Introduction
Myelodysplastic syndromes (MDS), as clonal malignant
diseases of the hematopoietic stem cells, are characterized by
ineffective hemopoiesis of stem or progenitor cells, which leads to
peripheral blood cytopenias and may progress to acute myeloid
leukemia (AML) in 30% of MDS patients (1). Although numerous types of therapy
including immunomodulatory agents, low-dose chemotherapy and
hematopoietic stem cell transplantation have been developed based
on the molecular mechanisms of MDS, the current treatment methods
only alleviate the symptoms, and, therefore, an in-depth
understanding of the disease pathogenesis as well as biological
alterations are necessary for the treatment of patients with
MDS.
microRNAs (miRNA/miRs) are short single-stranded
RNAs that serve roles in regulation of gene expression at the
post-transcriptional level (2). In
recent years, increasing number of studies have demonstrated that
miRNAs may act as oncogenes or tumor suppressors regulating
numerous biological processes including cell proliferation, cell
cycle and apoptosis (3–5). In addition, it has been reported that
dysregulation of miRNAs is involved in the pathogenesis of MDS
(6). For example, miR-22 as a
potent proto-oncogene is upregulated in MDS and contributes to the
onset of hematological malignancies by negatively regulating the
expression of methylcytosine dioxygenase 2 (7). Kuang et al (8) has reported that miR-378 inhibits cell
growth and enhances apoptosis in human MDS. In addition, miR-21 has
been demonstrated to be dysregulated in many types of cancer acting
as an oncogene promoting cell proliferation, migration and invasion
(9,10). Furthermore, miR-21 is overexpressed
and directly targets mothers against decapentaplegic (SMAD)-7 in
MDS (11). Therefore, the
expression levels of SMAD-7 are markedly reduced which leads to
ineffective hematopoiesis by overactivation of transforming growth
factor-β signaling in MDS. To date, the majority of functional
analyses of miR-21 focused on various human cancers, including
colon (12), renal (13), lung (14) and cervical cancers (15). However, the mechanism underlying
miR-21-mediated regulation of cell proliferation and apoptosis in
MDS/AML remains to be elucidated.
In the present study, downregulation of miR-21
expression inhibited cell proliferation, induced G1 arrest and
promoted apoptosis in SKM-1 cells. Furthermore, phosphatase and
tensin homolog (PTEN) is a downstream target of miR-21 and miR-21
inhibitor inhibited cell proliferation, induced G1 arrest and
promoted cell apoptosis by modulating the PTEN/protein kinase B
(AKT) pathway. These results suggest that miR-21 could be a
potential target for MDS therapy.
Materials and methods
Cell culture
SKM-1, SH-SY5Y, SRA01/04 and Kasumi-1 cell lines
were purchased from Cell Bank of Chinese Academy of Sciences
(Shanghai, China). The SKM-1 and SH-SY5Y cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100
µg/ml streptomycin and 100 U/ml penicillin. All cells were
incubated at 37°C with 5% CO2. The SRA01/04 cells were
cultured in modified Eagle's medium (MEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1% Non-Essential
Amino Acid Solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at 37°C in a humidified atmosphere containing 5% CO2 and
95% air. The Kasumi-1 cells were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 15% FBS, 100
µg/ml streptomycin and 100 U/ml penicillin at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air.
Lentiviral vector construction and
lentivirus transfection
To down-regulate miR-21 in SKM-1 cells, the
inhibitor of hsa-miR-21 lentivirus gene transfer vector encoding
green fluorescent protein (GFP) was constructed by Shanghai
GenePharma Co., Ltd. (Shanghai, China). The sequence of the
inhibitor of hsa-miR-21 5′-TAGCTTATCAGACTGATGTTGA-3′ was confirmed
by sequencing (data not shown). The recombinant lentivirus of
miR-21 inhibitor (LV-miR-21 inhibitor) and the control lentivirus
(LV-NC, 5′-TTCTCCGAACGTGTCACGT-3′) were prepared and tittered to
1×108 transfection unit (TU)/ml. A total of
~0.5×105 SKM-1 cells were plated in each well in 24-well
plates overnight at 37°C.
Following 24 h of culture, lentiviruses were diluted
in 0.4 ml Iscove's Modified Dulbecco Medium (IMDM; Gibco; Thermo
Fisher Scientific, Inc.) containing polybrene (5 µg/ml;
Sigma-Aldrich; Merck KGaA) and added to the cells and incubated at
37°C for an additional 24 h, followed by incubation in 0.5 ml of
fresh IMDM for another 24 h at 37°C, which was replaced with fresh
IMDM and the cells were cultured for 48 h at 37°C. The lentivirus
transduction efficiency of SKM-1 cells was determined by the
detection of GFP signals by fluorescence microscopy (magnification,
×100) and flow cytometry (FACSCalibur; BD Biosciences, San Jose,
CA, USA) CellQuest software version 2.0 (BD Biosciences) 96 h
following transduction. Data were analysed using CellQuest software
(BD Biosciences).
Cell viability assay
The proliferation of SKM-1 cells was measured using
the cell counting kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) assay. In brief, cells at a density of
1×104 cells/well were seeded into a 96-well plate and
incubated for 24 h at 37°C. Cells were transfected with LV-miR-21
inhibitor and LV-NC for a further 24 h, as described above. After 4
days of lentiviral infection, a total of 10 µl CCK-8 solution was
added to each well and incubated for 4 h at 37°C. The absorbance
value of each well was measured with a microplate reader (Multiskan
MK3; Thermo Fisher Scientific, Inc.) at a wavelength of 450 nm. All
experiments were performed thrice and the results are presented as
the mean ± standard deviation.
Cell cycle assay
The cell cycle distribution of SKM-1 cells
was analyzed by flow cytometry (BD Biosciences) using a
Propidium Iodide staining kit (BD Biosciences). Briefly, SKM-1
cells were seeded at 5×105−1×106 cells/well
in 6-well plates for 24 h at 37°C. Following 96 h of viral
transfection, as described above, the cell groups were collected by
centrifugation at 1,000 × g for 5 min at room temperature and fixed
in ice-cold 70% ethanol overnight. Subsequently, RNase A (60 µg/ml)
and propidium iodide (25 µg/ml) in PBS were added, and samples were
incubated for 30 min in the dark at room temperature. Finally,
cells were tested using flow cytometry (FACSCalibur) at 488 nm to
determine DNA content. Data were analysed using CellQuest software
version 2.0.
Measurement of apoptosis by flow
cytometry
Cell apoptosis was assessed using a
PE-Annexin-V/7-AAD apoptosis detection kit (BD Pharmingen, San
Jose, CA) and the apoptotic rate was analyzed by a flow cytometry
on FACS Calibur. Data were analysed using CellQuest software
version 2.0. A total of 5×105−1×106
cells/well were seeded in 6-well plates for 24 h at 37°C. Following
96-h of viral transfection, as described above, cells were
harvested and washed with PBS 2–3 times at 37°C for 5 min.
