Introduction
The mandible is an especially dynamic bone. Despite
its fundamental similarity to other bones, the mandible performs
distinct functions and responds differently to mechanical,
developmental, and homeostatic stimuli (1). Diverse differential and osteogenic
potentials have been confirmed between mandible- and long
bone-derived cells (2–4). Many bone defect diseases, such as
osteomyelitis, bone cyst, periodontitis and trauma, involve this
bone. Therefore, it is very important to evaluate the responses to
external stimuli and the remodelling and healing potential of the
mandible.
Icariin (ICA), the major effective ingredient of the
plant herb Epimedium, is used to improve kidney yang in
traditional Chinese medicine. Many reports have indicated that ICA
can attenuate inflammation (5) and
restore osteogenesis (6). ICA can
stimulate the proliferation and osteoblastic differentiation of
mesenchymal stem cells or long bone-derived cells through the
Wnt/β-catenin signalling pathway (6–9).
However, there are few reports about the effect and mechanism of
ICA on the differentiation and proliferation of osteoblastic cells
from the mandible.
Therefore, this study mainly aimed to determine the
effect of ICA on the proliferation and differentiation of
osteoblastic cells isolated from the rat mandible and to determine
whether the Wnt/β-catenin pathway participates in this effect.
Materials and methods
Materials
ICA was purchased from the National Institute for
the Control of Pharmaceutical and Biological Products (Beijing,
China); Dickkopf-1 (DKK-1) was purchased from PeproTech, Inc.
(Rocky Hill, NJ, USA); foetal bovine serum (FBS) was obtained from
Gibco, USA; high glucose Dulbecco's modified Eagle medium (H-DMEM),
penicillin, streptomycin and trypsin were purchased from Hangzhou
Jinuo Biotechnology Co., Ltd. (Hangzhou, China); all the plates and
flasks used for cell culture were purchased from Corning, USA; an
alkaline phosphatase (ALP) staining kit, Cell Counting kit-8
(CCK-8) and ALP activity detection kit were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China).
Isolation of osteoblastic cells from a
rat mandible
A new born C57 rat was sacrificed and immersed in
70% ethanol for 10 min; then, the mandible body was isolated from
the soft tissue, washed with phosphate-buffered saline (PBS;
containing 200 IU/ml penicillin and 200 µg/ml streptomycin) three
times and cut into small pieces with diameters between 1 and 2 mm.
Two millilitres of 0.25% trypsin with ethylenediaminetetraacetic
acid was added to the tube containing the mandible pieces, which
were incubated for 30 min in a 37°C water bath and then centrifuged
at 1,100 r/min. The pellet was collected, and PBS was added for 5
additional centrifugations. The pellet was finally resolved in
H-DMEM containing 15% FBS, 200 IU/ml penicillin and 200 µg/ml
streptomycin and cultured in an incubator (37°C, 5% CO2)
for 20 min. Rarefied osteoblastic cells were obtained after the
fibroblasts with differential attachment were removed. These cells
were maintained in H-DMEM with 15% FBS, 200 IU/ml penicillin, and
200 µg/ml streptomycin, and 5 mM β-glycerophosphate, 100 mM
L-ascorbic acid, and 0.1 mM dexamethasone were added for
differentiation experiments. The growth situation of the
osteoblastic cells was checked by cytometry. ALP staining and
alizarin red staining were used to check the differentiation and
mineralization of the isolated osteoblastic cells afterwards.
The present study was carried out in accordance with
the recommendations of the National Institutes of Health Guide for
the Care and Use of Experimental Animals. The protocol was approved
by the Ethics Committee of Beijing Stomatological Hospital
(Beijing, China).
Evaluation of the effects of ICA on
osteoblastic cell proliferation and differentiation
Twenty micrograms of ICA (powder) was dissolved in 1
ml of dimethyl sulfoxide (DMSO) and stored at −20°C. Later, a
series of working concentrations (0, 0.0015, 0.015, 0.15, 1.5, 15,
30, 60 and 100 µM) was obtained from this stock. Cells in the
logarithmic growth phase were digested by trypsin.
For CCK-8 measurement, cells were seeded in 96-well
plates at 4,000 cells per well. Forty-eight h later, different
working concentrations of ICA were added to the well, and the
control sample was treated with DMSO. All the cells were then
divided into three groups according to the incubation period,
namely, 24, 48 and 72 h. After a certain incubation time, the
medium was aspirated, and 100 µl of DMEM with 10 µl of CCK-8
solution was added. According to the manufacturer's instructions,
after 2 h, the optical density (OD) value of each sample was
determined by a microplate reader at a wavelength of 450 nm.
