Introduction
Astrocytes, are the most abundant type of glial cell
in the central nervous system (1).
Astrocytes promote nerve repair and regeneration, and serve
important roles in the maintenance of the blood-brain barrier,
nervous system stability and nervous system damage (2–4). Any
form of damage and lesions in the central nervous system can lead
to the activation of astrocytes, which has been associated with
astrocyte dysfunction and central nervous system disorders
(5). A previous study demonstrated
that the migration of astrocytes serves a key role in
neurodegenerative diseases (6).
Studies of patients with Parkinson's disease and animal models have
revealed that the activation of astrocytes is accompanied with
impaired mitochondrial function in astrocytes under pathological
conditions (7,8). An important feature of astrocyte
activation following nerve injury is the upregulation of glial
fibrillary acidic protein (GFAP) expression. Inhibition of GFAP
gene expression has been reported to delay the regeneration of
nerve axons and functional recovery following nerve injury
(9). Therefore, investigation of
the migration of astrocytes is of great importance in the treatment
of central nervous system diseases.
P38 signaling pathway is associated with the mitogen
(MAPK) family of proteins, which serves an important role in cell
apoptosis, cytokine production and transcriptional regulation
(10). It has been reported that
the activation of p38-MAPK serves an important role in the
pathological reaction of Alzheimer's disease. A large number of
phosphorylated p38-MAPK can be observed in the brain tissue of
patients with early Alzheimer's disease (11). The activation of p38-MAPK has been
associated with the damage of glial cells, which exhibit enhanced
protective effects when p38 MAPK is inhibited. It also regulates
the secretion of nerve growth factor and tumor necrosis factor-α
and cell migration of glial cells (11). Protein phosphatase 2A (PP2A) is a
serine/threonine protein phosphatase, with a total of 75 types of
heterotrimers, that is involved in various cell biological
functions (12). In
neurodegenerative diseases, the main function of PP2A has been
proposed to involve the dephosphorylation of highly phosphorylated
tau protein. During the development of the central nervous system,
PP2A can dephosphorylate brain retardation response regulatory
protein 2 and promote axonal polarization and growth (13,14).
A recent study demonstrated that elevated levels of PP2A in
astrocytes may alleviate neurotoxicity in APP/PS1 double transgenic
mice (15). In a rat model of
Alzheimer's disease (tg2576 rats), PP2A deficiency can lead to Aβ
aggregation by inhibiting the migration of astrocytes (16). In the present study, astrocytes
were isolated and cultured in vitro to investigate the
effect of PP2A on their migration in order to provide a theoretical
basis for the treatment of central nervous system diseases.
Materials and methods
Experimental animals
A total of 10 Sprague-Dawley rats of either gender,
(aged 1–3 days and weighed ~200+20 g), were purchased from the
Experimental Animal Center of Xinxiang Medical University
(Xinxiang, China). All rats were housed in sterile conditions at
19–21°C, with a relative humidity of 50–60%, ad libitum
intake of water and food, under 12 h of light/dark cycle. The
present study was performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (16). The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
Xinxiang Medical University.
Instruments and reagents
p38 (cat. no. 8690T), p-p38 (cat. no. 4511T), matrix
metalloproteinase (MMP)-2 (cat. no. 40994S), MMP-9 (cat. no.
13667T) and β-actin (cat. no. 3700T) monoclonal antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The inverted microscope was purchased from Olympus Corporation,
(Tokyo, Japan). The CO2 incubator was purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The PP2A
activity test kit was purchased from Promega Corporation (Madison,
WI, USA). The BCA Protein Concentration assay kit was purchased
from Beyotime Institute of Biotechnology (Haimen, China).
Isolation and culture of
astrocytes
Sprague-Dawley rats born within 48 h were sacrificed
by cervical dislocation and disinfected with 75% alcohol. The
neonatal brain tissue was removed and washed with PBS three times.
