Introduction
Bronchial asthma is a common respiratory disease and
one of the most frequent chronic airway diseases to affect children
(3–14 years) (1). Its incidence is
increasing each year in China and it poses a mental and economic
burden to patients and society (2). Bronchial asthma is an immunoglobulin
(Ig)E-mediated type I chronic allergic disease that involves
numerous inflammatory cells including eosinophils, mast cells, T
cells and airway epithelial cells and can cause recurrent wheezing,
shortness of breath, chest tightness or cough, and be
life-threatening (3). The
pathological mechanism of asthma is complex and associated with
immunity and genetic background (4). The aims of asthma treatment include
reducing the frequency of attacks, improving respiratory function
preventing acute attacks and recurrent exacerbations and preventing
death (5). Advances have been made
in the control of symptoms of asthma, but the incidence of
recurrent asthma remains high in China and the side effects
associated with use of asthma drugs can be severe (6).
Vitamin D has been previously reported to serve a
role in the pathogenesis of asthma (7). It was previously demonstrated to
regulate bone metabolism and calcium balance (8). Increasing number of epidemiological,
genetic and animal model studies have demonstrated that vitamin D
exhibits strong immunoregulatory function and it is associated with
innate and adaptive immune responses (9–13).
Vitamin D receptor (VDR) is the main receptor for
1,25-hydroxy-vitamin D, the biologically active form vitamin D
(14). Its polymorphism is closely
associated with the occurrence of asthma in children and adults. In
the present study, blood samples were collected from clinical
patients with asthma and animal models of asthma were established
to investigate the role of VDR in the disease in question. In
addition, bioinformatics methods were used to predict the B-cell
and T-cell epitopes of VDR and perform sequence analysis. The
results of the present study provide theoretical data that can be
further validated and in the future, may be used for prevention and
treatment of asthma, and development of epitope-based vaccines
against this disease.
Materials and methods
Patients and groups
A total of 65 children with asthma (31 males, 34
females; average age 3.45±1.36 years) who received treatment and 65
healthy children who acted as normal control (33 males, 32 females;
aged 6.11±1.79 years) in Shengjing hospital affiliated to China
Medical University between March and June 2017, were included in
the present study. The venous blood (1 ml) was collected in
children patients and the normal control group. The present study
was approved by the Ethics Committee of Shengjing Hospital
affiliated to the China Medical University (approval no.
2016PS86K). Inclusion criteria of these patients included: i)
Recurrent wheezing, cough, breathlessness and chest tightness, all
of which occurred due to contact with allergens, cold air, physical
or chemical stimuli, respiratory infections and exercise,
frequently worsening at night and/or in the early morning; ii)
diffuse or scattered airflow limitation in both lungs mainly during
the expiratory and prolonged expiratory phases; and iii) the above
symptoms and signs were alleviated by anti-asthma treatment or
self-mitigated. Exclusion criteria included wheezing, cough,
breathlessness and chest tightness, caused by other diseases.
Within 1 week prior to grouping, all patients did not use hormones
or suffer from acute infection. The included patients were randomly
divided into a normal control group (healthy children) and an
asthma group (asthma children). Healthy children in the normal
group received physical examination in Shengjing hospital and had
no history of allergic diseases. All normal controls did not use
hormone or suffer from acute infection within 1 week prior to
grouping. Written informed consent was obtained from the guardians
of the included children.
Animals and groups
Twenty BALB/c mice (6 weeks old, male, weight 20–23
g) were obtained from the Experimental Animal Center of China
Medical University (Shenyang, China) [production license no. SCXK
(Liao)-2016-2417; application license no. SYXK (Liao)-2016-2417].
Mice were given a normal diet and water and the housing conditions
were as follows: Ambient temperature of 20–26°C, 40–70% relative
humidity, 12-h light/dark cycle. The present study was approved by
the Animal Welfare and Ethics Committee of China Medical University
Laboratory (Shenyang, China) (approval no. 2016072). Mice were
randomly divided into two groups (according to random number
table), including the control group (n=10) and asthma group
(n=10).
Establishment of mouse models of
asthma
Mouse models of asthma were established as
previously described (15). On
days 2, 8 and 15, following the model establishment, model mice
were sensitized by intraperitoneal injection of 0.2 ml mixture of
ovalbumin (OVA, 257–264, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and aluminum hydroxide [2 mg/ml OVA+0.1 ml aluminum
hydroxide gel (1017502, Sigma-Aldrich; Merck KGaA)]. On the same
days, equal amount of normal saline was intraperitoneally
administered in normal control mice. On day 22, each mouse was
placed in a 30×30×20 cm-sized closed container. Mice from the
asthma group were sprayed with 2% OVA whereas mice from the normal
control group were sprayed with normal saliva, once a day, 45 min
each time, for 7 successive days. During this process, mouse
behaviors and symptomatic reactions were closely monitored.
Sample collection
On day 29 following asthma induction, following
anesthesia, mice were sacrificed with cervical prolapse and lung
tissue was obtained, fixed with 10% formaldehyde at room
temperature for at least 48 h and preserved at −80°C. Peripheral
blood samples (1 ml) were collected and preserved at −80°C.
Hematoxylin and eosin (H&E)
staining
The aforementioned 10% formaldehyde-fixed, mouse
lung tissue was placed in xylene for 10 min, dehydrated in gradient
ethanol (absolute, 95 and 75% ethanol) and infiltrated into
paraffin liquid at 65°C for 1–2 h, and paraffin-embedded and sliced
into 5-µm thick sections. Paraffin sections were dewaxed, stained
with hematoxylin for 5 min at room temperature, PBS washed,
differentiated with 1% hydrochloric acid-ethanol, stained with
eosin for 1 min at room temperature and mounted with 40% neutral
resin at room temperature. Finally, pathological alterations of
lung tissue were observed under the light microscope (NE950, Leica
Microsystems, Ltd., Milton Kaynes, UK).
