Introduction
Human endothelial progenitor cells (EPCs) serve an
important role in angiogenesis and endothelial repair (1,2).
However, it is difficult to obtain an adequate number of EPCs for
transplantation from one single donation. The function of EPCs is
impaired in the presence of various cardiovascular disease risk
factors, including age, hypertension and diabetes (3,4).
Therefore, increasing the number of EPCs obtained from donations
and improving their function is critical for EPC transplantation
optimization.
In addition to direct differentiation into mature
endothelial cells to repair endothelial injury, EPCs are capable of
secreting protective paracrine factors to accelerate repair, which
is another critical mechanism of EPC transplantation (5). There is increasing evidence to
indicate that EPCs secrete various paracrine factors, including
stromal cell-derived factor (SDF)-1, vascular endothelial growth
factor (VEGF) and hepatocyte growth factor (HGF), to improve
endothelial cell function and repair various types of wounds in
different animal models (6).
EPC-conditioned medium (EPC-CM) promotes angiogenesis in rat artery
loops, inhibits apoptosis in endothelial cells and increases the
capillary density of an ischemic limb (7). Kim et al (8) reported that an injection of EPC-CM
alone improves the recovery of diabetic wounds, and even requires a
smaller number of cells compared with EPC transplantation.
Thymosin β4 (Tβ4) is a small protein widely
distributed in numerous cells and tissues, which mediates multiple
biological reactions, including vessel formation and wound healing
(9). Previous studies indicated
that Tβ4 promotes EPC proliferation, migration and adhesion, and
inhibits apoptosis and senescence in vitro (10–12).
Since these actions may all contribute to angiogenesis, there has
been an increased interest in evaluating the role of Tβ4 in EPC
angiogenesis.
VEGF is an important paracrine factor secreted by
progenitor cells to promote angiogenesis. Barcelos et al
(13) reported that prominin 1
(CD133)+ progenitor cells and the cell-conditioned
medium (CCM) of CD133+ cells contain high levels of
VEGF-A and interleukin (IL)-8, and application of these cells or
CD133+ CCM accelerates the healing process of diabetic
ulcers in the lower limbs of rats. In addition, neutralizing
antibodies against VEGF-A or IL-8 inhibit the healing effect of
CD133+ CCM (13). Wnt
family member 7A expression is increased in CD133+
cell-treated wounds compared with either CD133− cell- or
collagen-treated wounds. Secreted frizzled-related proteins are Wnt
antagonists, and their application abolishes the facilitation of
wound closure and reparative angiogenesis by CD133+ CCM
(13). These findings indicate
that the promotion of angiogenesis is primarily mediated by
paracrine factors secreted by progenitor cells. However, whether
Tβ4 stimulates EPCs to secrete an increased amount of VEGF to
improve endothelial function requires further investigation. In the
present study, it was examined whether pre-treatment of Tβ4 was
able to augment the volume of VEGF mRNA and secretion of serum VEGF
in EPC-CM. Additionally, the present study aimed to determine
whether the increased amount of VEGF was able to further promote
the angiogenesis of endothelial cells, which may improve the
efficacy of therapeutic EPC transplantation.
Materials and methods
Cell culture
EPCs were isolated, cultured and characterized
according to previously described techniques (10–12).
The present study was approved by the Ethics Committee of Sir Run
Run Shaw Hospital of Zheijang University (Hangzhou, China) and
written informed consent was obtained from 30 healthy individuals
(20–55 years old; 50:50 male:female). The date range of the
recruitment was from September 2014 to December 2014. All the
samples were collected in the biomedical research center of Sir Run
Run Shaw Hospital. To obtain EPCs, mononuclear cells were isolated
from human peripheral blood via density-gradient centrifugation
(400 × g; 35 min; room temperature) with Ficoll separating solution
(Cedarlane Laboratories, Burlington, ON, Canada). The mononuclear
cells were subsequently placed on fibronectin (Merck KGaA,
Darmstadt, Germany)-coated plates and incubated with endothelial
growth medium-2 (EGM-2MV; Lonza Group, Ltd., Basel, Switzerland).
After 7 days of incubation at 37°C and 5% CO2,
106 of cells grew into EPCs, which exhibited a spindle
shape. The EPCs were detached using trypsin and subsequently
collected for further experiments. Human umbilical vein endothelial
cells (HUVECs) were obtained from ScienCell Research Laboratories,
Inc. (San Diego, CA, USA) and cultured in endothelial cell medium
supplemented with 10% fetal bovine serum (both ScienCell Research
Laboratories, Inc.). For experimental treatments, cells (passages
3–8) were grown to 70–90% confluence.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from EPCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). cDNA synthesis was performed with 1 µg
total RNA using a PrimeScriptTM RT Master Mix (Takara Biotechnology
Co., Ltd., Dalian, China), according to the manufacturer's
protocol. The reaction mixture was incubated under the following
condition: RT, 37°C for 15 min; and inactivation of RT with heat
treatment, 85°C for 5 sec. RT-qPCR was conducted using the
SsoFastTM EvaGreen Supermix with Low ROX (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The reaction mixture was incubated under
the following thermocycling condition: Polymerase activation, 95°C
for 30 sec; denaturation, 95°C for 5 sec; annealing/extension, 55°C
for 30 sec; melt curve analysis, 65–95°C in 0.5°C increments for 2
sec. Data analysis was performed with an ABI 7500 cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the
2−ΔΔCq method, as previously described (14). GAPDH was used as the endogenous
control for mRNA expression. The primers used were as follows:
GAPDH forward, 5′-GGGTGTGAACCATGAGAAGT-3′ and reverse,
5′-GACTGTGGTCATGAGTCCT-3′; VEGF forward, 5′-TCTTGGGTGCATTGGAGCCT-3′
and reverse, 5′-AGCTCATCTCTCCTATGTGC-3′; and IL-8 forward,
5′-CCTGATTTCTGCAGCTCTGT-3′ and reverse,
5′-AACTTCTCCACAACCCTCTG-3.
