Introduction
Cervical cancer ranks as the third most common
cancer and the fourth leading cause of cancer-associated mortality
among females worldwide (1).
Cervical cancer cases in developing countries account for >80%
of all cases of cervical cancer, given the absence of widespread
screening in these countries (2).
Approximately 58,000 cervical cancer cases are diagnosed and
>20,000 cervical cancer-associated mortalities are reported in
China each year (3). Surgical
resection combined with radiotherapy and chemotherapy remains the
major therapeutic strategy for cervical cancer treatment (4). Despite remarkable advances in
cervical cancer diagnosis and therapies, the prognosis of patients
at the advanced stage remains unsatisfactory, with a five-year
survival rate of <40% (5).
Recurrence, as well as local and distant metastases, are the
predominant reasons for poor patient prognosis (6). Therefore, a deeper understanding of
the molecular mechanisms associated with cervical cancer
development and progression is critical to develop novel and
effective therapeutic strategies for this malignancy.
MicroRNAs (miRNAs/miRs) are a large group of
evolutionarily conserved, non-coding, single-stranded RNAs, ~19–24
nucleotides in length (7). miRNAs
regulate gene expression through translational suppression and/or
mRNA degradation by completely or partially binding to the
3′-untranslated region (3′-UTR) of their target genes (8). A total of >1,000 mature human
miRNAs have been identified, and these may regulate the expression
of at least one-third of protein-coding genes in the human genome
(9). A growing number of miRNAs
have been identified to be dysregulated in numerous types of human
cancer, including cervical (10),
prostate (11), gastric (12) and lung cancer (13). The dysregulation of miRNAs is
involved in tumor onset and development by affecting various
biological processes, including the cell cycle, proliferation,
apoptosis, differentiation, metastasis and angiogenesis (14–16).
Aberrantly expressed miRNAs in human cancer may serve as either
tumor suppressors or promoters by downregulating the expression of
oncogenes or tumor suppressor genes, respectively (17). Therefore, miRNAs may be potential
therapeutic targets in cancer treatment strategies.
miR-485 is aberrantly expressed in several types of
human cancer, including glioblastoma (18,19),
gastric cancer (20) and lung
adenocarcinoma (21). However, to
the best of the authors' knowledge, the expression pattern,
biological functions and underlying mechanisms of miR-485 in
cervical cancer have not yet been investigated. Therefore, the
present study aimed to detect the expression of miR-485 in cervical
cancer tissues and cell lines. In addition, the clinical
significance of miR-485 in cervical cancer was evaluated, and the
effects of miR-485 overexpression on cancer cell proliferation and
invasion were analyzed. Furthermore, the molecular mechanisms
underlying the actions of miR-485 in cervical cancer were
investigated. The results of the present study provided further
insight into the molecular mechanisms underlying the pathogenesis
of cervical cancer and validated the potential of miR-485 as a
novel target in cervical cancer treatment.
Materials and methods
Clinical tissues and cell lines
A total of 49 paired cervical cancer and
corresponding adjacent non-tumorous tissues were collected from
patients (aged 46–71 years old) diagnosed with cervical cancer and
treated via surgical resection at Weifang People's Hospital
(Weifang, China) between May 2014 and October 2016. No patients had
received radiotherapy, chemotherapy or other treatments prior to
surgery. All tissue specimens were rapidly frozen in liquid
nitrogen and stored in a −80°C super-cold refrigerator until RNA
extraction. The present research was approved by the Ethics
Committee of Weifang People's Hospital. All participants provided
written informed consent.
A total of four human cervical cancer cell lines
were purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China): HeLa, SiHa, C33A and CaSki. The normal
human cervical epithelial cell line Ect1/E6E7 was obtained from the
American Type Culture Collection (Manassas, VA, USA). All cell
lines were maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 mg/ml
penicillin and 100 mg/ml streptomycin (all purchased from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), and were
cultured at 37°C in an incubator with 5% CO2 and 95%
air.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied to extract the total RNA from in
vitro cells and in vivo tissue specimens, according to
the manufacturer's protocol. For the analysis of miR-485
expression, a TaqMan miRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to synthesize
cDNA. The temperature protocol for reverse transcription was as
follows: 16°C for 30 min, 42°C for 30 min and 85°C for 5 min.
Following this, qPCR was performed using a TaqMan miRNA PCR kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions for qPCR were as follows: 50°C for 2 min
and 95°C for 10 min; followed by 40 cycles of denaturation at 95°C
for 15 sec; and annealing/extension at 60°C for 60 sec.
For the detection of metastasis associated in colon
cancer-1 (MACC1) mRNA expression, total RNA was converted into cDNA
using Moloney murine leukemia virus reverse transcriptase
(Fermentas; Thermo Fisher Scientific, Inc.), followed by qPCR with
SYBR Premix Ex Taq™ (Takara Biotechnology Co., Ltd., Dalian,
China). The reaction for reverse transcription included 1 µl Oligo
(dT)12-18 (500 µg/ml), 1 µl dNTP Mix (10 mM), 4 µl 5X First-Strand
Buffer, 2 µl DTT (0.1 M), 1 µl RNaseOUT™ Recombinant Ribonuclease
Inhibitor (40 units/µl), 1 µl Moloney murine leukemia virus reverse
transcriptase, 1 µg total RNA and distilled water. The temperature
protocol used for reverse transcription was as follows: 65°C for 5
min, 37°C for 2 min, 37°C for 50 min and 70°C for 15 min. The
cycling conditions for qPCR were as follows: 5 min at 95°C;
followed by 40 cycles of 95°C for 30 sec and 65°C for 45 sec. The
expression levels of miR-485 and MACC1 mRNA were normalized to the
expression level of U6 small nuclear RNA and GAPDH, respectively.
The primers were designed as follows: miR-485 forward,
5′-CCAAGCTTCACCCATTCCTAACAGGAC-3′ and reverse,
5′-CGGGATCCGTAGGTCAGTTACATGCATC-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; MACC1 forward,
5′-CACAACTTGCGGAGGTCAC-3′ and reverse, 5′-AAGCTGTGGGGTTTTTCC-3′;
and GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. Relative gene expression was
quantified by the 2−ΔΔCq method (22).
Oligonucleotide and plasmid
transfection
miR-485 mimics and corresponding negative control
miRNA (miR-NC) were acquired from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). MACC1 overexpression plasmid (pcDNA3.1-MACC1)
and empty pcDNA3.1 plasmid were synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). Cells were plated into 6-well plates
at a density of 6×105 cells/well and cultured at 37°C in
DMEM containing 10% FBS, 100 mg/ml penicillin and 100 mg/ml
streptomycin. Once cells had grown to 70% confluence, cells were
transfected with miR-485 mimics (100 pmol), miR-NC (100 pmol),
pcDNA3.1 (4 µg) or pcDNA3.1-MACC1 plasmids (4 µg) using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Cell Counting
kit-8 (CCK-8) and Transwell invasion assays were performed 24 h
post-transfection.
Cell Counting kit-8 (CCK-8) assay
Cell proliferation was evaluated with a CCK-8 assay.