Subsequently, cells were resuspended at a density of
1×106 cells/ml, stained with Annexin V-PE and
counterstained with 7-AAD in binding buffer (included in kit) at
room temperature for 15 min. The apoptotic cells were measured
using a flow cytometer with 488 nm excitation and 578 nm emission
for Annexin V-PE detection, and 488 nm excitation and 647 nm
emission for 7-AAD detection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from homogenized cell
samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and treated with DNase (Promega Corporation, Madison, WI,
USA). SKM-1 cells were analyzed following 4 days of culture post
lentiviral transfection, as described above. For each sample, 2 µg
of RNA was used for complementary (c)DNA synthesis with the
All-in-One™ miRNA First-Strand cDNA Synthesis kit (GeneCopoeia,
Inc., Rockville, MD, USA) containing universal qPCR primers, and
was reverse transcribed at 37°C for 60 min. The primers used were
as follows: miRNA-21, forward 5′-TAGCTTATCAGACTGATGTTGA-3′; U6,
forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-ACGCTTCACGAATTTGCGT-3′. were obtained from GeneCopoeia. The
expression of miRNA-21 was determined using All-in-One miRNA qPCR
Detection kit (GeneCopoeia, Inc., Rockville, MD, USA) and the U6
gene was used as a control for normalization. The PCR reaction was
conducted at 95°C for 10 min, followed by 40 cycles of denaturing
at 95°C for 10 sec, annealing at 60°C for 20 sec, and extension at
72°C for 10 sec. The relative level of miR-21 was calculated with
the comparative Cq method (2−ΔΔCq) (16). qPCR was performed with SYBR Green I
(included in kit) on ABI 7300 (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Western blot analysis
Cells were seeded in 6-well plates at a density of
2×106 cells/well with 2 ml complete DMEM (10% FBS, 100
µg/ml streptomycin and 100 U/ml penicillin) for 24 h at 37°C.
Following 96 h of viral transfection, as described above, the cells
were washed with PBS and lysed with radioimmunoprecipitation assay
lysis buffer (150 mmol/l NaCl, 50 mmol/l Tris-HCl, pH 7.4, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with 1 mM sodium
orthovanadate, 1 mM PMSF, and 1% cocktail of protease inhibitors
(Sigma-Aldrich; Merck KGaA). The protein concentration was
determined using the BCA method. Equal quantities of proteins (20
µg) were separated by 10–15% SDS-PAGE and transferred by
electroblotting onto a nitrocellulose membrane. After blocking with
5% nonfat milk for 2 h at room temperature, membranes were
incubated with various primary antibodies overnight at 4°C: AKT
(1:1,000 dilution; cat. no. 9272), phosphorylated (p)-AKT (1:1,000
dilution; cat. no. 4060), cyclin D1 (1:1,000 dilution; cat. no.
2978), cleaved caspase 3 (1:1,000 dilution; cat. no. 9661),
apoptosis regulator Bcl-2 (bcl-2; 1:1,000 dilution; ab182858),
bcl-associated protein X (bax; 1:1,000 dilution; ab32503) or GAPDH
(1:3,000 dilution; cat. no. 5174) followed by incubation with the
corresponding horseradish peroxidase-conjugated secondary antibody
(1:20,000 dilution; cat. no. 7074) for 2 h at room temperature.
Primary antibodies and secondary antibodies were obtained from
Abcam (Cambridge, UK) and Cell Signaling Technology, Inc. (Danvers,
MA, USA), respectively. Visualization was achieved using
SuperSignal West Pico chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.). Densitometry of western blots was
performed using ImageJ version 1.38× software (National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
All the data were expressed as the mean ± standard
deviation from three independent experiments. Differences between
groups were determined by one-way analysis of variance followed by
Dunnett's or Tukey's post hoc tests. P<0.05 was considered to
indicate a statistically significant difference. Data were analyzed
using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA,
USA).
Results
miR-21 is highly expressed in the
SKM-1 cell line
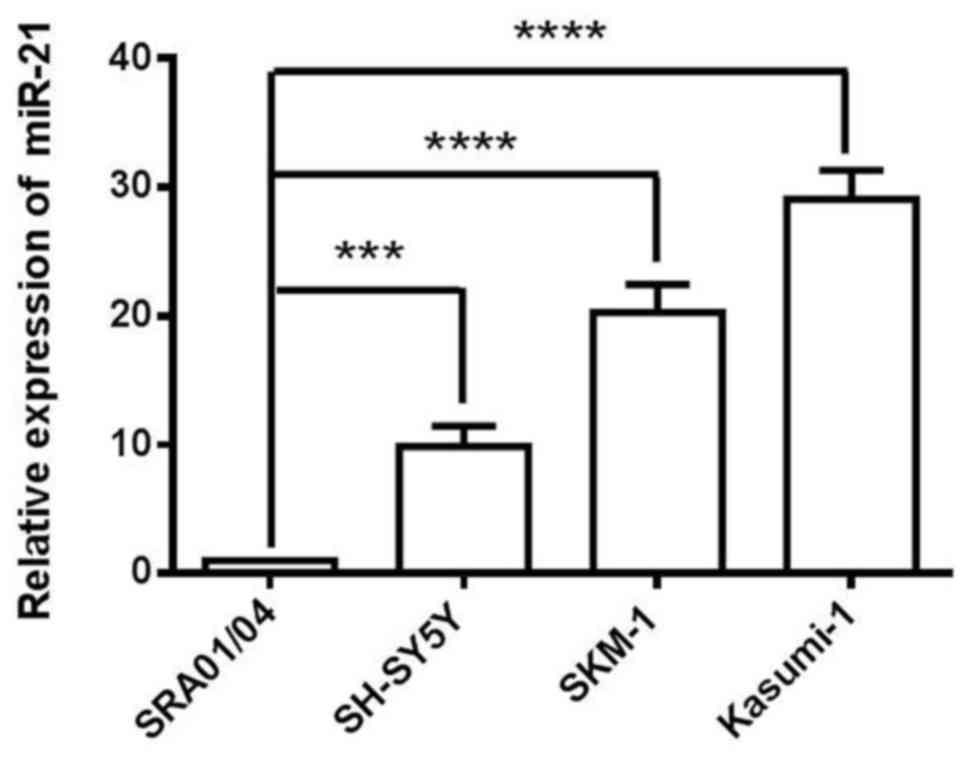
In the present study, SKM-1, Kasumi-1 and SH-SY5Y
cells were used as a model of cancer cells, and SRA01/04 cells as a
model of non-cancer cells. The expression level of miR-21 was
analyzed in SRA01/04, SKM-1, Kasumi-1 and SH-SY5Y four cell lines.
miR-21 expression was relatively increased in SKM-1 cells compared
with SRA01/04 and SH-SY5Y cells (Fig.