Small interfering (si)RNA
transfection
RAC-α serine/threonine-protein kinase (Akt) and
endothelial nitric oxide synthase (eNOS) siRNA, in addition to the
scramble sequence negative control siRNA, were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). When 60–80%
confluence was reached, cells were transfected with 150 pmol Akt
siRNA, eNOS siRNA or negative control siRNA with HiPerFect
Transfection Reagent (Qiagen GmbH, Hilden, Germany) incubated for
24 h (15–25°C). The effectiveness of Akt and eNOS knockdown was
confirmed with western blotting. The Akt siRNA sequence was as
follows: Forward, 5′-GCACUUUCGGCAAGGUGAUTT-3′ and reverse,
5′-AUCACCUUGCCGAAAGUGCTT-3′. The eNOS siRNA sequence was as
follows: Forward, 5′-CAGUACUACAGCUCCAUUATT-3′ and reverse,
5′-UAAUGGAGCUGUAGUACUGTT-3. The scramble siRNA sequence was as
follows: Forward, 5′-GACCCAAAUUUGACAUGAUTT-3′ and reverse, 5′- AUC
AUG UCA AAU UUG GGU CTT-3′.
ELISA
A density of 4×105 transfected EPCs were
seeded in 6-well plates and treated with 0, 10, 100 and 1,000 ng/ml
Tβ4 (ProSpec-Tany TechnoGene, Ltd., East Brunswick, NJ, USA) for
37°C 24 h. Conditioned media were subsequently collected for the
VEGF ELISA (cat. no. DVE00; Abcam, Cambridge, UK).
EPC tube formation assay
The tube formation assay was performed using a
Matrigel matrix™ basement membrane (BD Biosciences, Franklin Lakes,
NJ, USA) to investigate HUVEC angiogenesis under treatment with
different types of EPC-CM (8).
Matrigel solution was thawed at 4°C overnight and subsequently
diluted with EBM-2 (Clonetics™; Lonza Group, Ltd.,
Basel, Switzerland) to solidify in a 96-well plate at 37°C for 1 h.
A total of 2×104 HUVECs were seeded on the matrix for
further incubation with either 2 ml EPC-CM, Tβ4-EPC-CM or
Tβ4-EPC-CM with 0.4 µg/ml VEGF antibody (cat. no. MAB293; R&D
Systems, Inc., Minneapolis, MN, USA) at 37°C for 6 h. The CCM was
the supernatant of different group of EPCs obtained following
centrifugation at 150 × g for 5 min. Tube formation was assessed
using a light microscope (magnification, ×100) and five random
fields were selected for each assay. The average branch point
numbers that indicated tube formation were compared using Image Pro
Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Experimental animal model
Animal procedures were conducted in accordance with
the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MD, USA; 8th Edition, 2011) and
were approved by the Institutional Animal Care and Use Committee of
Zhejiang University (Hangzhou, China). The rat myocardial
infarction (MI) model was established as previously described
(10). A total of 40 male
Sprague-Dawley rats (8 weeks; weighing 200–250 g) were purchased
from the Experimental Animal Center of Zhejiang University
(Hangzhou, China). Experimental rats were anesthetized and
intubated for artificial ventilation. A left thoracotomy was
subsequently performed. The left anterior descending coronary
artery (LAD) was ligated with a 6-0 suture with the fourth
intercostal space open. Sham-operated (sham) rats received surgery
without LAD ligation. Rats in the EPC group (EPCs) were
intramyocardially injected with 1×106 human EPCs
re-suspended in 150 µl EGM-2, while rats in the Tβ4-EPC group
(Tβ4-EPCs) received the same amount of Tβ4 pretreated human EPCs
(EPCs were pretreated with 1,000 ng/ml Tβ4 for 24 h). Rats
receiving 150 µl blank EGM-2 without cells were set as the control
group. A total of three sites in the border zone of the infarcted
heart received three injections. The rats were sacrificed 4 weeks
following this procedure.
Immunohistochemical staining
Immunohistochemical analysis of VEGF (1:100; cat.
no. ab46154; Abcam) staining was performed, in which 5 µm fresh
frozen sections (−20°C) of rat cardiac tissue were incubated with
the primary antibody at 37°C for 2 h. The process was followed by
incubation with specific horseradish peroxidase (HRP)-conjugated
secondary antibodies at 37°C for 10 min (1:100; cat. no. SPN-9001;
OriGene Technologies, Inc., Beijing, China) and DAB at 37°C for 2
h. Sections were rinsed in PBS and subsequently counterstained with
0.5% hematoxylin (cat. no. ZLI-9608; OriGene Technologies, Inc.) at
room temperature for 1 min, dehydrated and mounted. A total of five
random fields were selected for assessment, and the area of
myocardial fibrosis and the expression of VEGF were quantified
using Image-Pro Plus 6.0.
