Introduction
Huntington's disease (HD) is a frequent and
incurable hereditary neurodegenerative disorder that impairs motor
and cognitive functions (1). With
autosomal dominant inheritance, typical mid-life onset, and
unrelenting, progressive motor, cognitive and psychiatric symptoms
over 15–20 years, the impact of HD on patients and their families
is devastating (2). Although
caused by a dominantly inherited CAG trinucleotide repeat expansion
in the HD gene on chromosome 4 (3), the pathogenesis of HD has not yet
been fully elucidated. Previous studies showed the number of CAG
repeats was associated with the age of onset; however, only 50 to
70% of the variation can be attributed to repeat size (4). In addition, significant variation in
clinical phenotypes is not well explained (5). Consequently, all these variations
indicate other pathogenic factors such as heredity affect the
disease progression. Recently, studies have focused on miRNAs, the
small non-coding RNAs that participate in transcriptional
regulation and translational repression of target genes (6). In HD, a neurodegenerative disease
caused by a trinucleotide repeat expansion, miRNAs can interact
with RelA/NFkB, p53 (7),
Mitofusin2 (8), TBP (9), REST, or RE1 (10,11).
Dysregulation of miRNA may also impact CAG length and affect the
progression or severity of HD (12). Conversely, a certain degree of
genetic heterogeneity of HD that may exhibit different miRNA
expression in some cases has also been reported (13). In addition to research on the
pathological mechanism, the role of miRNA in the treatment has been
a subject of interest. Currently, miRNA-based Huntingtin
(HTT)-lowering therapy is one of the most advanced gene strategies
(14), silencing the HD gene by
injecting artificial miRNA into the striatum of Q140/Q140 mice and
transgenic sheep models and achieving the expected effect (15,16).
However, bioinformatics studies focusing on miRNA and mRNA
expression profiles in HD patients and healthy controls have not
been published to date. Therefore, identifying the miRNA-mRNA
interactions, understanding their synergistic effects on the
pathogenesis, and exploring possible therapeutic approaches for HD
are important.
To elucidate the miRNAs and associated target genes
and pathways involved in HD, we downloaded miRNA and mRNA
expression profiles of HD patients and healthy controls from the
Gene Expression Omnibus (GEO) database. The differentially
expressed genes (DEGs) and differentially expressed miRNAs (DEMs)
target genes were identified and a miRNA-mRNA regulatory network
established.
Materials and methods
miRNA and mRNA expression
profiles
The mRNA and miRNA expression profiles of HD were
downloaded from the GEO database (www.ncbi.nlm.nih.gov/geo) and were termed GSE64810
(https://www.ncbi.nlm.nih.gov/gds/?term=GSE64810;
accessed August 8, 2017) and GSE64977 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64977;
accessed August 9, 2017), respectively (6,17).
In the GSE64810 dataset, 20 HD and 49 neurologically normal control
samples from post-mortem human subjects were included. In the
GSE64977 dataset, expression profiles were obtained from 28 HD and
36 neurologically normal control prefrontal cortex samples. Both
mRNA and miRNA profiling was performed using the Illumina HiSeq
2000 (Homo sapiens) platform (Illumina, Inc., San Diego, CA,
USA).
Differential expression analysis for
mRNA and miRNA expression profiling
Raw microarray data were first preprocessed
including background correction and normalization. The probes
corresponding to multiple genes were abandoned and multiple probes
corresponding to one gene or miRNA were used to calculate the
average expression level. Limma package (www.bioconductor.org/packages/release/bioc/html/limma.html)
was used to identify DEGs and DEMs between HD and control samples.
A two-tailed Student's t-test was used for statistical analysis.
The DEGs and DEMs were considered significantly differentially
expressed if the P-value was <0.05 and the log fold change (FC)
was >1. The miRNAs were further classified using hierarchical
clustering analysis.
Prediction of miRNAs associated with
DEGs and construction of miRNA gene regulatory network
Target genes regulated by DEMs were predicted using
TargetScan (http://www.targetscan.org/vert_71/), which is used to
predict biological targets of miRNAs by searching for the presence
of conserved 8mer, 7mer, and 6mer sites that match the seed region
of each miRNA (18). In mammals,
predictions are ranked based on the predicted efficacy of targeting
as calculated using cumulative weighted context ++ scores of the
sites (19). In the present study,
the miRNA target gene set with context ++ scores <-0.4 were then
used for further analysis. A Venn diagram of DEGs overlapping with
DEM target genes was constructed using FunRich (20). Furthermore, Cytoscape was utilized
to visualize the miRNA gene regulatory network (21).
Functional enrichment analyses
Functional enrichment analyses were performed using
the open software FunRich (http://funrich.org/faq), which is a stand-alone
software tool used mainly for functional enrichment and interaction
network analysis of genes and proteins (20). Statistical cut-off of enrichment
analyses in FunRich software was set to default with a P-value
<0.05 after Bonferroni correction.
Results
Differential expression analysis
Based on the gene expression analysis, 8 upregulated
and 2 downregulated DEMs were identified in HD compared with the
normal controls (Table I). Based
on the mRNA expression profile analysis, 1,612 DEGs were
identified, including 945 upregulated and 667 downregulated DEGs.