For the ALP activity measurement, cells were seeded
into 12-well plates at 50,000 cells per well. After a certain
incubation period with ICA, the cells were harvested into 1.5-ml
Eppendorf (EP) tubes and lysed by 1% Triton X-100 for 30 min. The
supernatant was then collected after centrifugation at 12,000 r/min
for 5 min, the concentration of the total protein was determined
using the bicinchoninic acid (BCA) method, and the ALP activity was
measured using the ALP activity detection kit according to the
manufacturer's procedure.
Expression of the mRNA of markers
related to the Wnt/β-catenin signalling pathway
Ten micrograms of DKK-1 was dissolved in 5 ml of PBS
(containing 10% FBS), and the working concentration of DKK-1 was
0.1 µg/ml. Osteoblastic cells were seeded in 35-mm dishes at
1×105/ml and cultured for 3 days. Then, the cells were
treated with 1.5 µM ICA, 0.1 µg/ml DKK-1 or their mixture, and
DMSO-treated cells were used as a control. The cells were again
divided into three groups with incubation times of 24, 48 and 72 h.
After each incubation period, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) was performed to determine the
gene expression level of the marker proteins, including β-catenin,
cyclin D1, runt-related transcription factor 2 (RUNX2) and ALP.
Total RNA was isolated using TRIzol reagent (Sunbio, Beijing,
China) according to the manufacturer's instructions. cDNA was
synthesized using a cDNA synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) following the manufacturer's
protocol. The cDNA was then amplified by RT-qPCR with the SYBR
Premix Ex Taq kit (Takara Biotechnology, Otsu, Japan) and the
following thermocycling conditions: 95°C for 10 min, followed by 45
cycles of 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The
primers used are shown in Table I.
Each sample was assessed in triplicate and analysed using the
2−ΔΔCq method (10).
 | Table I.Primers used to amplify β-catenin,
RUNX2, ALP and cyclin D1. |
Table I.
Primers used to amplify β-catenin,
RUNX2, ALP and cyclin D1.
| Primer | Sequence (5′-3′) |
|---|
| β-catenin | Forward:
TGCTGAAGGTGCTGTCTGTC |
|
| Reverse:
TCGCTGACTTGGGTCTGTC |
| RUNX2 | Forward:
CCTCTGACTTCTGCCTCTGG |
|
| Reverse:
ATGAAATGCTTGGGAACTGC |
| ALP | Forward:
CTTGCTGGTGGAAGGAGGCAGG |
|
| Reverse:
GGAGCACAGGAAGTTGGGAC |
| Cyclin D1 | Forward:
CAGAAGTGCGAAGAGGAGGT |
|
| Reverse:
GCAGTCAAGGGAATGGTCTC |
Data analysis and statistical
methods
The data were analysed using GraphPad 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Every type of
experiment was repeated 4 times, and the results were presented as
the mean ± standard deviation. The results were first checked for
normality and homogeneity using a Kolmogorov-Smirnov test. A t-test
was used to compare two independent groups. For experiments divided
into several groups, statistical analysis was performed by one-way
analysis of variance with Tukey's post hoc pairwise comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Isolation of osteoblastic cells from a
rat mandible
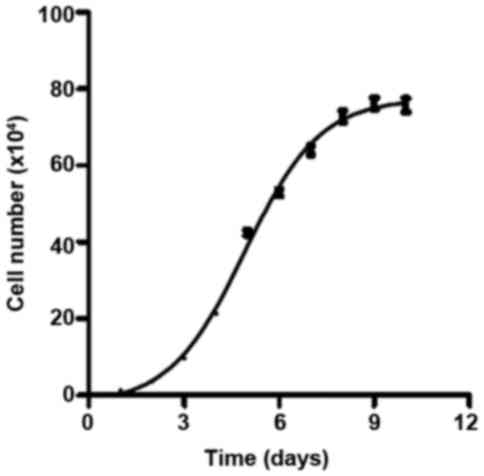
The growth curve of osteoblastic cells isolated from
a rat mandible was obtained (Fig.