When the washing liquid remained translucent, the cerebral cortex
tissue was removed and cut with an ophthalmic scalpel, 0.125%
trypsin was subsequently added at 37°C for 15 min and agitated
every 3 min. The digestion time of the tissue was different
depending on the size of the tissue blocks. The tissue was observed
until digested completely and Dulbecco's modified Eagle's
medium/F12 cell culture medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/ml penicillin-streptomycin was added to
terminate the digestion. Subsequently, the cell culture was sieved
with 200-mesh sieve and centrifuged at 300 × g for 10 min at room
temperature. The supernatant was discarded and the appropriate
amount of cell culture medium was added to suspend the cells. Cells
were subsequently seeded in the culture flask in a 37°C and 5%
CO2 incubator. The culture medium was replaced every 3
days. After 9 days, the cell culture flask was sealed and placed on
a shaker at 37°C overnight at 100 rpm. The isolated, cultured cells
expressed glial fibrillary acidic protein, by immunofluorescence
and were therefore identified as astrocytes (17). Fresh culture medium was added and
the cells were cultured until the second generation where they were
subsequently employed in experiments. Briefly, the cells were fixed
as follows: Cells were washed with PBS for 1–2 times; 4%
polyformaldehyde was added for 15–20 min at room temperature. The
cells were then rinsed with PBS for two times (5 min per wash) and
100 µl of 0.2% Triton X-100 was added to each hole to permeabilize
for 15 min at room temperature. Subsequently, the cells were
blocked for 30 min at room temperature by adding 100 µl of 3%
bovine serum albumin (BSA) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). A total of 100 µl of 1:2,000 1% BSA-diluted primary
antibody against GFAP 1 (cat. no. D1F4Q, Cell Signaling Technology,
Inc.) was added to the cells and incubated at 4°C overnight.
Following incubation, the cells were incubated for 1 h in the dark
at room temperature with secondary antibody goat-anti-rabbit IgG
H&L (1:1,000; ab150077, Abcam, Cambridge, MA, USA) diluted with
PBS. After washing, a laser-scanning confocal microscopy
(magnification, ×40, TCS SP8 CARS, Leica Microsystems, Inc.,
Buffalo Grove, IL, USA) was used for analysis and to capture images
(data not shown).
Experimental grouping
Astrocytes were divided into experimental groups:
Control group, PP2A inhibitor group and PP2A activation group.
Cells were seeded at concentration of 1×105 cells/ml. A
total of 10 µl dimethyl sulfoxide was added to the control cells,
while 15 nM PP2A inhibitor, okadaic acid (OA) (Beyotime Institute
of Biotechnology) was added to the inhibitor group and 15 nM PP2A
activator, D-erythro-Sphingosine (DES) (Beyotime Institute of
Biotechnology) was added to the activated group. Each group of the
cells was treated for 48 h under normal cell culture
conditions.
PP2A enzyme activity test
The cells in each group were cultured for 48 h
following the aforementioned treatments, land subsequently lysed on
ice for 20 min. The lysates were transferred to Eppendorf (EP)
tubes and centrifuged at 15,000 × g for 10 min at 4°C. The protein
supernatant was extracted and transferred into EP tubes. A protein
concentration detection kit was used to determine the concentration
of the extracted protein. A 50 µg protein sample was used to detect
the activity of PP2A in the sample according to the PP2A activity
assay kit. Calculated the linear regression equation of the
standard curve according to the concentration of the standard
product, and the PP2A activities were calculated according to the
OD value of the sample based on the regression equation.
Cell migration by Transwell assay
The second generation of astrocytes in each group
was collected, digested by trypsin and the corresponding cell
culture medium DMEM/F12 containing 1% FBS was added to resuspend
cells. The cell concentration was adjusted to 1×105
cells/ml. A total of 500 µl cell suspension was added to the upper
chamber of the poly-D-lysine pre-coated Transwell chamber (Corning
Incorporated, Corning, NY, USA) and 700 µl cell culture medium
DMEM/F12 containing 10% FBS was added to the lower chamber. The
cells were placed on a 12-well plate and incubated for 8 h in a 5%
CO2 incubator at 37°C, and the excess cells were removed
using a cotton swab. The cells were fixed with methanol for 5 min
at room temperature and stained with Giemsa dye for 15 min at room
temperature. The number of cells that migrated after 8 h was
observed under light microscope (magnification, ×100). The total
number of cells was the sum of the cells in the upper and the lower
layer of the membrane. Cell mobility=Number of cells in the lower
layer of the membrane/total cells.
Western blotting of p38,
phosphorylated (p)-p38, MMP-2 and MMP-9 protein levels in
astrocytes
Using radioimmunoprecipitation buffer
(Sigma-Aldrich; Merck KGaA), total proteins were extracted from the
cells of the respective groups after culture for 48 h. Following
quantification with a Bicinchoninic Acid kit, 20 µg of each
denatured protein sample was loaded into the SDS-PAGE gel wells
with 6% stacking gel and 10% removal gel. When the bromophenol blue
moved to the junction of the stacking and removing gels,
electrophoresis with 100 V was performed for 90 min. The proteins
were transferred to a nitrocellulose membrane with transferring
current of 275 mA and transfer time of 60 min. The nitrocellulose
membrane was blocked in 4% skimmed milk powder for 2 h at room
temperature. Subsequently, membranes were incubated with the
primary antibodies (1:800) at 4°C overnight, followed by secondary
antibody incubation (1:1,000) at 37°C for 120 min. Membranes were
then exposed after addition of the visualization enhanced
chemiluminescent reagent (Beyotime Institute of Biotechnology) and
analyzed via Tanon 4600 Chemiluminescence Image Analysis System
version 3.0 (Tannon, Shanghai, China). The relative expression of
proteins was calculated.