Transfected cells were collected at 24 h post-transfection, a
single cell suspension was prepared and seeded into 96-well plates
at a density of 3×103 cells/well. Following incubation
at 37°C for 0, 24, 48 and 72 h, the CCK-8 assay was performed, in
accordance with the manufacturer's instructions. Briefly, 10 µl
CCK-8 solution (Dojindo Molecular Technologies, Inc., Shanghai,
China) was added into each well. The plates were subsequently
incubated at 37°C with 5% CO2 for a further 2 h. Optical
density was detected at a wavelength of 450 nm with a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transwell invasion assay
Transwell chambers (pore size, 8 µm) coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) were used to
determine cell invasion capacity. A total of 1×105
transfected cells were suspended in FBS-free DMEM and plated into
the upper Transwell chambers. The lower Transwell chambers were
filled with 500 µl DMEM, containing 20% FBS as a chemoattractant.
After 24 h incubation at 37°C with 5% CO2, the cells
remaining on the upper surfaces of the Transwell chambers were
carefully removed with cotton swabs. Invasive cells were fixed with
4% paraformaldehyde at room temperature for 15 min and stained with
0.05% crystal violet solution at room temperature for 15 min. The
number of invasive cells was counted under an inverted microscope
(magnification, ×200; IX31; Olympus Corporation, Tokyo, Japan) with
five randomly selected fields for each chamber.
Bioinformatics prediction
The following online miRNA target prediction
algorithms were utilized to predict the potential targets of
miR-485: TargetScan (release 7.1; www.targetscan.org/vert_71/) and miRanda (2010
release; 34.236.212.39/microrna/home.do).
Luciferase reporter assay
The 3′-UTR of MACC1 containing the putative
wild-type (Wt) miR-485 binding sites or mutant (Mut) miR-485
binding sites was synthesized (Shanghai GenePharma Co., Ltd.) and
cloned into the pGL3-control vector (Ambion; Thermo Fisher
Scientific, Inc.), and designated as pGL3-MACC1-3′-UTR Wt or
pGL3-MACC1-3′-UTR Mut, respectively. Cells were plated into 24-well
plates at a density of 1.5×105 cells/well. Subsequently,
cells were co-transfected with miR-485 mimics or miR-NC and
pGL3-MACC1-3′-UTR Wt or pGL3-MACC1-3′-UTR Mut, using Lipofectamine
2000 according to the manufacturer's instructions. Cells were
harvested and subjected to luciferase activity detection using a
dual-luciferase reporter assay system (Promega Corporation,
Madison, WI, USA) 48 h post-transfection, according to the
manufacturer's protocol. Firefly luciferase activity was normalized
to Renilla luciferase activity.
Western blot analysis
Total protein was extracted from in vitro
cells and in vivo tissue specimens with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), and protein concentration was
determined using a bicinchoninic acid protein assay kit. Equal
amounts of protein (30 µg) were separated through 10% SDS-PAGE and
transferred to polyvinylidene fluoride membranes (Beyotime
Institute of Biotechnology). Membranes were blocked at room
temperature for 1 h with 5% fat-free milk dissolved in TBS
containing 0.1% Tween-20 (TBST). Following this, the membranes were
incubated overnight at 4°C with the following primary antibodies:
Anti-human polyclonal MACC1 (cat. no. ab106579; 1:1,000 dilution;
Abcam, Cambridge, UK), rabbit anti-human monoclonal MET
proto-oncogene, receptor tyrosine kinase (Met; cat. no. ab51067;
1:1,000 dilution; Abcam), mouse anti-human monoclonal RAC-α
serine/threonine-protein kinase (AKT; cat. no. sc-81434; 1:1,000
dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse
anti-human monoclonal phosphorylated (p)-AKT (cat. no. sc-271966;
1:1,000 dilution; Santa Cruz Biotechnology, Inc.) and mouse
anti-human monoclonal GAPDH (cat. no. ab9484; 1:1,000 dilution;
Abcam). GAPDH served as a loading control. Subsequently, the
membranes were washed with TBST and incubated with the
corresponding horseradish peroxidase-conjugated secondary antibody
(cat. no. sc-2005; 1:5,000 dilution; Santa Cruz Biotechnology,
Inc.) at room temperature for 2 h. Finally, enhanced
chemiluminescence reagents (Bio-Rad Laboratories, Inc.) were added
to visualize the protein signals. Protein expression was quantified
using Quantity One software (version 4.62; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation from three independent experiments. Data were analyzed
using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The
independent two-tailed Student's t-test and one-way analysis of
variance (ANOVA) were utilized to compare the differences between
groups. Student-Newman-Keuls test was used as the post-hoc test
following ANOVA. The association between miR-485 and the
clinicopathological characteristics of patients with cervical
cancer was assessed and determined using the χ2 test.
Spearman's correlation analysis was performed to assess the
correlation between miR-485 and MACC1 expression. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-485 is underexpressed in cervical
cancer tissues and cell lines
To elucidate the expression pattern of miR-485 in
cervical cancer, its expression was initially detected in 49 paired
cervical cancer and corresponding adjacent non-tumor tissues.
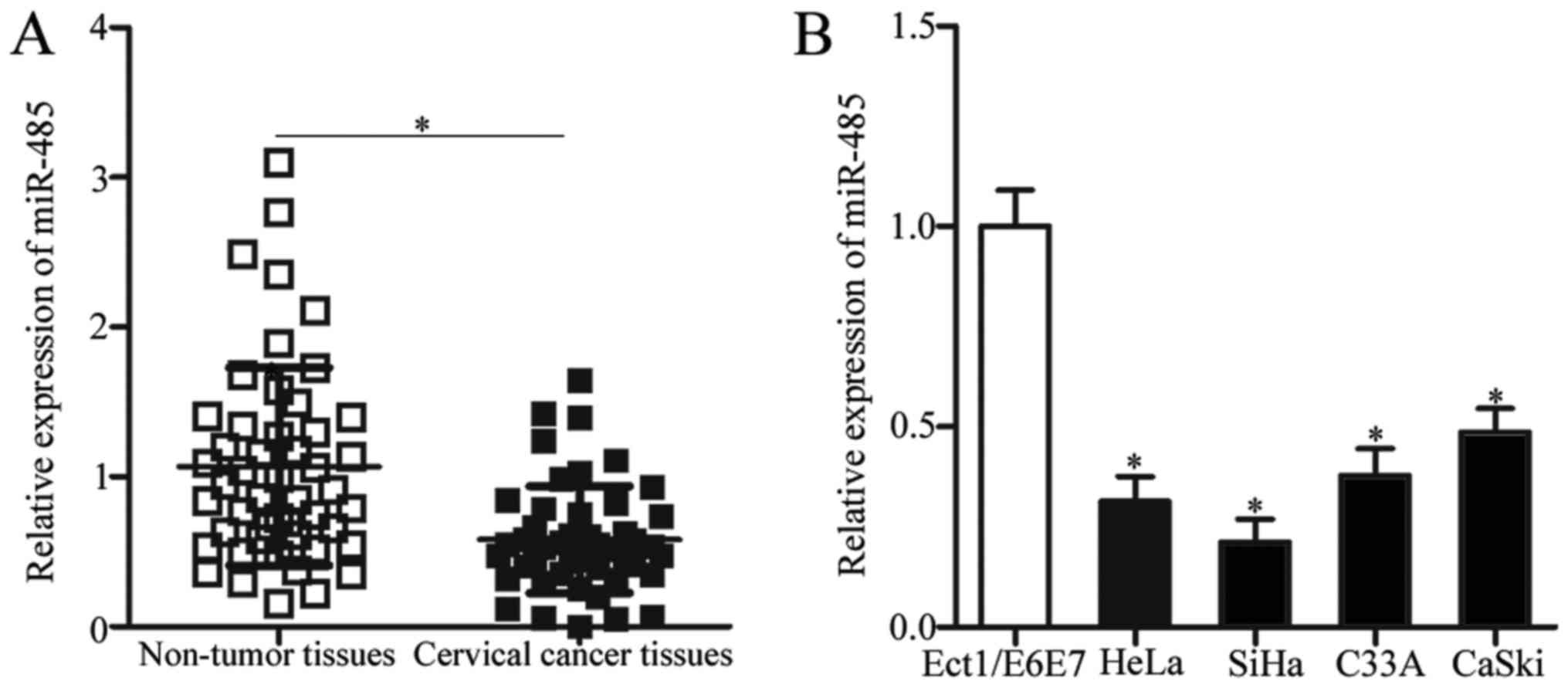
RT-qPCR analysis revealed that miR-485 expression in cervical
cancer tissues was significantly decreased compared with adjacent
non-tumor tissues (Fig. 1A;
P<0.05). To investigate the clinical significance of miR-485
expression in cervical cancer, all recruited patients with cervical
cancer were divided into either the low miR-485 expression group
(n=25) or the high miR-485 expression group (n=24) on the basis of
the median cut-off level (0.514) of miR-485 expression. Low miR-485
expression was associated with International Federation of
Gynecology and Obstetrics (FIGO) stage (P=0.001) and lymph node
metastasis (P=0.015). However, miR-485 expression and other
clinicopathological characteristics, including age, tumor size and
family history of cancer, were not significantly associated
(P>0.05; Table I). Furthermore,
miR-485 expression was compared in four human cervical cancer cell
lines and Ect1/E6E7, a normal human cervical epithelial cell line.