1). The curve met the criteria for a classic cell growth curve,
which contains four phases: lag phase, log phase, stationary phase
and death phase. The cells were in the log phase between day 2 and
day 6; therefore, this period was selected for cell treatments.
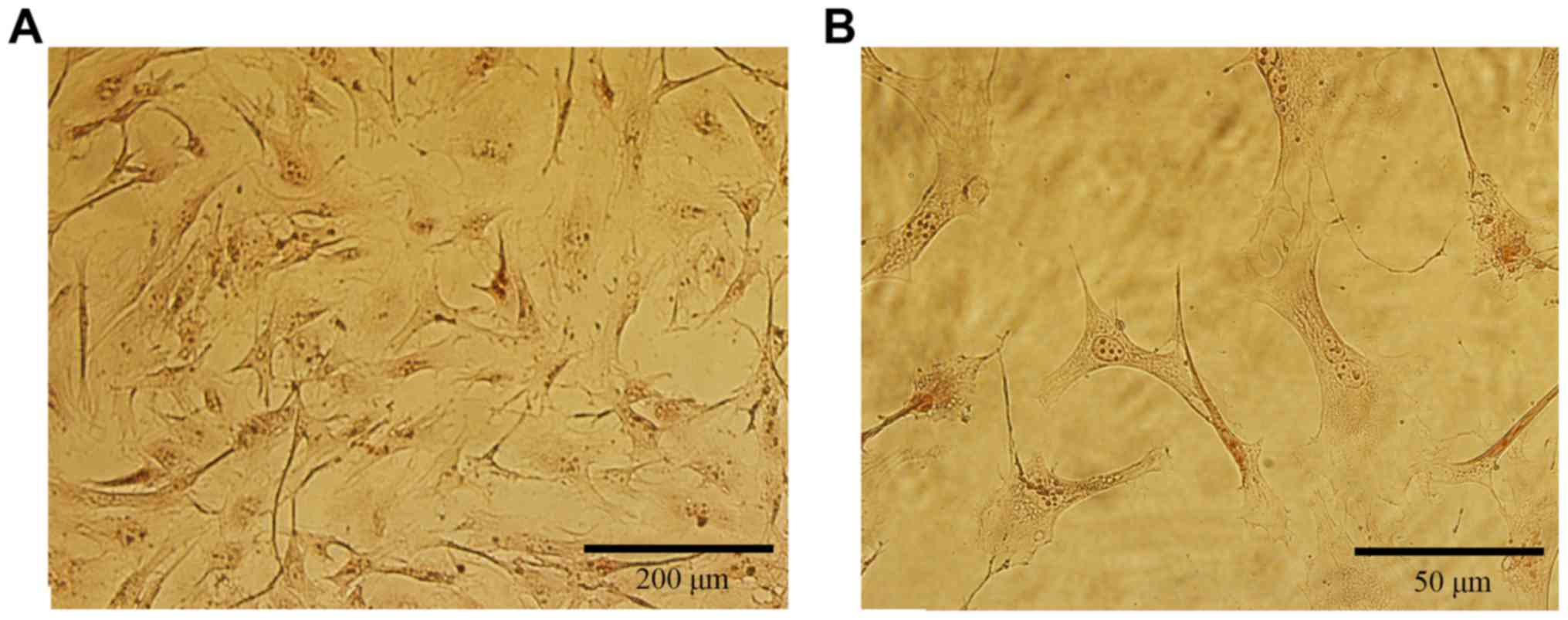
We performed ALP staining by using the ALP activity
detection kit. Over 95% of the osteoblasts were stained by the ALP
marker (Fig. 2), indicating that
these cells were healthy enough to express ALP. The osteoblastic
cells showed a typical morphology with a fibroblast appearance:
Most showed an irregular fusiform shape. These cells also firmly
adhered to the plate.
