Introduction
At present, lung cancer is the most common cause of
cancer-associated mortality worldwide (1). Lung adenocarcinoma is most frequently
diagnosed, which has a high 5-year relative mortality rate in the
range 51–99%, due to the presence of metastasis at the time of
diagnosis (2). Studies of relative
risk factors and a deeper understanding of adenocarcinoma
pathogenesis are required to improve prevention strategies and
targeted treatments.
Recent epidemiological and experimental studies have
identified adipose tissue and associated metabolic abnormalities as
negative prognostic predictors (3,4).
Adipocytes represent the primary percentage of cells within adipose
tissue (5). Previous studies
suggested that an interaction between adipocytes and certain cancer
types, including melanoma, prostate, ovarian and breast cancer, is
critical for tumor cell growth and invasion (6–9).
Adipocytes are a source of adipokines, including leptin,
adiponectin and visfatin, in addition to growth, immune,
inflammatory and angiogenic factors. Numerous studies have
demonstrated that adipocytes are involved in the regulation of
systemic energy and metabolic homeostasis (6–8,10,11).
In addition, research has demonstrated that in the presence of
cancer cells, which may act as metabolic parasites, adipocytes
become highly metabolically active and secrete significant
quantities of metabolic substrates, including glycerol and fatty
acids. These metabolites are utilized as the macromolecular
building blocks to support cancer cell proliferation and provide
the energy required for biomass synthesis, migration and invasion
(9).
The Warburg effect describes the high levels of
aerobic glycolysis observed in cancer cells. This provides their
primary energy source, by incompletely utilizing glucose and
switching to upstream intermediates for biosynthesis, providing
cancer cells with materials for the synthesis of essential cellular
components, including macromolecules (12,13).
In addition, the Warburg effect is thought to accelerate lactate
secretion, thereby acidifying the surrounding extracellular matrix
and facilitating angiogenesis and tumor metastasis (14). Furthermore, previous studies have
suggested that lipids may enhance the Warburg effect in certain
cancer cells (15,16).
The interaction between cancer cells and adipocytes
in lung cancer progression remains unclear. Although lung cancer is
not considered to be associated with obesity, previous studies have
demonstrated that obesity induced by a high-fat diet increases
cancer-associated mortality and the likelihood of lung carcinoma in
mice (17,18). Bone, in which adipocytes may
increase due to aging or metabolic disorder, is a common site of
metastasis in lung cancer (19).
Other common sites of metastasis in lung cancer include the liver,
the central organ of lipid metabolism and catabolism (20), and the brain, ~60% of which is
composed of lipids (21,22). The role of adipocytes and their
metabolic substrates should be considered in the progression of
lung cancer metastasis, according to the seed and soil theory,
which was first proposed by Stephen Paget, and suggests that
metastatic homing of tumor cells is not a stochastic event;
however, is governed by the interaction between metastatically
competent cancer cells (the ‘seed’) and the permissive
microenvironment of specific organs (the ‘soil’) (23). In the present study, the potential
capacity of adipocytes to promote the growth and metastasis of lung
adenocarcinoma A549 cells was determined and the mechanisms
responsible for the observed effects were investigated. The present
study of the association between adipocytes and lung cancer cells
may provide insight to the underlying progression mechanism, and a
novel target to cure lung cancer.
Materials and methods
Cell lines and reagents
3T3-L1 pre-adipose and human lung adenocarcinoma
A549 cells were obtained from Fudan University (Shanghai, China).
Dulbecco's modified Eagle's medium (DMEM) was purchased from
Hyclone (GE Healthcare Life Sciences, Logan, UT, USA). Fetal bovine
serum (FBS) and newborn calf serum (NBCS) were purchased from
Zhejiang Tianhang Biotechnology Co., Ltd. (Huzhou, China);
penicillin/streptomycin was purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Dexamethasone,
3-isobutyl-1-methylxanthine (IBMX) and dimethyl sulfoxide (DMSO)
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Insulin was purchased from Beijing Solarbio Science &
Technology Co., Ltd. (Shanghai, China). Crystal violet, MTT and
bovine serum albumin (BSA) were purchased from Amresco, LLC (Solon,
OH, USA). The reagents associated with western blot analysis were
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Polyvinylidene difluoride (PVDF) membranes were purchased from EMD
Millipore (Billerica, MA, USA).
Cell cultures and adipocyte
differentiation
A549 cells were maintained in DMEM supplemented with
10% FBS and 1% penicillin/streptomycin. 3T3-L1 cells were cultured
in DMEM supplemented with 10% NBCS and 1% penicillin/streptomycin.
Cultures were maintained in a humidified atmosphere of 95% air and
5% CO2 at 37°C. 3T3-L1 pre-adipocytes were harvested and
allowed to reach 100% confluence. Following 2 days, cell
differentiation was induced by the addition of a hormonal mixture
composed of 10 µg/ml insulin, 1 µM dexamethasone and 0.5 mM IBMX in
DMEM with 10% FBS. Following a further 2 days, the induction medium
was replaced by DMEM supplemented with 10% FBS and 10 µg/ml insulin
only. The medium was subsequently replaced at 2-day intervals.
Production of conditioned medium
(CM)
CM was collected from adipocytes cultured alone at
37°C with serum-free DMEM containing 1% BSA for 24 h (Ad-CM), or
obtained from adipocytes previously co-cultured during 48 h with
A549 cells in complete medium, cells were subsequently incubated in
serum-free DMEM containing 1% BSA at 37°C for 24 h (Ad-CCM). The CM
was subsequently harvested and centrifuged at 550 × g at room
temperature (RT) for 5 min to remove cellular debris, and stored at
−80°C until use.