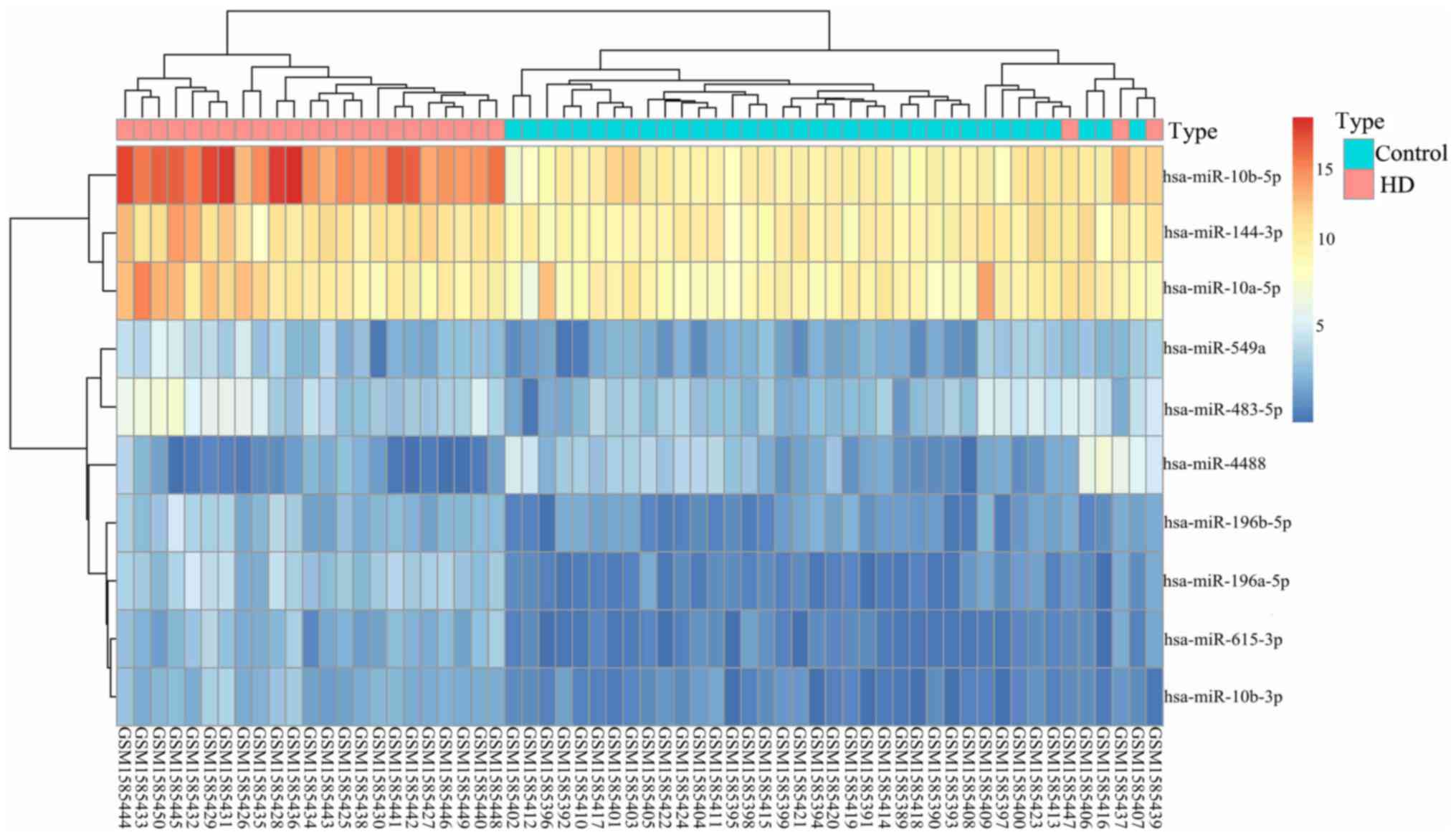
The top 10 DEGs are presented in Table II. The hierarchical clustering
heat map of miRNA shows the differences between HD and normal
controls (Fig. 1).
 | Table I.Differentially expressed miRNAs
between HD patients and healthy controls. |
Table I.
Differentially expressed miRNAs
between HD patients and healthy controls.
| miRNA | Log FC | P-value |
|---|
| hsa-miR-10b-5p | 4.175 | 9.10×10–23 |
|
hsa-miR-196a-5p | 2.430 | 5.85×10–22 |
| hsa-miR-615-3p | 1.678 | 2.12×10–17 |
| hsa-miR-10b-3p | 1.480 | 4.79×10–14 |
|
hsa-miR-196b-5p | 1.439 | 6.62×10–11 |
| hsa-miR-144-3p | 1.045 | 2.77×10-6 |
| hsa-miR-549a | 1.111 | 2.39×10-5 |
| hsa-miR-483-5p | 1.256 | 2.49×10-4 |
| hsa-miR-10a-5p | 1.042 | 6.0×10-4 |
| hsa-miR-4488 | −1.193 | 2.54×10-3 |
 | Table II.Top 10 most differentially expressed
mRNAs between HD patients and healthy controls. |
Table II.
