Introduction
Glioma is an aggressive subtype of primary brain
tumor with an extremely poor prognosis (1), in which glioblastoma is the most
severe manifestation. It is reported that only 3–5% patients with
glioblastoma survive for >5 years following diagnosis (2). Owing to the highly aggressive and
invasive nature of glioblastoma, current therapeutic strategies,
including neurosurgery, radiation therapy and chemotherapy, are
ineffective (3). Thus, it is
important to elucidate the key mechanisms underlying glioma
development and progression.
Long non-coding RNAs (lncRNAs) are RNA molecules
that are not translated into proteins. They have gained increasing
attention due to their diverse functions in various physiological
and pathological processes (4–6). The
dysregulation of lncRNAs has been demonstrated to have specific
roles in a variety of cancer types, including bladder, prostate and
kidney cancer (7–9). Furthermore, a number of lncRNAs may
be promising biomarkers or targets for the diagnosis and treatment
of cancer including breast and lung cancer (10,11).
In glioma, numerous lncRNAs, including metastasis associated lung
adenocarcinoma transcript 1 (12),
HOXA11 antisense RNA (13),
X-inactive specific transcript (14) and H19 imprinted maternally
expressed transcript (15),
function as key mediators in tumor development. Recently, lncRNA
ferritin heavy polypeptide 1 pseudogene 3 (FTH1P3), a member of the
FHC gene family, has been demonstrated to contribute to the
progression of oral squamous cell carcinoma and uveal melanoma by
functioning as a miR-224-5p sponge (16,17).
However, to the best of our knowledge, whether lncRNA FTH1P3
expression is dysregulated and involved in glioma development by
sequestering miR-224-5p has not yet been reported.
In the present study, the expression of lncRNA
FTH1P3 in low- and high-grade glioma tissues was detected.
Following this, FTH1P3 was overexpressed and suppressed in glioma
U251 cells in vitro to investigate its roles in regulating
cell proliferation and apoptosis. As discussed above, FTH1P3
functioned as an important regulator in the development and
progression of oral squamous cell carcinoma and uveal melanoma by
sequestering miR-224-5p (16,17),
and miR-224 inhibits the development of prostate cancer by
targeting tumor protein D52 (TPD52) (18). Therefore, the regulatory
association between FTH1P3 and the miR-224-5p/TPD52 axis was
investigated in glioma cells to further elucidate the potential
regulatory mechanism of FTH1P3. The findings of the present study
may provide a broader perspective for the treatment of this
disease.
Materials and methods
Tissue samples
Between March 2016 and April 2017, 20 patients (aged
27.3±9.6, male:female=12:8) with glioma were admitted to Tianjin
Medical University General Hospital (Tianjin, China). Fresh high-
and low-grade glioma samples (6 cases were high-grade and 14 were
low-grade) and adjacent normal tissues were obtained from these
patients during a temporal lobectomy procedure for epilepsy and
immediately snap-frozen in liquid nitrogen upon surgical removal.
The present study was approved by the ethics committee of Tianjin
Medical University General Hospital according to the criteria of
the Declaration of Helsinki. Informed consent was acquired from
each patient.
Cell culture and transfection
The human glioma cell line U251 was purchased from
the European Collection of Cell Cultures (09063001; ECACC, Porton
Down, Salisbury, UK; deposited by Shanghaisixin Biotech Co., Ltd,
Shanghai, China) and maintained in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), 100 µg/ml streptomycin and 100 U/ml penicillin in
a 5% CO2 incubator at 37°C.
U251 cells of an appropriate concentration
(approximately 80%) at 1×105 were seeded on 6-well
plates and were incubated at 37°C in the plates for a further 24 h
prior to transfection. Cells were subsequently transfected with
appropriate concentrations (200 µM for each) of pcDNA3.1-FTH1P3
(pc-FTH1P3) (Sangon Biotech Co., Ltd., Shanghai, China), small
interfering RNA (siRNA)-FTH1P3, miR-224-5p mimic, pcDNA3.1-TPD52
(pc-TPD52) and the corresponding controls using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Medium was replaced following 4 h of
transfection. The sequences for the target vectors were: FTH1P3
siRNA, 5′-CACCGCCAGCCCTCCGTCACCTCTTCGAAAAGAGGTGACGGAGGGCTGGC-3′;
miR-224-5p mimic sense, 5′-CAAGUCACUAGUGGUUCCGUU-3′ and antisense,
5′-CGGAACCACUAGUGACUUGUU-3′; siRNAcontrol sense,
5′-CACCGGGAGAATGCGATGGGAGAGCCGAAGCTCTCCCATCGCATTCTCCC-3′ and
antisense:
5′-AAAAGGGAGAATGCGATGGGAGAGCTTCGGCTCTCCCATCGCATTCTCCC-3′; miRNA
mimic control, sense 5′-AAAAUGGUGGUGCCCUAGUGACUACA-3′ and
antisense, 5′-UAGUCACCAUAAUAGGGCACCAUUUUUU-3′. Cells were harvested
for further experimentation at 24, 48 and 72 h following
incubation.
MTT assay
Cell viability was detected with a MTT assay.
Transfected cells were seeded in a 96-well plate at a density of
2×104 cells/well at 37°C and allowed to attach
overnight. MTT solution (20 µl; 0.5 mg/ml; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was subsequently added to each well for 4
h at 37°C. Medium was removed and 0.2 ml dimethyl sulfoxide was
added to each well for 30 min at 37°C. The optical density of each
well at 490 nm was read on an enzyme-labeled instrument (microplate
reader, Bio-Rad Laboratories, Inc., Hercules, CA, USA) to assess
cell viability.