Oil Red-O staining
The differentiated adipocytes, A549 cells,
co-cultured adipocytes and co-cultured A549 cells were washed twice
with 500 µl PBS and subsequently fixed with 400 µl 4%
paraformaldehyde at RT for 30 min. Cells were subsequently removed
and washed twice with PBS, prior to the addition of 400 µl Oil
Red-O staining solution to each well for 30 min at RT. Following
washing with 60% isopropyl alcohol, cells were observed under a
light microscope (Olympus Corporation, Tokyo, Japan; magnification,
×100 and ×400).
Indirect co-culture
Adipocytes were indirectly co-cultured with A549
cells using a Transwell culture system (pore size, 0.4 µm; Corning
Incorporated, Corning, NY, USA), which were maintained in DMEM with
10% FBS at 37°C. To assess adipocyte biology, the pre-adipocytes
were seeded at 1×105/ml in the bottom, and maintained
until the confluency reached 100% and differentiation occurred. The
adipocytes were obtained 8 days following differentiation. The
intact adipocytes were co-cultured with the A549 cells, which were
seeded in the upper chamber at 1×105 cells/ml. The time
of co-culture was 0, 3, 6 and 9 days, respectively. Conversely, for
experiments assessing cancer cell biology, adipocytes were grown in
the upper chamber, with A549 cells in the bottom. Adipocytes or
cancer cells cultured alone served as controls.
Cell proliferation assay
The proliferative ability of A549 cells was measured
by an MTT assay. A549 cells were seeded in 96-well plates at
2×103 cells/well, allowed to adhere (0 h) and
subsequently cultured in the presence of Ad-CM, Ad-CCM, or DMEM
with 10% FBS at 37°C for 24, 48 or 72 h. MTT (20 µl; 5 mg/ml) was
added to each well and incubated at 37°C for 4 h. The medium was
aspirated and cells were treated with 150 µl DMSO at RT for 10 min.
Following this, absorbance was measured with a microplate reader
(SpectraMax 190; Molecular Devices, LLC, Sunnyvale, CA, USA) at 490
nm.
Colony formation assay
For colony formation assays, A549 cells were plated
into 6-well plates (1×103 cells/well), maintained in
DMEM with 10% FBS and allowed to adhere for 24 h. Culture medium
was subsequently altered to 10% FBS in DMEM, Ad-CM or Ad-CCM and
the plate was incubated in a 5% CO2 incubator at 37°C.
Subsequently, the culture medium was replaced every 4 days at 50%.
Following 18 days, cells were washed twice with PBS and fixed with
4% paraformaldehyde at RT for 20 min. Colonies were stained with
0.1% crystal violet at RT for 15 min. Colonies were counted under a
light microscope (Olympus Corporation; magnification, ×100).
Western blot analysis
Following treatment, the A549 cells were harvested
and lysed using lysis buffer (Beyotime Institute of Biotechnology)
for 30 min on ice. The total proteins were isolated by centrifuging
at 12,000 × g for 10 min at 4°C and quantified using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). A total of 30 µg total protein was separated on 10%
SDS-PAGE and transferred to PVDF membranes. The membranes were
subsequently blocked in 5% non-fat milk at RT for 2 h. Following
washing with Tris-buffered saline with 0.05% Tween-20 (TBST), the
membranes were incubated with the corresponding primary antibodies,
including Rabbit anti-E-cadherin (1:10,000; cat. no. ab40772),
anti-Vimentin (1:1,000; cat. no. ab92547) and anti-β-catenin
(1:5,000; cat. no. ab32572; all Abcam, Cambridge, UK) at 4°C
overnight. Subsequently, the membranes were probed with the
appropriate horseradish peroxidase (HRP)-conjugated secondary
antibodies, goat anti-rabbit immunoglobulin G heavy and light HRP
(1:2,500; cat. no. ZB2301; OriGene Technologies, Inc., Rockville,
MD, USA) for 2 h at room temperature. Following washing in TBST,
the immunoreactive proteins were visualized using an enhanced
chemiluminescent kit (Pierce; Thermo Fisher Scientific, Inc.).
Wound closure assay
A wound closure assay was conducted to assess the
migration of A549 cells. When cell confluence reached 80%, a wound
was made in the center of the cell monolayer using a 10 µl sterile
pipette tip. Following co-culture, cell migration was observed
using a light microscope (Olympus Corporation; magnification,
×100). Images were captured at 0 and 24 h following when the wound
was made and the ImageJ version 1.44p software (National Institutes
of Health, Bethesda, MD, USA) was used to quantify the wound width
(µm). The wound-healing rate was calculated as follows: (width at 0
h -width following 24 h)/width at 0 h ×100.
Transwell migration and invasion
assay
To detect cell migration, Transwell chambers
(Corning Incorporated) with a polycarbonate membrane (24 wells;
pore size, 8 µm) were used. A549 cells were cultured in DMEM, Ad-CM
or Ad-CCM with 10% FBS for 48 h at 37°C, trypsinized and
resuspended in serum-free DMEM at a density of 1×105
cells/ml. Cell suspension (200 µl) was added to the upper chamber
and DMEM supplemented with 10% FBS (600 µl) was added to the lower
chamber as a chemoattractant. A549 cells cultured alone in similar
conditions served as a control. To detect cell invasion, filters
were coated with 50 µg/ml Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) solution. Matrigel was not used for cell migration assays.
Following 24 h, cells were washed twice with PBS, fixed in 4%
paraformaldehyde at RT for 30 min, and stained with 0.1% crystal
violet for 30 min at RT. The number of migrated or invaded cells
were counted under a microscope (Olympus Corporation;
magnification, ×200). Means were obtained from five randomly
selected fields in each well.