Western blot assay
Lung tissues were triturated and lysed using
radioimmunoprecipitation assay lysis (89900, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) buffer containing protease
inhibitor for 30 min on ice, and 4,000 × g, centrifuged for 10 min
at 4°C, and the supernatant was collected. Protein concentration
was determined using a BCA protein assay. Protein samples were
subjected to 10% SDS-PAGE and transferred to a polyvinylidene
fluoride membrane. The membrane was blocked by 5% skim milk for 2 h
and incubated overnight at 4°C with VDR antibody (1:1,000; cat. no.
ab109234; Abcam, Cambridge, UK) and GAPDH antibody (1:2,000; cat.
no. ab8245; Abcam). Following addition of horseradish
peroxidase-labeled secondary antibody (goat anti-rabbit IgG/HRP
antibody, 1:2,000, cat. no. bs-0295G-HRP, Bioss, Beijing, China),
protein samples were incubated at room temperature for 2 h. Protein
bands were visualized using an enhanced chemiluminescence detection
kit (32209, Thermo Fisher Scientific, Inc.) and a gel imaging
system. Absorbance analysis was performed using Image J software
(Image J 1.8.0, National Institutes of Health, Bethesda, MD,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Mouse lung tissues were harvested. Total RNA was
extracted using TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), dried by sedimentation and incubated with 50 µl
diethylpyrocarbonate-treated water. RNA purity and concentration
were determined using an ultraviolet spectrophotometer. Total RNA
was reserve transcribed into cDNA using the High-Capacity
RNA-to-cDNA™ kit (K1266, Invitrogen; Thermo Fisher Scientific,
Inc.). According to the manufacturer's protocol of the RT-qPCR kit
(Qiagen GmbH, Hilden, Germany), VDR expression was detected. RNA
primers were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.). The following primer sequences were used for the PCR: VDR
forward, 5′-GTAAGTACAGGGAGCTATT-3′ and reverse,
5′-GATCTGAATGAAGAAGGCT-3′; GAPDH forward, 5′-AAGATCGAGAATTGACA-3′
and reverse, 5′-GTCGGTGTGAACGGATTTG-3′.
Protein secondary structure prediction
in silico
Secondary structure of VDR (genbank ID: NM_009504.4)
was predicted using Self-Optimized Prediction Method with Alignment
(SOPMA) tool (npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html).
B-cell epitope prediction
The online prediction resources, including immune
epitope database and analysis resource (IEDB; tools.iedb.org/bcell/) and linear epitope prediction
based on propensity scale and support vector machines (LEPS)
(leps.cs.ntou.edu.tw/index.php)
together with hydrophilicity, antigenicity index, and flexibility
parameters, as provided by the prediction websites, were used to
predict B-cell epitopes. This online prediction software gives the
amino acid sequence that may form the epitope.
T-cell epitope prediction
The online prediction resources SYFPEITHI
(www.syfpeithi.de) and IEDB (tools.iedb.org/mhci/) were used to predict major
