Introduction
As a global problem, cataracts are the leading cause
of blindness in the elderly population and account for almost half
of visually disabled people (1).
Surgery has become an effective treatment method for cataracts.
However, risks of surgical complications exist (2,3).
Understanding the mechanisms of cataracts may allow the development
of cataracts to be prevented, and subsequently reduce risks of
surgical complications and the burden of cataracts on medical care
worldwide.
In recent decades, emerging research has enriched
our molecular understanding of the various forms of programmed cell
death, which includes several endogenous genetically defined
pathways (4). Apoptosis is the
most widely recognized form of programmed cell death and involves
particular caspases (cysteine-dependent aspartate-specific
proteases), which lead to coordinated cell disassembly (5,6). It
has been established that apoptosis of lens epithelial cells is a
common cellular basis for the initiation and progression for
non-congenital cataracts (7).
Additional forms of programmed cell death include autophagy,
oncosis and pyroptosis (also termed caspase 1-dependent programmed
cell death) (8).
Pyroptosis is associated with rapid plasma membrane
rupturing and the release of proinflammatory intracellular contents
(9), and may be initiated by
numerous pathological stimuli, including brain injury (10), myocardial infarction (11) or cancer (12), and has an important function in the
control of microbial infections (13). Pyroptosis is closely associated
with oxidative stress (14,15).
Several studies have demonstrated that oxidative stress has a key
role in cataractogenesis in vivo and in vitro
(16,17). However, whether pyroptosis is
implicated in the initiation and progression of non-congenital
cataracts remains to be established.
The present study investigated the involvement of
pyroptosis in cataract formation in a human lens epithelium cell
line, and also characterized the expression of caspase-1 and
interleukin (IL)-1β in cataract lens anterior capsule tissue
samples.
Materials and methods
Materials
The SV40 T-antigen-transformed human lens epithelial
cell line (SRA01/04 cells) was obtained from the American Type
Culture Collection (Manassas, VA, USA). Fetal bovine serum (FBS)
and Dulbecco's modified Eagle's medium/F12 (DMEM/F12) were obtained
from Biological Industries (Kibbutz Beit-Haemek, Israel).
H2O2 was purchased from Shanghai Zhongshi
Chemistry Industry Co., Ltd. (Shanghai, China). MTT was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). TRIzol and
polymerase chain reaction (PCR) primers were purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
NanoDrop spectrophotometer was obtained from NanoDrop Technologies
(Thermo Fisher Scientific, Inc.). Primary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Fluorochrome-labeled secondary antibody (Alexa Fluor 800) was
obtained from Thermo Fisher Scientific, Inc.
Human tissue samples
A total of 40 cataract lens anterior capsule samples
were obtained from patients with cataracts that were free from
other types of ocular diseases (age range, 68–80 years) in the
operation room of the Eye Hospital of Harbin Medical University
(Harbin, China), between October 2015 and March 2016. In addition,
40 control lens anterior capsular samples were collected from
healthy donor eyes (age range, 54–69 years) that were free of any
ocular diseases and donated to the Eye Bank of Heilongjiang
Province (Harbin, China), between July 2014 to March 2016. Samples
were either embedded in paraffin or immediately snap-frozen and
stored at −80°C until RNA extraction. All samples were collected
with informed consent and the study was approved by the Research
Ethics Committee of Harbin Medical University. Patient information
is listed in Table I.
 | Table I.Patient and volunteer
information. |
Table I.
Patient and volunteer
information.
| Tissue sample
type | Mean age, years (±
SD) | Age range,
years | Males (%) | Females (%) |
|---|
| Cataracts,
n=40 | 74.4 (±3.84) | 68-80 | 17
(42.5) | 23
(57.5) |
| Normal, n=40 | 62.4 (±4.04) | 54-69 | 20 (50) | 20 (50) |
Cell culture
SRA01/04 cells (1×105) were cultured in
DMEM/F12 supplemented with 20% FBS at 37°C in 5% CO2
overnight. Cells in the logarithmic growth phase were collected and
treated with 0, 25, 50 or 100 µM H2O2 for 0,
24 or 48 h at 37°C in 5% CO2. Control cells were treated
with DMEM/F12 containing 20% FBS alone. Caspase-1 inhibitor for
downregulation was synthesized by Cayman Chemical Company (item no.
10014; Ann Arbor, MI, USA). After the cell density reached 80%, 100
µM caspase-1 inhibitor was added simultaneously with
H2O2 to the designated well. After 0, 24 or
48 h of treatment at 37°C, cells were harvested for subsequent
experiments.
Cell proliferation assay
MTT were used in accordance with the manufacturer's
protocol. SRA01/04 cells were seeded in 96-well plates at
1×104 cells/well and treated with 0, 25, 50, 100 or 200
µM H2O2 and maintained for 24 or 48 h at
37°C. MTT solution (10 µl) was added to each well and cells were
incubated in 37°C for 2 h. DMSO (150 µl) was added to each well.
The absorbance at a wavelength of 450 nm was evaluated using a
microplate reader. The data are representative of three individual
experiments in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
Caspase-1 and IL-1β mRNA expression was determined
by RT-qPCR using SYBR Green detection reagents. Primer sequences
are listed in Table II. Total RNA
was extracted from each sample with TRIzol reagent, according to
the manufacturer's protocol. Total isolated RNA (500 ng) was used
to synthesize cDNA in a 20 µl reaction mixture containing 2 µl RNA,
4 µl 5X RT buffer, 1 µl primer mix, 1 µl RT enzyme and 12 µl
H2O (Toyobo Life Science, Osaka, Japan). qPCR
amplification was performed in a 20 µl reaction volume containing 2
µl cDNA, 6 µl diethyl pyrocarbonate, 10 µl SYBR Master mix (Toyobo
Life Science), 1 µl forward primer and 1 µl reverse primer. qPCR
was performed using the ABI PRISM 7500 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and PCR
conditions were as follows: 95°C for 60 sec, followed by 40 cycles
of 95°C for 15 sec, 60°C for 15 sec and 72°C for 45 sec. The
housekeeping gene GAPDH was used as an internal positive control
standard for quantitative analysis in triplicate. Cq values
obtained via the 2−ΔΔCq method (18) were used to quantify mRNA
expression.