5-bromo-2-deoxyuridine (BrdU)
incorporation assay
Following transfection, cells at a density of
1×105 cells/well were incubated with 10 µM BrdU
(Sigma-Aldrich; Merck KGaA) at 37°C for 48 h and fixed in 4%
paraformaldehyde at 37°C for 30 min. Cells were subsequently
incubated with 0.1% Triton X-100 for 10 min, 2 M HCl for 5 min and
0.1 M borate buffer (pH 8.5) for 5 min in the proper order, as
listed, at 37°C. To detect the BrdU-positive cells, cells were
blocked with 2% bovine serum albumin (FBS; Gibco; Thermo Fisher
Scientific, Inc.), probed with an anti-BrdU antibody (1:1,000;
ab1893; Abcam, Cambridge, UK) overnight at 4°C, followed by
incubation with a fluorescein isothiocyanate (FITC)-labeled
anti-mouse immunoglobulin G secondary antibody (1:1,000; ab157532;
Abcam). The percentage of cells with positive staining was examined
in three randomly selected high-power fields at ×400 magnification
using a fluorescence microscope and Cellquest software (version
3.0; BD Biosciences, Franklin Lakes, NJ USA).
Flow cytometry detection
The apoptosis rate of each group was examined with
an Annexin V-FITC apoptosis detection kit (Beijing Biosea
Biotechnology Co., Ltd, Beijing, China), according to the
manufacturer's protocol. Briefly, cells (5×106) were
harvested, seeded in a 6-well plate and mixed with 10 µl Annexin
and 5 µl propidium iodide (10 mg/l) for 15 min in the dark.
Apoptosis was detected with a flow cytometer (BD Biosciences) and
the apoptotic percentages were analyzed with BD CellQuest 3.0
software (BD Biosciences). Annexin V-positive cells were regarded
as apoptotic cells.
Luciferase reporter assay
TargetScanHuman version 7.1 (http://www.targetscan.org/vert_72/) was used to
predict the target sequences of miR-224-5p in TPD52. To construct
the wild-type (WT) luciferase reporter vectors, the TPD52
3′-untranslated region (3′-UTR), which included the miR-224-5p seed
binding sites, was cloned into the pGL3-promotor vector (Sangon
Biotech Co., Ltd., Shanghai, China). In the mutation-type (MUT)
luciferase reporter vector, the predicted seed zones in miR-224-5p
were replaced by nonsense sequences. For the luciferase reporter
assay, 100 ng TPD52 3′UTR luciferase construct and 400 ng
miR-224-5p mimics were co-transfected into U251 cells using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The relative luciferase activity of the
luciferase construct was subsequently detected using a Dual-Glo
Luciferase assay kit (E2940; Promega Corporation, Madison, WI, USA)
following 48 h transfection. Firefly luciferase activity was
normalized to Renilla activity. The β-galactosidase
expression vector was used as a control for transfection
efficiency.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and cDNA was synthesized at 70°C using a Maxima First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol [after heating to 94°C for 2 min, the
volume (50 µl) was subjected to 30 cycles of 94°C for 30 sec, 61°C
for 30 sec, and 72°C for 30 sec]. The gene expression levels of
FTH1P3, miR-224-5p and TPD52 were determined by SYBR green qPCR kit
(Thermo Fisher Scientific, Inc.) and calculated using the
2−ΔΔCq method (19).
The primer sequences used were as follows: FTH1P3 forward,
5′-TCCATTTACCTGTGCGTGGC-3′ and reverse, 5′-GAAGGAAGATTCGGCCACCT-3′;
miR-224-5p forward, 5′-CTGGTAGGTAAGTCACTA-3′ and reverse,
5′-TCAACTGGTGTCGTGGAG-3′; U6 forward,
5′-CTGGTAGGGTGCTCGCTTCGGCAG-3′ and reverse,
5′-CAACTGGTGTCGTGGAGTCGGC-3′; TPD52 forward,
5′-CGTTGTATTTGTTATTTATCAAGTTGT-3′ and reverse,
5′-TCCCCAAGTAAATCTAGTCATGC3′; GAPDH forward,
5′-AAGACCTTGGGCTGGGACTG-3′ and reverse, 5′-ACCAAATCCGTTGACTCCGA-3′.
PCR product was produced under the following parameters: 1
Predenaturation cycle of 2 min at 94°C, 32 cycles of 95°C for 15
sec, 64°C for 30 sec, and 72°C for 2 min, with a final extension at
72°C for 5 min. GAPDH was used as the endogenous control for FTH1P3
and TPD52, whereas U6 was used as an internal control for
miR-224-5p expression.
Western blot analysis
Total protein was extracted from cells with
radioimmunoprecipitation assay lysis buffer (Sangon Biotech Co.,
Ltd., Shanghai, China) and the concentration was measured with a
bicinchoninic acid protein assay kit. Equal amounts of protein (50
µg per lane) were subjected to 10% SDS-PAGE and blotted onto
polyvinylidene fluoride membranes. Membranes were blocked in
Tris-buffered saline with Tween-20 containing 5% non-fat milk at
4°C overnight, and subsequently incubated with primary rabbit
monoclonal antibodies against apoptosis regulator Bcl-2 (BCL2;
1:1,000; cat. no. ab32124), apoptosis regulator BAX (BAX; 1:1,000;
cat. no. ab32503), TPD52 (1:1,000; cat. no. ab181260) and GAPDH
(1:1,000; cat. no. ab8245; Abcam, Cambridge, UK) overnight at 4°C.