histocompatibility complex (MHC) class I HLA-A0201 T-cell epitopes.
This online prediction software gives the score of the amino acid
sequence that may form the epitope, get 15 higher score of T-cell
epitopes.
Prediction of tertiary structure of
VDR
The tertiary structure of VDR was predicted using
3DLigandSite (16), a web server
available at www.sbg.bio.ic.ac.uk/~3dligandsite/. RasMol software
(RasMol 2.7.5.2; www.rasmol.org/software/RasMol_2.7.5_Manual.html) was
used to analyze different models of the tertiary structure. The
tertiary structure was displayed in the models of structure and
group, in order to observe the three-dimensional structure of VDR
molecules.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp., Armonk, NY, USA). An independent samples
t-test was used. Data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
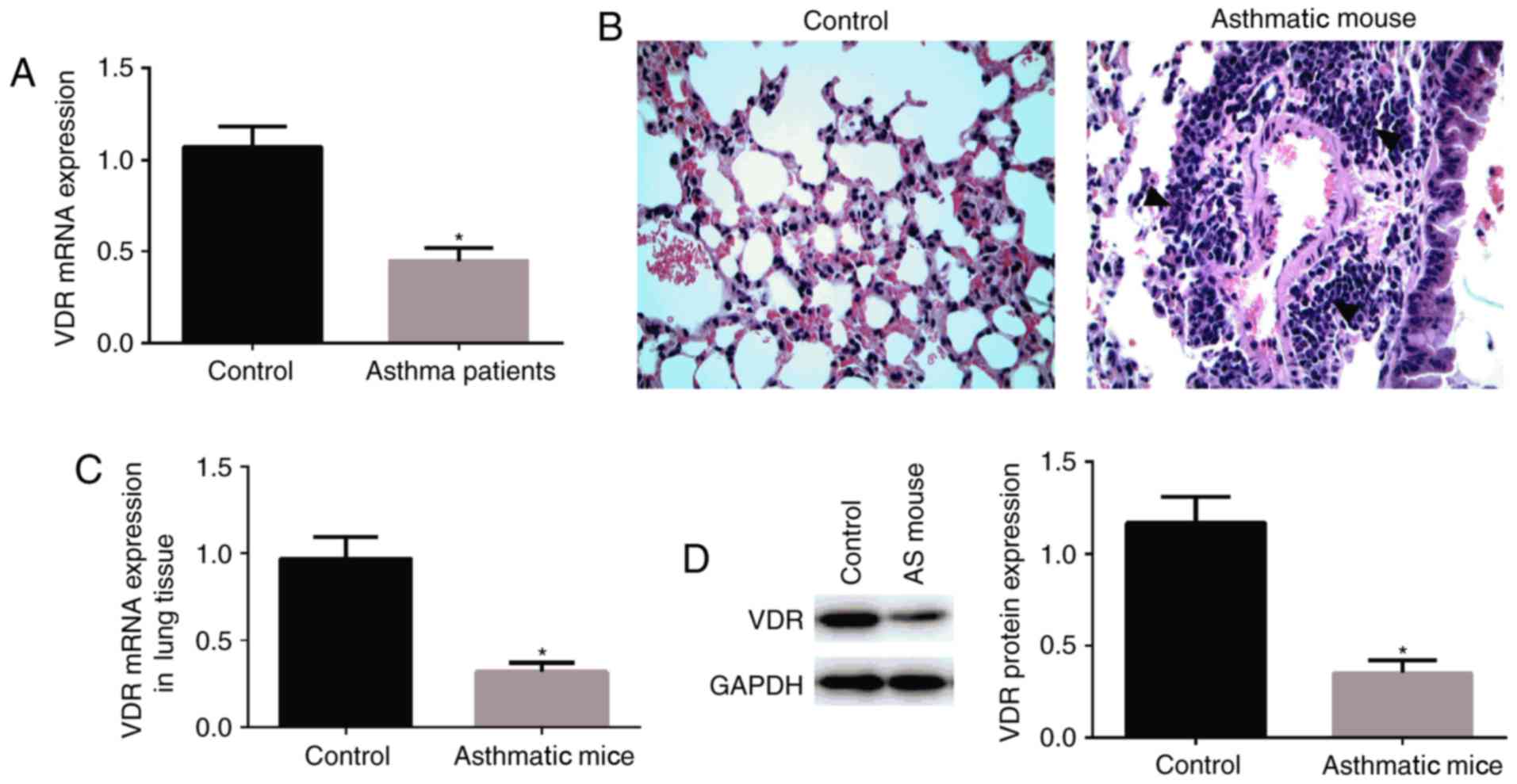
VDR gene expression is decreased in
children patients with asthma
RT-qPCR was used to detect VDR expression in the
peripheral blood. VDR expression in the peripheral blood was
significantly decreased in children patients with asthma compared
with normal controls (P<0.05; Fig.
1A).
Behavior of mice during
excitation
During excitation, behavior of mice was observed.
The mental state of mice was worsening, reduced activities, even
gathering together and nodding (17) rapid and deep breathing, lip
cyanosis, head and face itching, forelimb lifting and scratching
the nose (17) dark gray hair and
urinary or fecal incontinence. H&E staining of the lung tissue
revealed that there were a large number of inflammatory cells in
and around the pulmonary bronchial vessels, in the pulmonary
interstitium and pulmonary alveolar cavity. In addition, the
structure of airway was disordered, and airway epithelial
hyperplasia and hypertrophy were also observed. In the normal
control group, lung tissue structure of the mice was clear, the
respiratory epithelium was complete and a small number of
inflammatory cells infiltrated (Fig.
1B).
VDR expression is decreased in the
lungs of mouse models of asthma
RT-qPCR (Fig. 1C)
and western blot assay (Fig. 1D),
were performed to detect VDR expression in the lung tissue of mice.
VDR expression in the lung tissue was significantly decreased in
mice with asthma compared with normal control mice.
VDR gene-encoded amino acid
sequences
According to the VDR protein provided in the
GenBank, the sequence of 427 amino acids was as follows:
MEAMAASTSLPDPG DFDRNVPRICGVCGDRATGFHFNAMTCEGCKGFFRRS
MKRKALFTCPFNGDCRITKDNRRHCQACRLKRCVDIGMMKEFILTDEEVQRKREMILKRKEEEALKDSLRPKLSEEQQRIIAILLDAHHKTYDPTYSDFCQFRPPVRVNDGGGSHPSRPNSRHTPSFSGDSSSSCSDHCITSSD
MMDSSSFSNLDLSEEDSDDPSVTL ELS QLSMLPHLADL VSY SIQ KVI GFA KMI PGF
RDL TSEDQIVLLKSSAIEVIMLRS NESF TMD DMS WTC GNQ DYKYRVSDVTKAGHSLEL
IEP LIK FQV GLK KLNLHE EEH VLLM ICIVSPDRP GVQ DAA LIE AIQ DRL SNT
LQT YIRCRHPPPGSHLLYAK MIQ KLADLRSLNEEHSKQYRCLSFQPESMKLTPLVLEV FGN
EIS.