Western blot analysis
Rat cardiac tissue was harvested for western blot
analysis. The protein was extracted using radioimmunoprecipitation
buffer (Thermo Fisher Scientific, Inc.) and protein concentration
was determined with a bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology, Haimen, China). A total of 50 µg
protein sample was loaded per lane on a 10% Tris-glycine gradient
gel, and subsequently transferred onto polyvinylidene difluoride
membranes and blocked with 5% non-fat milk for 1 h at room
temperature. Following this, membranes were incubated with the
following primary antibodies (1:1,000) overnight at 4°C: VEGF (cat.
no. Ab46154; Abcam), eNOS [cat. no. 32027; Cell Signaling
Technology (CST), Inc., Danvers, MA, USA], Akt (cat. no. 2920; CST,
Inc.) and GAPDH (cat. no. 5174; CST, Inc.). The membrane was
subsequently incubated with goat anti-rabbit IgG-HRP secondary
antibodies (1:5,000; cat. no. 7074; CST, Inc.) at room temperature
for h. Proteins were visualized with enhanced chemiluminescence
reagent (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
and Image Quant LAS-4000 (GE Healthcare Life Sciences, Shanghai,
China). The grayscale values of the bands were examined using Image
Multi-Gauge software 2.0 (Fujifilm, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of least three independent experiments. Statistical
analyses between groups were performed using SPSS software version
19.0 (IBM Corp., Armonk, NY, USA) using one-way analysis of
variance with Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Tβ4 increases the secretion of VEGF
from human EPCs
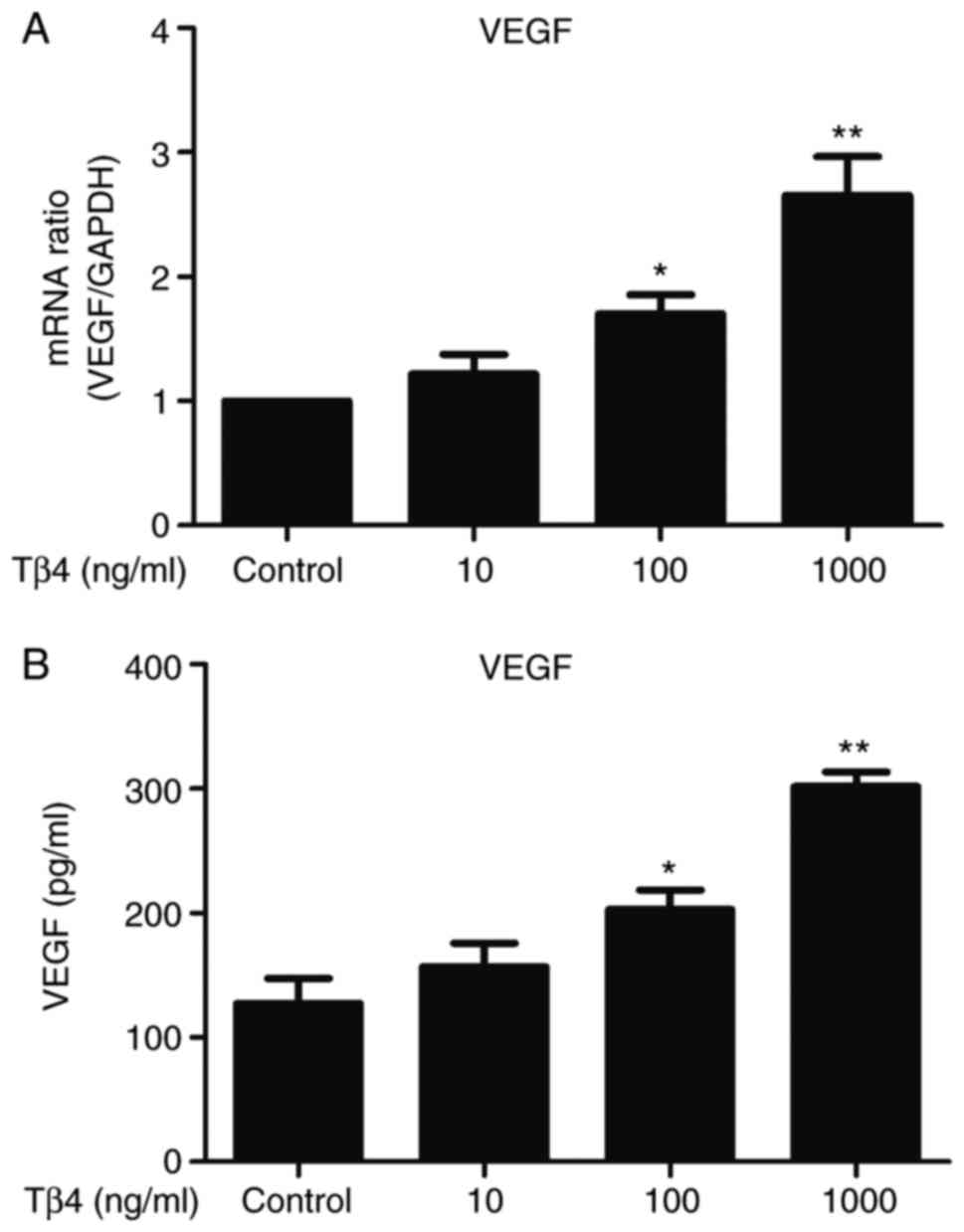
The present study investigated the effect of Tβ4 on
the expression of VEGF in EPCs. Treatment with Tβ4 significantly
increased the expression of VEGF in EPCs at the transcriptional
(Fig. 1A) and protein (Fig. 1B) levels. This pro-secretory effect
was dose-dependent, the highest VEGF mRNA/protein concentrations
were observed at 1,000 ng/ml Tβ4 for 24 h. At the transcriptional
and protein levels, the fold changes in expression were 2.65 and
2.54-fold, respectively, compared with the control group.