Compared with Ect1/E6E7, miR-485 expression was significantly
downregulated in all cervical cancer cell lines (Fig. 1B; P<0.05). These results
suggested that miR-485 is underexpressed in cervical cancer and may
be associated with cervical cancer progression.
 | Table I.Association between miR-485
expression and the clinicopathological characteristics of patients
with cervical cancer. |
Table I.
Association between miR-485
expression and the clinicopathological characteristics of patients
with cervical cancer.
|
|
| miR-485
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.376 |
|
<50 | 13 | 8 | 5 |
|
|
≥50 | 36 | 17 | 19 |
|
| Tumor size, cm |
|
|
| 0.308 |
|
<4 | 27 | 12 | 15 |
|
| ≥4 | 22 | 13 | 9 |
|
| Family history of
cancer |
|
|
| 0.444 |
| No | 30 | 14 | 16 |
|
|
Yes | 19 | 11 | 8 |
|
| FIGO stage |
|
|
| 0.001a |
|
I–II | 23 | 6 | 17 |
|
|
III–IV | 26 | 19 | 7 |
|
| Lymph node
metastasis |
|
|
| 0.015a |
|
Negative | 24 | 8 | 16 |
|
|
Positive | 25 | 17 | 8 |
|
miR-485 inhibits the proliferation and
invasion of cervical cancer cells
To identify the biological functions of miR-485 in
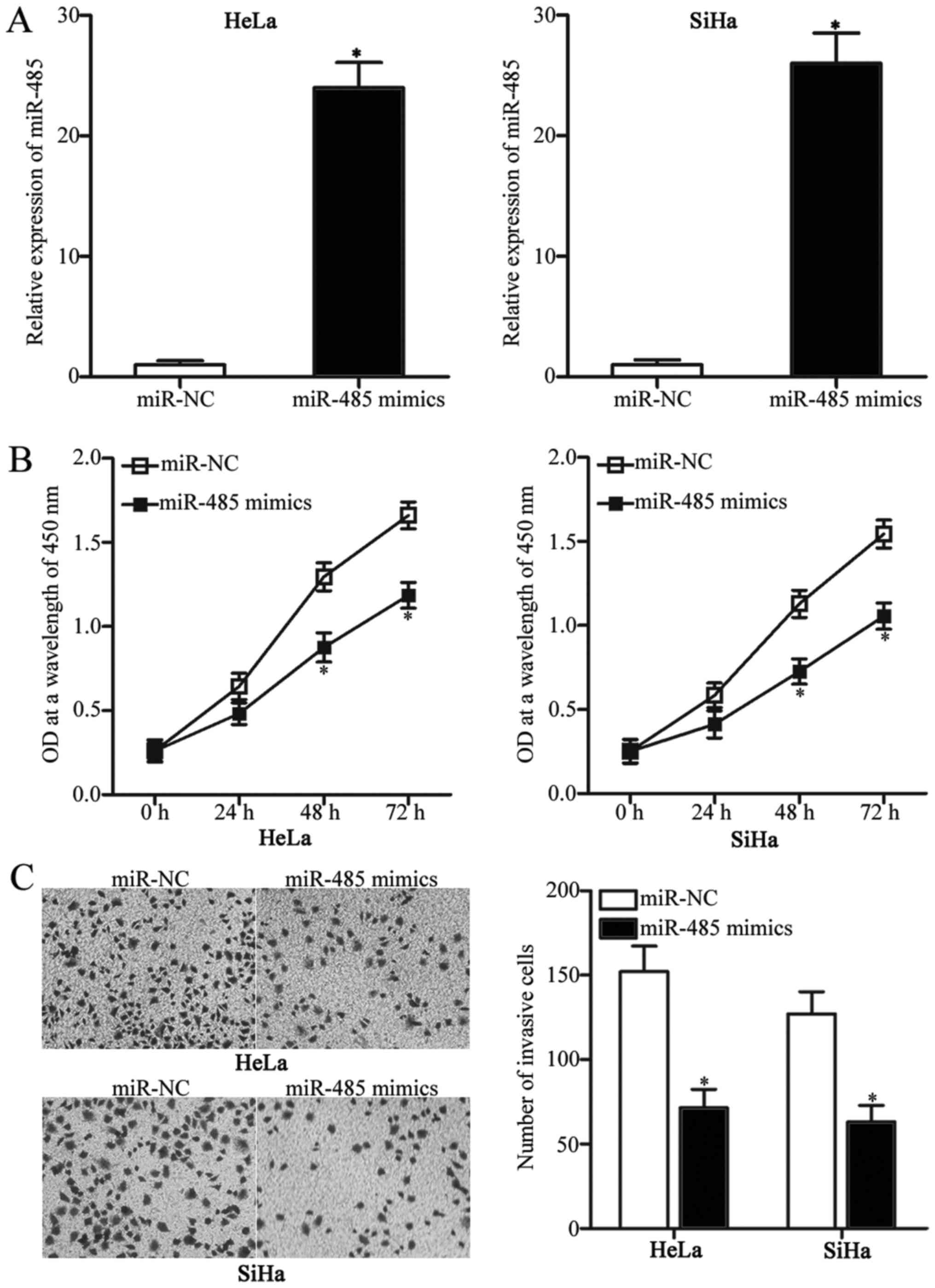
cervical cancer, miR-485 mimics were transfected into HeLa and SiHa
cells, which exhibited relatively low miR-485 expression among the
four cervical cancer cell lines. At 48 h post-transfection, miR-485
was significantly overexpressed in HeLa and SiHa cells transfected
with miR-485 mimics compared with cells transfected with miR-NC
(Fig. 2A; P<0.05). CCK-8 and
Transwell invasion assays were subsequently performed to
investigate the effects of miR-485 overexpression on cervical
cancer cell proliferation and invasion, respectively. The results
revealed that restored expression of miR-485 reduced the
proliferation (Fig. 2B; P<0.05)
and invasion (Fig. 2C; P<0.05)
of HeLa and SiHa cells. These results suggested that miR-485 may
have an inhibitory role in cervical cancer progression.