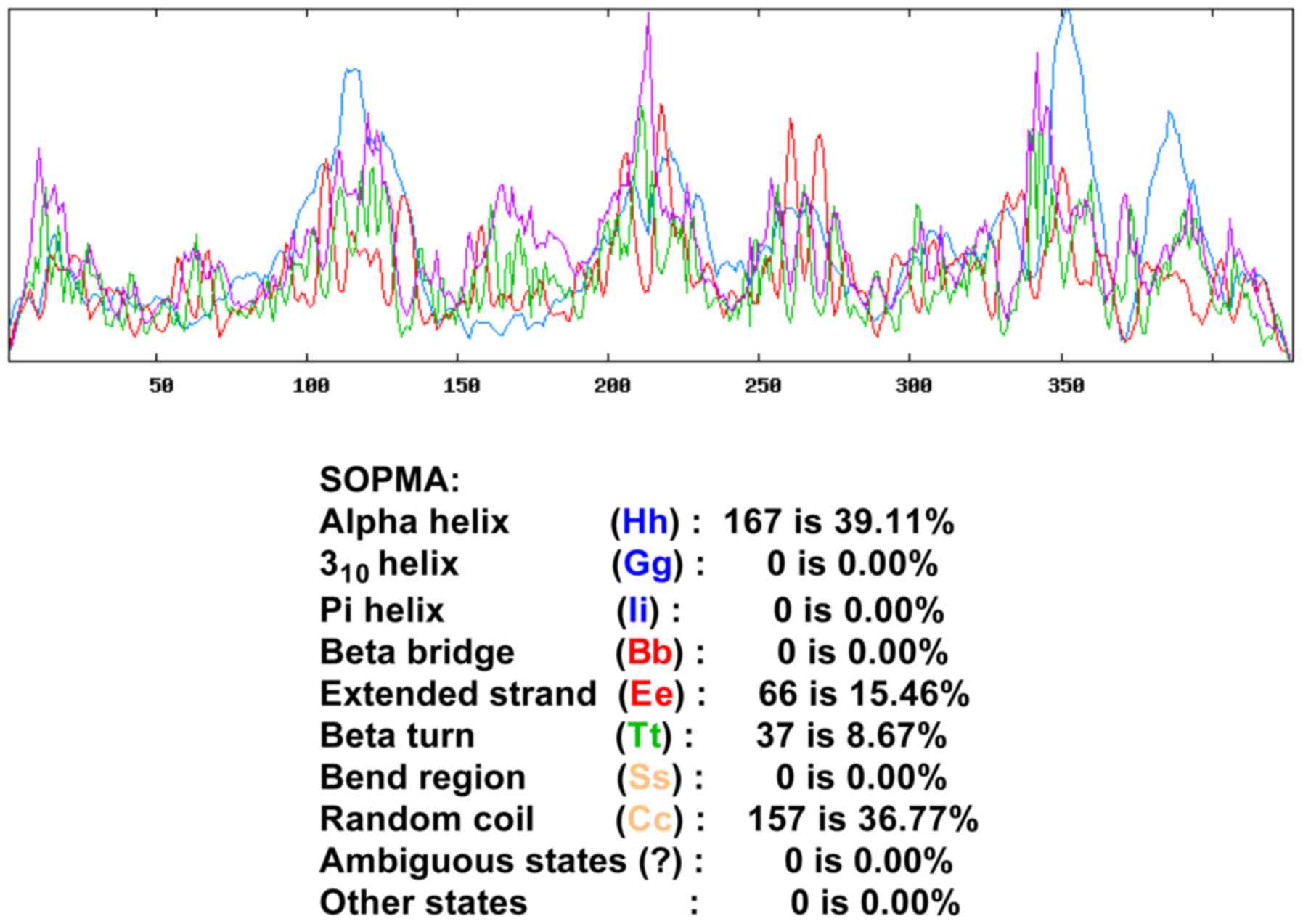
Prediction of the secondary structure
of the protein encoded by VDR gene
SOPMA was used to predict the secondary structure of
VDR protein. Among all amino acids, the α helix accounted for
39.11% of the sequence, β-fold accounted for 8.67%, irregular curl
for 16.76% and the extended strand accounted for 15.46% (Fig. 2).
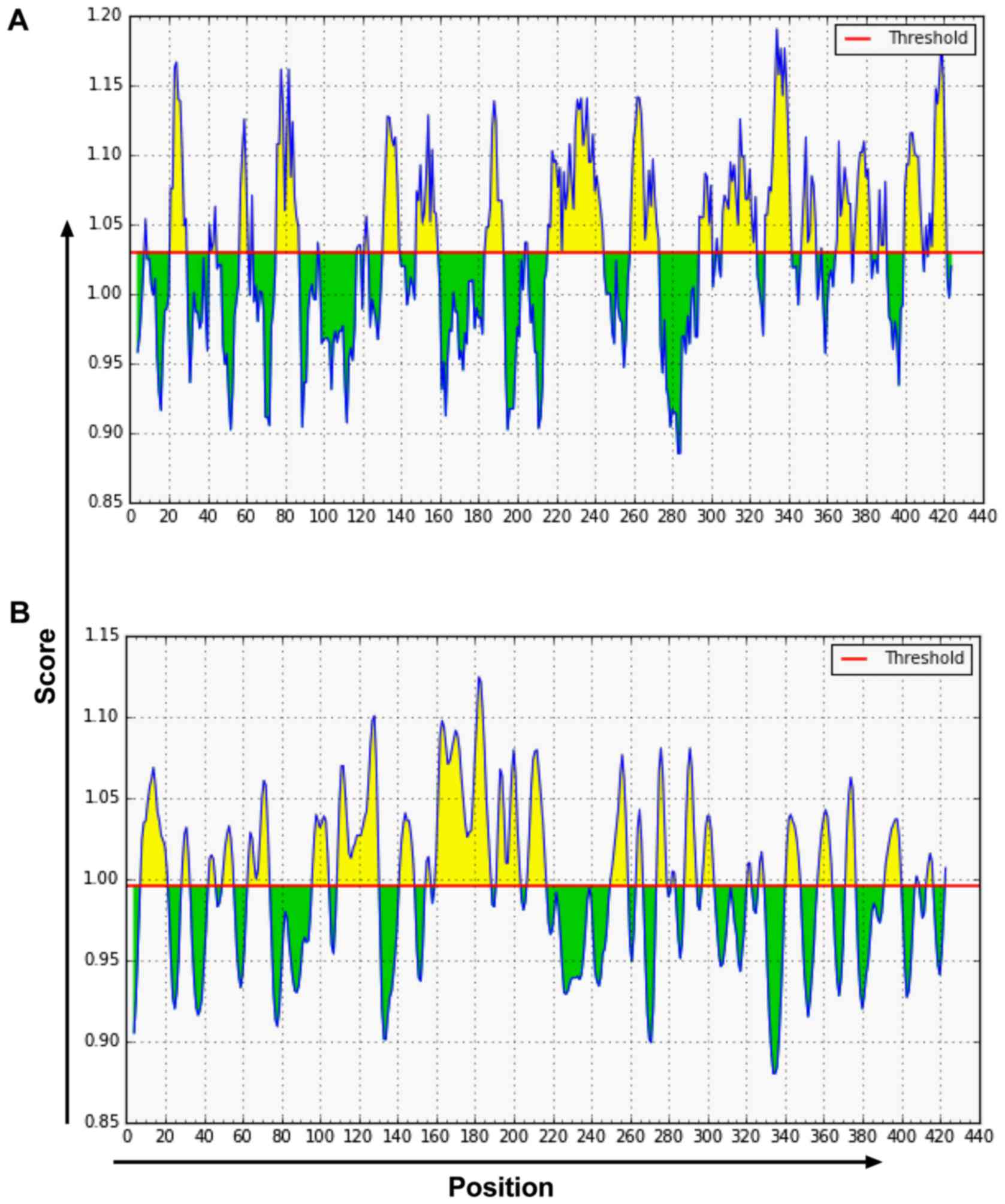
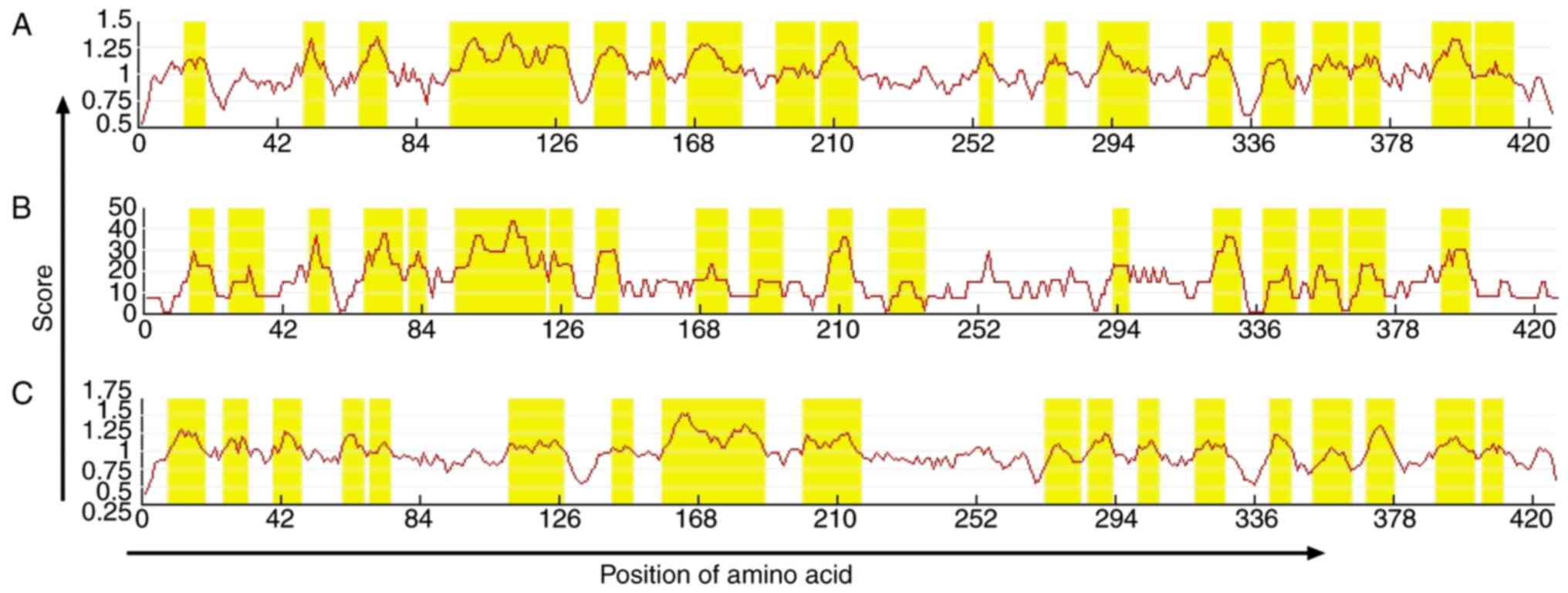
Prediction of VDR B-cell epitopes
The online prediction resources IEDB (Fig. 3) and LEPS (Fig. 4) together with hydrophilicity,
antigenicity index, and flexibility parameters were used to predict
B-cell epitopes. According to the prediction results, amino acid
sequences were determined. A total of six VDR B-cell epitope areas
were determined, including 37–45, 88–94, 123–131, 231–239, 286–294,
342–350 amino acid sequences.
Prediction of VDR T-cell epitopes
The online prediction resources SYFPEITHI and IEDB
were used to predict MHC class I HLA-A0201 T-cell epitopes
(Table I). A total of 15 T-cell
antigen epitopes with high scores were selected. A total of three
T-cell epitope areas with high scores were determined, including
125–130, 231–239, 265–272 amino acid sequences.
 | Table I.SYFPEITHI and IEDB software predicted
T-cell epitopes. |
Table I.
