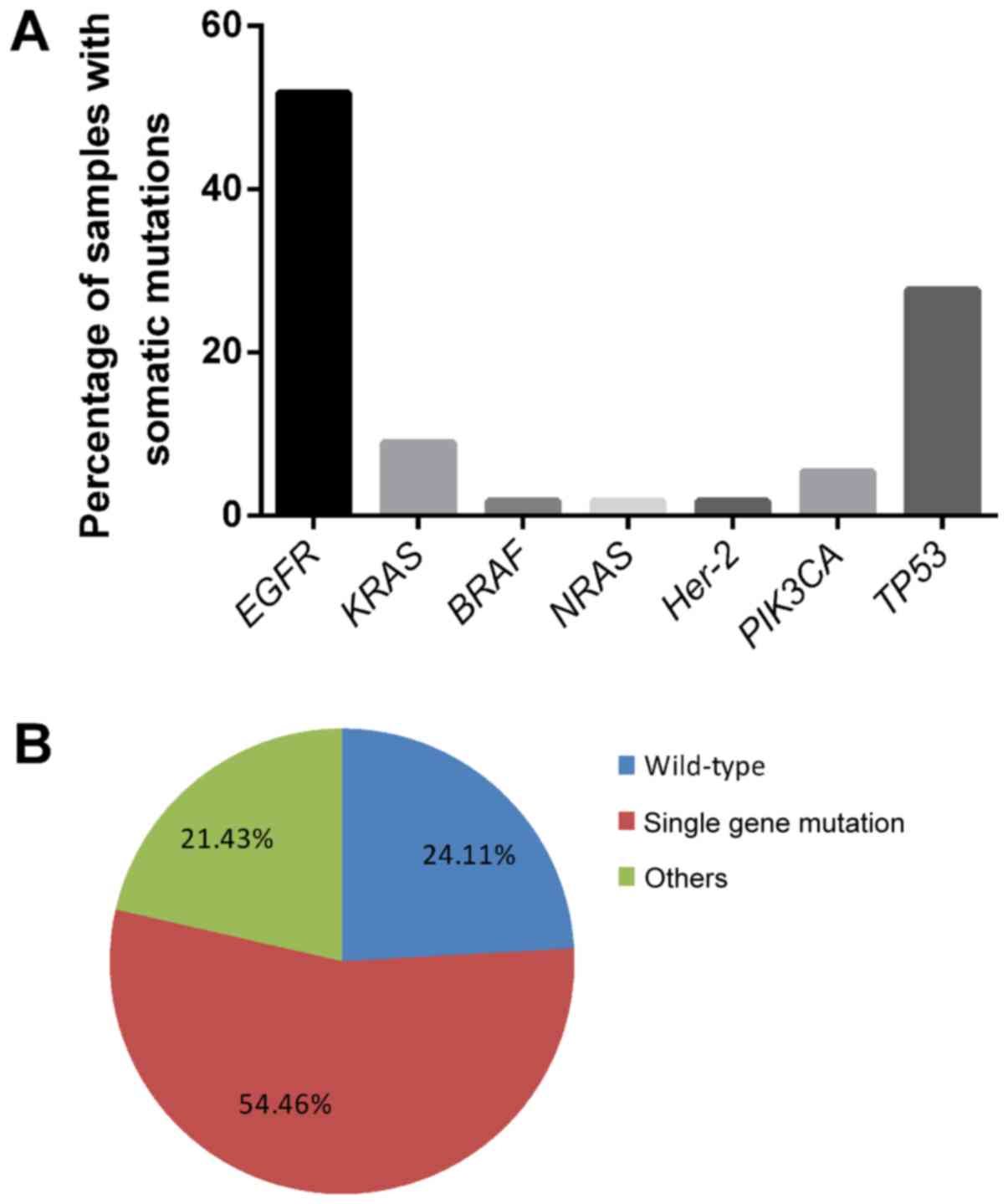
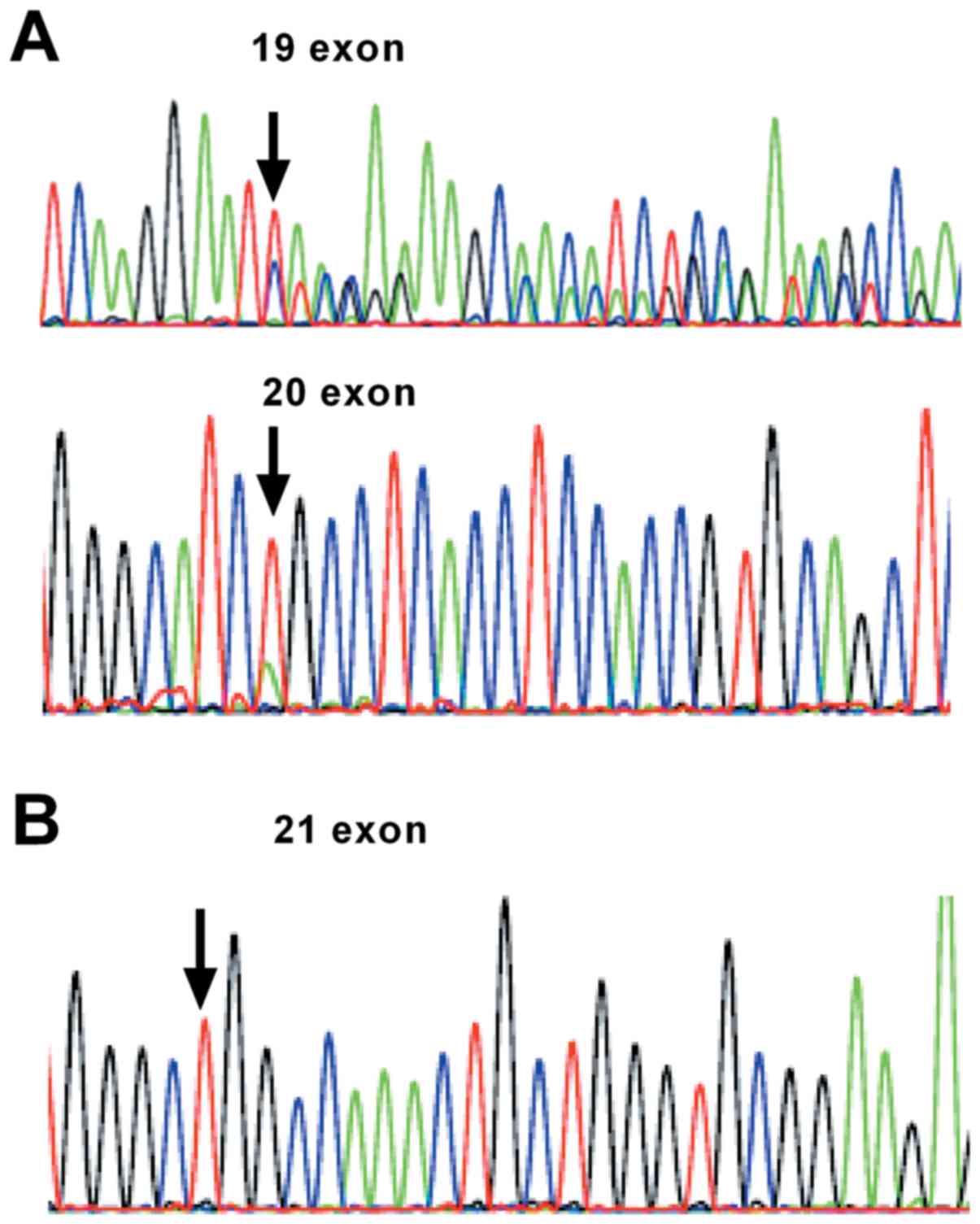
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baek JH, Sun JM, Min YJ, Cho EK, Cho BC,
Kim JH, Ahn MJ and Park K: Efficacy of EGFR tyrosine kinase
inhibitors in patients with EGFR-mutated non-small cell lung cancer
except both exon 19 deletion and exon 21 L858R: A retrospective
analysis in Korea. Lung Cancer. 87:148–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomasini P, Serdjebi C, Khobta N, Metellus
P, Ouafik L, Nanni I, Greillier L, Loundou A, Fina F, Mascaux C and
Barlesi F: EGFR and KRAS Mutations Predict the incidence and
outcome of brain metastases in non-small cell lung cancer. Int J
Mol Sci. 17:pii: E2132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smit E: BRAF mutations in non-small-cell
lung cancer. J Thorac Oncol. 9:1594–1595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheffler M, Bos M, Gardizi M, König K,
Michels S, Fassunke J, Heydt C, Künstlinger H, Ihle M, Ueckeroth F,
et al: PIK3CA mutations in non-small cell lung cancer (NSCLC):
Genetic heterogeneity, prognostic impact and incidence of prior
malignancies. Oncotarget. 6:1315–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SY, Jeon HS, Hwangbo Y, Jeong JY, Park
JY, Lee EJ, Jin G, Shin KM, Yoo SS, Lee J, et al: The influence of
TP53 mutations on the prognosis of patients with early stage
non-small cell lung cancer may depend on the intratumor
heterogeneity of the mutations. Mol Carcinog. 54:93–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu G, Ye X, Dong Z, Lu YC, Sun Y, Liu Y,
McCormack R, Gu Y and Liu X: Highly sensitive droplet digital PCR
method for detection of EGFR-activating mutations in plasma