Free fatty acid and triglyceride (TG)
detection
To detect the free fatty acid levels in each media,
CM was obtained from adipocytes cultured alone or indirectly
co-cultured with A549 cells at 37°C and subsequently analyzed using
a non-esterified fatty acid quantification kit (cat. no. A042-1;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China) under a
microplate reader (SpectraMax 190; Molecular Devices, LLC) at 440
nm, according to the manufacturer's protocol. To detect
intracellular TGs, following indirect co-culture, adipocytes or
A549 cells were centrifuged at 550 × g for 10 min at RT, and
sonicated at 300 W, 5 sec/beat, 30 sec of interval, 3–5 repeats in
an ice bath. The intracellular TG content was determined using a
glycerol-3-phosphate oxidase/phenol + aminophenazone enzymatic kit
(cat. no. A110-1; Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's protocol. The TG and FFA content
were determined at 510 and 440 nm, respectively, under a microplate
reader (SpectraMax 190; Molecular Devices, LLC).
Detection of glucose consumption and
lactate production
For the detection of glucose and lactate
concentrations, 1×105 A549 cells/ml were seeded in
6-well plates and cultured in DMEM with 10% FBS at 37°C. When cells
had reached 60–70% confluence, the culture medium was subsequently
altered to 10% FBS in DMEM, Ad-CM or Ad-CCM and the plate was
incubated in a 5% CO2 incubator at 37°C. Following 24 h,
the culture supernatant was collected for the detection of glucose
and lactate. Glucose (cat. no. F006) and lactate (cat. no. A019-2)
concentrations were determined using corresponding kits (Nanjing
Jiancheng Bioengineering Institute) and a microplate reader
(SpectraMax 190; Molecular Devices, LLC) at 505 nm and 530 nm,
respectively, according to the respective manufacturer's protocol.
The number of cells in each well was simultaneously counted using
the blood cell counting chamber. Glucose consumption and lactate
production were normalized to cell number.
Statistical analysis
All experimental data are presented as the mean ±
standard error of the mean from at least three independent
experiments. Statistical analysis of the data was performed using
GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Student's t-test was used for comparisons between two groups, while
multiple groups were compared using one-way analysis of variance
followed by Tukey's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of adipocytes on the
proliferation and colony formation of lung adenocarcinoma A549
cells
In the present study, a 3T3-L1 pre-adipose cell line
was used to generate mature adipocytes, according to a standard
differentiation protocol (24).
Following induction and differentiation, a large number of lipid
droplets had accumulated and were clearly visible in the cytoplasm
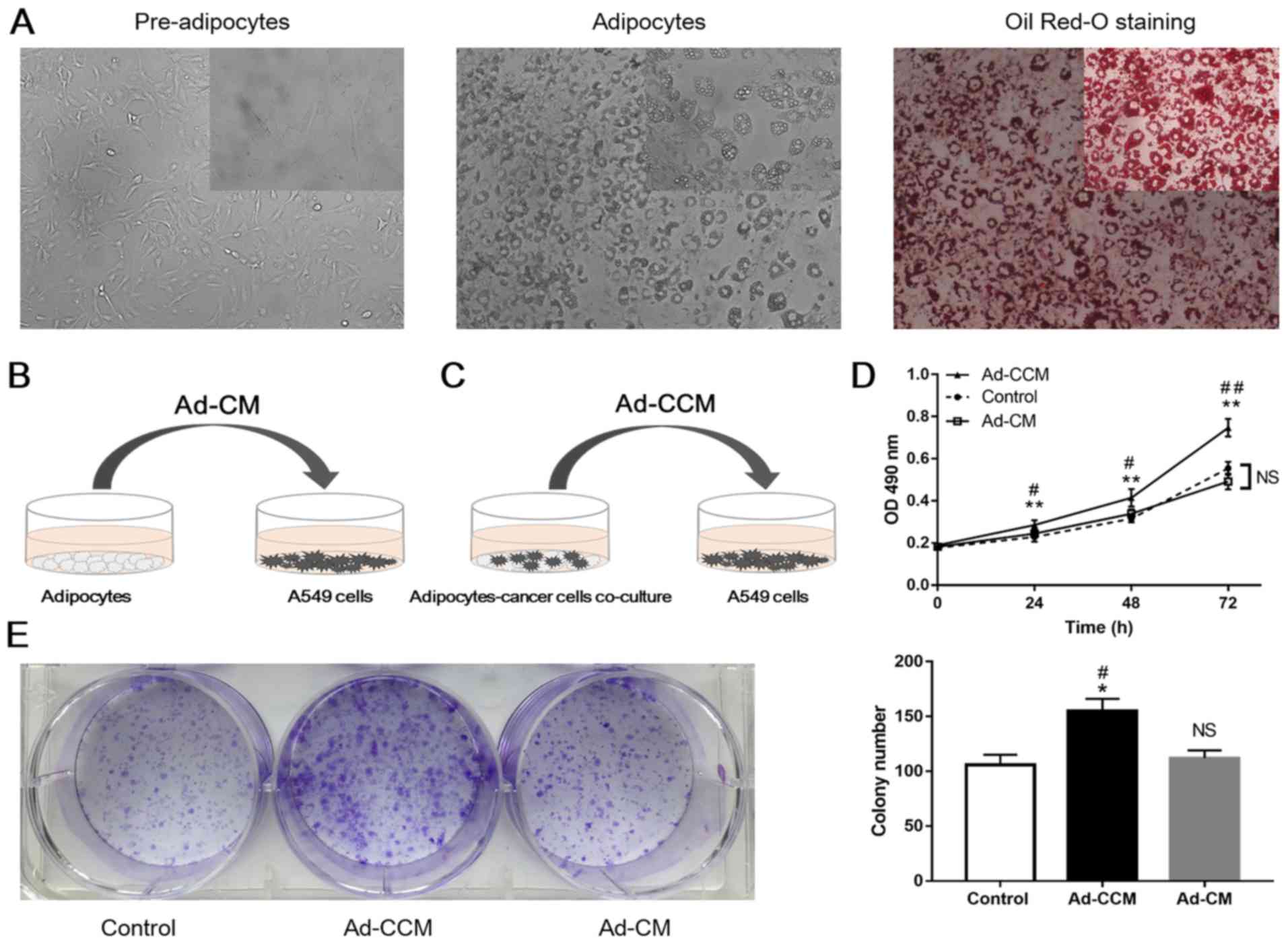
of adipocytes when stained with Oil Red-O (Fig. 1A). To assess the potential role of
adipocytes in A549 cell growth, MTT and colony formation assays
were conducted to examine alterations in A549 cell proliferation.