Effect of p38 signaling pathway
inhibition on astrocyte migration
For a different group of experiments, second
generation astrocytes were divided into untreated, SB202190 and DES
+ SB202190 groups. Cells were seeded at a density of
1×105 cells/ml in DMEM/F12. Cells in the SB202190 group
were incubated at 37°C for 48 h with cell culture medium containing
the p38 signal pathway inhibitor SB202190 (Cell Signaling
Technology, Inc.) at a final concentration of 30 µmol/l. Cells in
the DES + SB202190 group were treated with p38 signal pathway
inhibitor SB202190 at a final concentration of 30 µmol/l and 15 nM
PP2A activator DES at 37°C for 48 h, while the untreated group
received no treatment. The levels of p38, p-p38, MMP-2 and MMP-9
were detected by western blotting and migration was assessed by a
Transwell assay.
Statistical analysis
The experimental data were analyzed by SPSS 22.0
statistical software (IBM, Corp., Armonk, NY, USA). Data are
presented as the mean ± standard deviation of the mean. One-way
analysis of variance was used to compare multiple groups. Multiple
comparisons between the groups were performed using Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed at least
three times and all samples were tested in triplicate.
Results
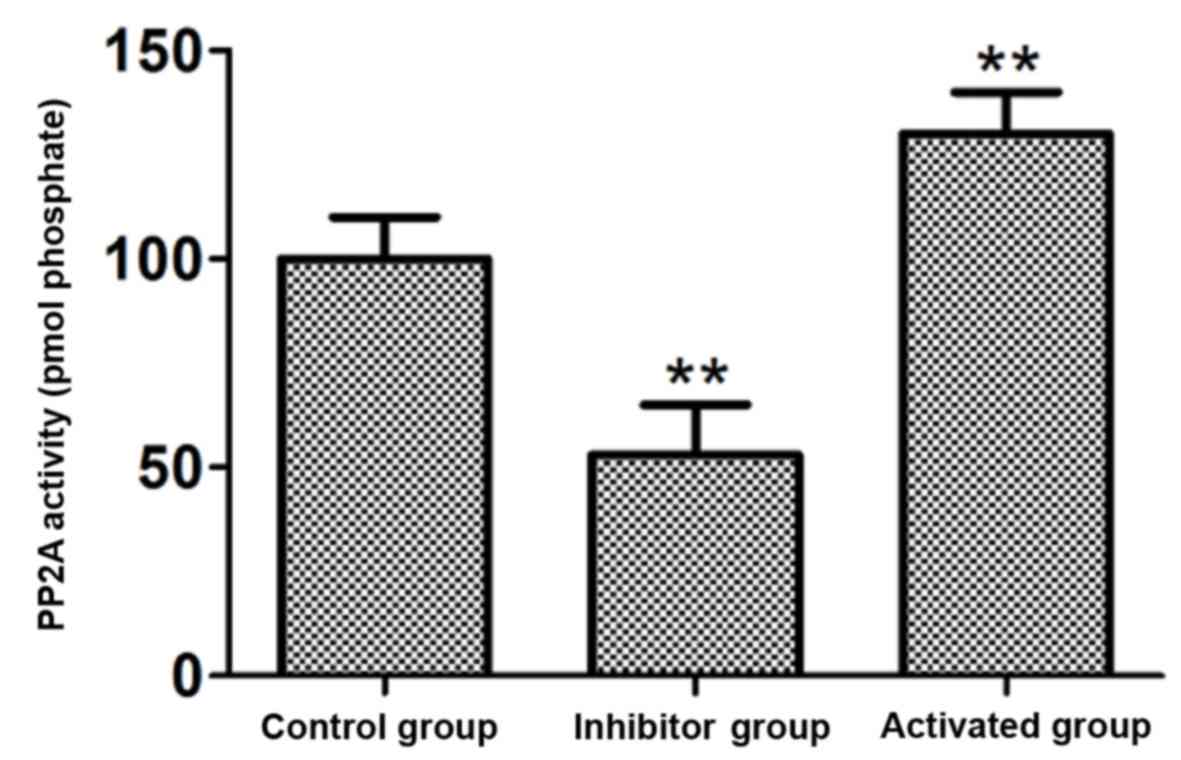
PP2A activity
A total of 15 nM PP2A inhibitor OA and 15 nM PP2A
activator DES were used to treat the astrocytes, and the PP2A
activity in cells was measured. The results demonstrated that the
activity of PP2A in the cells treated with the PP2A inhibitor OA
was significantly decreased compared with the control group
(P<0.01; Fig. 1). The activity
of PP2A in the cells treated with the PP2A activator DES was
significantly increased compared with the control group (P<0.01;
Fig. 1).