1). Kasumi-1 cells exhibited the highest expression levels of
miR-21; however, this cell line is often used as a model of AMl.
Therefore, based on the expression profile of the analysed cell
lines, SKM-1 cells as a MDS cell model were selected for the
following loss-of-function studies.
miR-21 inhibitor downregulates miR-21
in SKM-1 cells
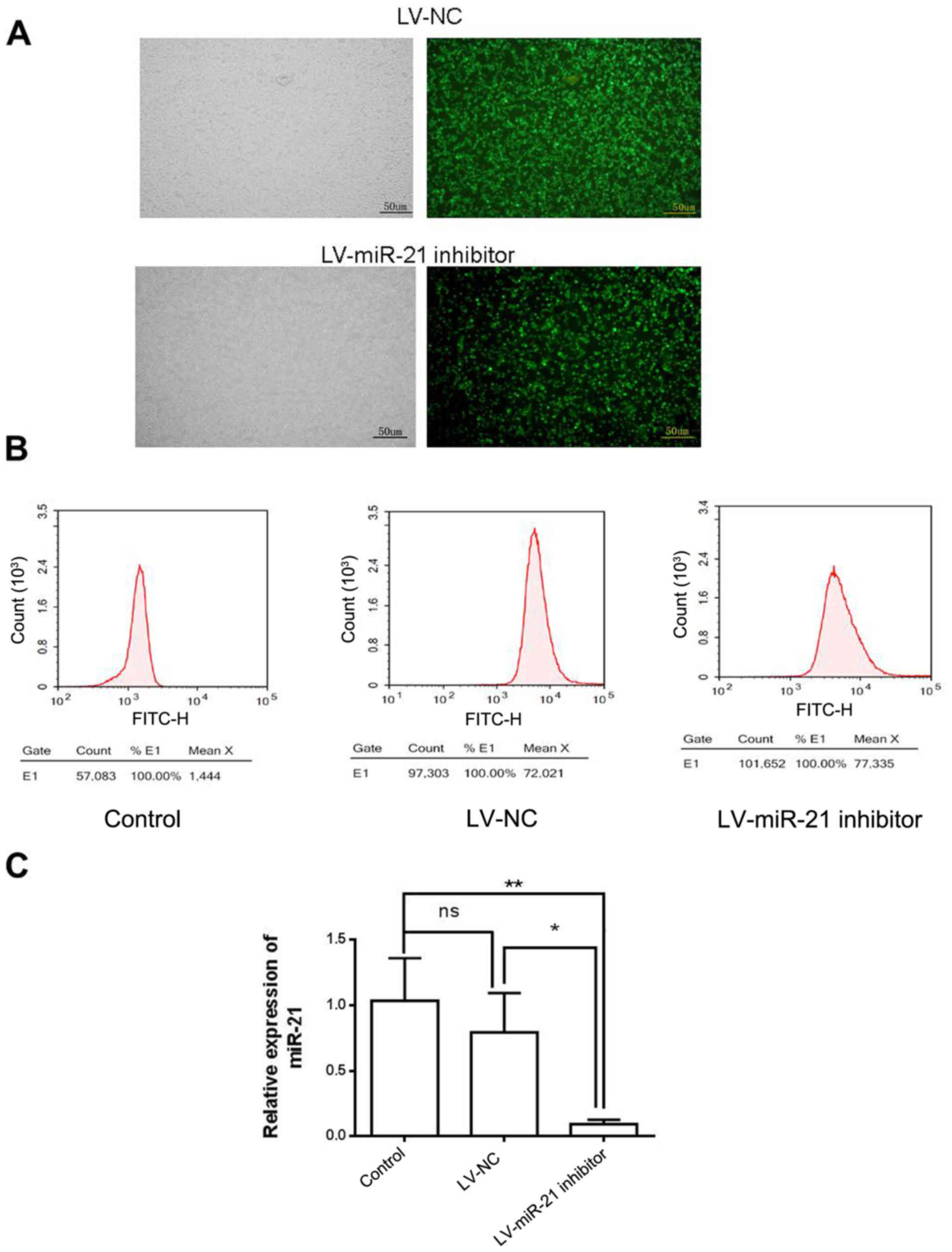
To investigate the molecular role of miR-21 in SKM-1
cells, a lentiviral vector system (LV-miR-21 inhibitor), which
incorporated GFP as a reporter gene, was used to downregulate the
expression of miR-21 (Fig. 2A).
Following lentiviral transfection, a high percentage of cells in
the LV-NC group (71.5%) and LV-miR-21 inhibitor group (73.5%)
expressed GFP (Fig. 2B),
indicating efficient transfection. RT-qPCR demonstrated that the
expression level of miR-21 was similar in LV-NC and blank control
groups. However, miR-21 expression level was lower in the LV-miR-21
inhibitor group compared with LV-NC and blank control groups,
indicating that miR-21 was successfully downregulated in SKM-1
cells (Fig. 2C).