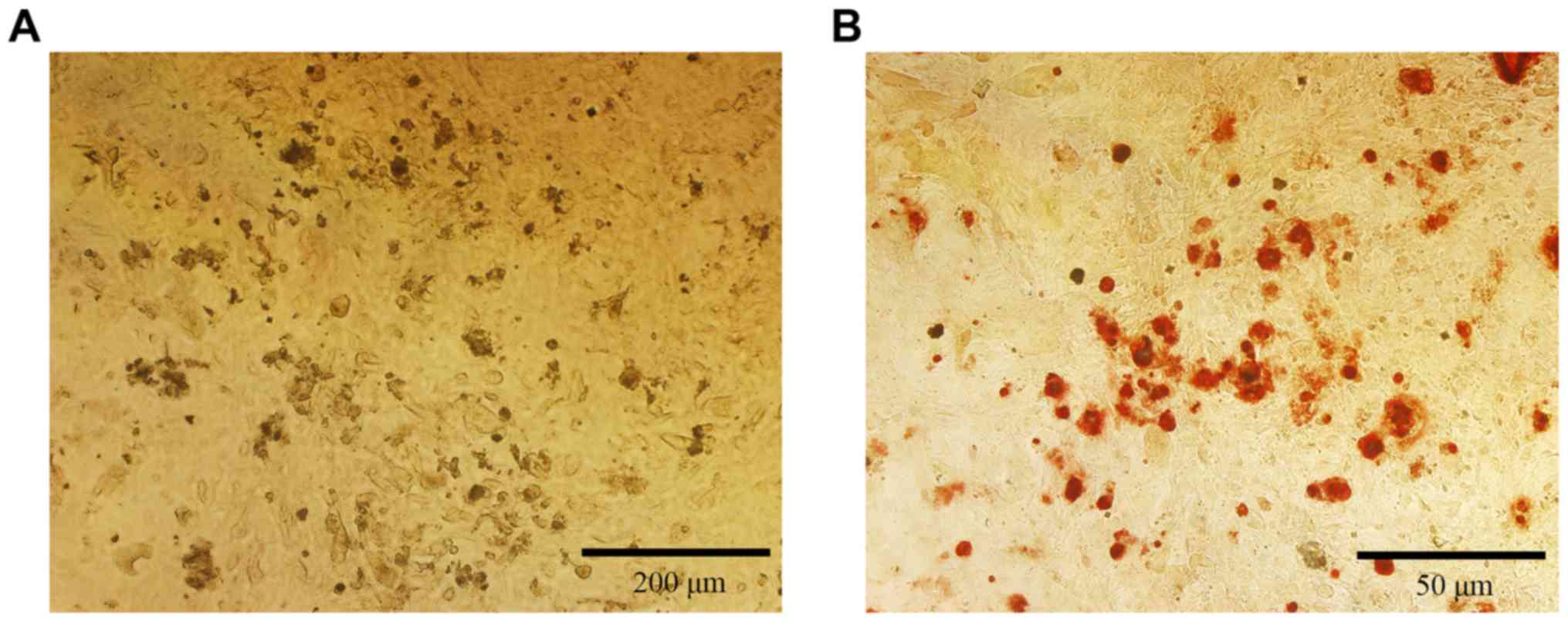
The mineralization of the isolated osteoblastic
cells was checked by alizarin red staining. After 21 days of
culture, many red dots were visible under a microscope (Fig. 3). Those dots were the mineralized
calcium nodules, suggesting that the osteoblasts can grow well
until mineralization and maturation.
ICA influences the proliferation and
differentiation of osteoblastic cells
The proliferation level of osteoblastic cells in
groups with different stimuli and treatment times was determined by
the CCK-8 method (Fig. 4). The
cells treated with DMSO were used as a blank control under this
circumstance. ICA just can promote the proliferation of
osteoblastic cells modestly after treatment for 24 h at
concentrations between 0.0015 and 60 µM. In regard to 48 h of
incubation, 0.0015 µM ICA promoted the proliferation of
osteoblastic cells significantly. With continued culture to 72 h,
ICA at concentrations between 0.15 and 15 µM significantly promoted
the proliferation of osteoblastic cells. However, 100 µM ICA
significantly suppressed the proliferation of osteoblastic cells at
all time points.