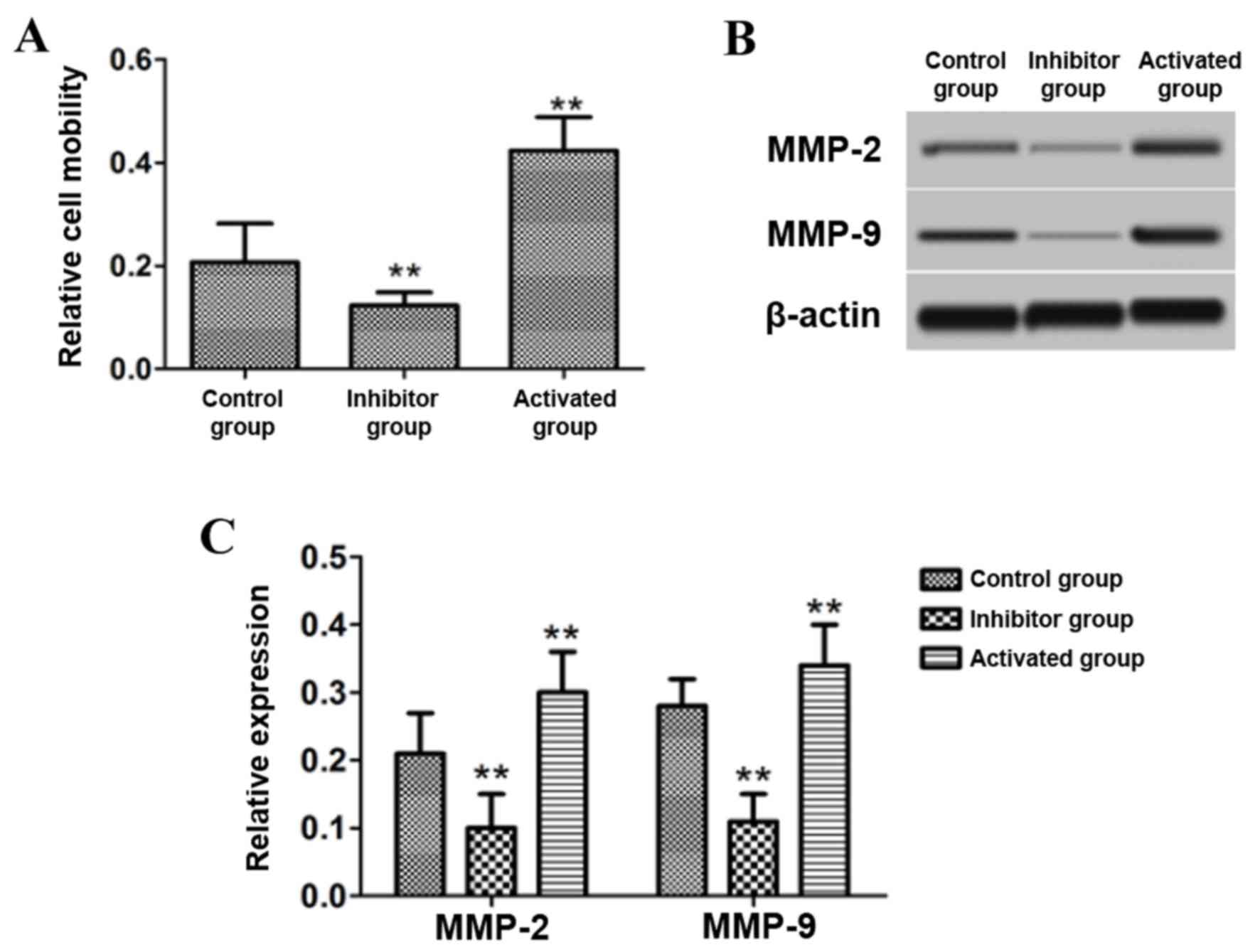
Effect of PP2A on cell migration
Following treatment of the astrocytes with 15 nM
PP2A inhibitor OA and 15 nM PP2A activator DES, cell migration was
measured using a Transwell chamber. The protein expression of MMP-2
and MMP-9 in the cells was also detected by western blotting. The
results demonstrated that the cell migration ability, and the
protein levels of MMP-2 and MMP-9, in astrocytes treated with OA
were significantly decreased compared with the control group
(P<0.01), while significant increases in migration and the
expression of MMP-2 and MMP-9 were observed compared with the
control group in those treated with DES (P<0.01; Fig. 2).