A549 cells were grown in the presence of CM obtained from
adipocytes cultured alone (Ad-CM; Fig.
1B), or CM obtained from adipocytes previously co-cultured with
A549 cells (Ad-CCM; Fig. 1C). The
proliferation rate of A549 cells cultured in Ad-CM was not
significantly different from that of A549 cells grown in normal
medium (Fig. 1D and E). Notably,
as presented in Fig. 1D, Ad-CCM
induced a time-dependent increase in A549 cell proliferation.
Proliferation was significantly increased compared with cancer
cells induced by control medium (P<0.01) and Ad-CM (P<0.05).
In addition, there was a 1.47-fold and 1.38-fold increase in colony
formation in lung adenocarcinoma A549 cells cultured in Ad-CCM,
compared with cancer cells cultured in control medium and Ad-CM,
respectively (Fig. 1E; P<0.05).
These results suggested that crosstalk between these two cell
populations may serve an important role in the proliferation of
lung adenocarcinoma A549 cells.
Effects of adipocytes on the EMT,
migration and invasion of lung adenocarcinoma A549 cells
To assess the influence of adipocytes on cancer cell
metastasis, A549 cells were incubated with CM as described above.
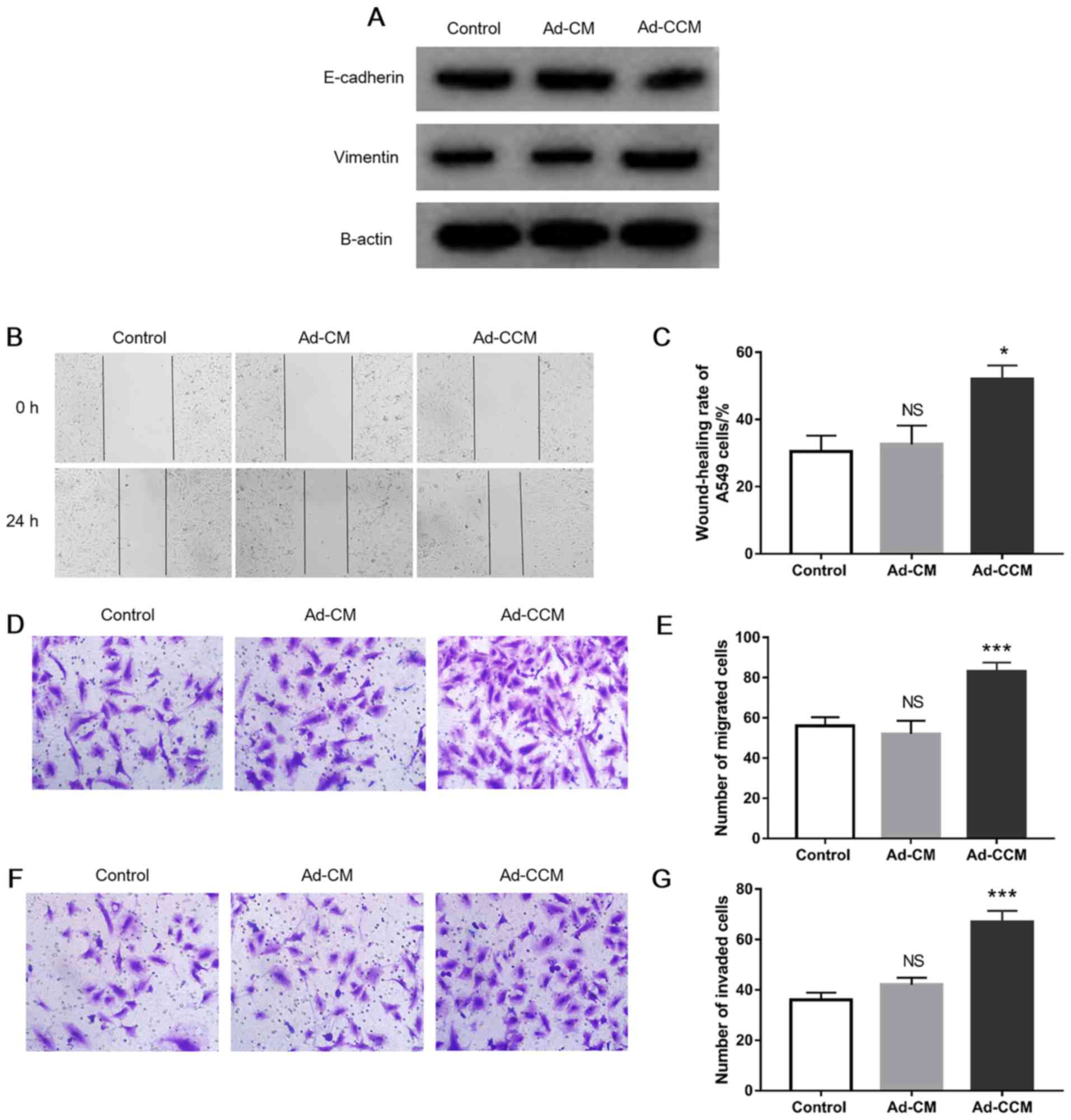
The results indicated that following induction with Ad-CCM for 48
h, the expression level of epithelial cell protein marker
E-cadherin was decreased and the mesenchymal protein marker
vimentin was increased in A549 cells (Fig. 2A). Furthermore, compared with the
control group, a ~1.7-fold increase in the wound-closure rate of
A549 cells was observed in the presence of Ad-CCM (Fig. 2B and C; P<0.05). Similarly,
Ad-CCM promoted cancer cell migration studied as compared with the
control, as evidenced by the increased numbers of migrated cells in
the Transwell migration assay (Fig. 2D
and E; P<0.001). In the invasion assay, a 1.86-fold increase
in the number of invaded A549 cells was observed in the Ad-CCM
cultured with cancer cells, compared with cells grown in control
medium (Fig. 2F and G;
P<0.001). Nevertheless, A549 cell metastasis was not
significantly increased when grown in the presence of Ad-CM
(Fig. 2). Collectively, these
observations clearly demonstrated that the metastatic phenotype of
lung adenocarcinoma A549 cells was enhanced following a reciprocal
interaction between adipose and cancer cells.