miR-485 directly targets MACC1 in
cervical cancer
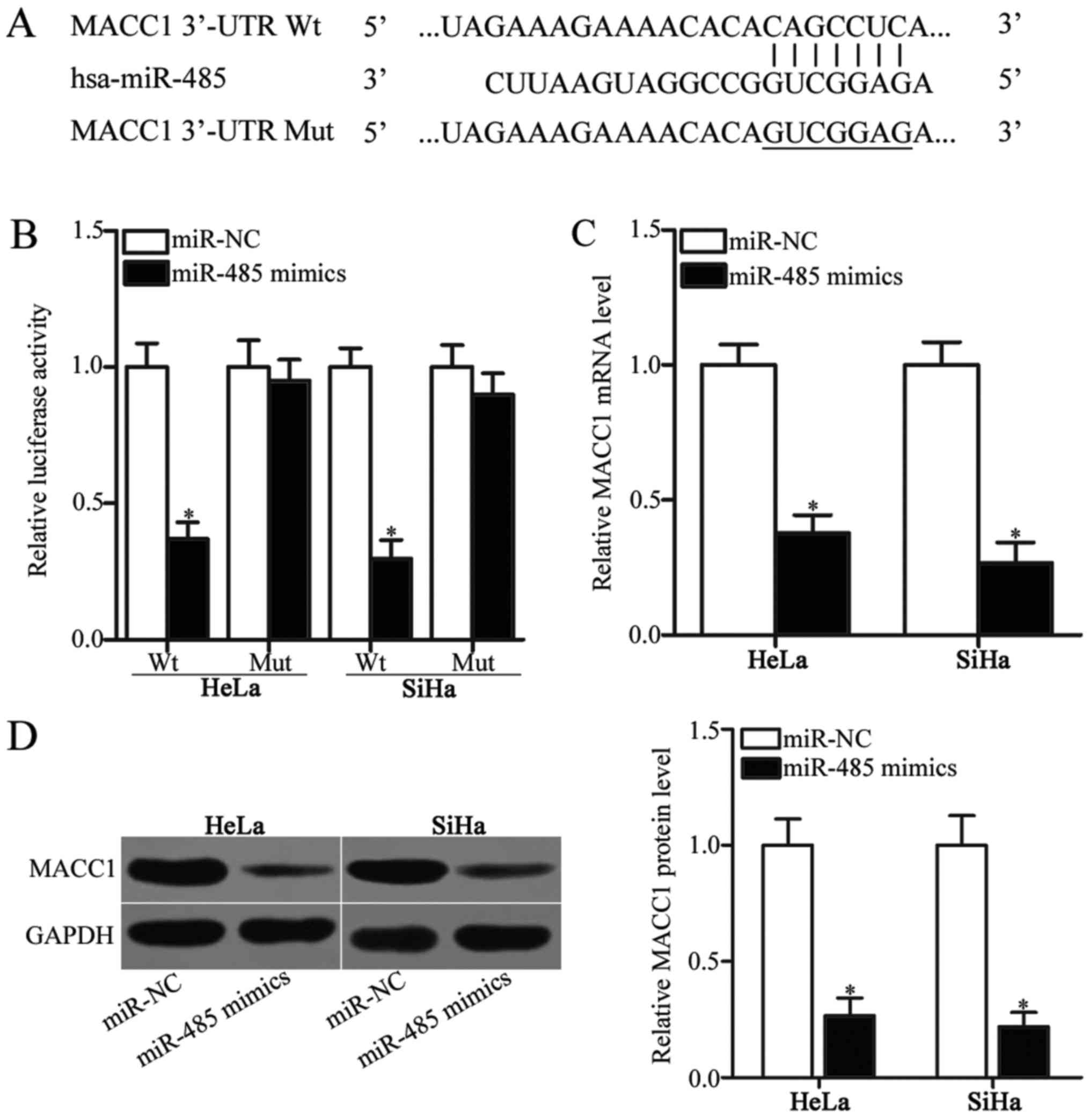
miRNAs regulate cellular processes by interacting
with the 3′-UTR sites of their target genes (8). Therefore, bioinformatics analysis was
conducted in order to identify the target genes of miR-485. MACC1
(Fig. 3A) was predicted as a
candidate target of miR-485 and was selected as the focus of the
present study, as it has been implicated in the occurrence and
development of cervical cancer (23–27).
To confirm this hypothesis, a luciferase reporter assay was
performed to confirm the binding of miR-485 to the 3′-UTR of MACC1.
HeLa and SiHa cells were transfected with miR-485 mimics or miR-NC
along with pGL3-MACC1-3-UTR Wt or pGL3-MACC1-3′-UTR Mut. As
presented in Fig. 3B, the
upregulation of miR-485 expression reduced the luciferase activity
of pGL3-MACC1-3′-UTR Wt (P<0.05). However, altering miR-485
expression did not affect the luciferase activity of
pGL3-MACC1-3′-UTR Mut in HeLa and SiHa cells (Fig. 3B). To further examine
miR-485-mediated MACC1 expression in cervical cancer, RT-qPCR and
western blot analysis was performed to detect MACC1 mRNA and
protein expression in HeLa and SiHa cells transfected with miR-485
mimics or miR-NC. The restored expression of miR-485 significantly
decreased the expression of MACC1 in HeLa and SiHa cells at the
mRNA (Fig. 3C; P<0.05) and
protein (Fig. 3D; P<0.05)
levels compared with the miR-NC-transfected cells. These results
suggested that MACC1 was a direct target gene of miR-485 in
cervical cancer.