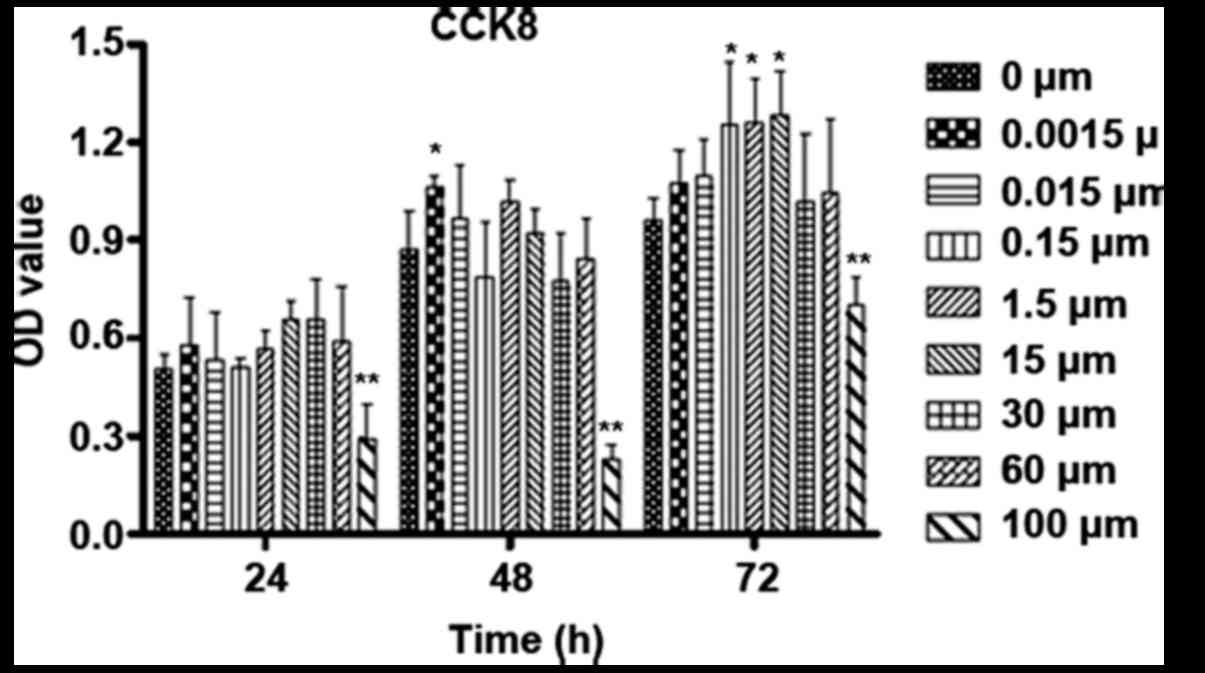 | Figure 4.Influence of time and varied
concentrations of ICA on the proliferation of osteoblastic cells.
Osteoblastic cells were cultured for 24, 48 and 72 h with 0,
0.0015, 0.015, 0.15, 1.5, 15, 30, 60 and 100 µM ICA. The
proliferation was checked by CCK-8. *P<0.05 and **P<0.01 vs.
0 µM ICA. CCK-8, Cell Counting kit-8; ICA, icariin; OD, optical
density. |
The ALP activity was also determined to evaluate the
effect of ICA on osteoblast differentiation (Fig. 5). ICA at certain concentrations
significantly improved the ALP activity after the osteoblastic
cells were treated for 24 or 48 h, and ICA at concentrations
between 0.015 and 15 µM increased the ALP activity significantly
after the cells were treated for 72 h.
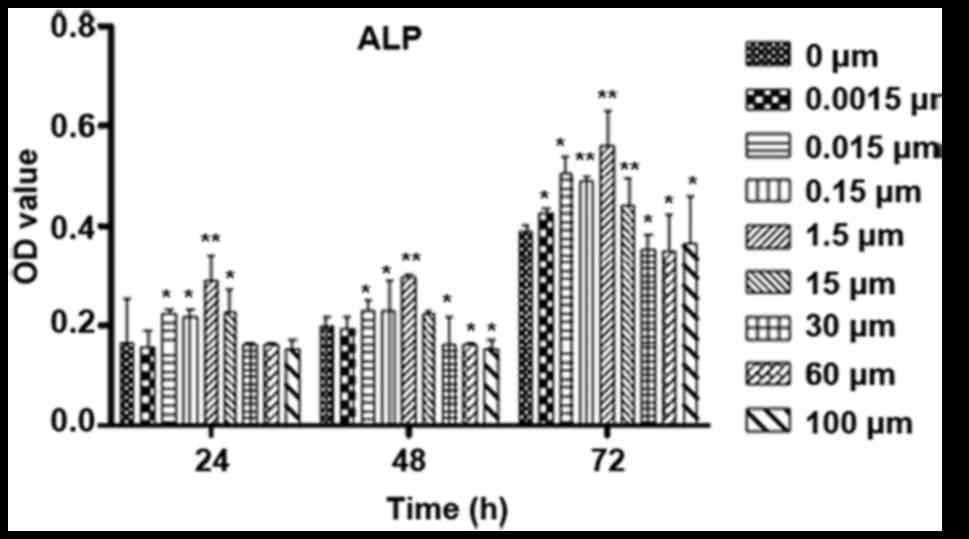 | Figure 5.Influence of time and varied
concentrations of ICA on the differentiation of osteoblastic cells.