miR-21 inhibitor reduces proliferation
of SKM-1 cells
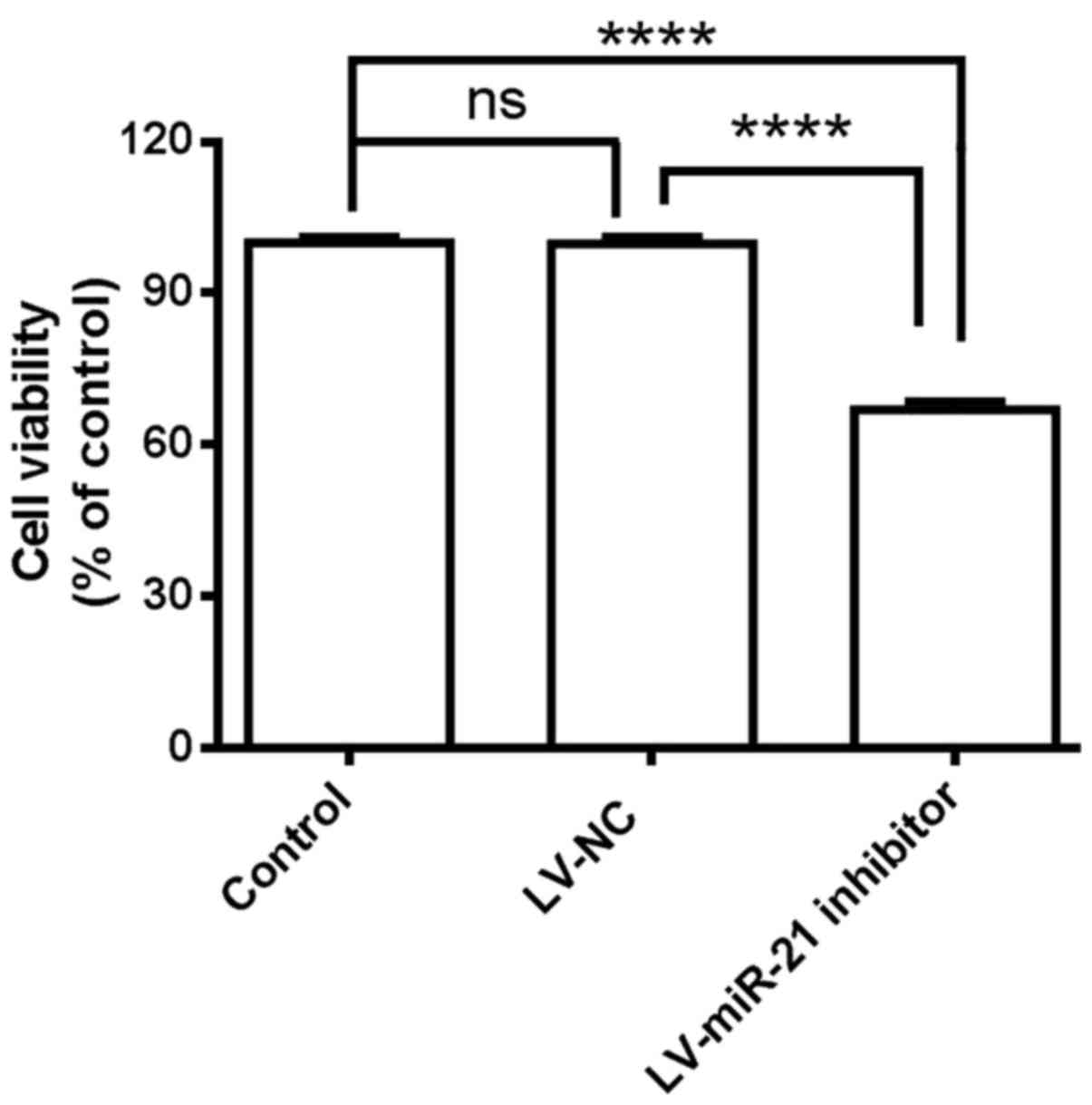
Based on the above results, an association between
miR-21 and SKM-1 proliferation was hypothesized. miR-21 was
inhibited in SKM-1 cells and cell viability was measured with CCK-8
assay. Suppression of miR-21 by transfection with the LV-miR-21
inhibitor significantly decreased the proliferation of SKM-1 cells
compared with LV-NC and blank control cells (Fig. 3). These results indicated that
miR-21 inhibitor exhibits inhibitory effects on the proliferation
of SKM-1 cells.
miR-21 inhibitor induces G1 arrest in
SKM-1 cells
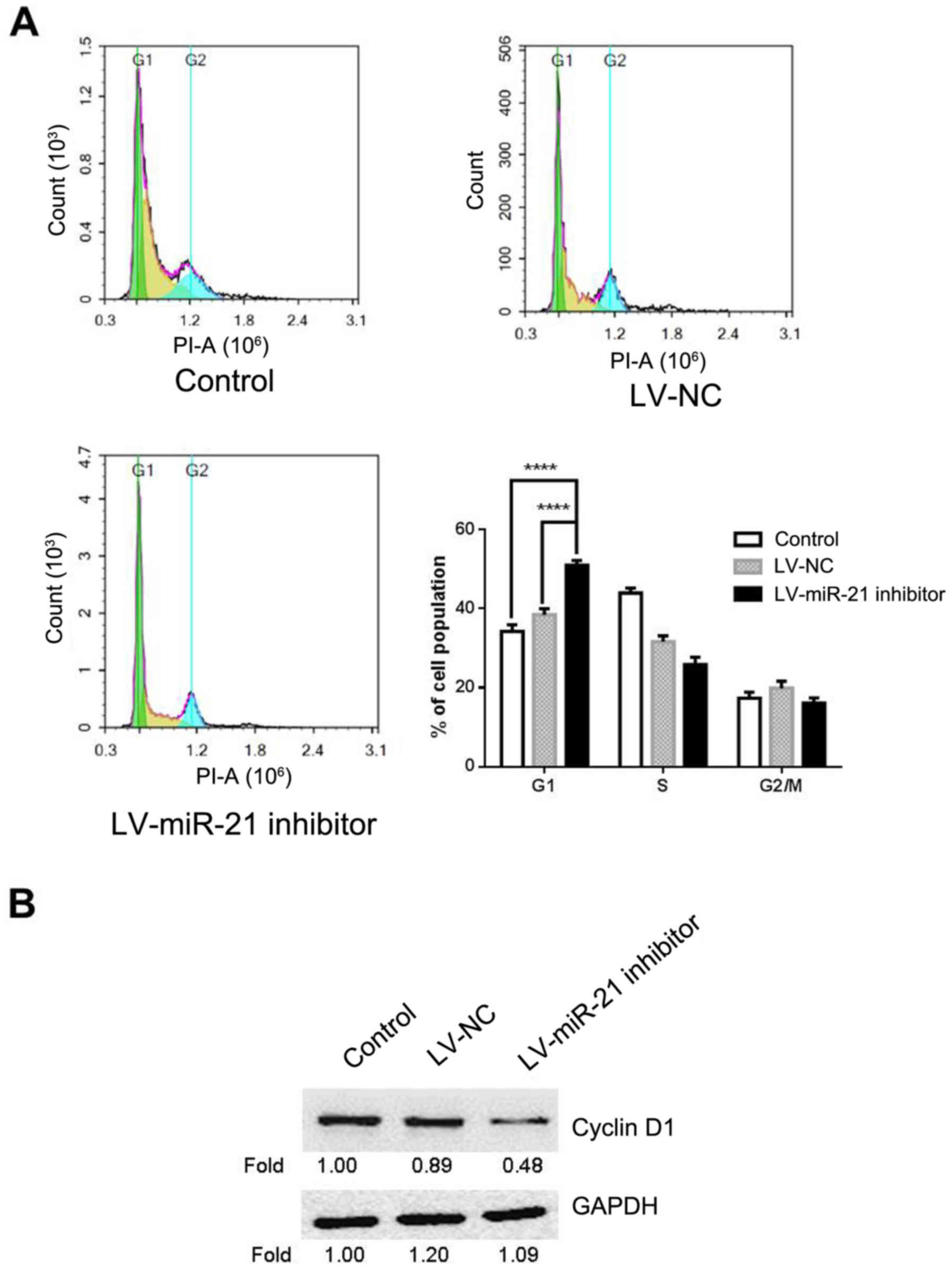
Given the suppressive effect of miR-21 inhibitor on
cell proliferation, miR-21 inhibitor may affect cell cycle.