SYFPEITHI and IEDB software predicted
T-cell epitopes.
| SYFPEITHI
software | IEDB software |
|---|
|
|
|---|
| Initiation site | Amino acid
sequence | Score | Initiation site | Amino acid
sequence | Score |
|---|
| 378 | LLYAKMIQKL | 29 | 96 | TDEEVQRKRE | 100 |
| 350 | ALIEAIQDRL | 26 | 68 | ITKDNRRHCQ | 99 |
| 115 | ALKDSLRPKL | 25 | 97 | DEEVQRKREM | 99 |
| 232 | DLVSYSIQKV | 25 | 396 | EHSKQYRCLS | 99.0 |
| 261 | VLLKSSAIEV | 25 | 156 | PVRVNDGGGS | 98.5 |
| 225 | SMLPHLADLV | 24 | 265 | SHPSRPNSRH | 98.5 |
| 229 | HLKDNRRHCQ | 24 | 167 | PSRPNSRHTP | 98.5 |
| 254 | LTSEDQIVLL | 24 | 180 | GDSSSSCSDH | 98.5 |
| 324 | NLHEEEHVLL | 24 | 231 | KDNRRHCQAC | 98 |
| 354 | AIQDRLSNTL | 24 | 120 | LRPKLSEEQQ | 98 |
| 218 | TLELSQLSML | 23 | 136 | LDAHHKTYDP | 98 |
| 262 | LKSHPSRPNS | 23 | 160 | NDGGGSHPSR | 98 |
| 107 | ILKRKEEEHV | 22 | 64 | GDCRITKDNR | 97.5 |
| 322 | KLNLHEEEHV | 22 | 66 | CRITKDNRRH | 97.5 |
| 417 | LVLEVFGNEI | 21 | 155 | PPVRVNDGGG | 97.5 |
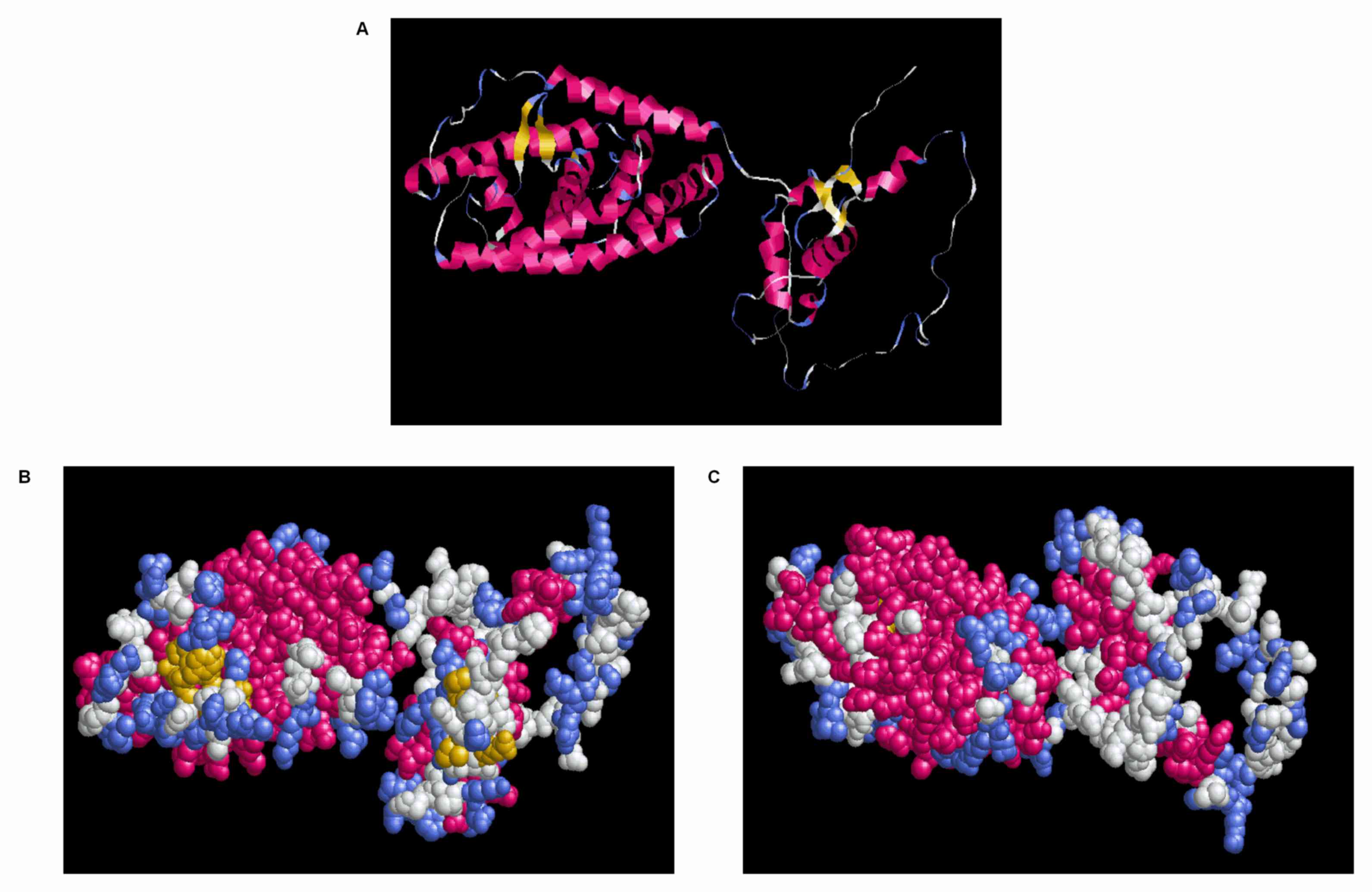
Display and analysis of tertiary
structure of VDR gene
RasMol software was used to analyze the tertiary
structure of 3DLigandSite-predicted VDR. The structure and group
models were used to demonstrate the specific position of each amino
acid on the three-dimensional structure (Fig. 5). The blue region of the structure
and group models (Fig. 5B and C)
was more distributed on the surface of spatial structure and was
the potential binding portion of an antigen to antibody.