Following this, the membrane was probed with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000;
cat. no. ab97051; Abcam) for 2 h at room temperature. An enhanced
chemiluminescence kit (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) was used to visualize the protein bands. Western blot
quantification was performed using ImageJ v1.48 software (National
Institutes of Health, Bethesda, MD, USA). GAPDH was used as the
reference protein.
Statistical analysis
All experiments were repeated at least three times
and the obtained data are presented as the mean ± standard
deviation. Student's t-tests were used for the analysis of
statistical significance between two groups, and one-way analysis
of variance followed by Dunnett's post hoc test was applied to
analyze statistical significance among three groups or more. All
statistical analysis was conducted using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
LncRNA FTH1P3 is upregulated in glioma
tissues, and its upregulation promotes the proliferation and
inhibits the apoptosis of glioma cells
The expression of FTH1P3 in glioma tissues was
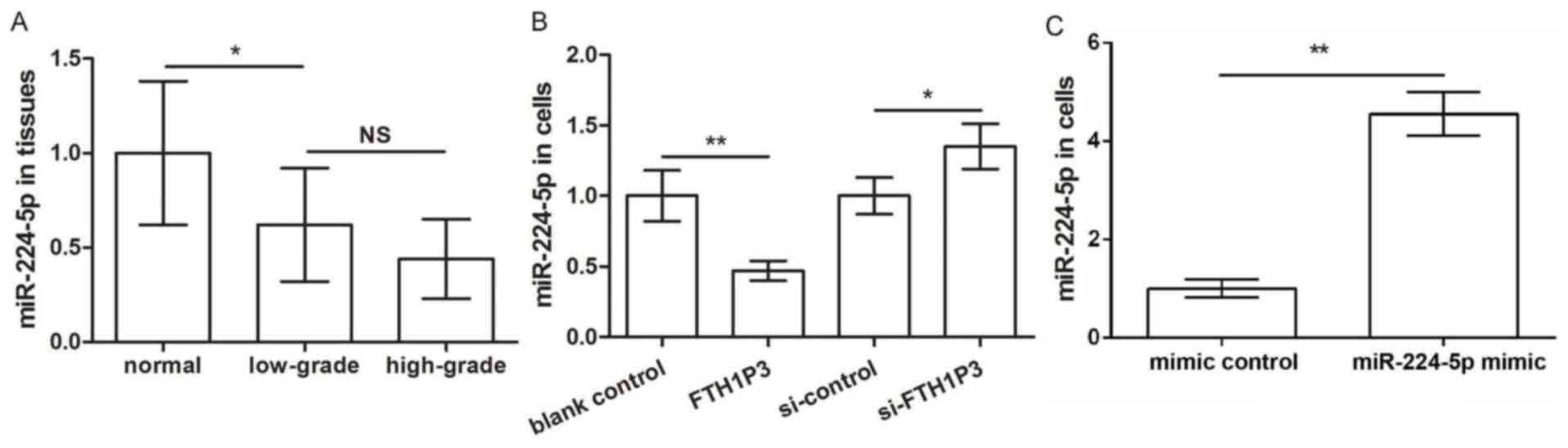
initially determined. As presented in Fig. 1A, FTH1P3 expression in glioma
tissues was significantly increased compared with that in normal
tissues (P<0.001); furthermore, TH1P3 expression in high-grade
glioma tissues was significantly higher compared with low-grade
glioma tissues (P<0.05), indicating that the upregulation of
FTH1P3 may be associated with the development and progression of
glioma. Thus, FTH1P3 was overexpressed and suppressed in glioma
U251 cells to investigate the potential role of FTH1P3 in glioma
in vitro. Following transfection with pc-FTH1P3 and
siRNA-FTH1P3, FTH1P3 expression was successfully overexpressed and
suppressed, compared with transfection with the corresponding
control (P<0.01, FTH1P3 vs. blank control; P<0.001 si-FTH1P3
vs. si-control; Fig. 1B). The
results of the MTT assay demonstrated that overexpression of FTH1P3
markedly increased cell viability after 24, 48 and 72 h, whereas
suppression of FTH1P3 had the opposite effect (P<0.05 at 24 h,
P<0.01 at 48 h, and P<0.001 at 72 h; Fig. 1C). Similar results were obtained
from BrdU assay; BrdU-positive cells were significantly increased
in the pc-FTH1P3 group compared with the blank group, whereas
positive cells were markedly decreased in the siRNA-FTH1P3 group
compared with the siRNA control group (P<0.05, FTH1P3 vs. blank
control; P<0.001 si-FTH1P3 vs. si-control; Fig. 1D). The results of MTT and BrdU
assays indicated that overexpression of FTH1P3 promoted glioma U251
cell proliferation. In addition, flow cytometry revealed that the
percentage of apoptotic cells in the pc-FTH1P3 group was
significantly lower compared with that of the blank group, whereas
the percentage in the siRNA-FTH1P3 group was significantly
increased compared with the siRNA-control group (P<0.01, FTH1P3
vs. blank control; P<0.01 si-FTH1P3 vs. si-control; Fig. 1E). Notably, western blot analysis
demonstrated that the alterations in BAX/BCL2 expression were in
line with the percentage of apoptotic cells in each transfected
group (P<0.05, FTH1P3 vs. blank control; P<0.001 si-FTH1P3
vs. si-control; Fig. 1F). These
data indicated that overexpression of FTH1P3 may have inhibited
glioma U251 cell apoptosis via regulation of BAX/BCL2
expression.