MACC1 upregulation is inversely
correlated with miR-485 expression in cervical cancer tissues
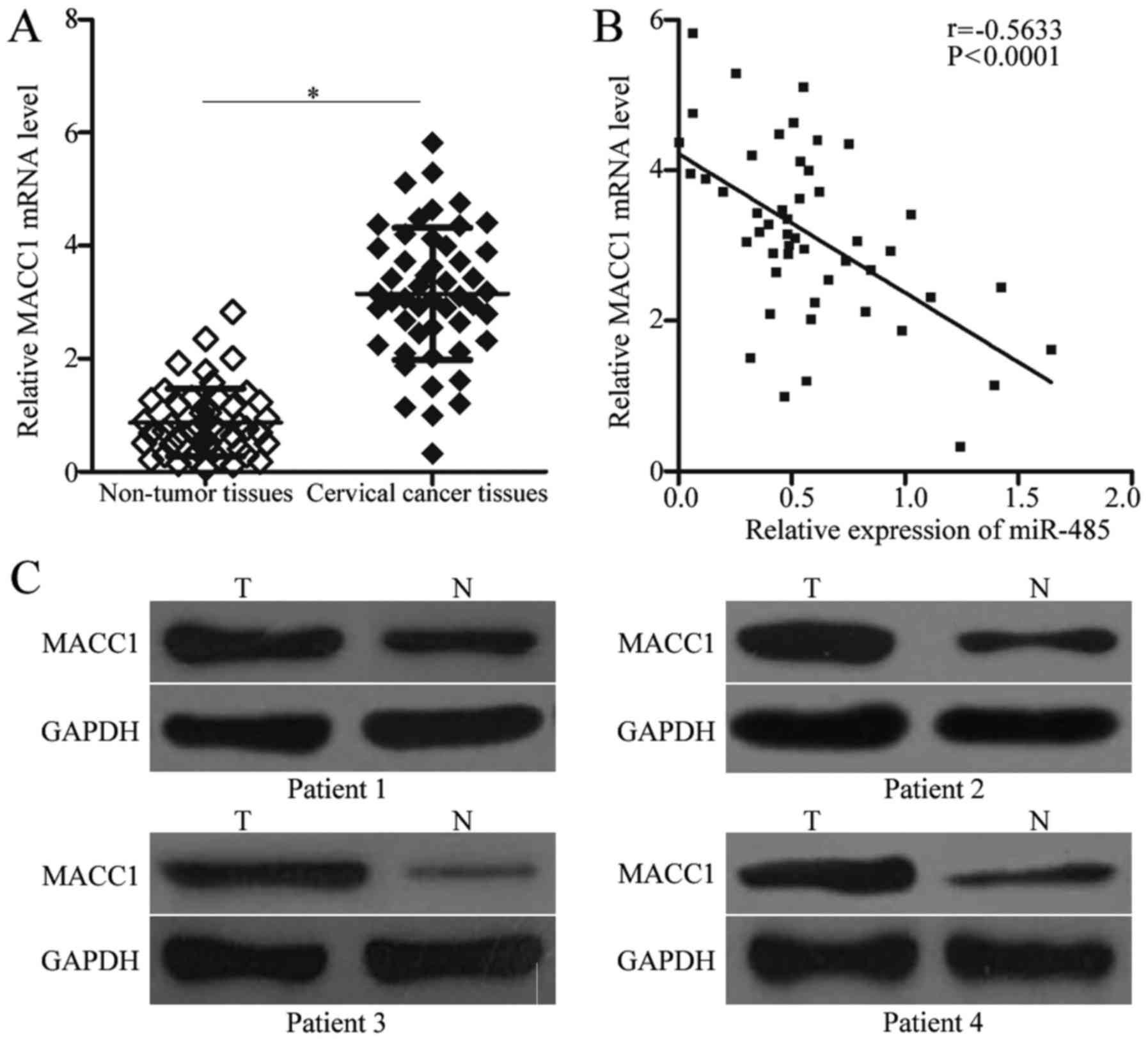
To further examine the association between miR-485
and MACC1 in cervical cancer, MACC1 expression was quantified in
cervical cancer tissues and corresponding adjacent non-tumor
tissues. RT-qPCR analysis revealed that MACC1 mRNA was
overexpressed in cervical cancer tissues compared with adjacent
non-tumor tissues (Fig. 4A;
P<0.05). Additionally, through Spearman's correlation analysis,
it was demonstrated that miR-485 and MACC1 mRNA expression was
inversely correlated in cervical cancer tissues (Fig. 4B; r=−0.5633; P<0.0001).
Furthermore, western blot analysis revealed that MACC1 protein
expression was upregulated in cervical cancer tissues, compared
with adjacent non-tumor tissues (Fig.
4C).
Restoration of MACC1 expression
reverses the suppressive effects of miR-485 overexpression in
cervical cancer
To further analyze whether the tumor-suppressive
role of miR-485 in cervical cancer was mediated by MACC1, HeLa and
SiHa cells were co-transfected with miR-485 mimics and
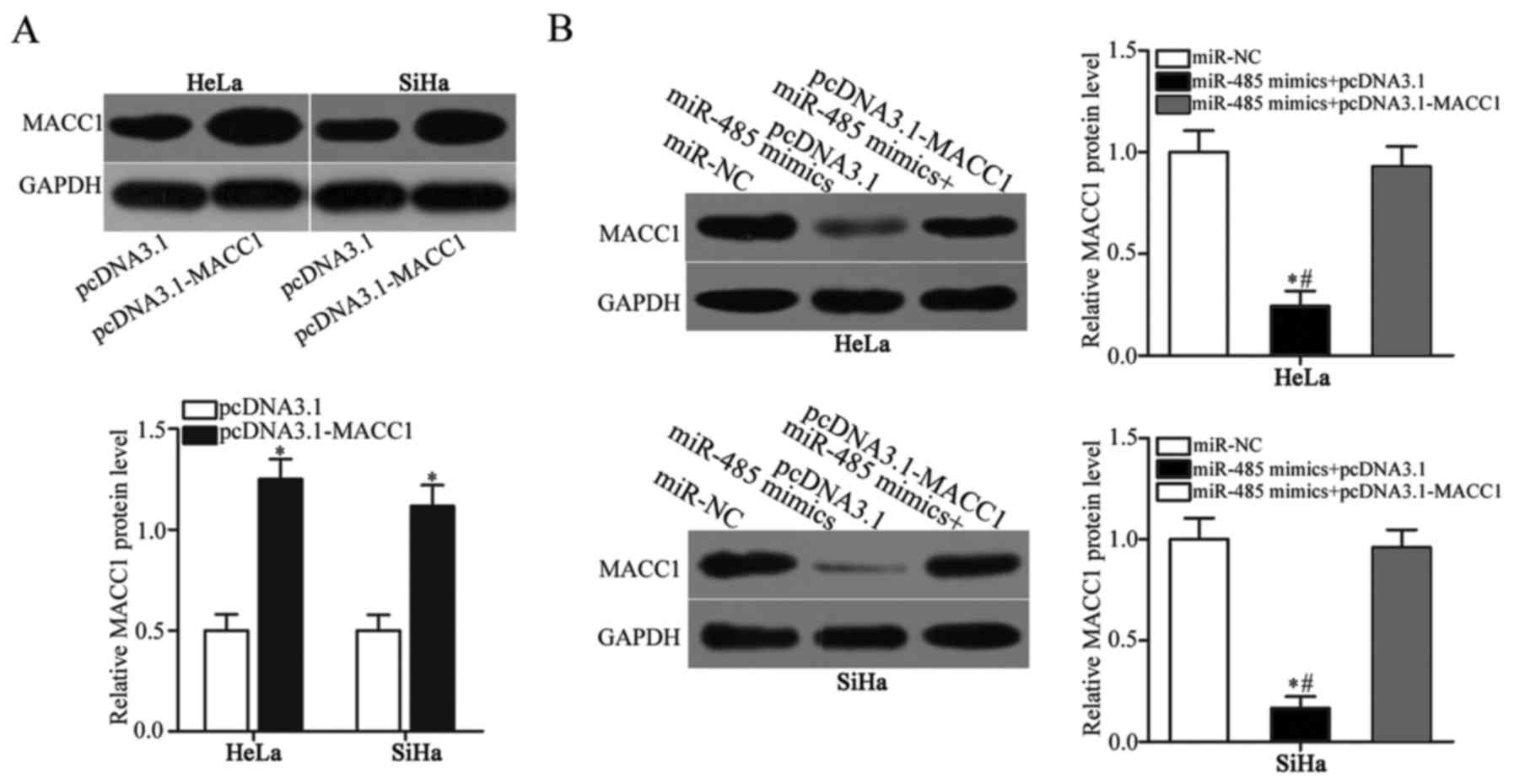
pcDNA3.1-MACC1 or pcDNA3.1. First, HeLa and SiHa cells were
transfected with pcDNA3.1-MACC1 or empty pcDNA3.1. Western blot
analysis demonstrated that MACC1 protein expression was markedly
upregulated in HeLa and SiHa cells following transfection with
pcDNA3.1-MACC1, compared with empty pcDNA3.1 (Fig. 5A; P<0.05). Co-transfection
confirmed that the downregulation of MACC1 protein expression by
miR-485 overexpression was reversed in HeLa and SiHa cells
following co-transfection with pcDNA3.1-MACC1 (Fig. 5B; P<0.05). CCK-8 and Transwell
invasion assays subsequently revealed that the restored expression
of MACC1 reversed the miR-485-mediated suppression of HeLa and SiHa
cell proliferation (Fig. 5C;
P<0.05) and invasion (Fig. 5D;
P<0.05). These results collectively indicated that miR-485 may
have inhibited the proliferation and invasion of cervical cancer
cells, at least partly through MACC1 expression downregulation.