VEGF is a critical mediator of
Tβ4-enhanced angiogenesis
The present study investigated the effect of EPC-CM
on the angiogenesis of HUVECs in vitro by performing a tube
formation assay (Fig. 2A). It was
demonstrated that EPC-CM enhanced the angiogenesis of HUVECs
compared with the control group, and pretreatment with 1,000 ng/ml
Tβ4 further augmented the effect of EPC-CM. Furthermore, the
presence of VEGF antibody reversed the pro-angiogenic effect of
Tβ4-pretreated EPC-CM (Fig.
2B).
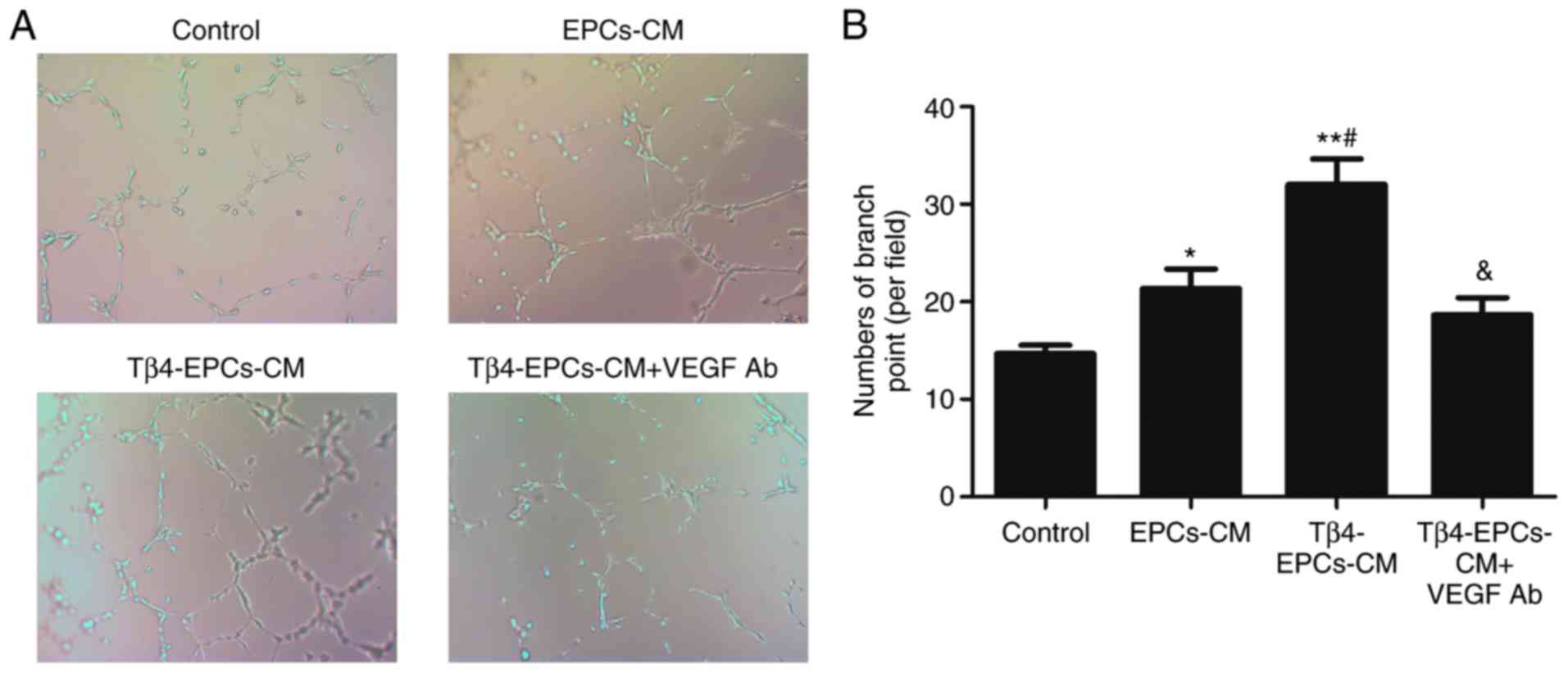 | Figure 2.VEGF is a critical mediator of
Tβ4-enhanced angiogenesis. (A) EPCs-CM, Tβ4-EPCs-CM and Tβ4-EPCs-CM
with VEGF Ab was added to test the angiogenic ability of HUVECs
in vitro at the concentration of 1,000 ng/ml for 24 h.