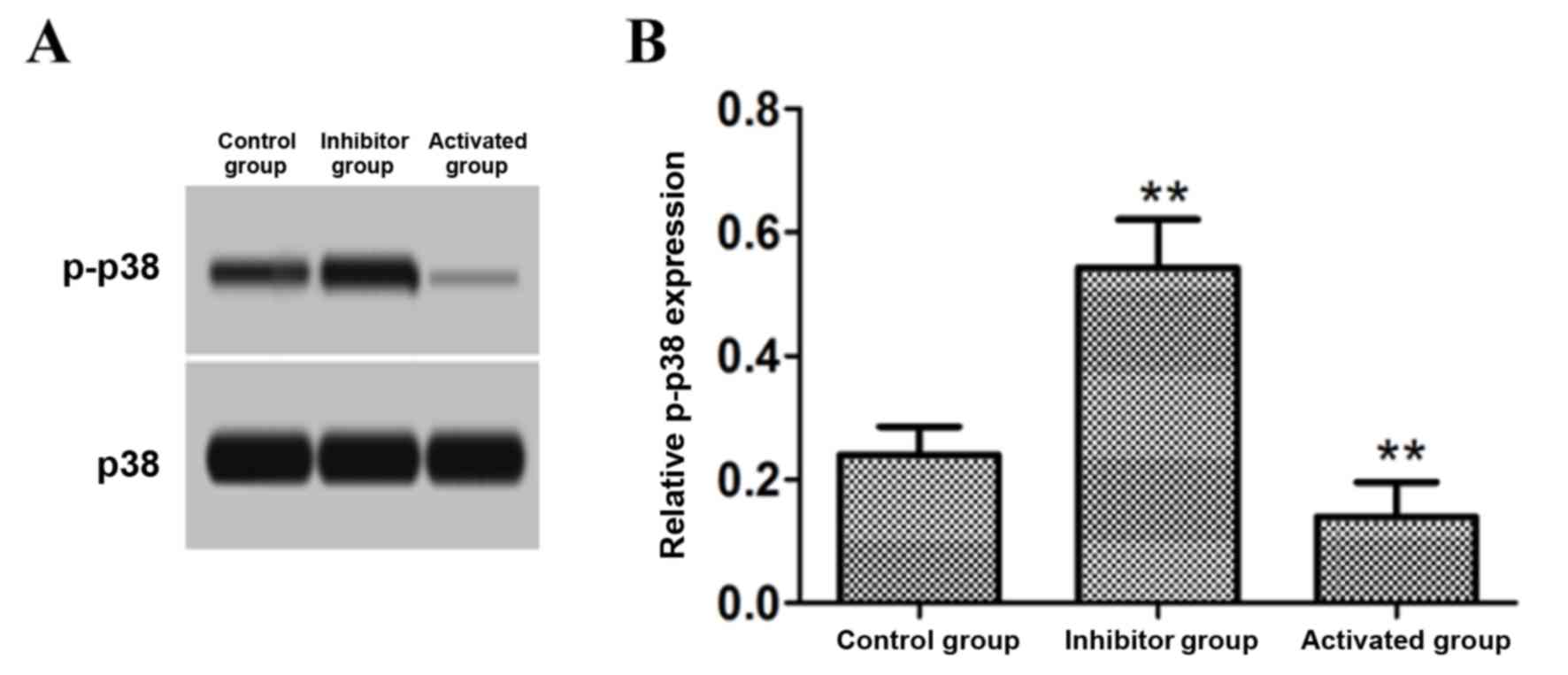
Effect of PP2A on p-p38 protein
expression
Astrocytes were treated with 15 nM PP2A inhibitor OA
and 15 nM PP2A activator DES, and the cell p-p38 level was detected
by western blotting. The results demonstrated that the level of
p-p38 protein in the cells treated with the PP2A inhibitor OA was
significantly increased compared with the control group
(P<0.01), while p-p38 protein levels in the cells treated with
PP2A activator DES were significantly decreased compared with the
control group (P<0.01; Fig.
3).