Top 10 most differentially expressed
mRNAs between HD patients and healthy controls.
| Gene | Log FC | P-value |
|---|
| PITX1 | 4.770 | 9.57×10–39 |
| POU4F2 | 3.962 | 3.42×10–23 |
| HAND1 | 3.703 | 1.46×10–17 |
| HOXD9 | 3.657 | 1.22×10–18 |
| SLC16A12 | 3.514 | 4.74×10–18 |
| PITX2 | 3.404 | 1.66×10–12 |
| BMP5 | 3.149 | 5.93×10–13 |
| OGN | 3.097 | 8.20×10–14 |
| SLC22A2 | 3.089 | 6.93×10–11 |
| IL1R2 | 3.038 | 3.35×10–12 |
Functional enrichment analysis
Using FunRich, 48 enriched GO terms were obtained
for DEGs. The 10 most significantly enriched GO terms are listed in
Table III, including integral
components of the plasma membrane, inflammatory response, plasma
membrane, and immune response. Furthermore, the most significantly
enriched GO terms including biological processes (BP), molecular
function (MF), and cellular component (CC) were analyzed (Fig. 2).
 | Table III.Top 10 GO functional annotation of
differentially expressed genes. |
Table III.
Top 10 GO functional annotation of
differentially expressed genes.
| GO ID | P-value | Term |