Compared with the control group, EPCs-CM enhanced the angiogenic
effect. This effect was further promoted in the 1,000 ng/ml
Tβ4-EPC-CM-treated group. VEGF Ab reversed the pro-angiogenic
effect of Tβ4-EPC-CM. (B) Angiogenesis was quantified by
determining the number of branch points in five random fields of
view. Magnification, ×100. n=6. *P<0.05, **P<0.01 vs. control
group. #P<0.05 vs. EPCs-CM treated group.
&P<0.05 vs. Tβ4-EPC-CM group. EPCs, endothelial
progenitor cells; Tβ4, thymosin β4; VEGF, vascular endothelial
growth factor; CM, conditioned medium; Ab, antibody. |
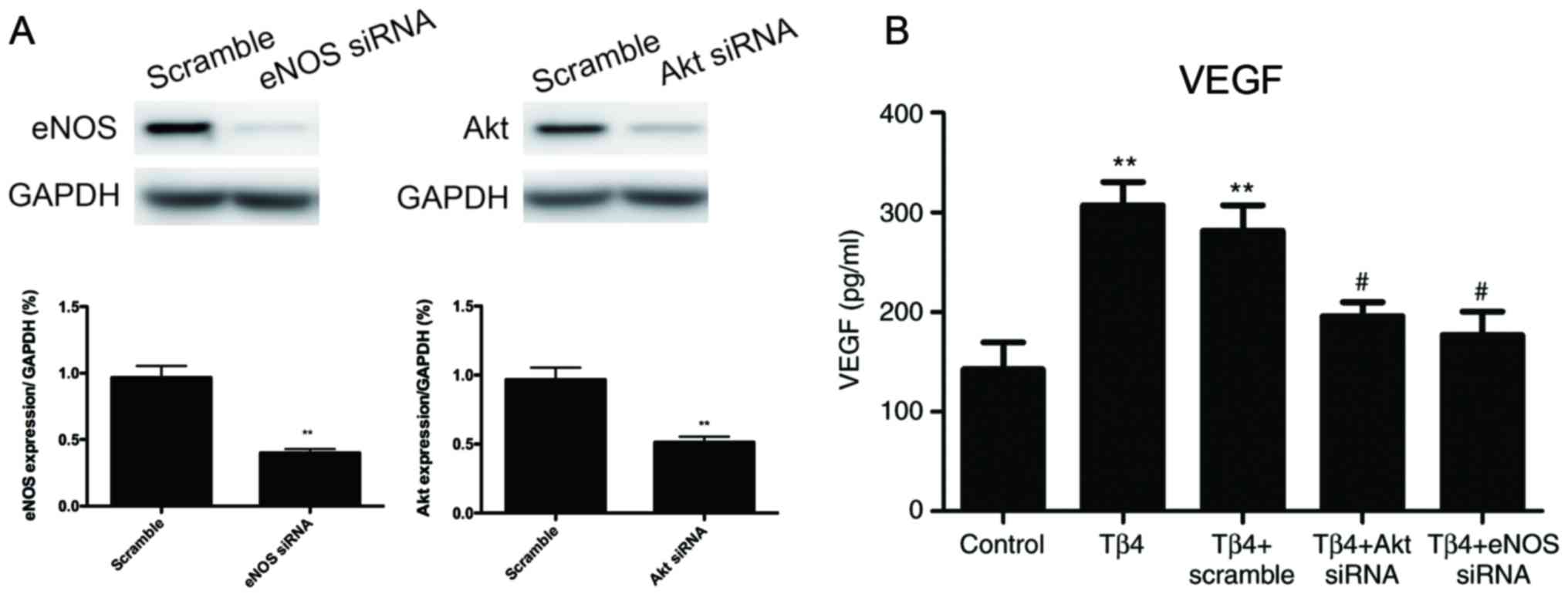
Akt/eNOS pathway signaling is involved
in Tβ4-induced VEGF secretion
To further investigate whether the Akt/eNOS pathway
was involved in Tβ4-induced VEGF secretion in EPCs, Akt and eNOS
siRNAs were used to knock down the expression of Akt and eNOS.
Western blot analysis was performed to confirm that Akt and eNOS
expression was effectively reduced compared with the scramble
control (Fig. 3A). In addition,
the levels of VEGF in EPC-CM were significantly decreased following
knockdown of Akt or eNOS, which indicated that the Akt/eNOS pathway
was involved in Tβ4-induced VEGF secretion (Fig. 3B).