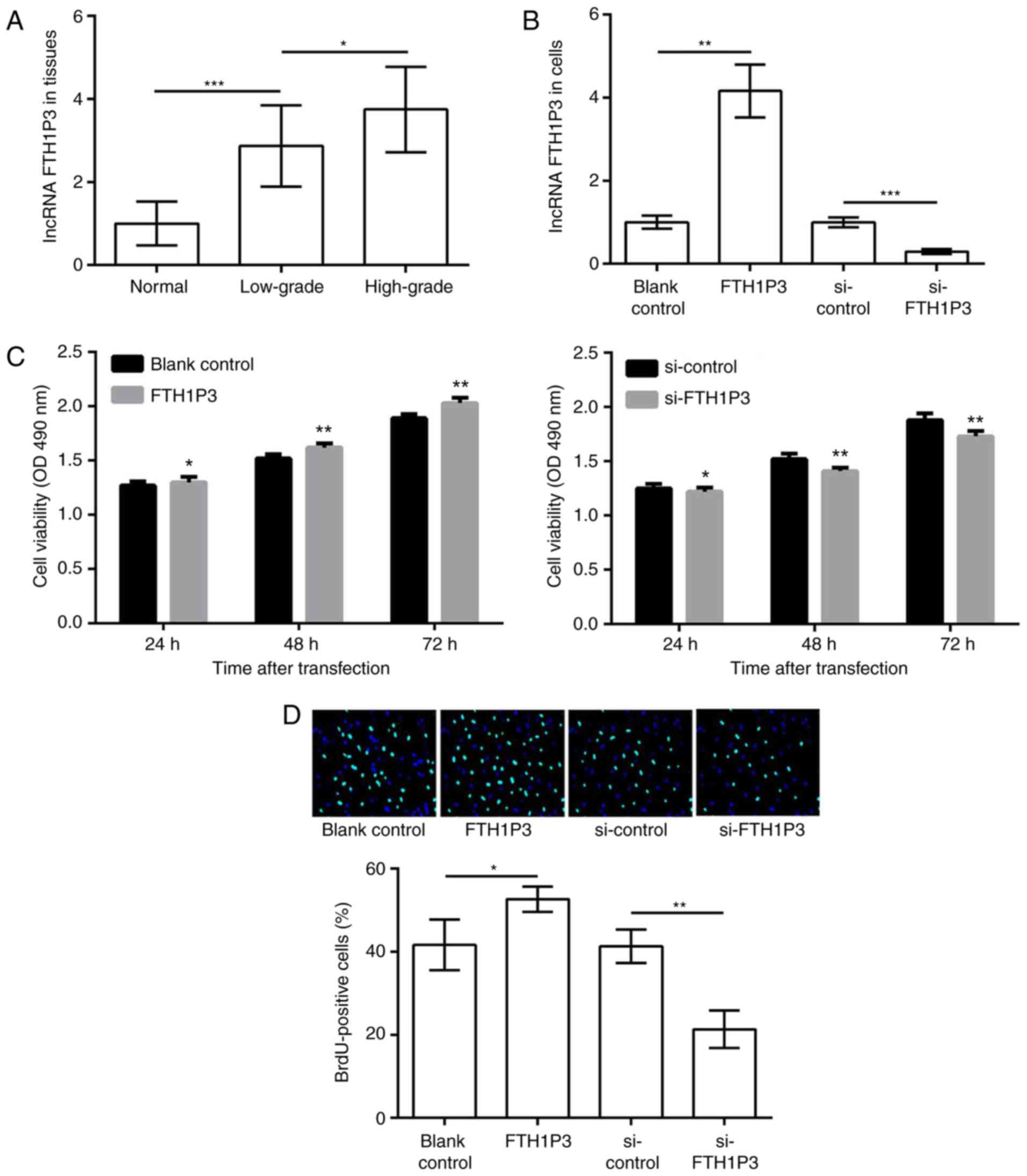 | Figure 1.LncRNA FTH1P3 is upregulated in glioma
tissues, and its upregulation promotes glioma cell proliferation
and inhibits apoptosis. (A) The expression of lncRNA FTH1P3 in low-
and high-grade glioma tissues and adjacent normal tissues. (B)
Expression of lncRNA FTH1P3 in glioma U251 cells transfected with
pc-FTH1P3, si-FTH1P3 and the corresponding controls. (C) The cell
viability of each group after 24, 48 h and 72 h was determined with
an MTT assay. (D) A BrdU incorporation assay was performed to
determine cell proliferation (magnification, ×400). (E) The
percentage of apoptotic cells in each group was determined by flow
cytometry. (F) Western blot analysis of BAX/BCL2 expression.
*P<0.05, **P<0.01 and ***P<0.001. lncRNA, long non-coding
RNA; FTH1P3, ferritin heavy polypeptide 1 pseudogene 3; pc,
pcDNA3.1; si, small interfering; BrdU, 5-bromo-2-deoxyuridine; BAX,
apoptosis regulator BAX; BCL2, B cell lymphoma 2; OD, optical
density; FITC, fluorescein isothiocyanate. |
LncRNA FTH1P3 inhibits miR-224-5p
expression
It has been reported that FTH1P3 facilitates the
progression of oral squamous cell carcinoma by inhibiting
miR-224-5p (16). Thus, the
regulatory association between FTH1P3 and miR-224-5p in U251 cells
was investigated. As presented in Fig.