Therefore, flow cytometry was carried out for cell cycle analysis
in LV-miR-21 inhibitor, LV-NC and blank control groups. The results
demonstrated that the percentage of cells at the G0/G1 phase in the
LV-miR-21 inhibitor group (50.93%) significantly increased compared
with the blank control (34.27%) and LV-NC groups (38.06%; Fig. 4A). To further elucidate the
molecular mechanism underlying miR-21 inhibitor-induced G1 cell
cycle arrest, the expression level of cyclin D1 in SKM-1 cells was
measured. This demonstrated that LV-miR-21 inhibitor transfection
markedly decreased the expression of cyclin D1 in SKM-1 cells
(Fig. 4B) compared with the LV-NC
and blank groups. The above results indicated that miR-21 inhibitor
reduces proliferation possibly by inducing G0/G1 cell cycle
arrest.
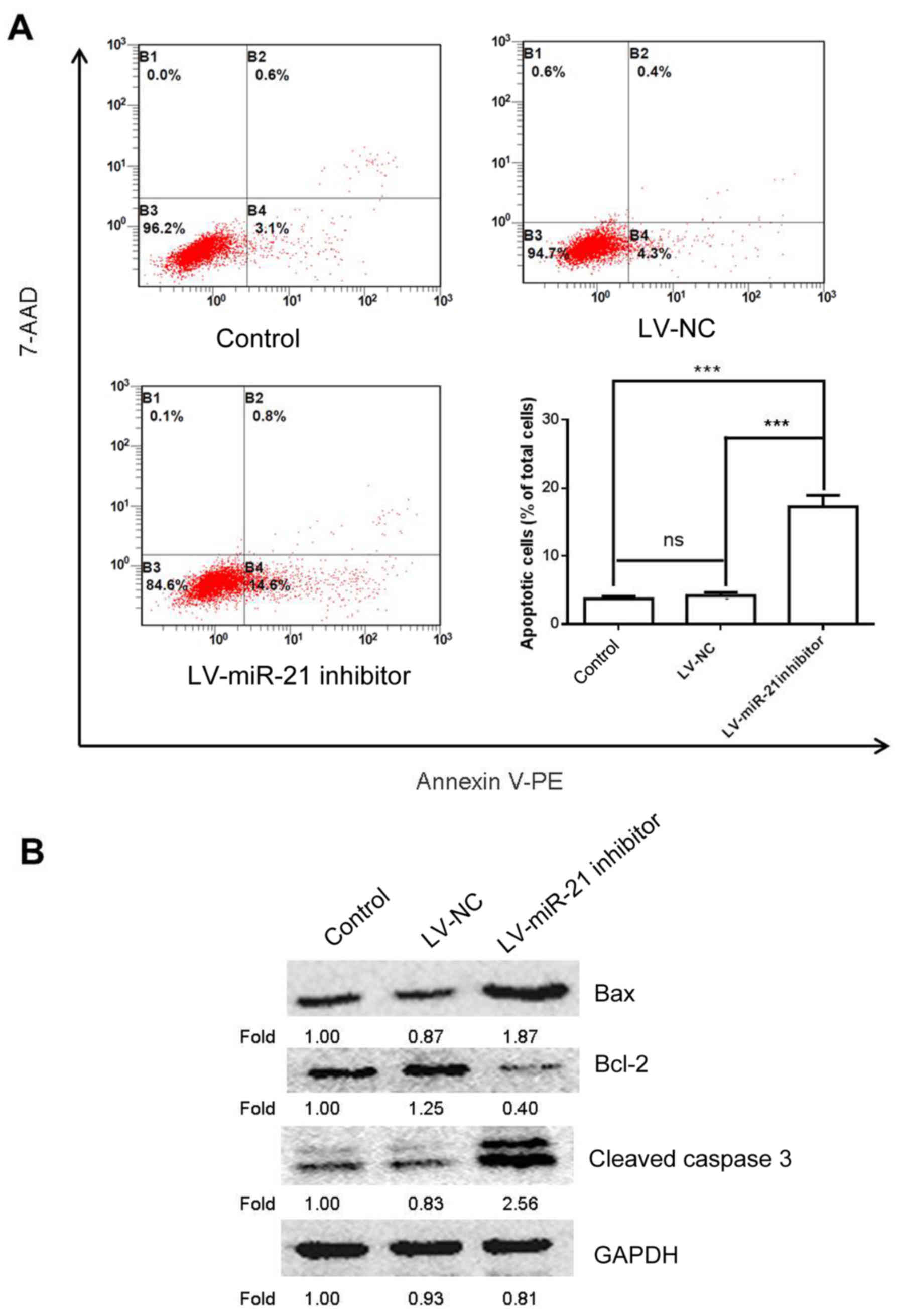
miR-21 inhibitor induces apoptosis in
SKM-1 cells
To further study the effects of miR-21 on SKM-1 cell
apoptosis a flow cytometry experiment was conducted. The results
demonstrated that cells transfected with the LV-miR-21 inhibitor
exhibited increased apoptosis compared with the blank and NC groups
(Fig. 5A). To confirm these
findings, western blotting was also performed to examine the
protein expression of bcl-2, bax and cleaved caspase 3, which are
apoptosis-associated markers (17). As expected, transfection with the
LV-miR-21 inhibitor elevated the expression level of bax and
cleaved caspase 3 and decreased the expression level of bcl-2
(Fig. 5B). These results
demonstrated proapoptotic effects of miR-21 inhibitor on SKM-1
cells.
miR-21 inhibitor regulates the
PTEN/AKT signaling pathway to induce apoptosis and cell cycle
arrest
An increasing amount of evidence demonstrated that
the PTEN/AKT pathway modulates tumor cell proliferation and
apoptosis (17,18), and miR-21 has been demonstrated to
control the expression and the activities of PTEN and AKT (19,20).
Therefore, the effect of miR-21 inhibitor on the expression of PTEN
and p-AKT was investigated. The result of western blotting revealed
that, compared with the blank control and LV-NC groups, miR-21
inhibitor promoted the expression of PTEN, but suppressed the
protein level of p-AKT (Fig. 6A).
These results indicated that miR-21 inhibitor may regulate the
PTEN/AKT pathway in SKM-1 cells. Subsequently, to further assess
whether the PTEN/AKT pathway was involved in the anticancer effects
of miR-21 inhibitor, the protein expression levels of cyclin D1 and
apoptosis-associated proteins (bcl-2, bax and cleaved caspase 3)
were examined by western blotting following treatment of SKM-1
cells with AKT siRNA. AKT knockdown markedly increased the
expression of bax and cleaved caspase 3, and reduced the expression
of cyclin D1, p-AKT, AKT and bcl-2 in SKM-1 cells (Fig. 6B). Numerous studies have revealed
that the G0/G1 arrest may be association with reducing the
expression of cyclin D1 (21–23).
Taken together, these results (Fig. 6A
and B) indicated that the PTEN/AKT pathway could serve an
important role in regulating miR-21 inhibitor-induced apoptosis and
G0/G1 cell cycle arrest in SKM-1 cells as reported previously
(21–23).