 | Table II.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
|
| Primer
sequence |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
5′-AAGAAGGTGGTGAAGCAGGC-3′ |
5′-TCCACCACCCTGTTGCTGTA-3′ |
| Caspase-1 |
5′-ACACGTCTTGCCCTCATTATCT-3′ |
5′-ATAACCTTGGGCTTGTCTTTCA-3′ |
| Interleukin-1β |
5′-CCTTGTCGAGAATGGGCAGT-3′ |
5′-TTCTGTCGACAATGCTGCCT-3′ |
Western blot analysis
Western blot analysis was performed to detect the
expression levels of certain proteins of interest. Samples were
lysed using radioimmunoprecipitation assay buffer (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). A
bicinchoninic acid assay (Beijing Solarbio Science & Technology
Co., Ltd.) was used to determine the protein concentration of the
samples. Protein (50 µg) per sample was separated using 10%
SDS-PAGE and transferred onto nitrocellulose membranes. Membranes
were blocked in 5% milk for 2 h at room temperature, and then
immunoblotted overnight with rabbit caspase-1 (1:1,000; cat. no.
PRS3459; Sigma-Aldrich; Merck KGaA), IL-1β (1:1,000; cat. no.
SAB4503272; Sigma-Aldrich; Merck KGaA) and GAPDH (1:800; cat no.
ab8245; Abcam, Cambridge, UK) primary antibodies at 4°C with gentle
shaking. Subsequently, membranes were incubated with goat
anti-rabbit fluorochrome-labeled secondary antibody for 2 h at room
temperature (1:5,000; Alexa Fluor 800; cat. no. A32735).
Immunoreactivity was detected with an Odyssey fluorescent scanning
system (LI-COR Biosciences, Lincoln, NE, USA) and analyzed by Image
Studio software version 4.0 (LI-COR Biosciences). GAPDH was used as
loading control.
Immunofluorescence and
immunohistochemistry staining
For immunofluorescence staining, SRA01/04 cells were
seeded in 24-well plates at a density of 3×105
cells/well and maintained for 24 h. Following treatment with 100 µM
H2O2 with or without caspase-1 inhibitor
treatment, cells were fixed with 4% paraformaldehyde for 1 h at
37°C, then permeabilized with 0.1% Triton for 15 min at room
temperature. After rinsing with PBS, cells were blocked with 10%
bovine serum albumin (Biosharp, China) 30 min at room temperature.
The cells were subsequently incubated with rabbit caspase-1 (1:200)
or IL-1β (1:200) antibodies overnight at 37°C. Cells were rinsed
three times in PBS and incubated with Cy3-conjugated goat
anti-rabbit IgG (1:100; cat. no. AP132C; Sigma-Aldrich; Merck KGaA)
secondary antibody 30 min at room temperature. Staining was
observed using an Observer A1 fluorescence microscope (Zeiss GmbH,
Jena, Germany), and ZEN software version 2 (Zeiss GmbH) was
used.
For immunohistochemistry staining, lens anterior
capsule tissue samples were placed on cover slides and fixed with
4% paraformaldehyde for 30 min at room temperature. After rinsing
with PBS, capsular samples were penetrated with 0.1% Triton X-100
15 min and blocked with 5% BSA 30 min at room temperature. Slides
were subsequently incubated with primary antibodies against
caspase-1 (1:200) and IL-1β (1:200) at 4°C overnight. Slides were
then washed using PBS and further incubated with secondary
antibodies conjugated to Cy3 (1:100; cat no. AP132C; Sigma-Aldrich;
Merck KGaA) for 2 h at room temperature, followed by staining with
3,3′-diaminobenzidine 15 sec at room temperature. Samples were
hydrated in graded ethanol. Staining was observed using an Observer
A1 fluorescence microscope (Zeiss GmbH, Jena, Germany), and ZEN
software version 2 (Zeiss GmbH) was used.
TUNEL staining
SRA01/04 cells (3×105 cells/well) were
plated onto coverslips in 24-well culture plates and an in
situ Cell Death Detection kit (Fluorescein; Roche, Diagnostics;
Indianapolis, IN, USA) was employed to detect DNA fragmentation of
individual cells, according to the manufacturer's protocol. The
nuclei were stained with DAPI (1 µg/ml) for 5 min at room
temperature. TUNEL staining was assessed by fluorescence microscopy
(Eclipse 80i; Nikon Co., Tokyo, Japan) at ×200 magnification in 6
fields of view. Nuclei that were double labeled with DAPI and TUNEL
were considered positive.