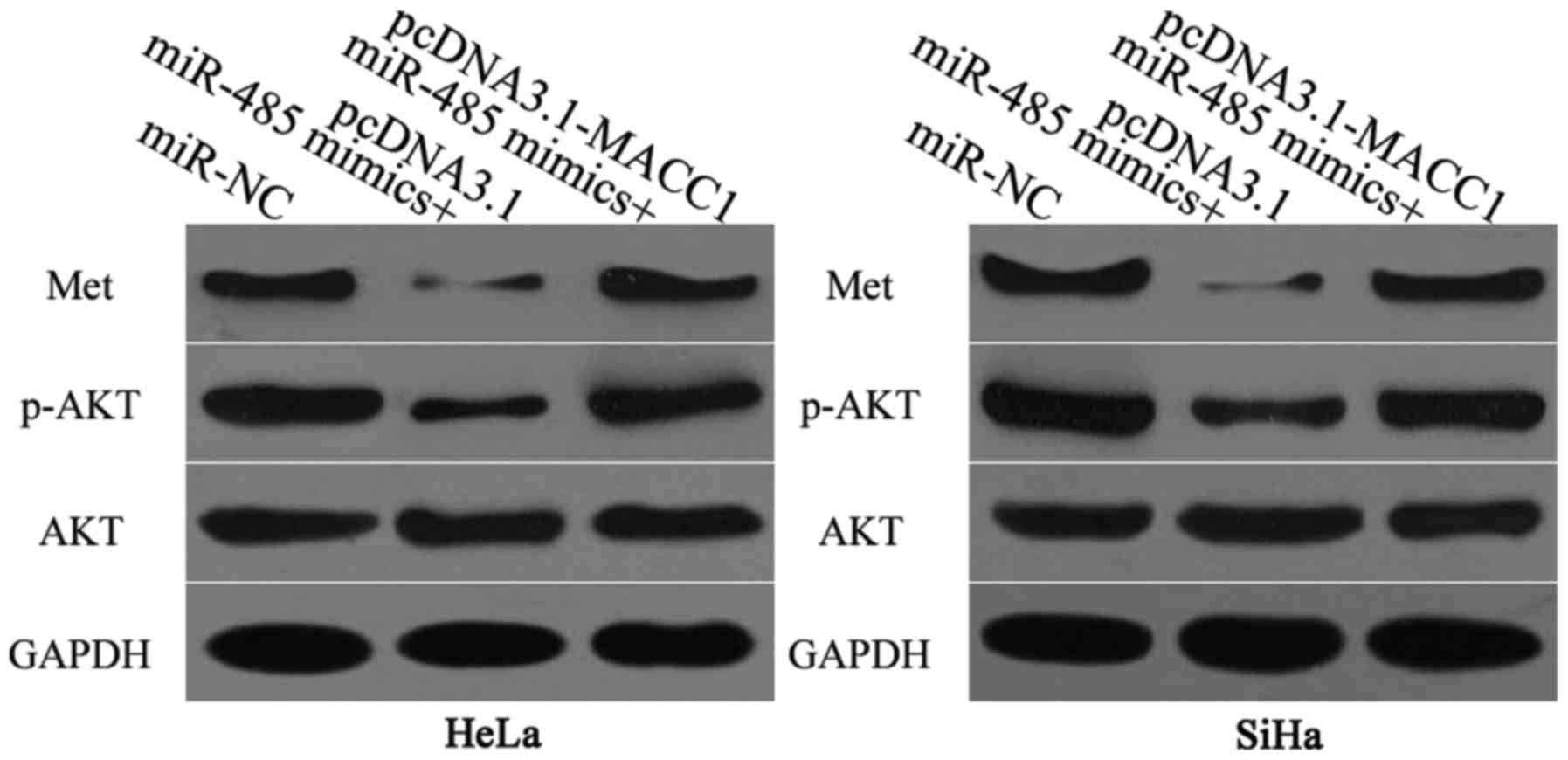
miR-485 affects the Met/AKT signaling
pathway in cervical cancer
MACC1 is involved in the regulation of the Met/AKT
pathway (28,29). Therefore, the effect of miR-485
expression on the Met/AKT signaling pathway in cervical cancer was
analyzed. Cells were transfected with miR-485 mimics along with
pcDNA3.1-MACC1 or pcDNA3.1. Western blot analysis was performed in
order to detect Met, p-AKT and AKT protein expression levels 72 h
post-transfection. The results demonstrated that miR-485
overexpression decreased Met and p-AKT expression without altering
the total AKT expression. In addition, Met and p-AKT protein
expression in HeLa and SiHa cells was restored following
co-transfection with pcDNA3.1-MACC1 (Fig. 6). These results suggested that
miR-485 suppressed the Met/AKT signaling pathway in cervical cancer
by inhibiting MACC1 expression.
Discussion
A large body of evidence indicates that miRNAs have
an essential role in the development and progression of cervical
cancer (30–32). Thus, miRNAs with dysregulated
expression in cervical cancer are potential biomarkers for the
diagnosis and prognosis of this disease. In the present study, it
was demonstrated that miR-485 expression was downregulated in
cervical cancer tissues and cell lines. Low miR-485 expression
levels in cervical cancer were correlated with FIGO stage and lymph
node metastasis. The ectopic expression of miR-485 inhibited the
proliferation and invasion of cervical cancer cells. Notably, it
was revealed that MACC1 was a direct target gene of miR-485 in
cervical cancer. MACC1 upregulation was inversely correlated with
miR-485 expression in cervical cancer tissues. Furthermore,
overexpression of MACC1 reversed the suppressive role of miR-485
overexpression in cervical cancer. miR-485 overexpression also
suppressed the Met/AKT signaling pathway in cervical cancer. The
results of the present study determined the expression pattern and
biological roles of miR-485 in cervical cancer, and suggested that
miR-485 may be a potential therapeutic target in the treatment of
cervical cancer.