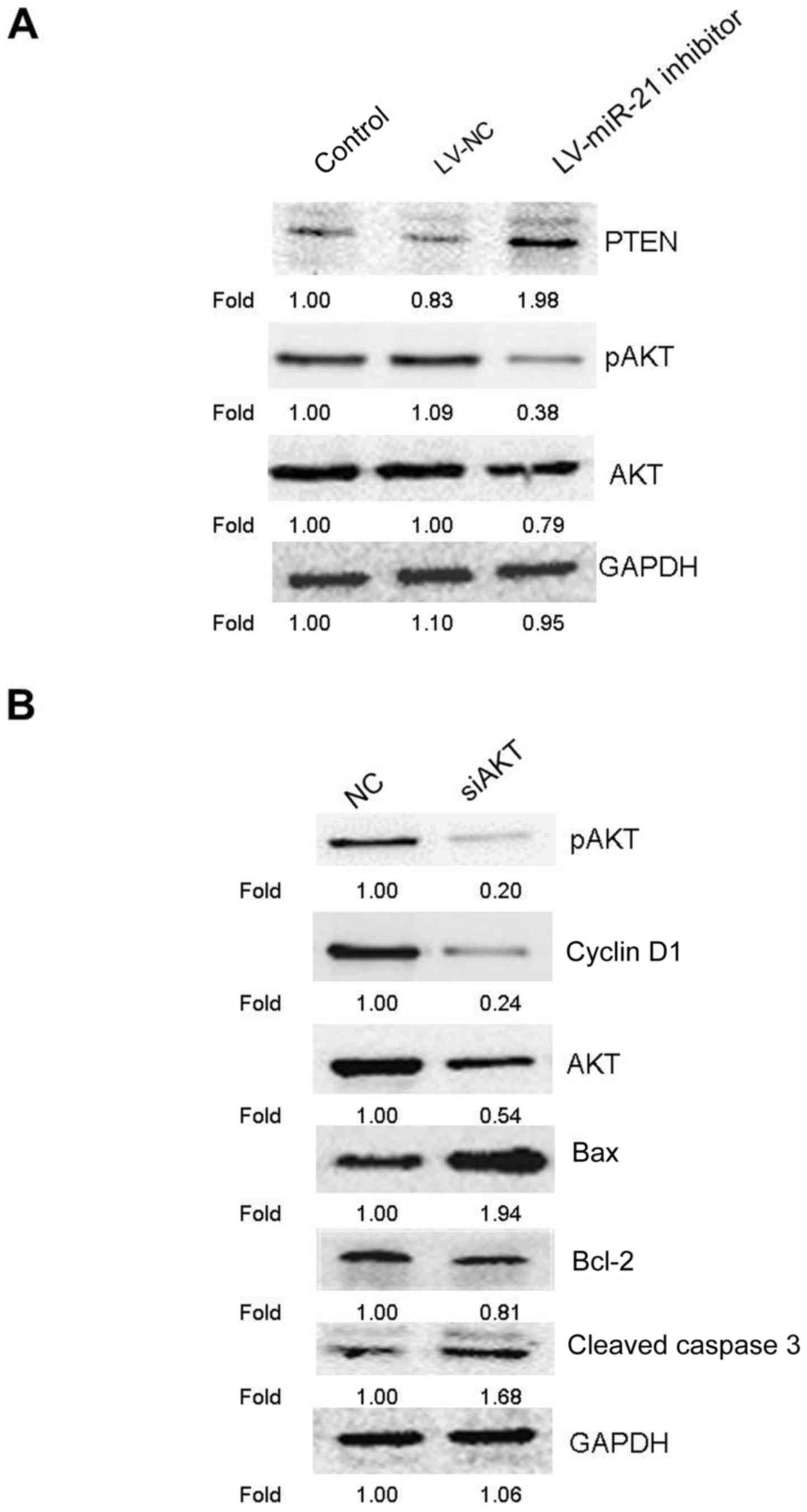 | Figure 6.Apoptosis-associated proteins were
detected by western blotting following AKT knockdown in SKM-1
cells. (A) Representative western blot images following knockdown
of miR-21 affecting expression of PTEN, pAKT and AKT. (B) Following
48 h of transfection with siAKT or non-targeted NC into SKM-1
cells, SKM-1 cells were collected and subjected to western blot
analysis for detection of apoptosis-associated protein levels. Bax,
bcl-associated protein X; bcl-2, apoptosis regulator Bcl-2; LV,
lentivirus; miR, microRNA; NC, negative control; ns, negative
control; p, phosphorylated; si, small interference. |
Discussion
Accumulated evidence has demonstrated that miRNAs
are aberrantly expressed in various physiological and pathological
processes, including carcinogenesis, and numerous miRNAs function
as tumor suppressors or oncogenes (24–26).
Previous studies have demonstrated that miR-21 is frequently
upregulated and serves a role in tumorigenesis and tumor
progression of glioblastoma (27),
head and neck cancer (28),
ovarian cancer (29), B-cell
lymphoma (30), hepatocellular
carcinoma (31), cervical cancer
(32), prostate cancer (33), lung cancer (34) and leukemia (35). However, the potential role of
miR-21 in MDS is relatively uncharacterized. SKM-1 is an acute
myeloid leukemia cell line established in the leukemic phase during
the progression of MDS to AML (MDS/AML) and has been used in
numerous studies on myelodysplastic syndromes as a MDS cell model
(8,36). In the present study, the SKM-1 cell
line was used and the effects of miR-21 on cell proliferation,
apoptosis, and cell cycle arrest were evaluated. SKM-1 cells were
used as an in vitro model of MDS/AML to further investigate
whether the cell viability and apoptosis of SKM-1 cells can be
modulated by miR-21 inhibitor.
In the present study, for the first time to the best
of our knowledge, it was demonstrated that miR-21 inhibitor exerts
tumor suppressive function in SKM-1 cells. Functional experiments
using SKM-1 cells further demonstrated that downregulation of
miR-21 expression lead to suppression of cyclin D1 activity, cell
cycle arrest at the G0/G1 checkpoint and inhibited cancer cell
proliferation. Furthermore, suppression of miR-21 expression in
SKM-1 cells could lead to upregulation of the bax expression and
cleaved caspase 3 and downregulation of the bcl-2 expression,
promoting cell apoptosis.