2A, miR-224-5p expression was significantly decreased in glioma
tissues compared with normal tissues (P<0.05); however, there
was no significant difference in miR-224-5p expression between
high- and low-grade glioma tissues. In addition, miR-224-5p
expression was significantly decreased in the pc-FTH1P3 group and
increased in the siRNA-FTH1P3 group, compared with the
corresponding control groups (P<0.01, FTH1P3 vs. blank control;
P<0.05 si-FTH1P3 vs. si-control; Fig. 2B). These data indicated that
miR-224-5p expression was inhibited by FTH1P3 in glioma U251 cells.
To further examine the functional role of miR-224-5p in glioma U251
cells, miR-224-5p was overexpressed. As presented in Fig. 2C, the results demonstrated that the
expression of miR-224-5p was significantly increased by
transfection with miR-224-5p mimic, indicating a high transfection
efficiency (P<0.01).
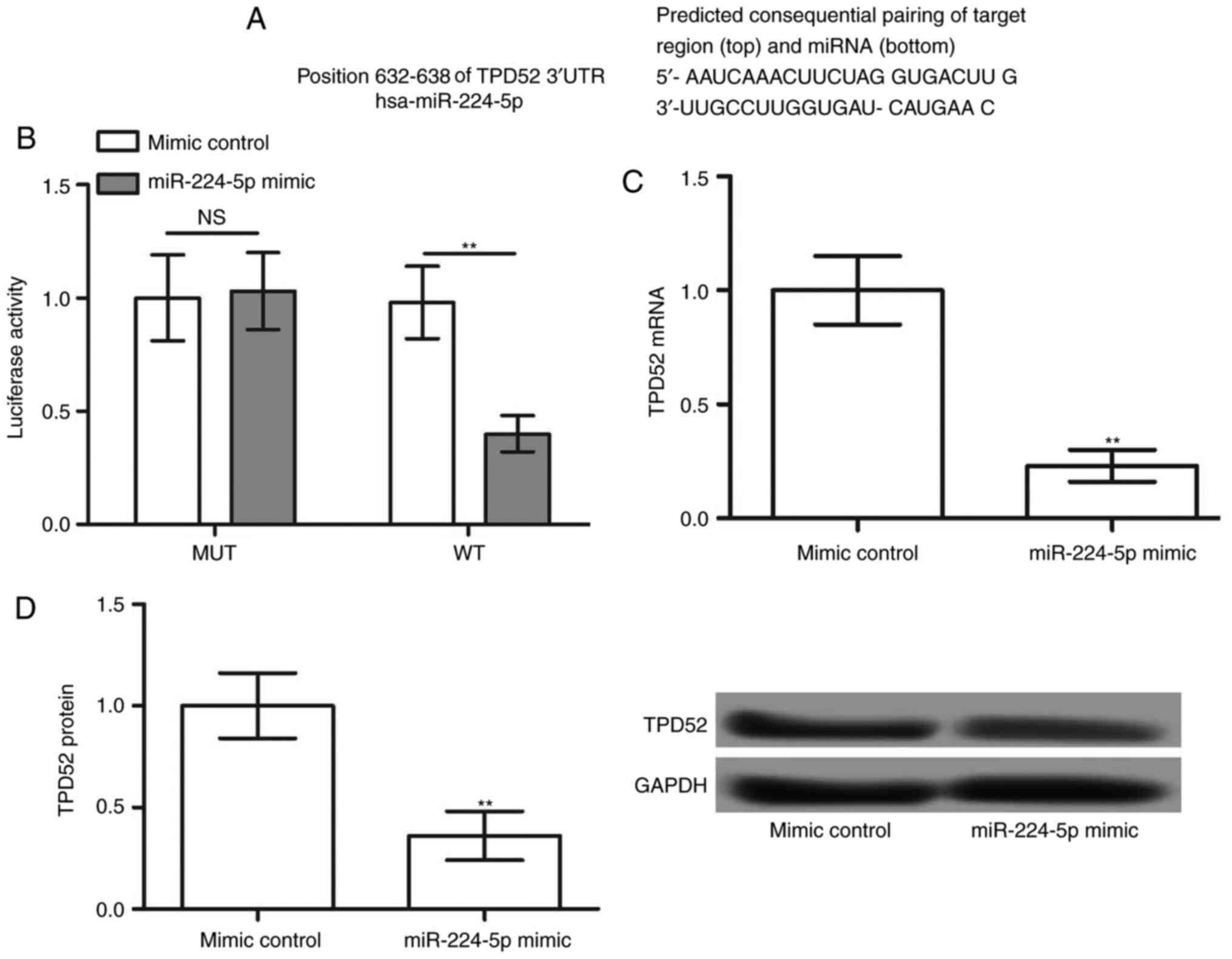
miR-244-5p negatively regulates TPD52
expression
To further examine the downstream regulatory
mechanism of miR-224-5p, the target genes of miR-224-5p were
predicted by TargetScanHuman. The results revealed that TPD52 was a
potential target of miR-224-5p (Fig.
3A). The luciferase reporter assay demonstrated that miR-224-5p
mimic decreased the luciferase activity of TPD52 3′-UTR-WT
(P<0.05), although not that of TPD52 3′-UTR-MUT (Fig. 3B). In addition, the mRNA and
protein expression of TPD52 was significantly decreased following
overexpression of miR-224-5p (P<0.05; Fig. 3C and D). These data indicated that
TPD52 was a target of miR-224-5p, and TPD52 expression was
negatively regulated by miR-224-5p.