Statistical analysis
Statistical significance was determined by a
two-tailed Student's t-test or one-way analysis of variance
followed by Tukey's post hoc test. Data are presented as the mean ±
standard deviation. The results were analyzed by SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
Pyroptosis is induced in
H2O2-treated SRA01/04 cells
Caspase-1 and IL-1β are established markers of
pyroptosis. To determine whether pyroptosis is involved in cataract
formation, SRA01/04 lens epithelial cells were exposed to various
concentrations of H2O2 (0, 25, 50 and 100 µM)
for 24 or 48 h. Pyroptosis was detected by morphology analyses,
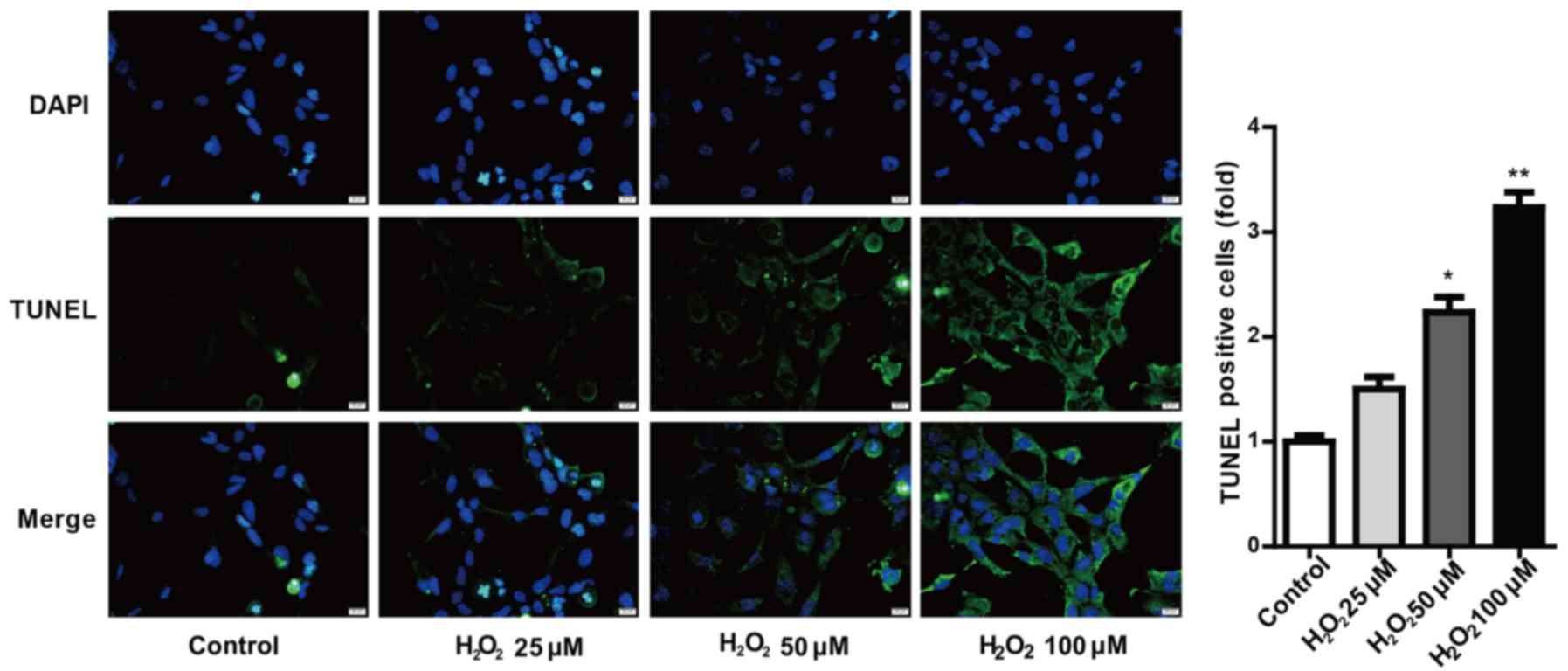
MTT, RT-qPCR and western blot assays, and TUNEL staining (Figs. 1 and 2, respectively). SRA01/04 cells became
swollen and the cell number decreased with increasing
H2O2 concentration. MTT assay results
demonstrate that SRA01/04 cell growth was inhibited by 25, 50, 100
and 200 µM H2O2 at 24 and 48 h (Fig. 1B). The mRNA and protein expression
of caspase-1 and IL-1β were increased in
H2O2-treated SRA01/04 cells in a
dose-dependent manner (Fig. 1C-F).
Compared with the control group, relative caspase-1 and IL-1β
expression increased >50% when the H2O2
concentration was 100 µM. Furthermore, TUNEL assay results provided
further evidence that H2O2-induced cell
pyroptosis was significantly increased in a dose-dependent manner
(Fig. 2). Based on these results,
100 µM H2O2 was selected for subsequent
experiments.
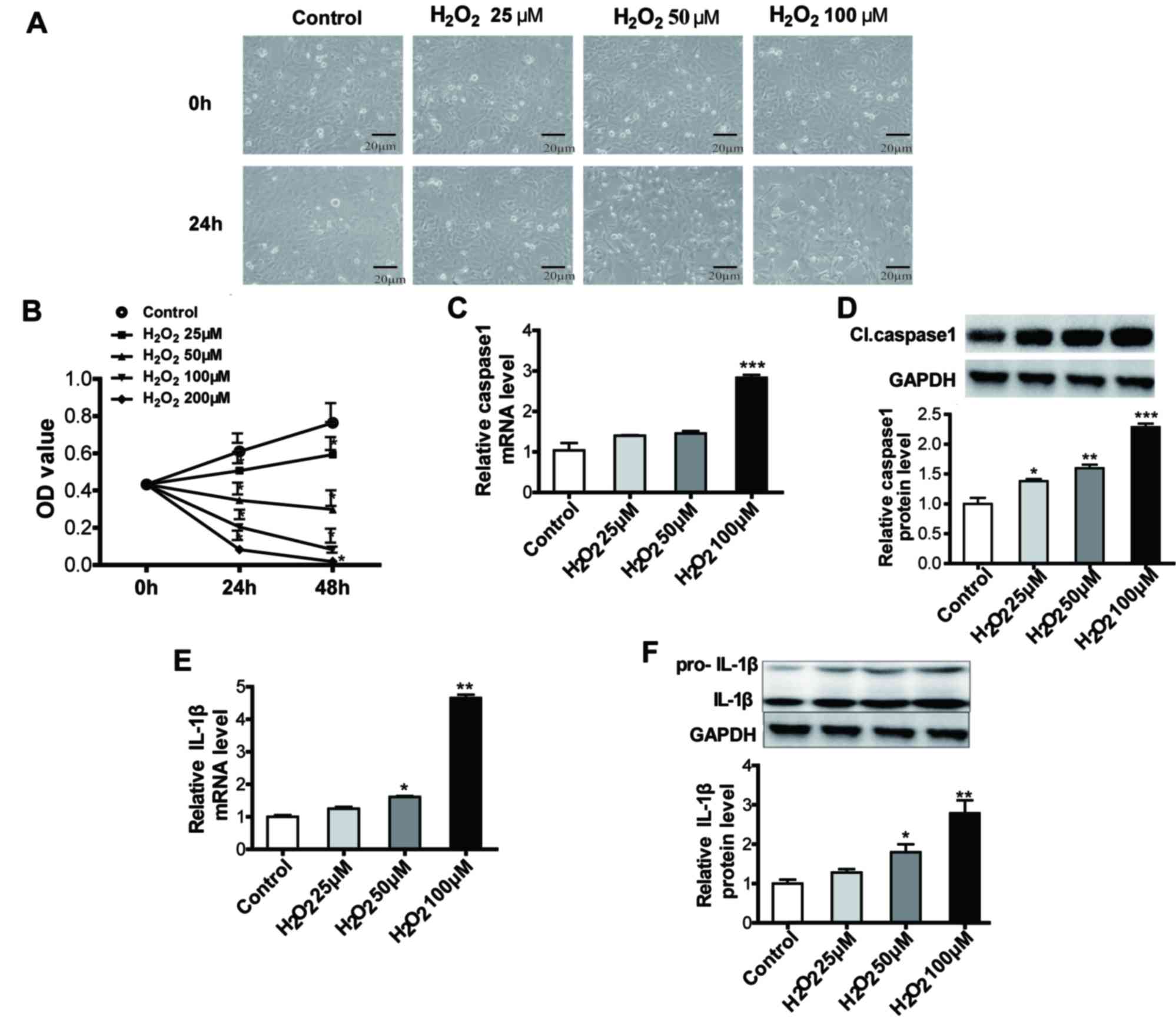 | Figure 1.Pyroptosis was increased in
H2O2 treated human lens epithelium cells. (A)
When exposed to 0, 25, 50 and 100 µM H2O2 for