PTEN is located on human chromosome 10 in region
10q23 and functions as a tumor suppressor gene (37). It has been reported that PTEN is an
important downstream target of miR-21 which regulates cancer
development and progression by targeting PTEN (38). For instance, miR-21 promotes tumor
growth and invasion by downregulation of PTEN in prostate cancer
(19). Zheng et al
(20) revealed that miR-21
modulates cisplatin sensitivity of gastric cancer by modulating the
PTEN/PI3K/AKT pathway. Wang et al (39) revealed that downregulation of
miR-21 enhanced imatinib-induced apoptosis, and that overexpression
of miR-21 conferred imatinib resistance by modulating PTEN
expression in acute lymphoblastic leukemia. In addition, loss of
PTEN facilitates cell proliferation and inhibits apoptosis
(40–43). It has been suggested that silencing
of the PTEN gene in A549 cells significantly enhanced cell
proliferation and inhibited cell apoptosis (40). Silencing PTEN expression may
promote cell proliferation, decrease the rate of apoptosis of
HCC827 cells and reduce the sensitivity of HCC827 cells to icotinib
(41). Loss of PTEN can
effectively inhibit glucocorticoid-induced apoptosis and induce
resistance to glucocorticoid therapy in acute lymphoblastic
leukemia (42). PTEN loss is also
likely to result in cancer progression and relapse in T-cell acute
lymphoblastic leukemia (43). To
verify the association between miR-21 and PTEN in SKM-1 cells,
western blot analysis was performed and the results demonstrated
that down-regulation of miR-21 markedly elevated levels of PTEN in
SKM-1 cells.
AKT is a serine/threonine kinase and AKT pathway has
been widely reported to be associated with cell growth,
proliferation and apoptosis in various types of tumor cells
(44,45). It has also been reported that
inactivation of PTEN results in constitutive activation of the
PI3K/AKT pathway and increased proliferation and survival of cancer
cells (18). Furthermore,
up-regulation of PTEN protein inactivated the AKT signaling
pathway, which further inhibited proliferation and induced
apoptosis in human acute T cell leukemia cells (46). miR-21 expression was demonstrated
to suppress cell proliferation and induce cell apoptosis in SKM-1
cells. Subsequently, whether AKT signaling pathway was involved in
the regulation of cell proliferation and apoptosis was
investigated. In response to inhibited miR-21 expression, p-AKT
protein level was downregulated. AKT may regulate carcinogenesis by
several downstream targets including cyclinD1, bax, bcl-2 and
caspase 3 (47,48). To further confirm the role of the
AKT signaling in cell proliferation and apoptosis, siAKT was used
to inhibit the expression of p-AKT in SKM-1 cells. It was
demonstrated that the protein levels of cyclin D1 and bcl-2 were
reduced and the protein levels of bax and cleaved caspase 3
increased following transfection of SKM-1 cells with siAKT. These
results indicated inactivation of Akt signaling pathway may involve
in miR-21 inhibitor-mediated cell proliferation and apoptosis of
SKM-1 cells.
In conclusion, the present study demonstrated that
miR-21 inhibitor may act as a tumor suppressor in SKM-1 cells.
Furthermore, downregulation of miR-21 expression inhibited SKM-1
cell proliferation and induced SKM-1 cell apoptosis by regulating
the miR-21/PTEN/AKT axis. These results suggested that miR-21 may
be a potential therapeutic target for the treatment of MDS;
however, the present study was performed in vitro. Therefore
the anti-tumor activity via the inhibitory effects of miR-21
requires further investigation in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Natural
Science Foundation of Shanxi (grant no. 2013JM4016).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL conducted the experiments and analyzed the data.
YS made substantial contributions to the design of the present
study and prepared the manuscript. GCL, JR, JX, YZ, FG, JM and JD
performed the western blotting and analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aggerholm A, Holm MS, Guldberg P, Olesen
LH and Hokland P: Promoter hypermethylation of p15INK4B, HIC1, CDH1
and ER is frequent in myelodysplastic syndrome and predicts poor
prognosis in early-stage patients. Eur J Haematol. 76:23–32. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massillo C, Dalton GN, Farre PL, De Luca P
and De Siervi A: Implications of microRNA dysregulation in the
development of prostate cancer. Reproduction. 154:R81–R97. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou K, Liu M and Cao Y: New insight into
microRNA functions in cancer: Oncogene-microRNA-tumor suppressor
gene network. Front Mol Biosci. 4:462017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sekar D, Krishnan R, Thirugnanasambantham
K, Rajasekaran B, Islam VI and Sekar P: Significance of microRNA 21
in gastric cancer. Clin Res Hepatol Gastroenterol. 40:538–545.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang SJ, Choi IS, Park G, Moon DS, Choi
JS, Nam MH, Yoon SY, Choi CH and Kang SH: MicroRNA-205-5p is
upregulated in myelodysplastic syndromes and induces cell
proliferation via PTEN suppression. Leuk Res. 47:172–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song SJ, Ito K, Ala U, Kats L, Webster K,
Sun SM, Jongen-Lavrencic M, Manova-Todorova K, Teruya-Feldstein J,
Avigan DE, et al: The oncogenic microRNA miR-22 targets the TET2
tumor suppressor to promote hematopoietic stem cell self-renewal
and transformation. Cell Stem Cell. 13:87–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuang X, Wei C, Zhang T, Yang Z, Chi J and
Wang L: miR-378 inhibits cell growth and enhances apoptosis in
human myelodysplastic syndromes. Int J Oncol. 49:1921–1930. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pfeffer SR, Yang CH and Pfeffer LM: The
role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhagat TD, Zhou L, Sokol L, Kessel R,
Caceres G, Gundabolu K, Tamari R, Gordon S, Mantzaris I, Jodlowski
T, et al: miR-21 mediates hematopoietic suppression in MDS by
activating TGF-β signaling. Blood. 121:2875–2881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Han B, Yu H, Cui Z, Li Z and Wang J:
Berberine regulates the microRNA-21-ITGBeta4-PDCD4 axis and
inhibits colon cancer viability. Oncol Lett. 15:5971–5976.
2018.PubMed/NCBI
|
|
13
|
DeMaria AN: Clinical research in the
United States-a threatened activity. J Am Coll Cardiol. 13:508–510.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Y, Zuo Y, Qian XL, Chen ZP, Wang SK,
Song L and Peng LP: Inhibition of MicroRNA-21-5p promotes the
radiation sensitivity of non-small cell lung cancer through HMSH2.