|---|
| GO:0005887 | 6.55×10–21 | Integral component
of plasma membrane |
| GO:0006954 | 1.54×10–19 | Inflammatory
response |
| GO:0005886 | 4.77×10–19 | Plasma
membrane |
| GO:0006955 | 3.86×10–19 | Immune
response |
| GO:0005576 | 4.51×10–18 | Extracellular
region |
| GO:0005615 | 1.43×10–17 | Extracellular
space |
| GO:0045087 | 1.65×10–15 | Innate immune
response |
| GO:0009952 | 1.61×10–14 | Anterior/posterior
pattern specification |
| GO:0043565 | 1.22×10–11 | Sequence-specific
DNA binding |
| GO:0042742 | 4.73×10–10 | Defense response to
bacterium |
Synergistic miRNA network
construction
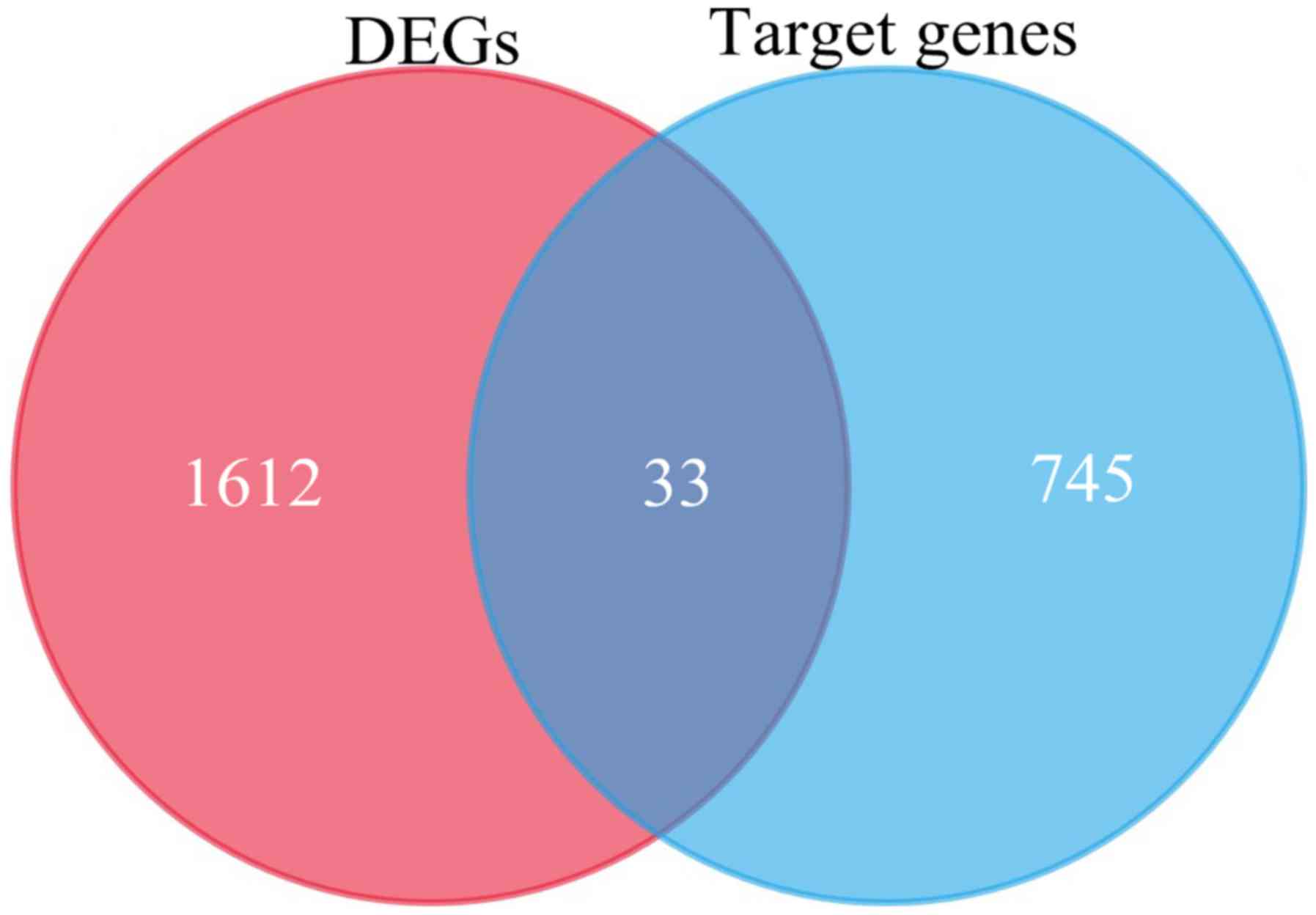
Utilizing TargetScan, 745 target genes of DEMs and
33 overlaps were identified between the target genes and DEGs
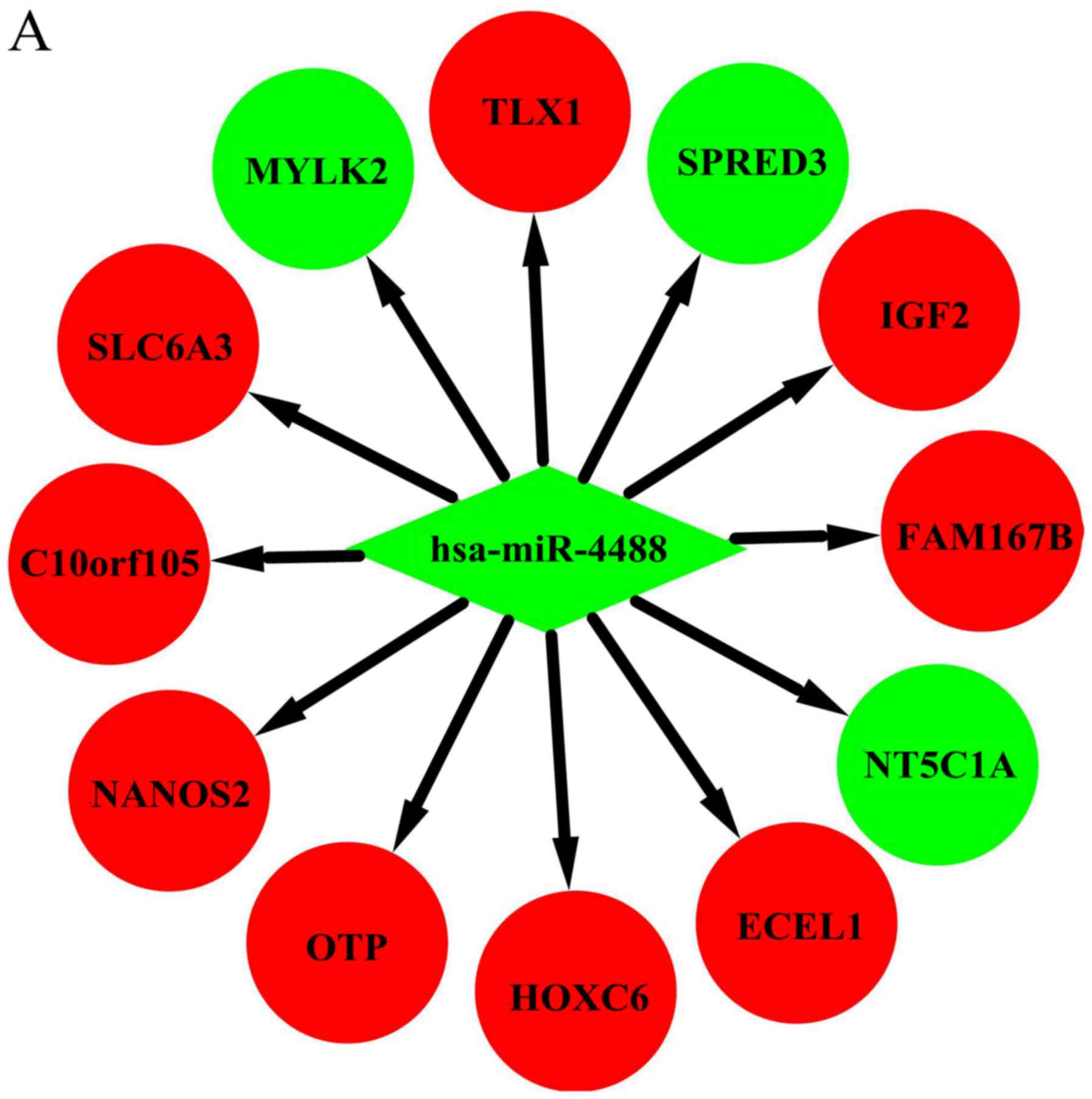
(Fig. 3). We identified 33
possible miRNA-mRNA target pairs. In the network, hsa-miR-4488,
hsa-miR-196a-5p, and hsa-miR-549a had a high degree and may be
involved in the pathogenesis and used as potential therapeutic
targets for HD (Fig. 4).