24 h, SRA01/04 cells became swollen and the cell number decreased
with increasing H2O2 concentration. Scale
bar, 20 µm. ×200 magnification. (B) MTT assay results demonstrated
that SRA01/04 cell growth was inhibited when treated with 25, 50,
100 and 200 µM H2O2 for 24 and 48 h. (C)
RT-qPCR results indicated that relative caspase-1 mRNA levels were
increased with increasing concentrations of
H2O2. (D) Cl. caspase-1 protein levels were
upregulated increasing H2O2 concentrations,
as demonstrated by western blot analysis. (E) RT-qPCR results
demonstrated that relative IL-1β mRNA levels were increased with
increasing concentrations of H2O2. (F) IL-1β
protein levels were upregulated with increasing
H2O2 concentrations, as demonstrated by
western blot analysis. *P<0.05, **P<0.01 and ***P<0.001
vs. control. RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; Cl., cleaved; IL, interleukin; OD, optical density;
pro-, precursor. |
Validation of pyroptosis in the lens
anterior capsules of patients with cataracts
To further validate these results, cataract lens
anterior capsules and normal lens anterior capsule samples were
collected and analyzed for caspase-1 and IL-1β expression using
immunohistochemistry staining, RT-qPCR and western blot analysis.
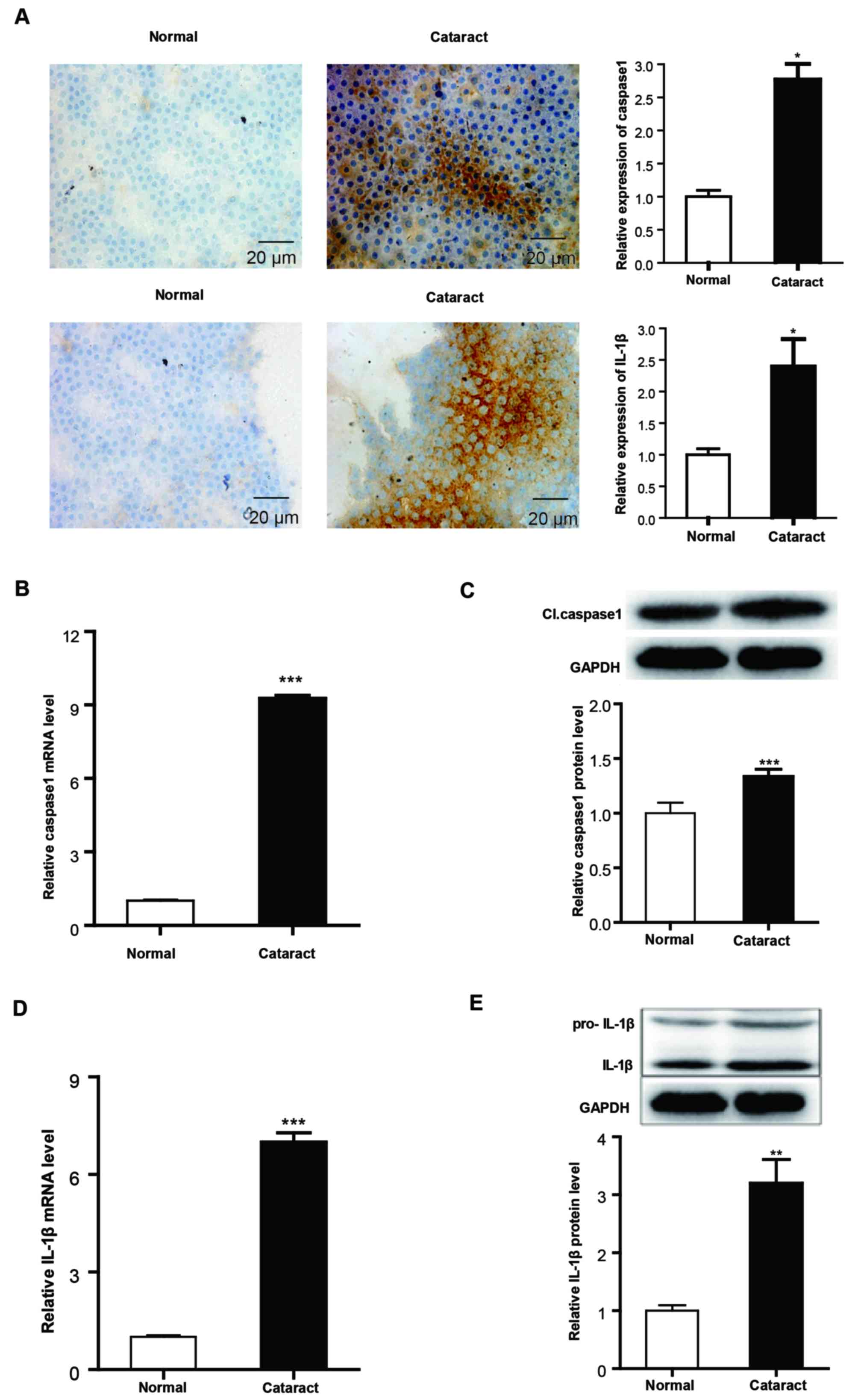
Immunohistochemistry staining results demonstrated increased
positive staining of caspase-1 and IL-1β in the cataract lens
anterior capsular specimens compared with normal samples (Fig. 3A). The mRNA and protein expression
levels of caspase-1 and IL-1β were also significantly upregulated
in cataract samples compared with normal anterior lens capsules
(Fig. 3B-E).