Co-cultured adipocytes exhibit a
lipolysis phenotype in vitro
Cancer cell proliferation and metastasis are
energy-intensive processes (25),
and the results of the present study indicated that Ad-CCM
conferred a proliferative advantage on lung adenocarcinoma A549
cells and enhanced its invasive phenotype. As adipocytes store
energy in the form of triglycerides (25), it was hypothesized that this may be
the result of energy-dense lipid or metabolic substrate transfer
from adipocytes to A549 cells to promote migration and invasion.
Therefore, to determine the effect of lung cancer cells on
adipocytes, adipocyte metabolism was assessed in a Transwell
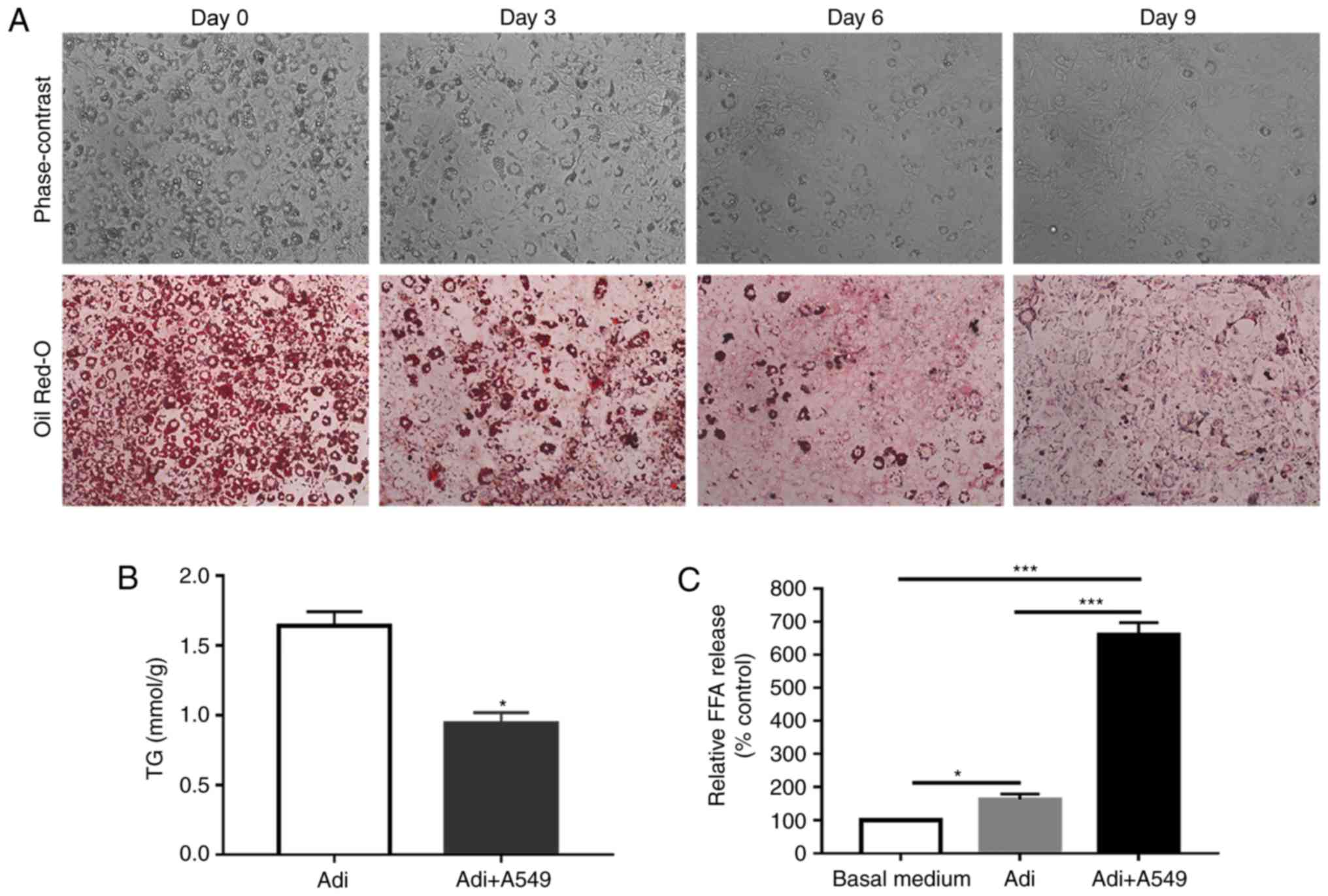
culture system. As presented in Fig.