Discussion
HD is an inherited progressive neurodegenerative
disorder that usually affects people in midlife (22). Previous studies have implicated
that gene expression may be altered at more than one stage of RNA
processing, translation, protein post-transcriptional trafficking
or modifications (23). miRNAs are
small non-coding RNAs that can influence diverse ranges of cellular
processes (24). Recently,
dysregulation of miRNAs has been reportedly associated with
neurological and neurodegenerative disorders (25) and several studies have explored the
functions of miRNAs in HD patients (10,26).
Although altered expression of miRNAs has been reported in cellular
models (10) and mouse models of
HD (27) using quantified
microarray technology, a bioinformatics study of miRNA and mRNA
expression profiles in HD patients and healthy controls has not yet
been performed. In the present study, we elucidated the synergistic
effects of miRNAs on the pathogenesis of HD by constructing a
miRNA-mRNA interaction network.
Using a bioinformatics approach to analyze
differential expression profiles of mRNA and miRNA, HD patients
showed significant differences in mRNA and miRNA expression when
compared with neurologically normal controls. In total, 1,612 DEGs
and 10 DEMs were identified. GO function (BP, CC, and MF) and
biological pathway analysis of DEGs demonstrated the majority of
genes were involved in some processes and pathways, such as signal
transduction in BP (28), plasma
membrane in CC (29),
transcription factor activity in MF (30), and immune system in biological
pathway (28). Changes in
Ca2+ signaling and/or transduction systems and misfolded
protein-plasma membrane interactions affected HD initiation and
progression as reported by Fan and Shrivastava (29,31).
Many previous studies showed that aberrant transcriptional
regulations play important roles in the molecular pathogenesis of
HD. The HTT gene interferes with some important transcription
factors (32); among these, Sp1
and TAFII130 were shown disrupted in the early stage of HD
progression (33). Furthermore,
both innate and adaptive immune systems have been previously
suggested to be activated during the progression of HD (34). Reportedly, HD is characterized by
cellular and molecular features of inflammation (cytokine
expression and microglia activation) and HTT mRNA expression in
immune cells is on average higher than that observed in most organs
(35). miRNAs may also influence
the dysregulated production of both type 1 and type 2 cytokines
observed at different stages of HD. This result is attracting
attention as pathogenetic mechanism and as possible therapeutic
approach with immunomodulation (36). Combined with the previous research
conclusions, the present functional analysis may provide novel
therapeutic targets or possible pathogenesis to be further
studied.
In the context of the miRNA-mRNA interaction
network, hsa-miR-4488, hsa-miR-196a-5p, and hsa-miR-549a had a high
degree in this study (Table IV).
Hsa-miR-4488 was downregulated and had 12 target genes in our
study. Lee et al also found that miR-4488 was differentially
downregulated in their HD patients (27). Among the target genes of
hsa-miR-4488 in the present study, SPRED3 (37), IGF2 (38), HOXC6 (39), NANOS2 (40), SLC6A3 (41), TLX1 (42), and NT5C1A (43) have been reported to play various
roles in the pathogenesis or development of HD. Although we could
not determine the association of FAM167B, ECEL1, OTP, C10orf105, or
MYLK2 with HD, further experimental studies are necessary to
elucidate their possible relationships with HD. Previous studies
also showed hsa-miR-196a plays an important role in the
pathogenesis and progression of HD (44), Fu et al suggested that
hsa-miR-196a altered the RIG-I-like receptor signaling pathway and
the immune system, as well as changed the expression of several
well-defined pathways of HD, such as apoptosis and cell adhesion
(45). A previous study showed
that HOX is indirectly involved in the neuroprotective response in
HD and increased expression of HOX genes can enhance H3K27me3 or
impair PcG repression (39). In
our present study, hsa-miR-196a-5p was found to mainly interact
with HOX genes, indicating hsa-miR-196a-5p may be involved in the
neuroprotective response in HD. Reportedly, miR-196a dominantly
altered the immune system or adaptive immune system (45). During the last decade, a
hyperreactive immune system has been recognized as an important
feature of HD pathogenesis. Macrophages in individuals with
manifested HD and even pre-manifested HD have been reported to
release more proinflammatory cytokines such as TNF-alpha (46) and prototypical anti-inflammatory
cytokines such as IL-10 (47) and
IL-13 (48). Similarly, Dobson
et al showed that at baseline, monocytes from HD subjects
released more cytokines than monocytes isolated from healthy
volunteers, and this abnormality could be modulated by laquinimod,
which exerts an immunomodulatory effect on isolated HD monocytes
(49).
 | Table IV.Some of the miRNAs associated with
HD. |
Table IV.