Cell Physiol Biochem. 43:1258–1272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu L, Xu Q, Li X and Zhang X: MicroRNA-21
regulates the proliferation and apoptosis of cervical cancer cells
via tumor necrosis factor-alpha. Mol Med Rep. 16:4659–4663. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang C and Hu G: Shikonin suppresses
proliferation and induces apoptosis in endometrioid endometrial
cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling
pathway. Biosci Rep. 38:BSR201715462018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Guo JX and Shao ZQ: miR-21 targets
and inhibits tumor suppressor gene PTEN to promote prostate cancer
cell proliferation and invasion: An experimental study. Asian Pac J
Trop Med. 10:87–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Zhou W, Wu J, Yao L, Xue L, Zhang
Q, Wang Z, Wang X, Dong S, Zhao J and Yin D: Antitumor activity of
nimotuzumab in combination with cisplatin in lung cancer cell line
A549 in vitro. Oncol Lett. 15:5280–5284. 2018.PubMed/NCBI
|
|
22
|
Yang L, Liu H, Long M, Wang X, Lin F, Gao
Z and Zhang H: Peptide SA12 inhibits proliferation of breast cancer
cell lines MCF-7 and MDA-MB-231 through G0/G1 phase cell cycle
arrest. Onco Targets Ther. 11:2409–2417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SL, Liu Z, Zhang LD, Zhu HQ, Guo JH,
Zhao M, Wu YL, Liu F and Gao FH: GSK3β-dependent cyclin D1 and
cyclin E1 degradation is indispensable for NVP-BEZ235 induced G0/G1
arrest in neuroblastoma cells. Cell Cycle. 16:2386–2395. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou B, Wang D, Sun G, Mei F, Cui Y and Xu
H: Effect of miR-21 on apoptosis in lung cancer cell through
inhibiting the PI3K/Akt/NF-κB signaling pathway in vitro and in
vivo. Cell Physiol Biochem. 46:999–1008. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang C, Tabatabaei SN, Ruan X and Hardy P:
The dual regulatory role of MiR-181a in breast cancer. Cell Physiol
Biochem. 44:843–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masoudi MS, Mehrabian E and Mirzaei H:
MiR-21: A key player in glioblastoma pathogenesis. J Cell Biochem.
119:1285–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang AJ, Li ZW, Hu MX, Wang SD and Leng M:
Ionic mechanism of noradrenaline-induced membrane potential changes
of neurones in toad dorsal root ganglion. Sheng Li Xue Bao.
41:145–152. 1989.(In Chinese). PubMed/NCBI
|
|
29
|
Echevarria-Vargas IM, Valiyeva F and
Vivas-Mejia PE: Upregulation of miR-21 in cisplatin resistant
ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 9:e970942014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munch-Petersen HD, Ralfkiaer U, Sjo LD,
Hother C, Asmar F, Nielsen BS, Brown P, Ralfkiaer E and Grønbæk K:
Differential expression of miR-155 and miR-21 in tumor and stroma
cells in diffuse large B-cell lymphoma. Appl Immunohistochem Mol
Morphol. 23:188–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He C, Dong X, Zhai B, Jiang X, Dong D, Li
B, Jiang H, Xu S and Sun X: MiR-21 mediates sorafenib resistance of
hepatocellular carcinoma cells by inhibiting autophagy via the
PTEN/Akt pathway. Oncotarget. 6:28867–28881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peralta-Zaragoza O, Deas J, Meneses-Acosta
A, De la O-Gómez F, Fernández-Tilapa G, Gómez-Cerón C,
Benítez-Boijseauneau O, Burguete-García A, Torres-Poveda K,
Bermúdez-Morales VH, et al: Relevance of miR-21 in regulation of
tumor suppressor gene PTEN in human cervical cancer cells. BMC
Cancer. 16:2152016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reis ST, Pontes-Junior J, Antunes AA,
Dall'Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR,
Nesrallah AJ, Piantino C, et al: miR-21 may acts as an oncomir by
targeting RECK, a matrix metalloproteinase regulator, in prostate
cancer. BMC Urol. 12:142012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue X, Liu Y, Wang Y, Meng M, Wang K, Zang
X, Zhao S, Sun X, Cui L, Pan L and Liu S: MiR-21 and MiR-155
promote non-small cell lung cancer progression by downregulating
SOCS1, SOCS6 and PTEN. Oncotarget. 7:84508–84519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jurkovicova D, Lukackova R, Magyerkova M,
Kulcsar L, Krivjanska M, Krivjansky V and Chovanec M: microRNA
expression profiling as supportive diagnostic and therapy
prediction tool in chronic myeloid leukemia. Neoplasma. 62:949–958.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng W, Dai H, Yan M, Cai X, Luo H, Ke M
and Liu Z: Decitabine-induced changes in human myelodysplastic
syndrome cell line SKM-1 are mediated by FOXO3A activation. J
Immunol Res. 2017:43023202017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu Y, Song Y, Xiong Y, Wang X, Xu K, Han
B, Bai Y, Li L, Zhang Y and Zhou L: MicroRNA-21 (Mir-21) promotes
cell growth and invasion by repressing tumor suppressor PTEN in
colorectal cancer. Cell Physiol Biochem. 43:945–958. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang WZ, Lin XH, Pu QH, Liu MY, Li L, Wu
LR, Wu QQ, Mao JW, Zhu JY and Jin XB: Targeting miR-21 sensitizes
Ph+ ALL Sup-b15 cells to imatinib-induced apoptosis through
upregulation of PTEN. Biochem Biophys Res Commun. 454:423–428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis
and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT
pathway in lung adenocarcinoma A549 cells. Biomed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhai Y, Zhang Y, Nan K and Liang X:
Reduced expression levels of PTEN are associated with decreased
sensitivity of HCC827 cells to icotinib. Oncol Lett. 13:3233–3238.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Piovan E, Yu J, Tosello V, Herranz D,
Ambesi-Impiombato A, Da Silva AC, Sanchez-Martin M, Perez-Garcia A,
Rigo I, Castillo M, et al: Direct reversal of glucocorticoid
resistance by AKT inhibition in acute lymphoblastic leukemia.
Cancer Cell. 24:766–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Clappier E, Gerby B, Sigaux F, Delord M,
Touzri F, Hernandez L, Ballerini P, Baruchel A, Pflumio F and
Soulier J: Clonal selection in xenografted human T cell acute
lymphoblastic leukemia recapitulates gain of malignancy at relapse.
J Exp Med. 208:653–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xue G, Zippelius A, Wicki A, Mandalà M,
Tang F, Massi D and Hemmings BA: Integrated Akt/PKB signaling in
immunomodulation and its potential role in cancer immunotherapy. J
Natl Cancer Inst. 107:djv1712015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Chen B, Wang Z, Zhang W, Hao K,
Chen Y, Li K, Wang T, Xie Y, Huang Z and Tong X: Marsdenia
tenacissimae extraction (MTE) inhibits the proliferation and
induces the apoptosis of human acute T cell leukemia cells through
inactivating PI3K/AKT/mTOR signaling pathway via PTEN enhancement.
Oncotarget. 7:82851–82863. 2016.PubMed/NCBI
|
|
47
|
Gao YH, Zhang HP, Yang SM, Yang Y, Ma YY,
Zhang XY and Yang YM: Inactivation of Akt by arsenic trioxide
induces cell death via mitochondrial-mediated apoptotic signaling
in SGC-7901 human gastric cancer cells. Oncol Rep. 31:1645–1652.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gu Y, Li A, Sun H, Li X, Zha H, Zhao J,
Xie J, Zeng Z and Zhou L: BCL6B suppresses proliferation and
migration of colorectal carcinoma cells through inhibition of the
PI3K/AKT signaling pathway. Int J Mol Med. 41:2660–2668.
2018.PubMed/NCBI
|