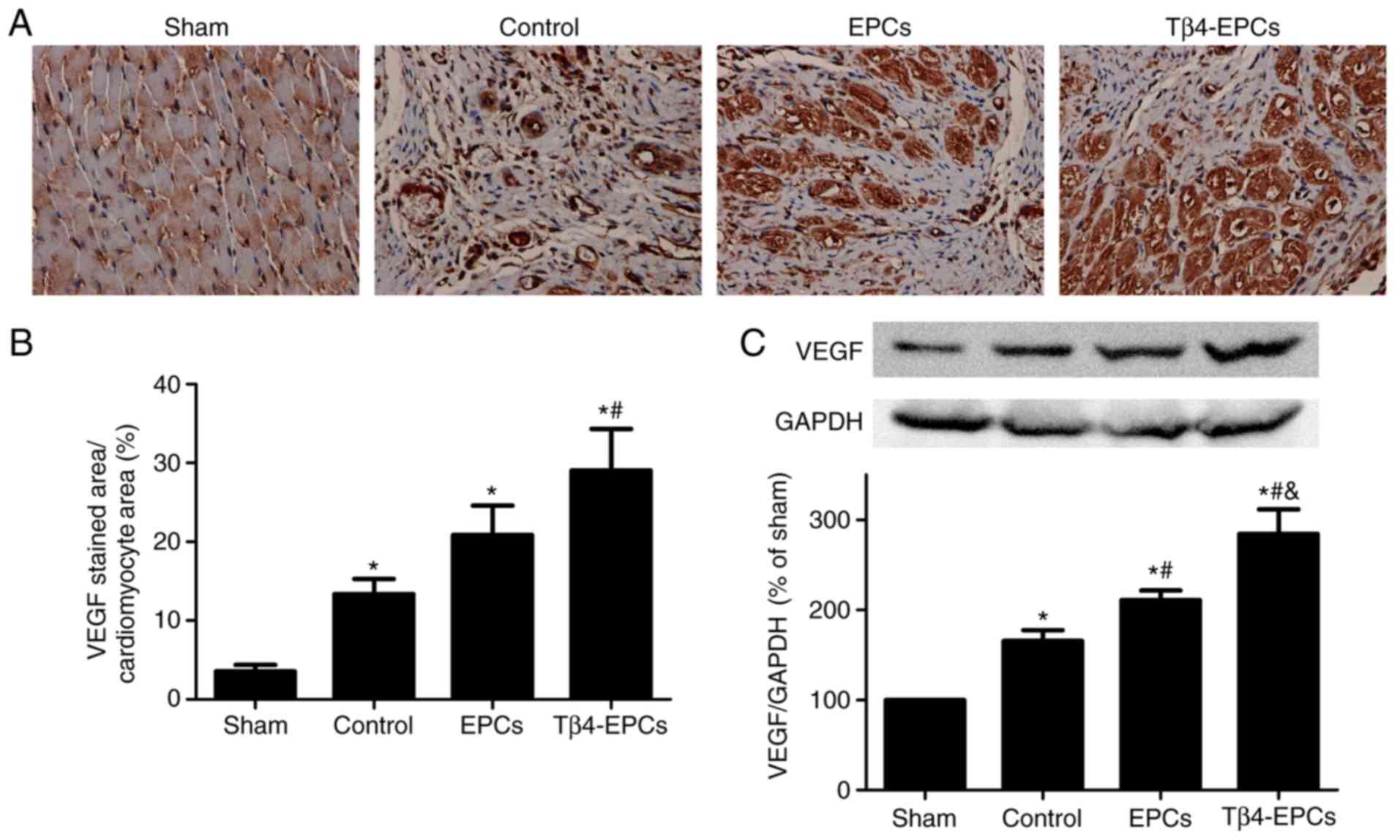
Tβ4-pretreated EPC transplantation
significantly augments VEGF expression in the border zone of
infarcted hearts
Immunohistochemical analysis demonstrated that VEGF
expression in cardiac tissue was significantly augmented at 4 weeks
post-MI compared with the sham group. The expression of VEGF was
significantly higher in the EPC transplantation alone group
compared with the untreated control group, and it was significantly
higher in the Tβ4-EPC-transplanted MI hearts at 4 weeks post-MI
compared with the control and EPC groups (Fig. 4A and B). Western blot analysis
indicated that VEGF expression was significantly augmented at 4
weeks post-MI. VEGF expression in the EPCs and Tβ4-EPCs groups
increased significantly compared with the control group, and the
expression of VEGF in the Tβ4-EPCs group was significantly higher
compared with the EPCs group (Fig.
4C).
Discussion
Angiogenesis is essential for the survival and
functional maintenance of the ischemic myocardium. Numerous known
methods of vessel formation have been investigated in normal tissue
and tumors, including the recruitment of EPCs that differentiate to
endothelial cells, intussusception and sprouting angiogenesis
(15). In the present study, it
was reported that Tβ4 promoted EPC angiogenesis by increasing the
secretion of VEGF, indicating that paracrine functions served an
important role in the regulation of angiogenesis. The Akt/eNOS
pathway was demonstrated to be involved in the regulation of VEGF
expression induced by Tβ4. Furthermore, in vivo, VEGF was
highly expressed near the border zone of infarcted hearts following
transplantation with Tβ4 pretreated EPCs, suggesting that the
ischemic myocardium may have benefited from angiogenesis mediated
by VEGF expression. Taken together, the experiments of the present
study revealed that Tβ4-pretreated EPCs promoted angiogenesis
through activation of the Akt/eNOS pathway, and may provide a novel
strategy for the effective transplantation of EPCs in MI.
Research has previously been conducted to determine
the way in which EPCs home to ischemic areas and become involved in
vasculogenesis, thereby increasing the blood flow to these tissues
and preserving cardiac function (16,17).