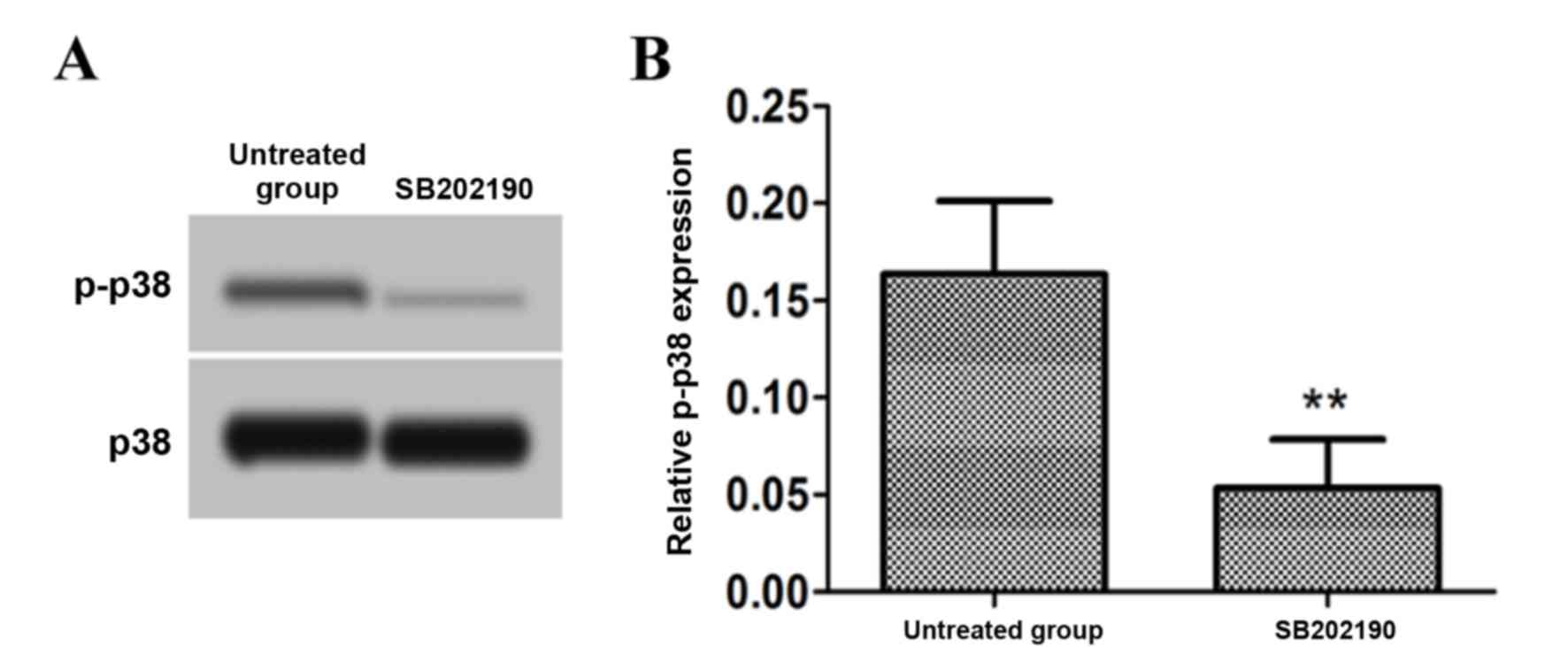
Effect of a p38 signal pathway
inhibitor on p-p38 protein expression
Following treatment of astrocytes with 30 µmol/l p38
signal pathway inhibitor SB202190, the levels of p-p38 were
measured by western blotting. The results demonstrated that the
level of p-p38 protein following treatment with the p38 signal
pathway inhibitor was significantly decreased compared with the
untreated group (P<0.01; Fig.
4).