Osteoblastic cells were cultured for 24, 48 and 72 h with 0,
0.0015, 0.015, 0.15, 1.5, 15, 30, 60 and 100 µM ICA. The
differentiation was evaluated by the levels of ALP activity.
*P<0.05 and **P<0.01 vs. 0 µM ICA. ALP, alkaline phosphatase;
ICA, icariin; OD, optical density. |
Participation of the Wnt/β-catenin
signalling pathway
To verify the role of the Wnt/β-catenin signalling
pathway in the effect of ICA, we determined the mRNA level of
several marker proteins, including β-catenin, RUNX2, cyclin D1 and
ALP (Fig. 6). The concentration of
ICA applied was 1.5 µM because of its significant proliferation and
differentiation effect, as shown above (Fig. 4). DKK-1 is an inhibitor of the
Wnt/β-catenin signalling pathway and was used as a negative
control. Compared with the mRNA levels of all the marker proteins
in the control group, those in the ICA group were significantly
increased at all time points of incubation; the one exception was
the mRNA level of cyclin D1, which was slightly but not
significantly increased at 24 h. The mRNA levels of all the marker
proteins in the control group were significantly suppressed by
DKK-1 treatment, except the mRNA level of cyclin D1, which was
slightly decreased at 24 h. The elevating effects of ICA on the
mRNA level of all the marker proteins were inhibited significantly
in the ICA and DKK-1 mixture group at all time points, except the
mRNA level of cyclin D1 at 24 h.
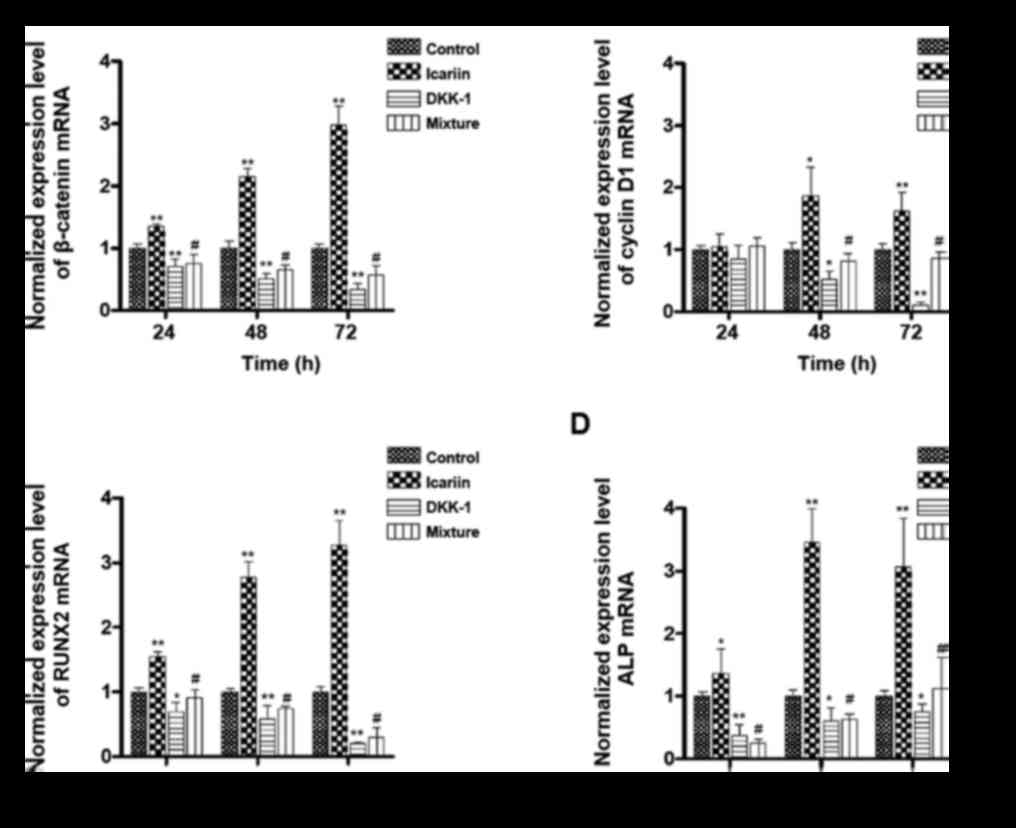 | Figure 6.Participation of the Wnt/β-catenin
signalling pathway in the effects of ICA. Osteoblastic cells were
treated with only medium (control), 1.5 µM ICA, 0.1 µg/ml of the
Wnt/β-catenin pathway inhibitor DKK-1, or a mixture of ICA and
DKK-1. All cells were incubated for 24, 48 and 72 h. Reverse
transcription-quantitative polymerase chain reaction was performed
to determine the gene expression level of (A) β-catenin, (B) cyclin
D1, (C) RUNX2 and (D) ALP. All of the expression levels were
normalized to the control group. *P<0.05 and **P<0.01 vs.