Some of the miRNAs associated with
HD.
| Author, year | miRNA | Change in
expression | Role in HD | Target genes | (Refs.) |
|---|
| Burgunder J-M et
al, 2014 | miR-144-3p | Upregulated | Not found | GPR183, MAP3K8,
MSX1 | (4) |
|
| miR-10b-3p | Upregulated | Influence CAG
length in HD | HOXD1, ZG16B |
|
|
| miR-10a-5p | Upregulated | Not found | HOXB3, TFAP2C |
|
|
| miR-483-5p | Upregulated | Not found | MZB1 |
|
| Traeger U et al,
2014; Shrivastava AN et al, 2017; van Hagen M et al, 2017; Fan MMY
et al, 2007; Moumne L et al, 2013; Dunah AW et al, 2002; Squitieri
F et al, 2006 | miR-4488 | Downregulated | Post-translational
protein modification; post-transcriptional regulation by preventing
translational initiation; | SPRED3, IGF2,
HOXC6, NANOS2, SLC6A3, TLX1, NT5C1A, FAM167B, ECEL1, OTP,
C10orf105, MYLK2 | (28–34) |
| Butland SL et al,
2014; Salem L et al, 2016; Hoss AG et al, 2014; Rokavec M et al,
2014 | miR-549a | Upregulated | Regulate
transcription factors, affect autophagy and endoplasmic reticulum
stress pathway | CCDC117, GMNN,
LAPTM5, RGS18, TNFAIP8 | (37–40) |
| Cheng P-H et al,
2013; Fu M-H et al, 2015; Zou J et al, 2015 | miR-196a | Upregulated | Neuroprotective
effect by interacting with HOX genes; affects the entire immune
system leading to disorder of cytokine secretion | GPCPD1, HAND1,
HOXA5, HOXA7, HOXA9, HOXB6, HOXB7, HOXC8 | (44–46) |
In addition, we found another miRNA, hsa-miR-549a,
was upregulated in HD patients and could regulate five target
genes. When reviewing the literature, we found that GMNN (50), TNFAIP18 (51), LAPTM5 (52), and RGS18 (53) played roles in the pathogenesis of
neurodegenerative diseases through various mechanisms, including
regulation of transcription factors and affecting autophagy and
endoplasmic reticulum stress pathways and might be involved in the
pathogenesis of HD. Other affected miRNAs found in our study
including hsa-miR-144-3p, hsa-miR-10b-3p, hsa-miR-10a-5p, and
hsa-miR-483-5p have been reported as differentially expressed in HD
samples; hsa-miR-10b-3p showed a significant association with CAG
length in HD (6). Regarding the
other three miRNAs, more research is be needed to identify their
exact relationships with HD.
Although extensive research has identified
aberrantly expressed miRNAs in HD, the molecular mechanisms
underlying the pathological implications remain largely unknown.
Using expression profile datasets, we compared the genomic
expression status of HD and revealed differentially expressed mRNAs
and DEMs. We identified DEGs and constructed a miRNA-mRNA
regulatory network. We found hsa-miR-4488, hsa-miR-196a-5p, and
hsa-miR-549a had a high degree and may be involved in the
pathogenesis of HD. Studies in this field could help improve the
understanding of how miRNAs mediate the etiopathological mechanisms
of HD. Since the neuroprotective effects of certain miRNAs have
been demonstrated in animal studies, the therapeutic potential of
miRNAs should be further investigated and followed by molecular
validation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371271) and was
sponsored by the Liaoning Bai Qian Wan Talents Program [grant no.
(2015)41].
Availability of data and materials
The datasets analyzed in the present study are
available in the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64977
and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64810.
Authors' contributions
XD designed the research, performed data and
statistical analysis, and drafted the manuscript. SC conceived and
designed the research, and revised the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Shengjing Hospital of China Medical University,
Liaoning, China.
Patient consent for publication
Written informed consent was obtained from all
volunteers for the publication of the associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HD
|
huntington disease
|
|
miRNA
|
microRNA
|
|
DEMs
|
differentially expressed miRNAs
|
|
DEGs
|
differentially expressed genes
|
|
PPI
|
protein-protein interaction
|
|
GEO
|
Gene Expression Omnibus
|
|
GO
|
gene ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
FC
|
fold change
|
|
MCODE
|
molecular complex detection
|
|
BP
|
biological processes
|
|
MF
|
molecular function
|
|
CC
|
cellular component
|
References
|
1
|
Huang WJ, Chen WW and Zhang X:
Huntington's disease: Molecular basis of pathology and status of
current therapeutic approaches. Exp Ther Med. 12:1951–1956. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghosh R and Tabrizi SJ: Clinical features
of Huntington's disease. Adv Exp Med Biol. 1049:1–28. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McColgan P and Tabrizi SJ: Huntington's
disease: A clinical review. Eur J Neurol. 25:24–34. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burgunder JM: Genetics of Huntington's
disease and related disorders. Drug Discov Today. 19:985–989. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Djoussé L, Knowlton B, Hayden M, Almqvist
EW, Brinkman R, Ross C, Margolis R, Rosenblatt A, Durr A, Dode C,
et al: Interaction of normal and expanded CAG repeat sizes
influences age at onset of Huntington disease. Am J Med Genet A.