Caspase-1 inhibitor reduces pyroptosis
in SRA01/04 cells
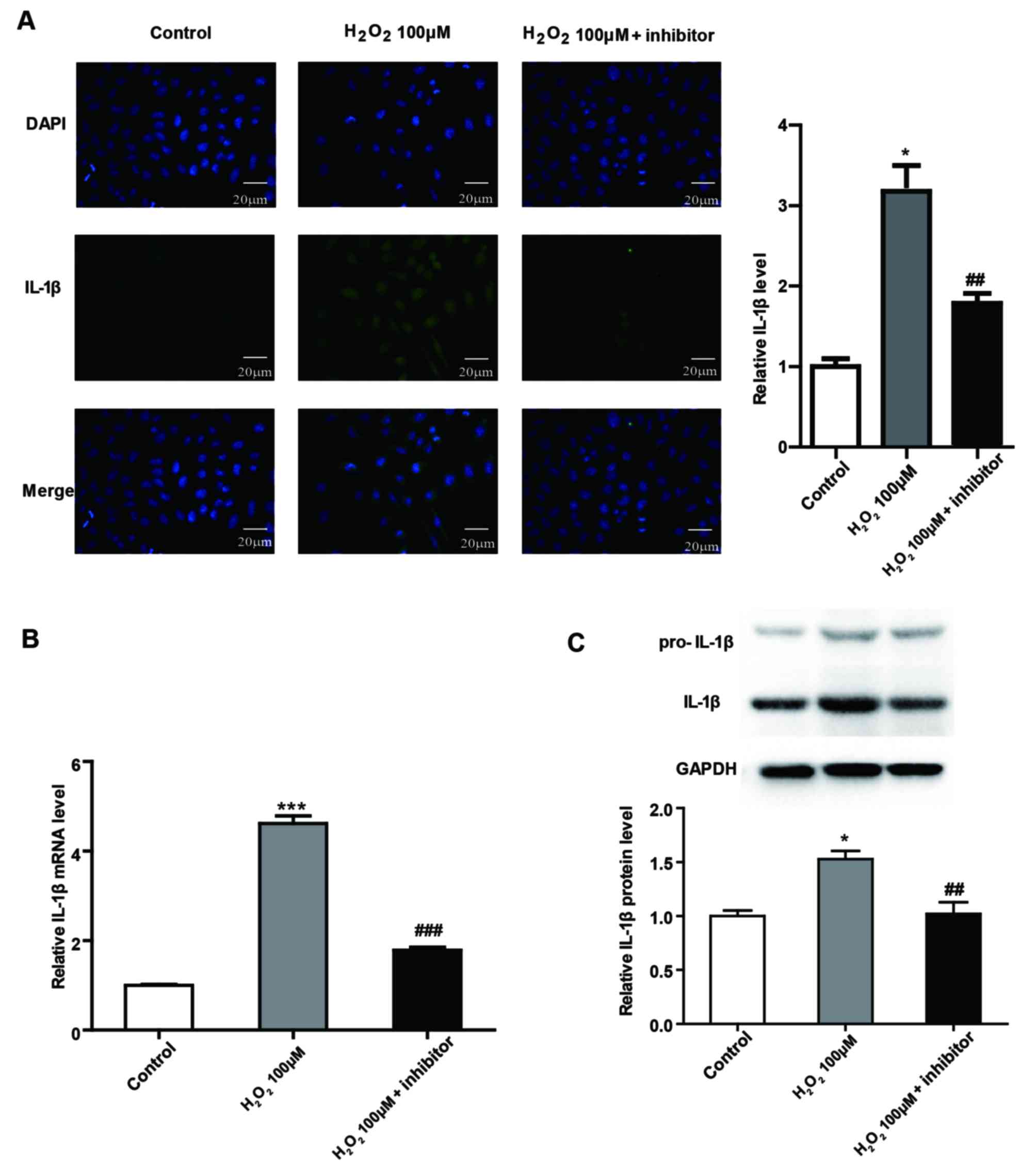
To confirm the role of caspase-1 in pyroptosis in
SRA01/04 cells, caspase-1 was downregulated by an irreversible
inhibitor. Caspase-1 inhibitor was incubated with SRA01/04 cells
and the efficacy was confirmed by immunofluorescence staining,
RT-qPCR and western-blot analysis (Fig. 4A-C). In addition, pyroptosis was
significantly reduced by caspase-1 inhibitor treatment compared
with the 100 µM H2O2-only group, as
demonstrated by TUNEL staining (Fig.