control group; #P<0.05 vs. ICA group. DKK-1,
Dickkopf-1; ICA, icariin; RUNX2, runt-related transcription factor
2; ALP, alkaline phosphatase. |
Discussion
The osteoblastic cell line that we established from
the rat mandible grew healthily and adherently. The cells expressed
ALP at a considerable level, as almost all cells showed a positive
ALP signal. Moreover, the osteoblastic cells had the classic
standard shape and grew to mineralization. Overall, this
osteoblastic cell line from the rat mandible was successfully
established and was suitable for scientific study.
ICA influenced the proliferation and differentiation
of the osteoblastic cells in a concentration- and time-dependent
manner, and compared with 24 and 48-h treatments, the 72-h
treatment had the largest effect. At this time point, the
concentration dependence was bell shaped; only the middle
concentrations, namely, 0.15, 1.5 and 15 µM, significantly
increased the proliferation and differentiation of the osteoblastic
cells. The ideal working concentration of ICA for the proliferation
of this osteoblastic cell line in vitro is between 0.15 and
15 µM. This finding was consistent with other reports, which
reported that the largest effect on osteoblastic cells from rat
calvaria and the hFOB 1.19 human osteoblastic cell line for
concentrations between 1 and 10 µM (6,11).
Treating the osteoblastic cells with 100 µM ICA inhibited the
proliferation and differentiation of the cells. The mechanism
behind this effect is still unknown. One possibility could be that
a high concentration of ICA can block some signalling pathways by
elevating the levels of the inhibitors of some nuclear receptors.
It will be interesting to pursue this idea in the future. One study
showed that ICA could suppress the inflammatory response mediated
by NF-κB, PPARα and PPARγ in rats (12), which indicated that ICA can
interact with multiple pathways via many signalling molecules.
The cell line we established came from the rat
mandible, and ICA at certain concentrations significantly increased
the proliferation and differentiation of these osteoblastic cells.
Thus, ICA could be used to treat mandible defect diseases,
including osteomyelitis, bone cyst, periodontitis and trauma.
Another feature of these diseases is the inflammatory response;
since ICA can inhibit the inflammatory response in rat brains
(12), ICA could be an ideal
treatment for these diseases. However, this possibility requires
more studies, especially in vivo studies, to develop this
treatment.
We found that ICA increased the mRNA level of
β-catenin, RUNX2, cyclin D1 and ALP. β-Catenin is the key protein
of the Wnt/β-catenin pathway, and RUNX2, cyclin D1 and ALP are
target genes of the Wnt/β-catenin pathway. Furthermore, this
upregulation was inhibited by DKK-1, which inhibits the
Wnt/β-catenin pathway. Therefore, our study indicates that the
Wnt/β-catenin pathway participates in the effect of ICA on
osteoblastic cells from the rat mandible. This finding is
consistent with other reports, which indicated that the
Wnt/β-catenin pathway plays a role in the ICA-induced promotion of
cells of other origins (7–9). Li et al (7) showed that the Wnt/β-catenin pathway
is important for improving osteoblast differentiation through ICA
in bone marrow stromal cells obtained from the rat femur and
bilateral tibia. Another article by Wang et al (9) determined that ICA induced osteogenic
differentiation of mouse mesenchymal stem cells and promoted new
bone formation in rat calvaria at a titanium-particle-induced
osteolytic site via activation of the Wnt/β-catenin signalling
pathway. Recently, Liu et al (8) suggested that ICA promotes the
proliferation and differentiation of mouse MC3T3-E1 cells exposed
to overload by activating the Wnt/β-catenin pathway. As a a
shortcoming of our study, we did not examine the expression levels
of proteins associated with the Wnt/β-catenin pathway and the
effect that ICA has on these. Therefore, further related study is
needed to supplement it.