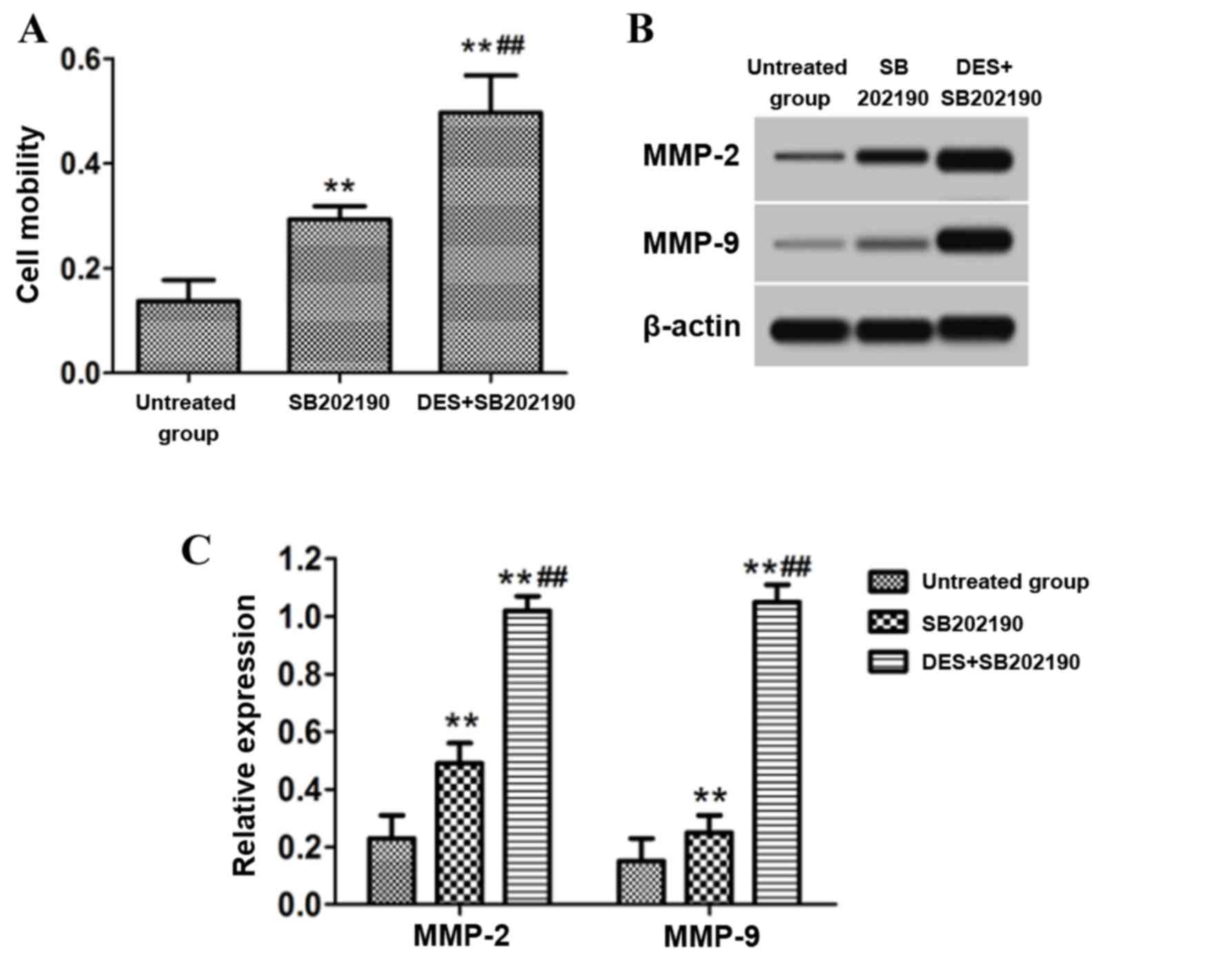
Effect of inhibition of p38 signaling
pathway on cell migration
Following treatment of astrocytes with 30 µmol/l p38
signaling inhibitor SB202190 with or without 15 nM PP2A activator
DES, the cell migration was detected using a Transwell chamber. The
protein expression of MMP-2 and MMP-9 in the cells was detected by
western blotting. The results demonstrated that the cell migratory
ability and the protein levels of MMP-2 and MMP-9 in astrocytes
following treatment with the p38 signal pathway inhibitor SB202190
were significantly increased compared with the untreated group
(P<0.01; Fig. 5). Furthermore,
the cell migratory ability and the protein levels of MMP-2 and
MMP-9 in astrocytes that received combined treatment with the p38
signal pathway inhibitor SB202190 and the PP2A activator DES were
significantly increased compared with those treated with SB202190
alone (P<0.01; Fig. 5).
Discussion
Astrocytes are a class of cells that are closely
associated with the central nervous system. Astrocytes serve a
supporting role in providing neurons with nutrition (18). Evidence has indicated that
astrocytes exhibit a wide range of biological functions in the
nervous system (19). Various
effects have been reported for astrocytes within the central
nervous system, including the transmission of neurotransmitters and
the maintenance of synaptic biological functions (20–22).
In addition, astrocytes have been reported to have an important
role in central nervous system disorders. For example, a large
number of astrocytes aggregated around the senile plaques in the
brain of patients with Alzheimer's disease (AD) (23). Investigation of the migration of
astrocytes may therefore be important to the pathogenesis of
AD.
PP2A possesses A, B and C subunits that contain a
large number of trimers, and these trimers affect intracellular
proteins in various ways, therefore participating in a numerous
biological function processes (24). Previous studies have demonstrated
that PP2A may serve an important role in the central nervous
system, and reduced PP2A activity was reported to disrupt the
function of neuronal axons (25,26).