4D). Furthermore, the mRNA and protein expression of IL-1β,
which functions downstream of caspase-1, was significantly
decreased in caspase-1 inhibitor-treated SRA01/04 cells compared
with the 100 µM H2O2-only group (Fig. 5).
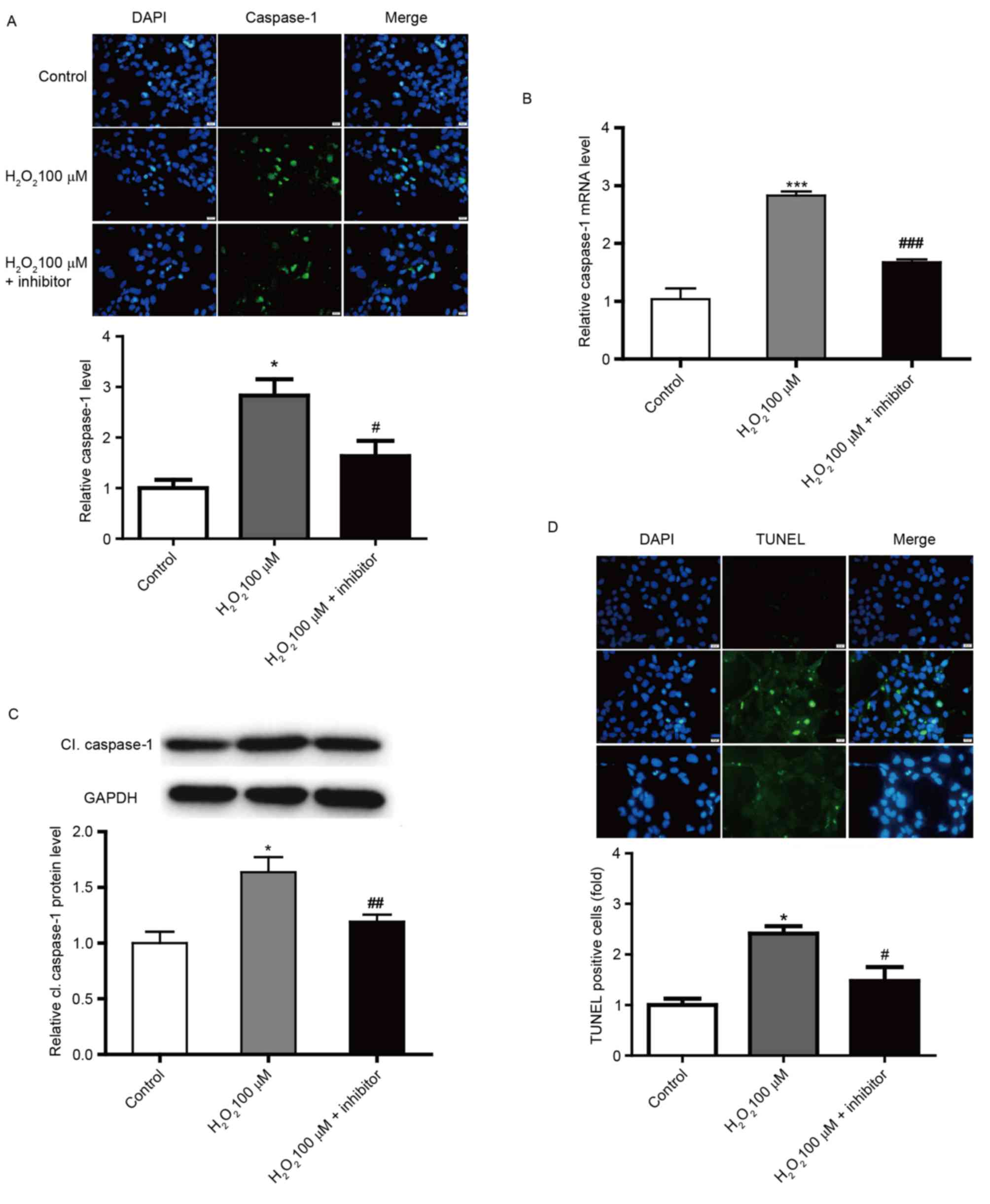 | Figure 4.Downregulation of caspase-1 in human
lens epithelium cells. (A) Immunofluorescence images demonstrating
the expression of caspase-1 in SRA01/04 cells in control, 100 µM
H2O2 and 100 µM H2O2 +
caspase-1 inhibitor groups. Blue, nuclear staining with DAPI;
green, caspase-1 staining. Scale bar, 20 µm. Relative (B) mRNA
levels of caspase-1 and (C) protein levels of cl. caspase-1 in
SRA01/04 cells in control, 100 µM H2O2 and
100 µM H2O2 + caspase-1 inhibitor groups. (D)
TUNEL staining images of SRA01/04 cells in control, 100 µM
H2O2 and 100 µM H2O2 +
caspase-1 inhibitor groups. Blue, nuclear staining with DAPI;
green, TUNEL staining. Scale bar, 20 µm. *P<0.05 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. 100 µM
H2O2-only group. cl., cleaved. |
Discussion
In recent years, increasing studies have focused on
understanding the mechanisms of pyroptosis in various diseases, and
identifying the genes and pathways that are implicated in this
process. The present study reported that pyroptosis may have an
important role in human lens epithelial cells under oxidative
stress. To investigate the effect of pyroptosis in cataract
formation, the present study demonstrated that caspase-1 expression
was increased in lens epithelium cells treated with
H2O2. In addition, the results also confirmed
that caspase-1 was significantly upregulated in cataract lens
anterior capsule samples compared with normal lens anterior capsule
samples. Furthermore, pyroptosis was decreased when caspase-1 was
downregulated by using a caspase-1 inhibitor. Due to the relative
low knockout efficiency of caspase-1 by the inhibitor that was
designed and used in the present study, certain limitations are
associated with our conclusions and further experimental
verification is required.