cell-free DNA from patients with advanced non-small cell lung
cancer. J Mol Diagn. 17:265–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu X, Yang Y, Li H, Chen Z, Jiang G and
Fei K: Assessment of the clinical application of detecting EGFR,
KRAS, PIK3CA and BRAF mutations in patients with non-small cell
lung cancer using next-generation sequencing. Scand J Clin Lab
Invest. 76:386–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar
|
|
10
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ,
Bell DW, et al: Detection of mutations in EGFR in circulating
lung-cancer cells. Engl J Med. 359:366–377. 2008. View Article : Google Scholar
|
|
11
|
Jackman DM, Miller VA, Cioffredi LA, Yeap
BY, Jänne PA, Riely GJ, Ruiz MG, Giaccone G, Sequist LV and Johnson
BE: Impact of epidermal growth factor receptor and KRAS mutations
on clinical outcomes in previously untreated non-small cell lung
cancer patients: Results of an online tumor registry of clinical
trials. Clin Cancer Res. 15:5267–5273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Li L, Zhu Y, Huang C, Qin Y, Liu H,
Ren-Heidenreich L, Shi B, Ren H, Chu X, et al: Coexistence of EGFR
with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: A
comprehensive mutation profiling from 5125 Chinese cohorts. Br J
Cancer. 110:2812–2820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flaherty KT, Puzanov I, Kim KB, Ribas A,
McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K and
Chapman PB: Inhibition of mutated, activated BRAF in metastatic
melanoma. N Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brustugun OT, Khattak AM, Trømborg AK,
Beigi M, Beiske K, Lund-Iversen M and Helland Å: BRAF-mutations in
non-small cell lung cancer. Lung Cancer. 84:36–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cancer Genome Atlas Research Network:
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazières J, Peters S, Lepage B, Cortot AB,
Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban
T, et al: Lung cancer that harbors an HER2 mutation: Epidemiologic
characteristics and therapeutic perspectives. J Clin Oncol.
31:1997–2003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eng J, Woo KM, Sima CS, Plodkowski A,
Hellmann MD, Chaft JE, Kris MG, Arcila ME, Ladanyi M and Drilon A:
Impact of concurrent PIK3CA mutations on response to EGFR tyrosine
kinase inhibition in EGFR-mutant lung cancers and on prognosis in
oncogene-driven lung adenocarcinomas. J Thorac Oncol. 10:1713–1719.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen YS, Cai L, Zhang XW, Zhu JF, Zhang ZC,
Shao JY and Zhang LJ: Concurrent oncogene mutation profile in
Chinese patients with stage Ib lung adenocarcinoma. Medicine
(Baltimore). 93:e2962014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kris MG, Johnson BE, Berry LD, Kwiatkowski
DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson
SL, Su PF, et al: Using multiplexed assays of oncogenic drivers in
lung cancers to select targeted drugs. JAMA. 311:1998–2006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keam B, Kim DW, Park JH, Lee JO, Kim TM,
Lee SH, Chung DH and Heo DS: Rare and complex mutations of
epidermal growth factor receptor, and efficacy of tyrosine kinase
inhibitor in patients with non-small cell lung cancer. Int J Clin
Oncol. 19:594–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou C, et al: Phase II Single Arm Study
of AZD9291 to Treat NSCLC Patients in Asia Pacific (AURA17).
https://clinicaltrials.gov/ct2/show/study/NCT02442349April
25–2017
|