119A:279–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoss AG, Labadorf A, Latourelle JC, Kartha
VK, Hadzi TC, Gusella JF, MacDonald ME, Chen JF, Akbarian S, Weng
Z, et al: miR-10b-5p expression in Huntington's disease brain
relates to age of onset and the extent of striatal involvement. BMC
Med Genomics. 8:102015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghose J, Sinha M, Das E, Jana NR and
Bhattacharyya NP: Regulation of miR-146a by RelA/NFkB and p53 in
STHdh(Q111)/Hdh(Q111) cells, a cell model of Huntington's disease.
PLos One. 6:e238372011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bucha S, Mukhopadhyay D and Bhattacharyya
NP: Regulation of mitochondrial morphology and cell cycle by
microRNA-214 targeting Mitofusin2. Biochem Biophys Res Commun.
465:797–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinha M, Ghose J, Das E and Bhattarcharyya
NP: Altered microRNAs in STHdh(Q111)/Hdh(Q111) cells: miR-146a
targets TBP. Biochem Biophys Res Commun. 396:742–747. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson R, Zuccato C, Belyaev ND, Guest
DJ, Cattaneo E and Buckley NJ: A microRNA-based gene dysregulation
pathway in Huntington's disease. Neurobiol Dis. 29:438–445. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Packer AN, Xing Y, Harper SQ, Jones L and
Davidson BL: The bifunctional microRNA miR-9/miR-9* regulates REST
and CoREST and is downregulated in Huntington's disease. J
Neurosci. 28:14341–14346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Langfelder P, Gao F, Wang N, Howland D,
Kwak S, Vogt TF, Aaronson JS, Rosinski J, Coppola G, Horvath S and
Yang XW: MicroRNA signatures of endogenous Huntingtin CAG repeat
expansion in mice. PLoS One. 13:e01905502018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kartsaki E, Spanaki C, Tzagournissakis M,
Petsakou A, Moschonas N, Macdonald M and Plaitakis A: Late-onset
and typical Huntington disease families from Crete have distinct
genetic origins. Int J Mol Med. 17:335–346. 2006.PubMed/NCBI
|
|
14
|
Miniarikova J, Evers MM and Konstantinova
P: Translation of MicroRNA-based huntingtin-lowering therapies from
preclinical studies to the Clinic. Mol Ther. 26:947–962. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keeler AM, Sapp E, Chase K, Sottosanti E,
Danielson E, Pfister E, Stoica L, DiFiglia M, Aronin N and
Sena-Esteves M: Cellular analysis of silencing the Huntington's
disease gene using AAV9 mediated delivery of artificial micro rna
into the striatum of Q140/Q140 mice. J Huntingtons Dis. 5:239–248.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfister EL, DiNardo N, Mondo E, Borel F,
Conroy F, Fraser C, Gernoux G, Han X, Hu D, Johnson E, et al:
Artificial miRNAs reduce human mutant huntingtin throughout the
striatum in a transgenic sheep model of Huntington's disease. Hum
Gene Ther. Feb 23–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Labadorf A, Hoss AG, Lagomarsino V,
Latourelle JC, Hadzi TC, Bregu J, MacDonald ME, Gusella JF, Chen
JF, Akbarian S, et al: Correction: RNA sequence analysis of human
huntington disease brain reveals an extensive increase in
inflammatory and developmental gene expression. PLoS One.
11:e01602952016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar :
|
|
20
|
Pathan M, Keerthikumar S, Ang CS, Gangoda
L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim
A, et al: FunRich: An open access standalone functional enrichment
and interaction network analysis tool. Proteomics. 15:2597–2601.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lund E, Guttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cha JH: Transcriptional signatures in
Huntington's disease. Prog Neurobiol. 83:228–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Junn E and Mouradian MM: MicroRNAs in
neurodegenerative diseases and their therapeutic potential.
Pharmacol Ther. 133:142–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin J, Cheng Y, Zhang Y, Wood W, Peng Q,
Hutchison E, Mattson MP, Becker KG and Duan W: Interrogation of
brain miRNA and mRNA expression profiles reveals a molecular
regulatory network that is perturbed by mutant huntingtin. J
Neurochem. 123:477–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee ST, Chu K, Im WS, Yoon HJ, Im JY, Park
JE, Park KH, Jung KH, Lee SK, Kim M and Roh JK: Altered microRNA
regulation in Huntington's disease models. Exp Neurol. 227:172–179.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Träger U, Andre R, Lahiri N,
Magnusson-Lind A, Weiss A, Grueninger S, McKinnon C,
Sirinathsinghji E, Kahlon S, Pfister EL, et al: HTT-lowering
reverses Huntington's disease immune dysfunction caused by NFκB
pathway dysregulation. Brain. 137:819–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shrivastava AN, Aperia A, Melki R and
Triller A: Physico-pathologic mechanisms involved in
neurodegeneration: Misfolded protein-plasma membrane interactions.
Neuron. 95:33–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Hagen M, Piebes DGE, de Leeuw WC,
Vuist IM, van Roon-Mom WMC, Moerland PD and Verschure PJ: The
dynamics of early-state transcriptional changes and aggregate
formation in a Huntington's disease cell model. BMC Genomics.