Lens epithelial cells have a key role in stabilizing
the intracellular environment and maintaining a clear crystalline
lens. Consistent with various other degenerative ocular diseases,
cataract formation occurs when the production rate of reactive
oxygen species (ROS) exceeds the removal rate (19,20).
When lens epithelial cells are exposed to endogenous and exogenous
oxidative stress, including growth factors, UVB radiation and
inflammatory cytokines, they suffer oxidative injury and produce
large amounts of ROS. Activation of ROS generation has been
reported to induce NLR family pyrin domain containing 3/caspase-1
activation, which subsequently triggers IL-1β/IL-18 production and
cell death by pyroptosis and apoptosis, in astroglial cells
(21). H2O2
contains activated oxygen, which permeates cellular membranes and
causes injury inside cells. H2O2 is commonly
used for in vitro cellular oxidative damage models, such as
cataract formation (22,23). The present study employed
H2O2 to investigate the effects of pyroptosis
in cataracts.
Caspase-1 and IL-1β are important markers in the
process of pyroptosis (24). The
caspase family constitutes a family of cysteinyl aspartate
proteases (25). Caspase-1 was the
first caspase to be identified in mammalian cells and has an
important function in apoptosis. Caspase-1 also mediates
proinflammatory programmed cell death, also termed pyroptosis, in
response to exogenous and endogenous stimuli to protect cells.
Caspase-1 is not only activated in immune cells, but is also
activated in epithelial and mesenchymal cells (26), and dysfunction of caspase-1 is
closely associated with various diseases (27,28).
However, whether pyroptosis of lens epithelial cells is implicated
in the initiation and progression of non-congenital cataracts in
humans remains to be established. The results of the current study
demonstrate that caspase-1 expression was increased in
H2O2-treated lens epithelial cells in a
dose-dependent manner, as determined by RT-qPCR and western blot
analysis. These results were also validated in the lens anterior
capsules of cataract patients compared with normal lens anterior
capsule samples. Although the increased levels of caspase-1 may
indicate the involvement of pyroptosis in the process, it is
difficult to distinguish between apoptosis and pyroptosis as
apoptosis has also been reported to be involved in cataract
formation in H2O2-treated lens epithelial
cells (29). Therefore, further
studies are required to address this issue.
Caspase-1 was initially characterized as a protease
that converts the inactive precursors of IL-1β and IL-18
(pro-IL-1β/IL-18) into mature inflammatory cytokines, and was
originally termed interleukin IL-1β-converting enzyme (30). The present study also observed
IL-1β to be downstream of caspase-1 in SRA01/04 cells. When
caspase-1 inhibitor was introduced to 100 µM
H2O2-treated SRA01/04 cells, IL-1β expression
was markedly decreased. The superfamily of IL-1 (IL-18, IL-1β,
pro-IL-18 and pro-IL-1β) are the main proinflammatory cytokines
inside the cell. Activated caspase-1 leads to the activation of
pro-IL-1β, and IL-1β subsequently activates downstream nuclear
factor-κB (NF-κB) signaling, which enhances the release of
inflammatory cytokines (31). It
has been reported that NF-κB signaling is implicated in cataract
formation (32). We hypothesize
that H2O2-induced oxidative stress may
activate NF-κB signaling in human lens epithelial cells via
caspase-1 activation and maturation of IL-1β, which subsequently
contributes to the development of cataracts. Further investigation
is required to confirm this hypothesis. The authors of the present
study plan to explore the association between NF-κB signaling and
pyroptosis by IL-33/sT2 signalling. IL-33 stimulation may include
ERK, p38 MAPK and JNK as well as the activation of NF-κB. It has
been reported that caspase-1 can result in the release of IL-33
(33). It may be possible for
future studies to explore this phenomenon in cataracts.
In conclusion, the results of the current study
provide evidence that pyroptosis participates in the oxidation of
human lens epithelial cells and may be involved in the initiation
and progression of non-congenital cataracts. The results indicate a
potential role of pyroptosis in cataract formation, which enhances
our understanding of cataracts and may provide novel effective
therapeutic methods for this condition.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ analyzed and interpreted the patient data
regarding the cataracts and was a major contributor in writing the
manuscript. HJ and YS performed the histological examination of the
lens anterior capsule samples. HZ and YG were involved in the
design of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All samples were collected with informed consent and
the study was approved by the Research Ethics Committee of Harbin
Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HLECs
|
human lens epithelium cells
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Resnikoff S, Pascolini D, Etya'ale D,
Kocur I, Pararajasegaram R, Pokharel GP and Mariotti SP: Global
data on visual impairment in the year 2002. Bull World Health
Organ. 82:844–851. 2004.PubMed/NCBI
|
|
2
|
Meacock WR, Spalton DJ, Boyce J and
Marshall J: The effect of posterior capsule opacification on visual
function. Invest Ophthalmol Vis Sci. 44:4665–4669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandez V, Fragoso MA, Billotte C, Lamar
P, Orozco MA, Dubovy S, Willcox M and Parel JM: Efficacy of various
drugs in the prevention of posterior capsule opacification:
Experimental study of rabbit eyes. J Cataract Refract Surg.