The expression of miR-485 is dysregulated in a
number of human cancer types. For example, miR-485 is
underexpressed in glioblastoma tissues, cell lines and blood serum.
In addition, decreased miR-485 expression is correlated with poor
progression-free survival and overall survival in patients with
glioblastoma (18,19). In gastric cancer, the
downregulation of miR-485 is strongly associated with tumor size,
invasion depth, lymph node metastasis and tumor-node-metastasis
(TNM) stage. Patients with gastric cancer exhibiting low miR-485
expression have shorter survival times than those exhibiting high
miR-485 expression. In addition, low miR-485 expression is an
independent predictor of poor prognosis in patients with gastric
cancer (20). Furthermore, miR-485
expression is low in lung adenocarcinoma tumor tissues and cell
lines, and is significantly correlated with tumor metastasis
(21). In hepatocellular
carcinoma, miR-485 expression is downregulated in tumor tissues and
cell lines, and is associated with tumor size, TNM stage,
metastasis and tumor number (33,34).
miR-485 expression is also downregulated in bladder cancer
(35), breast cancer (36,37),
melanoma (38) and oral-tongue
squamous cell carcinoma (39).
These findings suggest that miR-485 is frequently downregulated in
human malignancies and is therefore a potential biomarker for the
detection and prognosis of specific types of human cancer.
The upregulation of miR-485 has been demonstrated to
decrease the proliferation, colony formation, migration and
invasion of glioblastoma cells, in addition to promoting apoptosis
in vitro and suppressing tumor growth in vivo
(18,40). Kang et al (41) and Duan et al (42) reported that miR-485 overexpression
attenuates cell proliferation and metastasis in vitro and
represses tumor growth in vivo. Mou and Liu (21) demonstrated that restored expression
of miR-485 significantly decreases cell metastasis and
epithelial-mesenchymal transition (EMT) in lung adenocarcinoma. Guo
et al (33) and Sun et
al (34) revealed that the
ectopic expression of miR-485 inhibits cell growth and metastasis
in vitro, as well as reducing tumor growth in vivo.
Chen et al (35) reported
that miR-485 overexpression suppresses cell metastasis and EMT in
bladder cancer. Lou et al (36) and Anaya-Ruiz et al (37) demonstrated that miR-485 decreases
cell proliferation, colony formation, mitochondrial respiration and
metastasis in breast cancer. In addition, miR-485 has a vital role
in the oncogenesis of melanoma (38) and oral tongue squamous cell
carcinoma (39). Taken together,
these findings indicate that miR-485 may be an effective target of
antineoplastic agents.
Numerous targets of miR-485 have been previously
identified. These targets include: p21 (RAC1) activated kinase 4
(PAK1) (18) and tumor protein D52
like 2 (40) in glioma; flotillin
(Flot)1 (41) and nudix hydrolase
(42) in gastric cancer; Flot2
(21) in lung adenocarcinoma;
stanniocalcin 2 (33) and
extracellular matrix metalloproteinase inducer (34) in hepatocellular carcinoma; high
mobility group AT-hook 2 (35) in
bladder cancer; peroxisome proliferator-activated receptor γ
coactivator 1-α (36) in breast
cancer; frizzled class receptor 7 (38) in melanoma; and PAK1 (39) in oral tongue squamous cell
carcinoma. In the present study, it was demonstrated that MACC1 is
a direct target of miR-485 in cervical cancer. Previous studies
have revealed that MACC1 is overexpressed in various malignant
tumors, including gastric cancer (43), colorectal cancer (44), hepatocellular carcinoma (45) and glioma (46). Furthermore, MACC1 is upregulated in
cervical cancer and is positively correlated with FIGO stage,
pelvic lymph node metastasis, recurrence and poor survival in
patients with cervical cancer (24). Additionally, patients with cervical
cancer exhibiting high MACC1 levels have a worse prognosis than
those with low MACC1 levels (23).
MACC1 is involved in the tumorigenesis and tumor development of
cervical cancer by regulating multiple biological behaviors,
including cell proliferation, apoptosis, migration, invasion,
metastasis and angiogenesis (24–27).
Therefore, targeting MACC1 may be a potentially effective strategy
for the treatment of patients with cervical cancer.
In conclusion, it was confirmed that miR-485 has
tumor suppressive roles in cervical cancer by directly targeting
MACC1 and inhibiting the Met/AKT signaling pathway. The findings of
the present study provided novel evidence for the development of
miR-485/MACC1/Met/AKT-targeted therapies for patients with cervical
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ and SW designed the present study. SW and YZ
performed reverse transcription-quantitative polymerase chain
reaction, Cell Counting kit-8 assays and Transwell invasion assays.
SY performed luciferase reporter assays and western blot analysis.
XJ performed data analysis. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present research was approved by the Ethics
Committee of Weifang People's Hospital. All participants provided
written informed consent.
Consent for publication
All participants provided written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan W, Xiaoyun H, Haifeng Q, Jing L,
Weixu H, Ruofan D, Jinjin Y and Zongji S: MicroRNA-218 enhances the
radiosensitivity of human cervical cancer via promoting radiation
induced apoptosis. Int J Med Sci. 11:691–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pareja R, Rendon GJ, Sanz-Lomana CM,
Monzon O and Ramirez PT: Surgical, oncological, and obstetrical
outcomes after abdominal radical trachelectomy - a systematic
literature review. Gynecol Oncol. 131:77–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith RA, Brooks D, Cokkinides V, Saslow D
and Brawley OW: Cancer screening in the united states, 2013: A
review of current american cancer society guidelines, current
issues in cancer screening, and new guidance on cervical cancer
screening and lung cancer screening. CA Cancer J Clin. 63:88–105.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, Xie H, Liu Y, Liu W, Liu M and Tang
H: miR-484 suppresses proliferation and epithelial-mesenchymal
transition by targeting ZEB1 and SMAD2 in cervical cancer cells.
Cancer Cell Int. 17:362017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong J, Huang R, Su Z, Zhang M, Xu M,
Gong J, Chen N, Zeng H, Chen X and Zhou Q: Downregulation of
miR-199a-5p promotes prostate adeno-carcinoma progression through
loss of its inhibition of HIF-1α. Oncotarget. 8:83523–83538. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Jin Y, Zhang H, Huang X, Zhang Y
and Zhu J: MicroRNA-599 inhibits metastasis and
epithelial-mesenchymal transition via targeting EIF5A2 in gastric
cancer. Biomed Pharmacother. 97:473–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Liu Z, Fang X and Yang H:
MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in
non-small cell lung cancer. Cell Physiol Biochem. 43:2505–2515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Subramanian S, Lui WO, Lee CH, Espinosa I,
Nielsen TO, Heinrich MC, Corless CL, Fire AZ and van de Rijn M:
MicroRNA expression signature of human sarcomas. Oncogene.