FTH1P3 may exert its effects in glioma
via the miR-224-5p/TPD52 axis
The expression of TPD52 in glioma tissues was
further determined by western blot analysis. The results revealed
that TPD52 expression in glioma tissues was significantly increased
compared with normal tissues, with the highest expression in
high-grade glioma tissues (P<0.05; Fig. 4A). To further analyze the
functional role of TPD52, U251 cells were transfected with
pcDNA-TPD52. The results demonstrated that the protein expression
of TPD52 was significantly increased by pcDNA-TPD52 (P<0.001;
Fig. 4B). Additionally, compared
with the mimic control group, TPD52 expression was significantly
downregulated in U251 cells following transfection with miR-224-5p
mimic. This effect was markedly reversed following co-transfection
of miR-224-5p mimic and pc-TPD52 (P<0.01; Fig. 4C). Furthermore, the results of the
MTT and BrdU assays revealed that cell viability and the number of
BrdU-positive cells was significantly inhibited following
miR-224-5p overexpression. These effects were also markedly
reversed following co-transfection with miR-224-5p mimic and
pc-TPD52 (P<0.05, at 24 h; P<0.01, at 48 h and P<0.01 at
72 h; Fig. 4D and E). Finally,
miR-224-5p mimic transfection significantly induced cellular
apoptosis and increased the expression of BAX/BCL2 in U251 cells,
which was significantly reversed following co-transfection of
miR-224-5p and TPD52 (P<0.01; Fig.
4F and P<0.001 miR-224-5p mimic vs. mimic control,
P<0.01, miR-224-5p mimic+TPD52 vs. miR-224-5p mimic + blank
control; Fig. 4G). Taken together,
these data indicated that FTH1P3 may act through the
miR-224-5p/TPD52 axis in glioma (Fig.
4H).
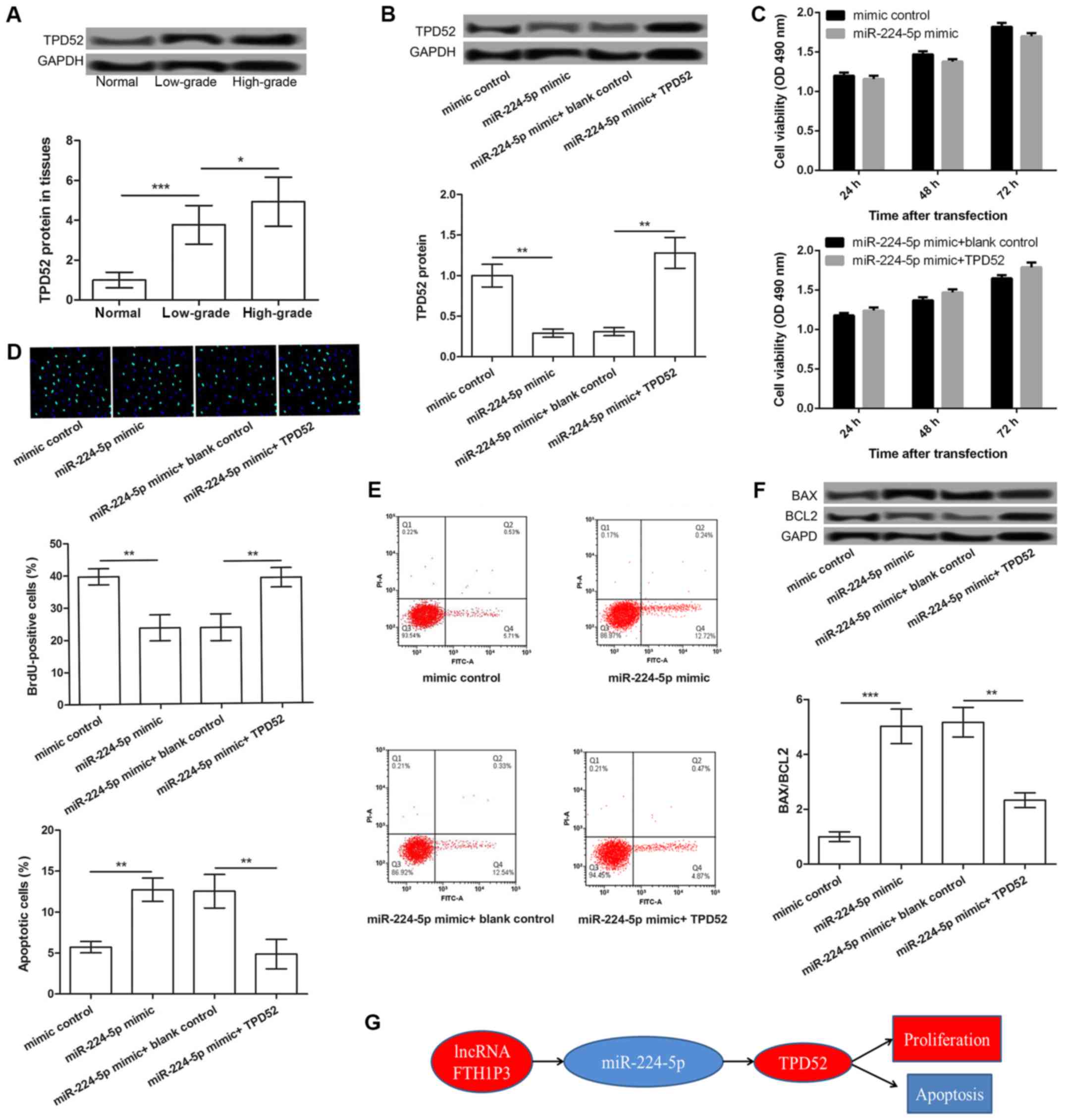 | Figure 4.The miR-224-5p/TPD52 axis may be a
functional mechanism of FTH1P3 in glioma. (A) TPD52 expression in
low- and high-grade glioma tissues and adjacent normal tissues. (B)
The expression of TPD52 in glioma U251 cells treated with mimic
control, miR-224-5p mimic, miR-224-5p mimic + blank control,
miR-224-5p mimic + pc-TPD52. (C) The cell viability of each group
after 24, 48 and 72 h was determined by MTT assay. (D) A BrdU
incorporation assay showed BrdU-positive cells in different
transfected groups (magnification, ×400). (E) The percentage of
apoptotic cells in each group was detected by flow cytometry. (F)
Western blot analysis showed the expression levels of BAX/BCL2 in
different transfected groups. (G) Schematic representation of the
potential mechanism of action of lncRNA FTH1P23 in glioma.