3A, mature adipocytes were indirectly co-cultured with lung
cancer cells for a prolonged period. As presented in Fig. 3A, the adipocytes exhibited a marked
reduction in the number and size of lipid droplets. Additionally, a
significant reduction in adipocyte TG content was observed
following co-culture with lung cancer cells (Fig. 3B; P<0.05). In addition, in the
presence of lung cancer cells, adipocytes released significantly
more free fatty acids, compared with adipocytes cultured alone
(Fig. 3C; P<0.001). Taken
together, these findings suggested that A549 cells may have induced
adipocyte lipolysis.
Co-cultured lung cancer cells exhibit
metabolic reprogramming in vitro
Based on the evidence presented above, cancer cells
appeared to have induced lipolysis in adipocytes. Thus, to
determine the metabolic interaction between adipocytes and lung
cancer cells, the metabolic alterations in lung cancer cells in the
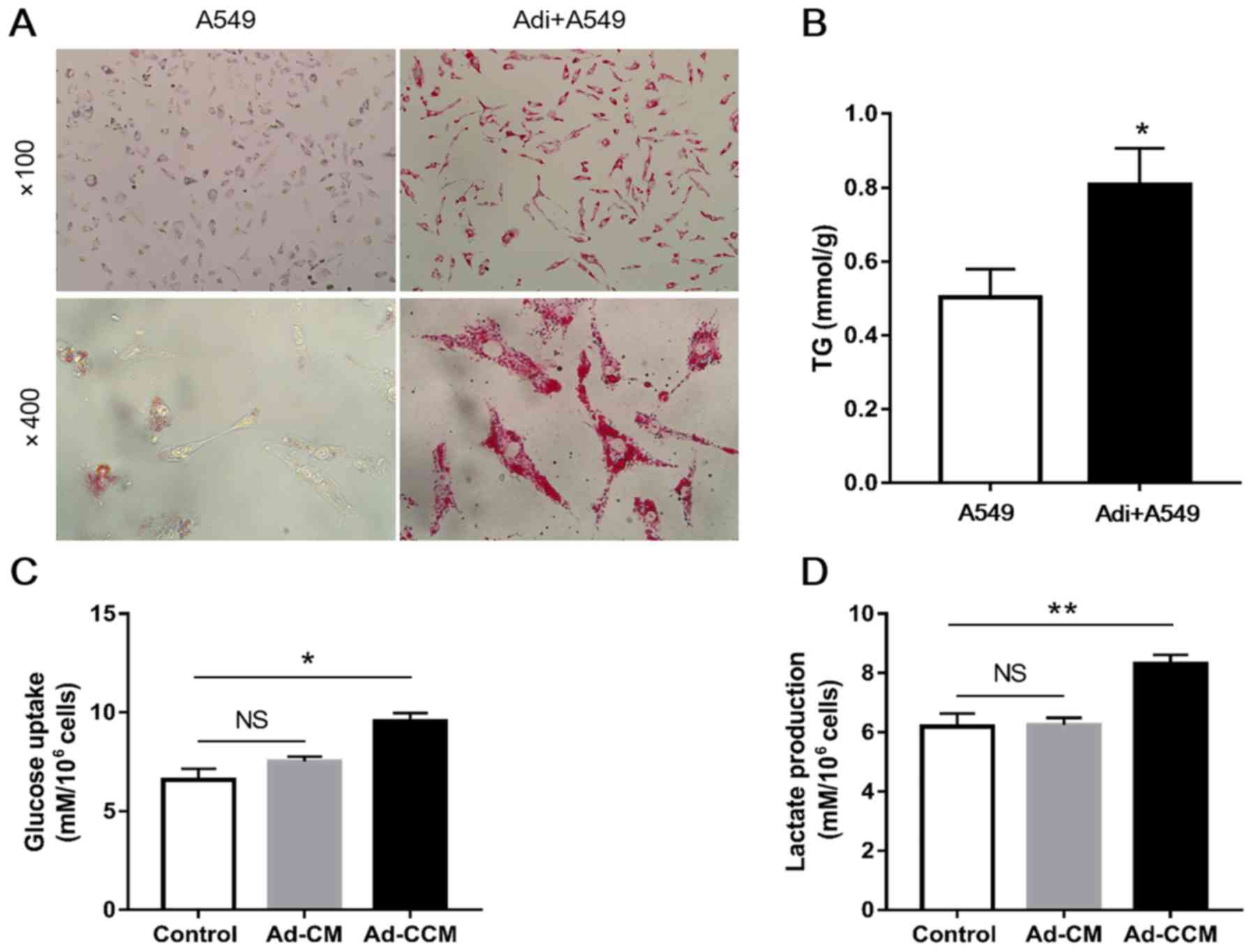
presence of adipocytes were studied. The results of the Oil Red-O
staining pointed to an increased accumulation of lipid droplets in
A549 cells following co-culture with adipocytes, suggesting that
adipocytes promoted lipid accumulation in lung cancer cells
(Fig. 4A). Similarly, compared
with cancer cells that were cultured alone, the TG content of A549
cells increased by approximately 1.6-fold in cells previously
co-cultured with adipocytes for 2 days (Fig. 4B; P<0.05). Furthermore, a
significant increase in glucose uptake and lactate production was
observed in A549 cells grown in the presence of Ad-CCM for 24 h,
compared with cells cultured in control medium. By contrast, Ad-CM
had no effects on the metabolic activity of A549 cells, compared
with the control medium cells (Fig. 4C
and D). These findings suggested that adipocytes may have
altered energy metabolism in lung cancer cells, particularly
through glycolysis.