Interestingly, the ICA mixed with DKK-1 group had
higher mRNA levels of marker proteins after the 72-h incubation
than the DKK-1 group, indicating that other signalling pathways may
participate in the effects of ICA on osteoblastic cells. Some
studies revealed that ICA affects the proliferation of osteoblasts
through the BMP-2/Smad4 signal transduction pathway (11,13),
which is consistent with our results.
In summary, our study is the first to show that ICA
promotes the proliferation and differentiation of osteoblastic
cells isolated from the rat mandible and that the Wnt/β-catenin
pathway might be involved in this effect. More experiments are
needed to confirm this mechanism and explore other signalling
pathways. Our study also showed that our isolation of an
osteoblastic cell line from the rat mandible was suitable for
scientific research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7122077) and the 4th High
Level Talents of the Beijing Health System (grant no. 201408).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
RW, YW and FZ participated in the research design,
conducted the experiments, and performed the data analyses. YW and
RW contributed to writing the manuscript. FZ contributed to
revising the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the recommendations of the National Institutes of Health Guide for
the Care and Use of Experimental Animals. The protocol was approved
by the Ethics Committee of Beijing Stomatological Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sodek J and McKee MD: Molecular and
cellular biology of alveolar bone. Periodontol 2000. 24:99–126.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aghaloo TL, Chaichanasakul T, Bezouglaia
O, Kang B, Franco R, Dry SM, Atti E and Tetradis S: Osteogenic
potential of mandibular vs. long-bone marrow stromal cells. J Dent
Res. 89:1293–1298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akintoye SO, Lam T, Shi S, Brahim J,
Collins MT and Robey PG: Skeletal site-specific characterization of
orofacial and iliac crest human bone marrow stromal cells in same
individuals. Bone. 38:758–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsubara T, Suardita K, Ishii M, Sugiyama
M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K,
et al: Alveolar bone marrow as a cell source for regenerative
medicine: Differences between alveolar and iliac bone marrow
stromal cells. J Bone Miner Res. 20:399–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Wang YS, Song SL, Liang H and Ji
AG: Icariin inhibits atherosclerosis progress in Apoe null mice by
downregulating CX3CR1 in macrophage. Biochem Biophys Res Commun.
470:845–850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icariin is more potent than genistein in
promoting osteoblast differentiation and mineralization in vitro. J
Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XF, Xu H, Zhao YJ, Tang DZ, Xu GH, Holz
J, Wang J, Cheng SD, Shi Q and Wang YJ: Icariin augments bone
formation and reverses the phenotypes of osteoprotegerin-deficient
mice through the activation of Wnt/β-catenin-BMP signaling. Evid
Based Complement Alternat Med. 2013:6523172013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Huang L, Hao B, Li H, Zhu S, Wang
Q, Li R, Xu Y and Zhang X: Use of an osteoblast overload damage
model to probe the effect of icariin on the proliferation,
differentiation and mineralization of MC3T3-E1 cells through the
Wnt/β-catenin signalling Pathway. Cell Physiol Biochem.
41:1605–1615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Tao Y, Ping Z, Zhang W, Hu X, Wang
Y, Wang L, Shi J, Wu X, Yang H, et al: Icariin attenuates
titanium-particle inhibition of bone formation by activating the
Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep.
6:238272016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang W, Lin M, Li X, Li C, Gao B, Gan H,
Yang Z, Lin X, Liao L and Yang M: Icariin promotes bone formation
via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19
human osteoblastic cell line. Int J Mol Med. 30:889–895. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong D, Deng Y, Huang B, Yin C, Liu B,
Shi J and Gong Q: Icariin attenuates cerebral ischemia-reperfusion
injury through inhibition of inflammatory response mediated by
NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol. 30:157–162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsieh TP, Sheu SY, Sun JS, Chen MH and Liu
MH: Icariin isolated from Epimedium pubescens regulates osteoblasts
anabolism through BMP-2, SMAD4, and Cbfa1 expression.
Phytomedicine. 17:414–423. 2010. View Article : Google Scholar : PubMed/NCBI
|