*P<0.05, **P<0.01 and ***P<0.001. lncRNA, long non-coding
RNA; FTH1P3, ferritin heavy polypeptide 1 pseudogene 3; BrdU,
5-bromo-2-deoxyuridine; BAX, apoptosis regulator BAX; BCL2, B cell
lymphoma 2; TPD52, tumor protein D52; miR-224-5p,
microRNA-224-5p. |
Discussion
lncRNAs have emerged as key regulators in cancer
biology (9). In the present study,
the role and regulatory mechanism of lncRNA FTH1P3 in glioma was
investigated. The results revealed that FTH1P3 was upregulated in
glioma tissues, and its upregulation promoted glioma cell
proliferation and inhibited apoptosis. In addition, FTH1P3
inhibited miR-224-5p expression, which in turn negatively regulated
TPD52 expression. Thus, the miR-224-5p/TPD52 axis may be a
downstream mechanism by which FTH1P3 may regulate glioma cell
proliferation and apoptosis. These findings suggested that the
FTH1P3/miR-224-5p/TPD52 axis may have a key role in glioma
progression.
Aberrant expression of miR-224 has been associated
with the development of numerous cancer types. Xia et al
(20) demonstrated that the
upregulation of miR-224 promotes cell proliferation and migration
in gastric cancer (20). Zhang
et al (21) confirmed that
miR-224 is upregulated in colorectal cancer and promotes cell
proliferation and invasion. In addition, dysregulation of
miR-224-5p is correlated with cisplatin resistance in ovarian
papillary serous carcinoma (22).
Notably, upregulation of miR-224 is also correlated with poor
prognosis in glioma patients (23). In the present study, FTH1P3 was
overexpressed and its upregulation is correlated to glioma cell
proliferation and apoptosis. FTH1P3 inhibited miR-224-5p
expression, and overexpression of miR-224-5p inhibited U251 cell
proliferation and induced cellular apoptosis. Given the key role of
miR-224-5p in numerous types of cancer, it was speculated that
upregulation of FTH1P3 may promote cell proliferation and inhibit
apoptosis in glioma via negative regulation of miR-224-5p
expression.
Furthermore, TPD52 was identified as a target of
miR-224-5p in the current study. Nearly 20 years ago, TPD52 was
originally identified as an overexpressed gene in human breast
cancer (24). Accumulating
evidence has confirmed that TPD52 contributes to the development
and progression of various human cancer types (25–28).
Decreased TPD52 expression is correlated with poor prognosis in
primary hepatocellular carcinoma (29). Additionally, miR-218 may function
as a tumor suppressor in lung squamous cell carcinoma by targeting
TPD52 (30). Li et al
(31) demonstrated that miR-34a
inhibits the metastasis of breast cancer by targeting oncogenic
TPD52. Notably, miR-224 suppresses the migration and invasion of
prostate cancer cells via TPD52 inhibition (18). In the present study, FTH1P3
inhibited miR-224-5p expression, which negatively regulated TPD52
expression. Overexpression of miR-224-5p significantly inhibited
U251 cell proliferation and induced cellular apoptosis; this effect
was clearly reversed following co-transfection of miR-224-5p and
TPD52. Taken together, these findings indicated that miR-224-5p may
control the proliferation and apoptosis of glioma cells via TPD52
inhibition, and FTH1P3 may exert its effects on glioma cells via
the miR-224-5p/TPD52 axis.
In conclusion, the data revealed that upregulation
of FTH1P3 may promote glioma cell proliferation and inhibit
apoptosis via regulation of the miR-224-5p/TPD52 axis. FTH1P3 may
provide a novel perspective on the pathogenesis of glioma. Future
experiments aiming at exploring the possible regulatory mechanism
of FTH1P3 in the other kinds of glioma cells are required in order
to further investigate the findings of the present study. The
findings of the present study may aid the provision of a
theoretical basis for the clinical therapy of glioma.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed this study, wrote the manuscript and
performed the MTT assay, RNA pull-down assay, western blotting and
RT-qPCR; YL performed the cell apoptosis analysis, JW contributed
the data analysis and PL helped to collect data.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Tianjin Medical University General Hospital according
to the criteria of the Declaration of Helsinki. Informed consent
was acquired from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. Jama. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gallego O: Nonsurgical treatment of
recurrent glioblastoma. Curr Oncol. 22:e273–e281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boon RA, Jaé N, Holdt L and Dimmeler S:
Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets.