27:2015–2026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banno K, Iida M, Yanokura M, Kisu I, Iwata
T, Tominaga E, Tanaka K and Aoki D: MicroRNA in cervical cancer:
OncomiRs and tumor suppressor miRs in diagnosis and treatment.
ScientificWorldJournal. 2014:1780752014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ and Hammond SM: A microRNA polycistron as a potential
human oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao K, Lei D, Zhang H and You C:
MicroRNA-485 inhibits malignant biological behaviour of
glioblastoma cells by directly targeting PAK4. Int J Oncol.
51:1521–1532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZQ, Zhang MY, Deng ML, Weng NQ, Wang
HY and Wu SX: Low serum level of miR-485-3p predicts poor survival
in patients with glioblastoma. PLoS One. 12:e01849692017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jing LL and Mo XM: Reduced miR-485-5p
expression predicts poor prognosis in patients with gastric cancer.
Eur Rev Med Pharmacol Sci. 20:1516–1520. 2016.PubMed/NCBI
|
|
21
|
Mou X and Liu S: MiR-485 inhibits
metastasis and EMT of lung adenocarcinoma by targeting Flot2.
Biochem Biophys Res Commun. 477:521–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo L, Lu W, Zhang X, Luo D and Zhang H:
Metastasis-associated colon cancer-1 is a novel prognostic marker
for cervical cancer. Int J Clin Exp Pathol. 7:4150–4155.
2014.PubMed/NCBI
|
|
24
|
Zhou X, Xu CJ, Wang JX, Dai T, Ye YP, Cui
YM, Liao WT, Wu XL and Ou JP: Metastasis-associated in colon
cancer-1 associates with poor prognosis and promotes cell invasion
and angiogenesis in human cervical cancer. Int J Gynecol Cancer.
25:1353–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hua F, Xia Y, Wang H, Chen R, Ren Y, Yang
J and Liang W: Effects of small interfering RNA silencing MACC-1
expression on cell proliferation, cell cycle and invasion ability
of cervical cancer SiHa cells. Zhonghua Zhong Liu Za Zhi.
36:496–500. 2014.(In Chinese). PubMed/NCBI
|
|
26
|
Chen XP, Ren XP, Lan JY, Chen YG and Shen
ZJ: Analysis of HGF, MACC1, C-met and apoptosis-related genes in
cervical carcinoma mice. Mol Biol Rep. 41:1247–1256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chai H and Yang Y: Effects of MACC1 siRNA
on biological behaviors of HeLa. Arch Gynecol Obstet.
289:1271–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma
H, Xia J, Bin J, Liao Y and Liao W: MiR-338-3p inhibits
epithelial-mesenchymal transition in gastric cancer cells by
targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget.
6:15222–15234. 2015.PubMed/NCBI
|
|
29
|
Yao Y, Dou C, Lu Z, Zheng X and Liu Q:
MACC1 suppresses cell apoptosis in hepatocellular carcinoma by
targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem.
35:983–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang P, Xi J and Liu S: MiR-139-3p
induces cell apoptosis and inhibits metastasis of cervical cancer
by targeting NOB1. Biomed Pharmacother. 83:850–856. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong P, Xiong Y, Watari H, Hanley SJ,
Konno Y, Ihira K, Suzuki F, Yamada T, Kudo M, Yue J and Sakuragi N:
Suppression of iASPP-dependent aggressiveness in cervical cancer
through reversal of methylation silencing of microRNA-124. Sci Rep.
6:354802016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng F, Zhang J, Luo S, Yi J, Wang P,
Zheng Q and Wen Y: miR-143 is associated with proliferation and
apoptosis involving ERK5 in HeLa cells. Oncol Lett. 12:3021–3027.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo GX, Li QY, Ma WL, Shi ZH and Ren XQ:
MicroRNA-485-5p suppresses cell proliferation and invasion in
hepatocellular carcinoma by targeting stanniocalcin 2. Int J Clin
Exp Pathol. 8:12292–12299. 2015.PubMed/NCBI
|
|
34
|
Sun X, Liu Y, Li M, Wang M and Wang Y:
Involvement of miR-485-5p in hepatocellular carcinoma progression
targeting EMMPRIN. Biomed Pharmacother. 72:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Z, Li Q, Wang S and Zhang J: miR4855p
inhibits bladder cancer metastasis by targeting HMGA2. Int J Mol
Med. 36:1136–1142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lou C, Xiao M, Cheng S, Lu X, Jia S, Ren Y
and Li Z: MiR-485-3p and miR-485-5p suppress breast cancer cell
metastasis by inhibiting PGC-1α expression. Cell Death Dis.
7:e21592016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anaya-Ruiz M, Bandala C and Perez-Santos
JL: miR-485 acts as a tumor suppressor by inhibiting cell growth
and migration in breast carcinoma T47D cells. Asian Pac J Cancer
Prev. 14:3757–3760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu J, Li J, Ren J and Zhang D:
MicroRNA-485-5p represses melanoma cell invasion and proliferation
by suppressing Frizzled7. Biomed Pharmacother. 90:303–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin XJ, He CL, Sun T, Duan XJ, Sun Y and
Xiong SJ: Hsa-miR-485-5p reverses epithelial to mesenchymal
transition and promotes cisplatin-induced cell death by targeting
PAK1 in oral tongue squamous cell carcinoma. Int J Mol Med.
40:83–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu J, Wu SW and Wu WP: A tumor-suppressive
microRNA, miRNA-485-5p, inhibits glioma cell proliferation and
invasion by down-regulating TPD52L2. Am J Transl Res. 9:3336–3344.
2017.PubMed/NCBI
|
|
41
|
Kang M, Ren MP, Zhao L, Li CP and Deng MM:
miR-485-5p acts as a negative regulator in gastric cancer
progression by targeting flotillin-1. Am J Transl Res. 7:2212–2222.
2015.PubMed/NCBI
|
|
42
|
Duan J, Zhang H, Li S, Wang X, Yang H,
Jiao S and Ba Y: The role of miR-485-5p/NUDT1 axis in gastric
cancer. Cancer Cell Int. 17:922017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Koh YW, Hur H and Lee D: Increased MACC1
expression indicates a poor prognosis independent of MET expression
in gastric adenocarcinoma. Pathol Res Pract. 212:93–100. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang J, Chen JX, Chen L, Tang JY, Cui Z,
Liu CH and Wang Z: Metastasis associated in colon cancer 1 (MACC1)
promotes growth and metastasis processes of colon cancer cells. Eur
Rev Med Pharmacol Sci. 20:2825–2834. 2016.PubMed/NCBI
|
|
45
|
Sun DW, Zhang YY, Qi Y, Liu GQ, Chen YG,
Ma J and Lv GY: Prognostic and clinicopathological significance of
MACC1 expression in hepatocellular carcinoma patients: A
meta-analysis. Int J Clin Exp Med. 8:4769–4777. 2015.PubMed/NCBI
|
|
46
|
Yang T, Kong B, Kuang YQ, Cheng L, Gu JW,
Zhang JH, Shu HF, Yu SX, He WQ, Xing XM and Huang HD:
Overexpression of MACC1 protein and its clinical implications in
patients with glioma. Tumour Biol. 35:815–819. 2014. View Article : Google Scholar : PubMed/NCBI
|