30:2598–2605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: Mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samali A, Zhivotovsky B, Jones D, Nagata S
and Orrenius S: Apoptosis: Cell death defined by caspase
activation. Cell Death Differ. 6:495–496. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Zhao LM, Dai SL, Cui WX, Lv HL, Chen
L and Shan BE: Periplocin extracted from cortex periplocae induced
apoptosis of gastric cancer cells via the ERK1/2-EGR1 pathway. Cell
Physiol Biochem. 38:1939–1951. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li WC, Kuszak JR, Dunn K, Wang RR, Ma W,
Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, et al: Lens
epithelial cell apoptosis appears to be a common cellular basis for
non-congenital cataract development in humans and animals. J Cell
Biol. 130:169–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan SH, Wang YY, Lu J, Zheng YL, Wu DM, Li
MQ, Hu B, Zhang ZF, Cheng W and Shan Q: Luteoloside suppresses
proliferation and metastasis of hepatocellular carcinoma cells by
inhibition of NLRP3 inflammasome. PLoS One. 9:e899612014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis BK, Wen H and Ting JP: The
inflammasome NLRs in immunity, inflammation, and associated
diseases. Annu Rev Immunol. 29:707–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Wang Z, Wei X, Han H, Meng X,
Zhang Y, Shi W, Li F, Xin T, Pang Q and Yi F: NLRP3 deficiency
ameliorates neurovascular damage in experimental ischemic stroke. J
Cereb Blood Flow Metab. 34:660–667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5:e14792014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu B, Elinav E, Huber S, Booth CJ, Strowig
T, Jin C, Eisenbarth SC and Flavell RA: Inflammation-induced
tumorigenesis in the colon is regulated by caspase-1 and NLRC4.
Proc Natl Acad Sci USA. 107:21635–21640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fann DY, Lee SY, Manzanero S, Tang SC,
Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL,
Thundyil J, et al: Intravenous immunoglobulin suppresses NLRP1 and
NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell
Death Dis. 4:e7902013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Lu Y, Cao Z, Ma Q, Pi H, Fang Y,
Yu Z, Hu H and Zhou Z: Cadmium induces NLRP3 inflammasome-dependent
pyroptosis in vascular endothelial cells. Toxicol Lett. 246:7–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Celkova L, Doyle SL and Campbell M: NLRP3
inflammasome and pathobiology in AMD. J Clin Med. 4:172–192. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Isai M, Sakthivel M, Ramesh E, Thomas PA
and Geraldine P: Prevention of selenite-induced cataractogenesis by
rutin in Wistar rats. Mol Vis. 15:2570–2577. 2009.PubMed/NCBI
|
|
17
|
Mok JW, Chang DJ and Joo CK: Antiapoptotic
effects of anthocyanin from the seed coat of black soybean against
oxidative damage of human lens epithelial cell induced by H2O2.
Curr Eye Res. 39:1090–1098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paz ML, Maglio Gonzalez DH, Weill FS,
Bustamante J and Leoni J: Mitochondrial dysfunction and cellular
stress progression after ultraviolet B irradiation in human
keratinocytes. Photodermatol Photoimmunol Photomed. 24:115–122.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Babizhayev MA: Mitochondria induce
oxidative stress, generation of reactive oxygen species and redox
state unbalance of the eye lens leading to human cataract
formation: Disruption of redox lens organization by phospholipid
hydroperoxides as a common basis for cataract disease. Cell Biochem
Funct. 29:183–206. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alfonso-Loeches S, Ureña-Peralta JR,
Morillo-Bargues MJ, Oliver-De La Cruz J and Guerri C: Role of
mitochondria ROS generation in ethanol-induced NLRP3 inflammasome
activation and cell death in astroglial cells. Front Cell Neurosci.
8:2162014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z
and Liu P: Resveratrol protects human lens epithelial cells against
H2O2-induced oxidative stress by increasing catalase, SOD-1, and
HO-1 expression. Mol Vis. 16:1467–1674. 2010.PubMed/NCBI
|
|
23
|
Ma T, Chen T, Li P, Ye Z, Zhai W, Jia L,
Chen W, Sun A, Huang Y, Wei S and Li Z: Heme oxygenase-1 (HO-1)
protects human lens epithelial cells (SRA01/04) against hydrogen
peroxide (H2O2)-induced oxidative stress and apoptosis. Exp Eye
Res. 146:318–329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin X, Jin H, Shi Y, Guo Y and Zhang H:
Long non-coding RNA KCNQ1OT1 promotes cataractogenesis via miR-214
and activation of the caspase-1 pathway. Cell Physiol Biochem.
42:295–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brydges SD, Broderick L, McGeough MD, Pena
CA, Mueller JL and Hoffman HM: Divergence of IL-1, IL-18, and cell
death in NLRP3 inflammasomopathies. J Clin Invest. 123:4695–4705.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yazdi AS, Drexler SK and Tschopp J: The
role of the inflammasome in nonmyeloid cells. J Clin Immunol.
30:623–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Zhao Y, Zhang P, Li Y, Yang Y,
Yang Y, Zhu J, Song X, Jiang G and Fan J: Hemorrhagic shock primes
for lung vascular endothelial cell pyroptosis: Role in pulmonary
inflammation following LPS. Cell Death Dis. 7:e23632016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zorman J, Sušjan P and Hafner-Bratkovič I:
Shikonin suppresses NLRP3 and AIM2 inflammasomes by direct
inhibition of caspase-1. PLoS One. 11:e01598262016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai J, Yang F, Dong L and Zheng Y: Ghrelin
protects human lens epithelial cells against oxidative
Stress-induced damage. Oxid Med Cell Longev. 2017:19104502017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fantuzzi G and Dinarello CA:
Interleukin-18 and interleukin-1 beta: Two cytokine substrates for
ICE (caspase-1). J Clin Immunol. 19:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, He F, Meng Q, Sun Y, Wang W and
Wang C: Inhibiting HIF-1α decreases expression of TNF-α and
caspase-3 in specific brain regions exposed kainic acid-induced
status epilepticus. Cell Physiol Biochem. 38:75–82. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia Z, Song Z, Zhao Y, Wang X and Liu P:
Grape seed proanthocyanidin extract protects human lens epithelial
cells from oxidative stress via reducing NF-κB and MAPK protein
expression. Mol Vis. 17:210–217. 2011.PubMed/NCBI
|
|
33
|
Kakkar R and Lee RT: The IL-33/ST2
pathway: Therapeutic target and novel biomarker. Nat Rev Drug
Discov. 7:827–840. 2008. View Article : Google Scholar : PubMed/NCBI
|