J Am Coll Cardiol. 67:1214–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Fan S and Song E: Noncoding RNAs:
New players in cancers. Adv Exp Med Biol. 927:1–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martens-Uzunova ES, Böttcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatima R, Akhade VS, Pal D and Rao SM:
Long noncoding RNAs in development and cancer: Potential biomarkers
and therapeutic targets. Mol Cell Ther. 3:52015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma KX, Wang HJ, Li XR, Li T, Su G, Yang P
and Wu JW: Long noncoding RNA MALAT1 associates with the malignant
status and poor prognosis in glioma. Tumor Biol. 36:3355–3359.
2015. View Article : Google Scholar
|
|
13
|
Wang Q, Zhang J, Liu Y, Zhang W, Zhou J,
Duan R, Pu P, Kang C and Han L: A novel cell cycle-associated
lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA
transcript and is a biomarker of progression in glioma. Cancer
Lett. 373:251–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang X, Yan Y, Hu M, Chen X, Wang Y, Dai
Y, Wu D, Wang Y, Zhuang Z and Xia H: Increased level of H19 long
noncoding RNA promotes invasion, angiogenesis and stemness of
glioblastoma cells. J Neurosurg. 124:129–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CZ: Long non-coding RNA FTH1P3
facilitates oral squamous cell carcinoma progression by acting as a
molecular sponge of miR-224-5p to modulate fizzled 5 expression.
Gene. 607:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X, Tang H, Zhao X, Sun Y, Jiang Y
and Liu Y: Long non-coding RNA FTH1P3 facilitates uveal melanoma
cell growth and invasion through miR-224-5p. PLoS One.
12:e01847462017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goto Y, Nishikawa R, Kojima S, Chiyomaru
T, Enokida H, Inoguchi S, Kinoshita T, Fuse M, Sakamoto S, Nakagawa
M, et al: Tumour-suppressive microRNA-224 inhibits cancer cell
migration and invasion via targeting oncogenic TPD52 in prostate
cancer. Febs Lett. 588:1973–1982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia M, Wei J and Tong K: MiR-224 promotes
proliferation and migration of gastric cancer cells through
targeting PAK4. Pharmazie. 71:460–464. 2016.PubMed/NCBI
|
|
21
|
Zhang GJ, Zhou H, Xiao H, Li Y and Zhou T:
Up-regulation of miR-224 promotes cancer cell proliferation and
invasion and predicts relapse of colorectal cancer. Cancer Cell
Int. 13:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao H, Bi T, Qu Z, Jiang J, Cui S and
Wang Y: Expression of miR-224-5p is associated with the original
cisplatin resistance of ovarian papillary serous carcinoma. Oncol
Rep. 32:1003–1012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu S, Wang S, Geng S, Ma S, Liang Z and
Jiao B: Upregulation of microRNA-224 confers a poor prognosis in
glioma patients. Clin Transl Oncol. 15:569–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Byrne JA, Tomasetto C, Garnier JM, Rouyer
N, Mattei MG, Bellocq JP, Rio MC and Basset P: A screening method
to identify genes commonly overexpressed in carcinomas and the
identification of a novel complementary DNA sequence. Cancer Res.
55:2896–2903. 1995.PubMed/NCBI
|
|
25
|
Byrne JA, Frost S, Chen Y and Bright RK:
Tumor protein D52 (TPD52) and cancer-oncogene understudy or
understudied oncogene? Tumor Biol. 35:7369–7382. 2014. View Article : Google Scholar
|
|
26
|
Moritz T, Venz S, Junker H, Kreuz S,
Walther R and Zimmermann U: Isoform 1 of TPD52 (PC-1) promotes
neuroendocrine transdifferentiation in prostate cancer cells. Tumor
Biol. 37:10435–10446. 2016. View Article : Google Scholar
|
|
27
|
Ummanni R, Teller S, Junker H, Zimmermann
U, Venz S, Scharf C, Giebel J and Walther R: Altered expression of
tumor protein D52 regulates apoptosis and migration of prostate
cancer cells. FEBS J. 275:5703–5713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Z, Liu H, Hou J, Li T, Du X, Zhao X,
Xu W, Xu W and Chang J: Tumor protein D52 (TPD52) inhibits growth
and metastasis in renal cell carcinoma cells through the PI3K/Akt
signaling pathway. Oncol Res. 25:773–779. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Chen CL, Pan QZ, Wu YY, Zhao JJ,
Jiang SS, Chao J, Zhang XF, Zhang HX, Zhou ZQ, et al: Decreased
TPD52 expression is associated with poor prognosis in primary
hepatocellular carcinoma. Oncotarget. 7:63232016.PubMed/NCBI
|
|
30
|
Kumamoto T, Seki N, Mataki H, et al:
Tumor-Suppressive MicroRNA-218 Inhibits Cancer Cell Migration And
Invasion Directly Targeting TPD52 In Lung Squamous Cell Carcinoma.
A71. Oncogenes and Angiogenesis In Lung Tumors. Am Thoracic Soc.
A23712017.
|
|
31
|
Li G, Yao L, Zhang J, Li X, Dang S, Zeng
K, Zhou Y and Gao F: Tumor-suppressive microRNA-34a inhibits breast
cancer cell migration and invasion via targeting oncogenic TPD52.
Tumor Biol. 37:7481–7491. 2016. View Article : Google Scholar
|