By using TEK tyrosine-protein kinase receptor (Tie-2)/LacZ
b-galactosidase (β-gal) transgenic mice, Ii et al (18) observed that EPCs, from donor mice
expressing β-gal driven by the endothelial gene promoter Tie-2, are
incorporated into the microvasculature following myocardial
ischemia (18). This provides
direct evidence that EPCs contribute to neovascularization.
Furthermore, EPCs activate angiogenesis through indirect
mechanisms, including through the secretion of proangiogenic
factors, including VEGF, HGF and SDF-1 (5). The present study demonstrated the
ability of EPCs to induce the formation of neovasculature, and the
critical role of VEGF in EPC-mediated angiogenesis in infarcted rat
hearts, supporting the in vitro findings.
A number of studies have reported that two types of
EPCs (early and late) may be isolated from peripheral blood
(19–21). The tube formation abilities of
these two cell types are different. Early EPCs with a spindle shape
secrete more angiogenic cytokines, including VEGF, compared with
late EPCs, which have a cobblestone shape. However, late EPCs form
capillary tubes more efficiently compared with early EPCs. Previous
studies demonstrated that following treatment with Tβ4, late EPCs
exhibit increased migration (11),
and reduced senescence (12) and
apoptosis (10). Considering these
findings, the present study used late EPCs to assess angiogenesis
under treatment with Tβ4. It was demonstrated that the Akt/eNOS
pathway is a critical component in regulating the signaling of
multiple biological processes (22,23).
Through the activation of this pathway, the migration and
anti-apoptotic ability of late EPCs increased, and finally promoted
angiogenesis to aid ischemic myocardial survival.
Tβ4 is required in the development of the coronary
vasculature. Knocking down Tβ4 expression in the developing heart
results in congenital coronary artery anomalies, demonstrating its
pivotal role in vasculogenesis (24). By using embryonic EPCs, Kupatt
et al (25) revealed that
Tβ4, prothymosin α and Tβ10 are among the most abundantly secreted
factors of early EPCs, suggesting that Tβ4 may be involved in
EPC-regulated angiogenesis. The present study provided direct
evidence that Tβ4 promoted EPC-mediated angiogenesis by increasing
the secretion of VEGF. In the border zone of the infarcted hearts,
VEGF expression was highly induced following Tβ4-pretreated EPC
transplantation. However, the mechanism underlying Tβ4-induced
angiogenesis is not well defined. The upstream kinases targeting
Akt include the phosphoinositide 3-kinase (PI3K), phosphorylated
(p)-phosphatase and tensin homolog, p-pyruvate dehydrogenase kinase
1 and the p-serine/threonine-protein kinase mTOR pathways (26). Our previous studies demonstrated
that Tβ4 predominantly activates PI3K as a principal upstream
component of the Akt pathway (11,12).
By using siRNAs to target Akt and eNOS, the present study
demonstrated that the AKT/eNOS pathway was involved in Tβ4-mediated
VEGF expression. Notably, Tβ4 and VEGF are upregulated following
the induction of Akt overexpression in bone marrow-derived
mesenchymal stem cells (27). This
suggests that a positive feedback loop may exist between the Akt
pathway and Tβ4. In addition, hypoxia-inducible factor (HIF)-1α
protein stability is directly increased by the expression of VEGF
induced by Tβ4 (28). It is
therefore plausible that under ischemic conditions, Tβ4 induces the
expression of VEGF in a HIF-1α-dependent manner.
The secretion of VEGF contributes to endothelial
cell differentiation (29).
However, the role of VEGF in arterial and venous specification
remains unclear and further study is required.
In conclusion, the results of the present study
demonstrated that the angiogenesis of EPCs was strongly enhanced
following treatment with Tβ4. The Akt/eNOS pathway mediated VEGF
expression, which was important for this process. The present study
demonstrated a novel way to improve EPC function and may be
critical for the optimization of EPC transplantation.
Acknowledgements
The authors would like to thank the Biomedical
Research (Therapy) Center, Sir Run Run Shaw Hospital, School of
Medicine, Zhejiang University (Hangzhou, China) for the use of
equipment.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81200114
and 81570246).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ contributed to the study conception and design.
JS acquired, analyzed and interpreted the data. XB was involved in
drafting the manuscript and interpreting the data. JG and ZS
revised the article critically for important intellectual content
and interpreted the data. JZ and GF gave the final approval of the
version to be published and contributed to the study conception.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Sir Run Run Shaw Hospital of Zheijang University
(Hangzhou, China) and written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C,
Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, et al: Evidence
for circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
2
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Kawamoto A and Masuda H:
Concise review: Circulating endothelial progenitor cells for
vascular medicine. Stem Cells. 29:1650–1655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urbich C, Aicher A, Heeschen C, Dernbach
E, Hofmann WK, Zeiher AM and Dimmeler S: Soluble factors released
by endothelial progenitor cells promote migration of endothelial
cells and cardiac resident progenitor cells. J Mol Cell Cardiol.
39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Santo S, Yang Z, von Ballmoos Wyler M,
Voelzmann J, Diehm N, Baumgartner I and Kalka C: Novel cell-free
strategy for therapeutic angiogenesis: In vitro generated
conditioned medium can replace progenitor cell transplantation.