18:3732017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan MM and Raymond LA:
N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in
Huntington's disease. Prog Neurobiol. 81:272–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moumne L, Betuing S and Caboche J:
Multiple aspects of gene dysregulation Huntington's disease. Front
Neurol. 4:1272013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dunah AW, Jeong H, Griffin A, Kim YM,
Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N and
Krainc D: Sp1 and TAFII130 transcriptional activity disrupted in
early Huntington's disease. Science. 296:2238–2243. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Squitieri F, Cannella M, Sgarbi G,
Maglione V, Falleni A, Lenzi P, Baracca A, Cislaghi G, Saft C,
Ragona G, et al: Severe ultrastructural mitochondrial changes in
lymphoblasts homozygous for Huntington disease mutation. Mech
Ageing Dev. 127:217–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crotti A and Glass CK: The choreography of
neuroinflammation in Huntington's disease. Trends Immunol.
36:364–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Björkqvist M: lmmunomodulation-a
disease-modifying avenue for treatment of Huntington's disease? J
Neurochem. 137:670–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Butland SL, Sanders SS, Schmidt ME,
Riechers SP, Lin DT, Martin DD, Vaid K, Graham RK, Singaraja RR,
Wanker EE, et al: The palmitoyl acyltransferase HIP14 shares a high
proportion of interactors with huntingtin: implications for a role
in the pathogenesis of Huntington's disease. Hum Mol Genet.
23:4142–4160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salem L, Saleh N, Désaméricq G, Youssov K,
Dolbeau G, Cleret L, Bourhis ML, Azulay JP, Krystkowiak P, Verny C,
et al: Insulin-like growth factor-1 but not insulin predicts
cognitive decline in Huntington's disease. PLoS One.
11:e01628902016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoss AG, Kartha VK, Dong X, Latourelle JC,
Dumitriu A, Hadzi TC, Macdonald ME, Gusella JF, Akbarian S, Chen
JF, et al: MicroRNAs located in the Hox gene clusters are
implicated in Huntington's disease pathogenesis. PLoS Genet.
10:e10041882014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rokavec M, Li H, Jiang L and Hermeking H:
The p53/miR-34 axis in development and disease. J Mol Cell Biol.
6:214–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shapshak P: Molecule of the month: miRNA
and proteins DARPP-32, DRD1, SLC6A3, and CK2. Bioinformation.
9:274–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ooi L and Wood IC: Regulation of gene
expression in the nervous system. Biochem J. 414:327–341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lloyd TE, Christopher-Stine L,
Pinal-Fernandez I, Tiniakou E, Petri M, Baer A, Danoff SK, Pak K,
Casciola-Rosen LA and Mammen AL: Cytosolic 5′-nucleotidase 1A As a
target of circulating autoantibodies in autoimmune diseases.
Arthritis Care Res (Hoboken). 68:66–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng PH, Li CL, Chang YF, Tsai SJ, Lai
YY, Chan AW, Chen CM and Yang SH: miR-196a ameliorates phenotypes
of Huntington disease in cell, transgenic mouse, and induced
pluripotent stem cell models. Am J Hum Genet. 93:306–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu MH, Li CL, Lin HL, Tsai SJ, Lai YY,
Chang YF, Cheng PH, Chen CM and Yang SH: The potential regulatory
mechanisms of miR-196a in Huntington's disease through
bioinformatic analyses. PLoS One. 10:e01376372015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zou J, Guo P, Lv N and Huang D:
Lipopolysaccharide-induced tumor necrosis factor-α factor enhances
inflammation and is associated with cancer (Review). Mol Med Rep.
12:6399–6404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gerard C, Bruyns C, Marchant A, Abramowicz
D, Vandenabeele P, Delvaux A, Fiers W, Goldman M and Velu T:
Interleukin 10 reduces the release of tumor necrosis factor and
prevents lethality in experimental endotoxemia. J Exp Med.
177:547–550. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nicoletti F, Mancuso G, Cusumano V, Di
Marco R, Zaccone P, Bendtzen K and Teti G: Prevention of
endotoxin-induced lethality in neonatal mice by interleukin-13. Eur
J Immunol. 27:1580–1583. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dobson L, Träger U, Farmer R, Hayardeny L,
Loupe P, Hayden MR and Tabrizi SJ: Laquinimod dampens hyperactive
cytokine production in Huntington's disease patient myeloid cells.
J Neurochem. 137:782–794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee HK, Lee HS and Moody SA: Neural
transcription factors: From embryos to neural stem cells. Mol
Cells. 37:705–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stefani IC, Wright D, Polizzi KM and
Kontoravdi C: The role of ER stress-induced apoptosis in
neurodegeneration. Curr Alzheimer Res. 9:373–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Glowacka WK, Alberts P, Ouchida R, Wang JY
and Rotin D: LAPTM5 protein is a positive regulator of
proinflammatory signaling pathways in macrophages. J Biol Chem.
287:27691–27702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Raju HB, Tsinoremas NF and Capobianco E:
Emerging putative associations between non-coding RNAs and
protein-coding genes in neuropathic pain: Added value from reusing
microarray data. Front Neurol. 7:1682016. View Article : Google Scholar : PubMed/NCBI
|