Discussion
Bronchial asthma is a chronic inflammatory airway
disease with genetic susceptibility, involving a variety of
inflammatory cells, inflammatory mediators and complex cytokine
networks (18). This disorder is
characterized by airway hyperresponsiveness and airway remodeling
(19). Asthma is the most common
chronic lung disease in China, that is a public health concern
(20). The recurrent episodes of
asthma can weaken pulmonary function, which negatively affects
children's physical and mental health (21). Currently, glucocorticoid therapy is
still the primary method of controlling asthma, but glucocorticoid
therapy often has serious complications to children affecting their
development (22).
VDR is a member of steroid/thyroid hormone receptor
superfamily. Human VDR is located on the long arm of chromosome 12
and it is expressed in numerous immune cells and immune tissues,
including peripheral blood mononuclear cells, activated T and B
cells, dendritic cells, thymus and lymph nodes (23). Following binding to VDR, vitamin D
can mediate multiple biological effects including immunological
regulation. Poon et al and Bossé et al performed a
chromosome scan and proposed that VDR is a candidate gene
associated with asthma (24,25).
In animal experiments, pulmonary inflammation and asthmatic
symptoms were severe in C57/B6 mice, and in mice lacking VDR are
resistant to airway inflammation (26). In the present study, peripheral
blood samples were collected from asthmatic children and VDR
expression was detected using RT-qPCR. The results demonstrated
that VDR expression in peripheral blood was decreased in asthmatic
children. A mouse model of asthma was also established in the
present study and VDR expression was detected using western blot
assay and RT-qPCR. Results demonstrated that VDR expression was
significantly decreased. These results suggest that VDR may be
involved in the progression of asthma.
Epitopes are antigenic molecules that determine the
specificity of antigen-specific chemical groups, also known as
antigenic determinants (27).
Epitopes were initially identified in foot-and-mouth disease
viruses (28). Epitopes are
divided into conformational epitopes and linear epitopes based on
their structure and they are also divided into B-cell epitopes and
T-cell epitopes when present on different receptor-binding cells
(29–32). Linear epitopes are primarily
recognized by T-cell antigen receptors and they are also recognized
by B-cell antigen receptors, while conformational epitopes are only
recognized by B-cell antigen receptors (30). Lin et al (33) used phage display technology to
screen for immunodominant epitopes of leptospiral outer membrane
proteins, which avoids the limitation that a single diagnostic
antigen can be used to detect only a single serotype. The phage
display technology possesses the advantage of early diagnostic
value over other conventional detection methods. Mahajan et
al (34) constructed
polyvalent epitope vaccines with immunodominant B-cell and T-cell
epitopes of liver and erythrocyte antigens to immunize mice,
ultimately resulting in effective cellular and humoral immunity. In
the present study, bioinformatics technique was used to predict VDR
B-cell epitopes. It was determined that among all amino acids, α
helix, β-fold, irregular curl and extended strand accounted for
39.11, 8.67, 36.77 and 15.46% of the amino acid sequence,
respectively. Potential VDR B-cell epitope areas included 37–45,
88–94, 123–131, 231–239, 286–294 and 342–350 amino acid sequences.
SYFPEITHI and IEDB were used to predict HLA-A0201 T-cell epitopes
and it was determined that 125–130 subset, 231–239 and 265–272
amino acid sequences exhibit high scores and may form T-cell
epitopes.
In conclusion, prediction of the secondary and
tertiary structures of VDR as well as the B-cell and T-cell
epitopes to obtain highly scored amino acid sequences for
establishing an efficient vaccine against asthma epitopes. Future
studies should involve validation assays to support the results of
the present study. These may involve synthesizing the VDR peptides
identified in the present study for epitope-associated vaccine
research. The future studies based on the present study may provide
further data to be used for immunological diagnosis for asthma.
Acknowledgements
The present study was supported by the Liaoning
Natural Fund Project (grant no. 2013021017) and Shenyang Science
and Technology Plan Project (grant no. F15-139-9-35).
References
|
1
|
Vora AC: Bronchial asthma. J Assoc
Physicians India. 62 3 Suppl:S5–S6. 2014.
|
|
2
|
Zhou X and Hong J: Pediatric asthma
management in china: Current and future challenges. Paediatr Drugs.
20:105–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kay AB: Mediators of hypersensitivity and
inflammatory cells in the pathogenesis of bronchial asthma. Eur J
Respir Dis Suppl. 129:1–44. 1983.PubMed/NCBI
|
|
4
|
Kuna P: Contemporary views on the
pathological mechanism of asthma. Pol Merkur Lekarski. 14:519–521.
2003.(In Polish). PubMed/NCBI
|
|
5
|
Carr TF and Peters AT: Chapter 12: Asthma:
Principles of treatment. Allergy Asthma Proc. 33 Suppl 1:S39–S43.
2012. View Article : Google Scholar
|
|
6
|
Tang SP, Liu YL, Wang SB, Weng SF, Chen S,
Zhang MJ, Dong L, Guo YH, Lin DR, Hua YH and Wang DY: Trends in
prevalence and risk factors of childhood asthma in Fuzhou, a city
in Southeastern China. J Asthma. 52:10–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang H, Porpodis K, Zarogoulidis P,
Domvri K, Giouleka P, Papaiwannou A, Primikyri S, Mylonaki E,
Spyratos D, Hohenforst-Schmidt W, et al: Vitamin D in asthma and
future perspectives. Drug Des Devel Ther. 7:1003–1013.
2013.PubMed/NCBI
|
|
8
|
Anderson PH, Turner AG and Morris HA:
Vitamin D actions to regulate calcium and skeletal homeostasis.
Clin Biochem. 45:880–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myszka M and Klinger M: The
immunomodulatory role of Vitamin D. Postepy Hig Med Dosw (Online).