PLos One. 4:e56432009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Song SH, Kim KL, Ko JJ, Im JE, Yie
SW, Ahn YK, Kim DK and Suh W: Human cord blood-derived endothelial
progenitor cells and their conditioned media exhibit therapeutic
equivalence for diabetic wound healing. Cell Transplant.
19:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huff T, Müller CS, Otto AM, Netzker R and
Hannappel E: beta-Thymosins, small acidic peptides with multiple
functions. Int J Biochem Cell Biol. 33:205–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Qiu F, Xu S, Yu L and Fu G:
Thymosin beta4 activates integrin-linked kinase and decreases
endothelial progenitor cells apoptosis under serum deprivation. J
Cell Physiol. 226:2798–2806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu FY, Song XX, Zheng H, Zhao YB and Fu
GS: Thymosin beta4 induces endothelial progenitor cell migration
via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc
Pharmacol. 53:209–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Yu L, Zhao Y, Fu G and Zhou B:
Thymosin beta4 reduces senescence of endothelial progenitor cells
via the PI3K/Akt/eNOS signal transduction pathway. Mol Med Rep.
7:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barcelos LS, Duplaa C, Krankel N, Graiani
G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica
M, et al: Human CD133+ progenitor cells promote the healing of
diabetic ischemic ulcers by paracrine stimulation of angiogenesis
and activation of Wnt signaling. Circ Res. 104:1095–1102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kocher AA, Schuster MD, Szabolcs MJ,
Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM and Itescu S:
Neovascularization of ischemic myocardium by human
bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis,
reduces remodeling and improves cardiac function. Nat Med.
7:430–436. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalka C, Tehrani H, Laudenberg B, Vale PR,
Isner JM, Asahara T and Symes JF: VEGF gene transfer mobilizes
endothelial progenitor cells in patients with inoperable coronary
disease. Ann Thorac Surg. 70:829–834. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ii M, Nishimura H, Iwakura A, Wecker A,
Eaton E, Asahara T and Losordo DW: Endothelial progenitor cells are
rapidly recruited to myocardium and mediate protective effect of
ischemic preconditioning via ‘imported’ nitric oxide synthase
activity. Circulation. 111:1114–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ,
Hwang KK, Oh BH, Lee MM and Park YB: Characterization of two types
of endothelial progenitor cells and their different contributions
to neovasculogenesis. Arterioscler Thromb Vasc Biol. 24:288–293.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tagawa S, Nakanishi C, Mori M, Yoshimuta
T, Yoshida S, Shimojima M, Yokawa J, Kawashiri MA, Yamagishi M and
Hayashi K: Determination of early and late endothelial progenitor
cells in peripheral circulation and their clinical association with
coronary artery disease. Int J Vasc Med. 2015:6742132015.PubMed/NCBI
|
|
21
|
Cheng CC, Chang SJ, Chueh YN, Huang TS,
Huang PH, Cheng SM, Tsai TN, Chen JW and Wang HW: Distinct
angiogenesis roles and surface markers of early and late
endothelial progenitor cells revealed by functional group analyses.
BMC Genomics. 14:1822013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Zhu W, Zhang P, Chen K, Zhao L, Li
J, Wei M and Liu M: Apelin-13 stimulates angiogenesis by promoting
crosstalk between amp-activated protein kinase and akt signaling in
myocardial microvascular endothelial cells. Mol Med Rep.
9:1590–1596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohashi K, Enomoto T, Joki Y, Shibata R,
Ogura Y, Kataoka Y, Shimizu Y, Kambara T, Uemura Y and Yuasa D:
Neuron-derived neurotrophic factor functions as a novel modulator
that enhances endothelial cell function and revascularization
processes. J Biol Chem. 289:14132–14144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Kodolitsch Y, Ito WD, Franzen O, Lund
GK, Koschyk DH and Meinertz T: Coronary artery anomalies. Part I:
Recent insights from molecular embryology. Z Kardiol. 93:929–937.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kupatt C, Horstkotte J, Vlastos GA,
Pfosser A, Lebherz C, Semisch M, Thalgott M, Büttner K, Browarzyk
C, Mages J, et al: Embryonic endothelial progenitor cells
expressing a broad range of proangiogenic and remodeling factors
enhance vascularization and tissue recovery in acute and chronic
ischemia. FASEB J. 19:1576–1578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reyes-Gordillo K, Shah R, Popratiloff A,
Fu S, Hindle A, Brody F and Rojkind M: Thymosin-beta4 (Tbeta4)
blunts PDGF-dependent phosphorylation and binding of AKT to actin
in hepatic stellate cells. Am J Pathol. 178:2100–2108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gnecchi M, He H, Noiseux N, Liang OD,
Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS and Dzau
VJ: Evidence supporting paracrine hypothesis for Akt-modified
mesenchymal stem cell-mediated cardiac protection and functional
improvement. FASEB J. 20:661–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jo JO, Kim SR, Bae MK, Kang YJ, Ock MS,
Kleinman HK and Cha HJ: Thymosin beta4 induces the expression of
vascular endothelial growth factor (VEGF) in a hypoxia-inducible
factor (HIF)-1alpha-dependent manner. Biochim Biophys Acta.
1803:1244–1251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carmeliet P and Tessier-Lavigne M: Common
mechanisms of nerve and blood vessel wiring. Nature. 436:193–200.
2005. View Article : Google Scholar : PubMed/NCBI
|