Discussion
Although a number of potential mechanisms linking
adipocytes and cancer have been proposed (26), no conclusive evidence currently
exists with regards to the roles of adipocytes and associated
metabolic substrates in lung cancer progression. In the present
study, the effects of adipocytes on lung adenocarcinoma A549 cells
were evaluated using a combination of CM and co-culture approaches.
It was demonstrated that following adipocyte interaction, A549 cell
proliferation, migration, invasion and intermediary metabolism was
significantly altered.
Previous studies have demonstrated that cancer cell
proliferation, migration and invasion are altered when they are
indirectly co-cultured with adipocytes or exposed to adipocyte CM
(6–8,11).
Similar effects are observed in xenograft models (27) and 3D cultures (28). However, to the best of our
knowledge, no studies have investigated the potential role of
adipocytes in lung cancer progression. In the present study,
differentiated 3T3-L1 pre-adipocytes were utilized to investigate
the crosstalk between adipocytes and A549 lung adenocarcinoma
cells. The results demonstrated that the biological behavior of
A549 cells cultured in Ad-CM was not notably altered. By contrast,
A549 cell metastasis and growth was significantly promoted by
Ad-CCM. These findings suggested that crosstalk between adipocytes
and cancer cells enhanced the progression of lung cancer. The
3T3-L1 pre-adipocytes used in the present investigation were
derived from mouse embryos, not from adult fat tissue. This is due
to the limitations of the experimental conditions; at present, it
is technically difficult to obtain bone marrow adipocytes and other
associated specimens from humans for experimentation. In addition,
the functions of adipocytes differ depending on the type of tissue
(29). Thus, future research
utilizing human omental, subcutaneous and bone marrow adipocytes is
required to confirm whether crosstalk exists between adipocytes and
lung cancer cells.
Cancer cell proliferation and metastasis are
energy-intensive processes and adipocytes store energy in the form
of TGs (25). Thus, it was
hypothesized that adipocytes provided energy-dense lipids or
metabolic substrates for lung adenocarcinoma cells, thereby
promoting lung cancer progression. Previous research has
demonstrated that when cancer cells invade surrounding adipose
tissue, adipocytes exhibit morphological and functional
modifications due to enhanced lipolytic activity (5). These modifications include size
reduction, increased secretion of certain inflammatory and growth
factors, including tumor necrosis factor α, interleukin (IL)-6,
IL-8 and insulin-like growth factor-1, and decreases in
adipocyte-differentiation markers in various types of tumors
growing in adipose tissue-dominated microenvironments, including
melanoma, gastric, breast, colon, prostate and ovarian cancer
(5). In the present study, it was
clearly demonstrated that adipocytes co-cultured with A549 cells
exhibited a dramatic reduction in the number of lipid droplets and
a significant increase in the release of free fatty acids, compared
with adipocytes cultured alone. In addition, cytoplasmic lipid
droplets accumulated in cancer cells. The latter was consistent
with cancer cells acting as metabolic parasites, as demonstrated in
a previous study (30). Further
research is required to determine whether lipids detected in cancer
cells following co-culture are derived from adipocytes or de
novo lipogenesis.
Increased glucose uptake and lactate production are
characteristics of the Warburg effect, which is the fastest way for
cancer cells to produce adenosine 5′-triphosphate via glycolysis
(31), allowing them to
efficiently gain metabolic autonomy in the tumor microenvironment
(31). The resulting production of
lactate by highly glycolytic cells is known to enhance the growth
of invasive cancer cells (32). In
addition, lactate may serve as an alternative carbon source for the
surrounding oxygenated cells (32). Previous research has demonstrated
that bone marrow adipocytes promote the expression of glycolytic
enzymes, increase lactate production and decrease mitochondrial
oxidative phosphorylation (15).
The present study additionally demonstrated that adipocytes were
capable of altering the Warburg phenotype in A549 cells through
paracrine signaling: A549 cells cultured in Ad-CCM consumed more
glucose and produced more lactate, compared with A549 cells
cultured in Ad-CM or normal medium. A previous report indicated
that the interaction between adipocytes and cancer cells ultimately
affects cancer cell metabolism, allowing for adaptive survival in
the metastatic niche (33).
Previous literature and the findings of the present
study highlight the potential for characterization of
adipocyte-cancer cell interactions, to provide a better
understanding of lung cancer progression, and to identify avenues
for the development of novel therapeutic strategies.
Acknowledgements
The authors thank Professor Shan Gao (Department of
Pharmacology, Basic Medical College, Anhui Medical University) for
his technical assistance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402427) and the
Provincial Natural Science Foundation of Anhui (grant no.
1408085QH165).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FFL conceived, designed and supervised the study. HZ
made the experiment, analyzed and interpreted the data, and was a
principal contributor in writing the manuscript. JJL and YNC
performed the partial experiments. XD and CG performed the data
collection and participated in the writing of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chalela R, Curull V, Enriquez C, Pijuan L,
Bellosillo B and Gea J: Lung adenocarcinoma: From molecular basis
to genome-guided therapy and immunotherapy. J Thorac Dis.
9:2142–2158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Divella R, De Luca R, Abbate I, Naglieri E
and Daniele A: Obesity and cancer: The role of adipose tissue and
adipo-cytokines-induced chronic inflammation. J Cancer.