68:865–878. 2014.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iruretagoyena M, Hirigoyen D, Naves R and
Burgos PI: Immune response modulation by vitamin D: Role in
systemic lupus erythematosus. Front Immunol. 6:5132015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mann EH, Chambers ES, Pfeffer PE and
Hawrylowicz CM: Immunoregulatory mechanisms of vitamin D relevant
to respiratory health and asthma. Ann N Y Acad Sci. 1317:57–69.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karagün E, Ergin C, Baysak S, Erden G,
Aktaş H and Ekiz Ö: The role of serum vitamin D levels in vitiligo.
Postepy Dermatol Alergol. 33:300–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abelha-Aleixo J, Fonseca R, Bernardo A,
Mariz E and Costa L: Vitamin D - immunomodulatory actions and new
potentialities. Acta Reumatol Port. 39:355–356. 2014.PubMed/NCBI
|
|
14
|
Belorusova AY and Rochel N: Structural
studies of vitamin D nuclear receptor ligand-binding properties.
Vitam Horm. 100:83–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan Y, Learoyd J, Meliton AY, Clay BS,
Leff AR and Zhu X: Inhibition of Pyk2 blocks airway inflammation
and hyperresponsiveness in a mouse model of asthma. Am J Respir
Cell Mol Biol. 42:491–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wass MN, Kelley LA and Sternberg MJ:
3DLigandSite: Predicting ligand-binding sites using similar
structures. Nucleic Acids Res. 38:(Web Server Issue). W469–W473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haspeslagh E, Debeuf N, Hammad H and
Lambrecht BN: Murine models of allergic asthma. Methods Mol Biol.
1559:121–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balmasova IP, Sepiashvili RI, Sepiashvili
IaR and Malova ES: Bronchial asthma pathogenesis and genetic
prognosis development. Zh Mikrobiol Epidemiol Immunobiol. 3:60–67.
2014.(In Russian).
|
|
19
|
Cleary RA, Wang R, Wang T and Tang DD:
Role of Abl in airway hyperresponsiveness and airway remodeling.
Respir Res. 14:1052013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ladebauche P: Managing asthma: A growth
and development approach. Pediatr Nurs. 23:37–44. 1997.PubMed/NCBI
|
|
21
|
Tantisira KG, Litonjua AA, Weiss ST and
Fuhlbrigge AL: Childhood Asthma Management Program Research Group:
Association of body mass with pulmonary function in the Childhood
Asthma Management Program (CAMP). Thorax. 58:1036–1041. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grimfeld A, Just J and Bodart E: Role of
inhalation therapy in the management of childhood asthma. Rev Mal
Respir. 9:413–416. 1992.(In French). PubMed/NCBI
|
|
23
|
Taymans SE, Pack S, Pak E, Orban Z,
Barsony J, Zhuang Z and Stratakis CA: The human vitamin D receptor
gene (VDR) is localized to region 12cen-q12 by fluorescent in situ
hybridization and radiation hybrid mapping: Genetic and physical
VDR map. J Bone Miner Res. 14:1163–1166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poon AH, Gong L, Brasch-Andersen C,
Litonjua AA, Raby BA, Hamid Q, Laprise C, Weiss ST, Altman RB and
Klein TE: Very important pharmacogene summary for VDR.
Pharmacogenet Genomics. 22:758–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bossé Y, Lemire M, Poon AH, Daley D, He
JQ, Sandford A, White JH, James AL, Musk AW, Palmer LJ, et al:
Asthma and genes encoding components of the vitamin D pathway.
Respir Res. 10:982009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wittke A, Chang A, Froicu M, Harandi OF,
Weaver V, August A, Paulson RF and Cantorna MT: Vitamin D receptor
expression by the lung micro-environment is required for maximal
induction of lung inflammation. Arch Biochem Biophys. 460:306–313.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hruby S, Alvord EC Jr, Martenson RE,
Deibler GE, Hickey WF and Gonatas NK: Epitopes in myelin basic
protein reactive with monoclonal antibodies. Prog Clin Biol Res.
146:271–276. 1984.PubMed/NCBI
|
|
28
|
Ma X, Li P, Sun P, Bai X, Bao H, Lu Z, Fu
Y, Cao Y, Li D, Chen Y, et al: Construction and characterization of
3A-epitope-tagged foot-and-mouth disease virus. Infect Genet Evol.
31:17–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Augustin T, Cehlar O, Skrabana R, Majerova
P and Hanes J: Unravelling viral camouflage: Approaches to the
study and characterization of conformational epitopes. Acta Virol.
59:103–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Forsström B, Axnäs BB, Rockberg J,
Danielsson H, Bohlin A and Uhlen M: Dissecting antibodies with
regards to linear and conformational epitopes. PLoS One.
10:e01216732015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pinilla C, Appel JR, Judkowski V and
Houghten RA: Identification of B cell and T cell epitopes using
synthetic peptide combinatorial libraries. Curr Protoc Immunol
Chapter. 9:Unit9.5. 2012. View Article : Google Scholar
|
|
32
|
Amin MR, Siddiqui MS, Ahmed D, Ahmed F and
Hossain A: B- and T-cell epitope mapping of human sapovirus capsid
protein: an immunomics approach. Int J Bioinform Res Appl.
7:287–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin X, Chen Y and Yan J: Recombinant
multiepitope protein for diagnosis of leptospirosis. Clin Vaccine
Immunol. 15:1711–1714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mahajan B, Berzofsky JA, Boykins RA, Majam
V, Zheng H, Chattopadhyay R, de la Vega P, Moch JK, Haynes JD,
Belyakov IM, et al: Multiple antigen peptide vaccines against
Plasmodium falciparum malaria. Infect Immun. 78:4613–4624. 2010.
View Article : Google Scholar : PubMed/NCBI
|