IH, Choi JY, Kim AY, Lee EM, Kim JH,
Park JH, Choi SW and Jeong KS: Anti-rheumatoid arthritic effect of
fermented Adlay and Achyranthes japonica Nakai on collagen-induced
arthritis in mice. Food Agr Immunol. 28:14–26. 2017. View Article : Google Scholar
|
|
27
|
Zhou T, Zhang YJ, Xu DP, Wang F, Zhou Y,
Zheng J, Li Y, Zhang JJ and Li HB: Protective effects of lemon
juice on alcohol-induced liver injury in mice. Biomed Res Int.
2017:74635712017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong Z, Ye S, Xiong Y, Wu L, Zhang M, Fan
X, Li L, Fu Z, Wang H, Chen M, et al: Decreased expression of
mitochondrial aldehyde dehydrogenase-2 induces liver injury via
activation of the mitogen-activated protein kinase pathway. Transpl
Int. 29:98–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng S, Yang X, Lassus H, Liang S, Kaur S,
Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al: Distinct expression
levels and patterns of stem cell marker, aldehyde dehydrogenase
isoform 1 (ALDH1), in human epithelial cancers. PLoS One.
5:e102772010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee WH and Kim SG: AMPK-dependent
metabolic regulation by PPAR agonists. PPAR Res. 2010:pii: 549101.
2010. View Article : Google Scholar :
|
|
31
|
Pyper SR, Viswakarma N, Yu S and Reddy JK:
PPARalpha: energy combustion, hypolipidemia, inflammation and
cancer. Nucl Recept Signal. 8:e0022010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smathers RL, Chiang DJ, McMullen MR,
Feldstein AE, Roychowdhury S and Nagy LE: Soluble IgM links
apoptosis to complement activation in early alcoholic liver disease
in mice. Mol Immunol. 72:9–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Qu XN, Han Y, Zheng SW, Wang J and
Wang YP: Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF)
from Schisandra chinensis on alcoholic liver oxidative injury in
mice. Int J Mol Sci. 16:2446–2457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park HJ, Lee SJ, Song Y, Jang SH, Ko YG,
Kang SN, Chung BY, Kim HD, Kim GS and Cho GH: Schisandra chinensis
prevents alcohol-induced fatty liver disease in rats. J Med Food.
17:103–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ronis MJ, Korourian S, Blackburn ML,
Badeaux J and Badger TM: The role of ethanol metabolism in
development of ethanolic steatohepatitis in the rat. Alcohol.
44:157–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sousa BCD, Miguel CB, Rodrigues WF,
Machado JR, Silva MVD, Costa TAD, Lazo-Chica JE, Degasperi TDP,
Sales-Campos H, Bucek EU and Oliverira CGF: Effects of short-term
consumption of Morinda citrifolia (Noni) fruit juice on mice
intestine, liver and kidney immune modulation. Food Agr Immunol.
28:1–15. 2017. View Article : Google Scholar
|
|
37
|
Vidyashankar S, Nandakumar KS and Patki
PS: Alcohol depletes coenzyme-Q(10) associated with increased
TNF-alpha secretion to induce cytotoxicity in HepG2 cells.
Toxicology. 302:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shearn CT, Smathers RL, Jiang H, Orlicky
DJ, Maclean KN and Petersen DR: Increased dietary fat contributes
to dysregulation of the LKB1/AMPK pathway and increased damage in a
mouse model of early-stage ethanol-mediated steatosis. J Nutr
Biochem. 24:1436–1445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang F, Liu JC, Zhou RJ, Zhao X, Liu M, Ye
H and Xie ML: Apigenin protects against alcohol-induced liver
injury in mice by regulating hepatic CYP2E1-mediated oxidative
stress and PPARα-mediated lipogenic gene expression. Chem Biol
Interact. 275:171–177. 2017. View Article : Google Scholar : PubMed/NCBI
|