7:2346–2359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gucalp A, Iyengar NM, Hudis CA and
Dannenberg AJ: Targeting obesity-related adipose tissue dysfunction
to prevent cancer development and progression. Semin Oncol.
43:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laurent V, Guérard A, Mazerolles C, Le
Gonidec S, Toulet A, Nieto L, Zaidi F, Majed B, Garandeau D,
Socrier Y, et al: Periprostatic adipocytes act as a driving force
for prostate cancer progression in obesity. Nat Commun.
7:102302016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lazar I, Clement E, Dauvillier S, Milhas
D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S,
et al: Adipocyte exosomes promote melanoma aggressiveness through
fatty acid oxidation: A novel mechanism linking obesity and cancer.
Cancer Res. 76:4051–4057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balaban S, Shearer RF, Lee LS, van
Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC,
Fazakerley DJ, Grewal T, et al: Adipocyte lipolysis links obesity
to breast cancer growth: adipocyte-derived fatty acids drive breast
cancer cell proliferation and migration. Cancer Metab. 5:12017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajala MW and Scherer PE: Minireview: The
adipocyte-at the crossroads of energy homeostasis, inflammation,
and atherosclerosis. Endocrinology. 144:3765–3773. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dirat B, Bochet L, Dabek M, Daviaud D,
Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S,
et al: Cancer-associated adipocytes exhibit an activated phenotype
and contribute to breast cancer invasion. Cancer Res. 71:2455–2465.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
13
|
Manzi L, Costantini L, Molinari R and
Merendino N: Effect of dietary ω-3 polyunsaturated fatty acid DHA
on glycolytic enzymes and warburg phenotypes in cancer. Biomed Res
Int. 2015:1370972015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi SY, Collins CC, Gout PW and Wang Y:
Cancer-generated lactic acid: A regulatory, immunosuppressive
metabolite? J Pathol. 230:350–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diedrich JD, Rajagurubandara E, Herroon
MK, Mahapatra G, Hüttemann M and Podgorski I: Bone marrow
adipocytes promote the Warburg phenotype in metastatic prostate
tumors via HIF-1α activation. Oncotarget. 7:64854–64877. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baenke F, Peck B, Miess H and Schulze A:
Hooked on fat: The role of lipid synthesis in cancer metabolism and
tumour development. Dis Model Mech. 6:1353–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan L and DeMars LC: Effects of dietary
fat on spontaneous metastasis of Lewis lung carcinoma in mice. Clin
Exp Metastasis. 27:581–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao N, Ma X, Guo Z, Zheng Y, Geng S, Meng
M, Du Z, Lin H, Duan Y and Du G: Oral kanglaite injection (KLTI)
attenuates the lung cancer-promoting effect of high-fat diet
(HFD)-induced obesity. Oncotarget. 7:61093–61106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuchuk M, Addison CL, Clemons M, Kuchuk I
and Wheatley-Price P: Incidence and consequences of bone metastases
in lung cancer patients. J Bone Oncol. 2:22–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones JG: Hepatic glucose and lipid
metabolism. Diabetologia. 59:1098–1103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson AC, McNabb AR and Rossiter RJ:
Lipids of normal brain. Biochem J. 43:573–577. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Basinska J, Sastry PS and Stancer HC:
Lipid composition of human, bovine and sheep pineal glands. J
Neurochem. 16:707–714. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
24
|
Coelho P, Almeida J, Prudencio C,
Fernandes R and Soares R: Effect of adipocyte secretome in melanoma
progression and vasculogenic mimicry. J Cell Biochem.
117:1697–1706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shafat MS, Oellerich T, Mohr S, Robinson
SD, Edwards DR, Marlein CR, Piddock RE, Fenech M, Zaitseva L,
Abdul-Aziz A, et al: Leukemic blasts program bone marrow adipocytes
to generate a protumoral microenvironment. Blood. 129:1320–1332.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duong MN, Geneste A, Fallone F, Li X,
Dumontet C and Muller C: The fat and the bad: Mature adipocytes,
key actors in tumor progression and resistance. Oncotarget.
8:57622–57641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Gao C, Meng K, Qiao H and Wang Y:
Human adipocytes stimulate invasion of breast cancer MCF-7 cells by
secreting IGFBP-2. PLoS One. 10:e01193482015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lapeire L, Hendrix A, Lambein K, Van
Bockstal M, Braems G, Van Den Broecke R, Limame R, Mestdagh P,
Vandesompele J, Vanhove C, et al: Cancer-associated adipose tissue
promotes breast cancer progression by paracrine oncostatin M and
Jak/STAT3 signaling. Cancer Res. 74:6806–6819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ibrahim MM: Subcutaneous and visceral
adipose tissue: Structural and functional differences. Obes Rev.
11:11–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinez-Outschoorn UE, Pestell RG, Howell
A, Tykocinski ML, Nagajyothi F, Machado FS, Tanowitz HB, Sotgia F
and Lisanti MP: Energy transfer in ‘parasitic’ cancer metabolism:
Mitochondria are the powerhouse and Achilles' heel of tumor cells.
Cell Cycle. 10:4208–4216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiche J, Brahimi-Horn MC and Pouyssegur
J: Tumour hypoxia induces a metabolic shift causing acidosis: A
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sonveaux P, Végran F, Schroeder T, Wergin
MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C,
Jordan BF, et al: Targeting lactate-fueled respiration selectively
kills hypoxic tumor cells in mice. J Clin Invest. 118:3930–3942.
2008.PubMed/NCBI
|
|
33
|
Martinez-Outschoorn UE, Sotgia F and
Lisanti MP: Power surge: Supporting cells ‘fuel’ cancer cell
mitochondria. Cell Metab. 15:4–5. 2012. View Article : Google Scholar : PubMed/NCBI
|