PP2A activity in the brain of patients with AD has been reported to
be reduced (27); whether PP2A can
affect the migration of astrocytes is lack of sufficient evidence.
In the present study, astrocytes were isolated and cultured in
vitro, and treated with PP2A activators or inhibitors. The
results demonstrated that PP2A activators and inhibitors
effectively promoted PP2A activity and inhibited PP2A activity,
respectively. There were no obvious morphological alterations
following stimulation or inhibition of PP2A (data not shown).
Further detection of cell migration demonstrated that PP2A
activation had the ability to promote the migration of astrocytes,
consistent with the findings in a rat model (15,27).
Cell migration is a form of cellular movement that
serves an important role in angiogenesis, the immune response,
embryonic development, cancer, wound healing and neurodegenerative
diseases (28,29). Cell migration is a complex process
and is strictly regulated by various genes. MMPs are the most
investigated family of proteins associated with cell migration.
MMP-2 and MMP-9 have been reported to have a key role in the
process of cell migration (30).
The MMP-2 and MMP-9 protein expression levels were analyzed in the
present study and the results demonstrated that the PP2A activator
promoted the expression of the MMP-2 and MMP-9 proteins in
astrocytes, while the PP2A inhibitor inhibited the expression of
MMP-2 and MMP-9 in astrocytes. The results were consistent with
that of the cell migration assay and observations that OA can
activate MMP-9 (31).
p38 is a member of the MAPK family and serves a role
in cellular signal transduction (32). Studies have demonstrated that p38
is involved in inflammatory processes, physiological stress, cell
migration and neurological disorders (33,34).
In the present study, the phosphorylation of p38 in cells treated
with PP2A activators and inhibitors was measured. The results
demonstrated that the PP2A activator attenuated the phosphorylation
of p38, while the PP2A inhibitor enhanced the phosphorylation of
p38. In order to investigate the effect of the p38 signaling
pathway on astrocytes, a p38 signaling pathway-specific inhibitor
was used to treat astrocytes, and the results demonstrated that the
p38 signaling pathway-specific inhibitor enhanced the migratory
ability of astrocytes and enhanced the ability of the PP2A
activator to promote migration, which is consistent with reports in
stem cells and tumor cells (35,36).
These results indicated that PP2A activation may promote the
migration of astrocytes through negative regulation of the p38
signaling pathway.
In conclusion, the current study demonstrated that
PP2A activation promoted the migration of astrocytes and the
underlying mechanism may be associated with the p38 signaling
pathway. The results of the present study provide a basis for
further investigation of the effect of PP2A on the biological
activity of astrocytes and provide a theoretical basis for the
treatment of neurodegenerative diseases and potential therapeutic
targets. The heterogeneity of astrocytes in the CNS under normal
and pathological conditions, however, requires further
investigation. Therefore, relevant signaling pathways and possible
treatment options also need to be performed verification and
research in models in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Science
and Technology Project of Henan Province (grant no. 172102310685),
the Key Scientific Research Projects in Henan Universities (grant
no. 16B320019), Scientific and technological Projects of Health and
Family Planning Commission (grant no. 201303105), Henan Province,
China.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJ made substantial contributions to the design of
the present study. LZ, LS, LW and JZ performed the experiments of
enzyme activity test, Transwell assay and analyzed the data, and LZ
was a major contributor in writing the manuscript. LZ, PM, QG and
LM performed the analysis of astrocytes and western blotting. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal use protocol in the present study was
reviewed and approved by the Institutional Animal Care and Use
Committee of Xinxiang Medical University (Xinxiang, China). The
present study was performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Furihata T, Ito R, Kamiichi A, Saito K and
Chiba K: Establishment and characterization of a new conditionally
immortalized human astrocyte cell line. J Neurochem. 136:92–105.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maresca B, Spagnuolo MS and Cigliano L:
Haptoglobin modulates beta-amyloid uptake by U-87 MG astrocyte cell
line. J Mol Neurosci. 56:35–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajalakshmy AR, Malathi J and Madhavan HN:
Serum-derived hepatitis C virus 1a infection of human astrocyte
cell line SVG. J Viral Hepat. 23:211–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ono K, Suzuki H, Higa M, Tabata K and
Sawada M: Glutamate release from astrocyte cell-line GL261 via
alterations in the intracellular ion environment. J Neural Transm
(Vienna). 121:245–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor
DH, Ji J, Fan W, Huang Z and Hu J: Activation of α7 nicotinic
acetylcholine receptors protects astrocytes against oxidative
stress-induced apoptosis: Implications for Parkinson's disease.
Neuropharmacology. 91:87–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seyedzadeh MH, Safari Z, Zare A,
Gholizadeh Navashenaq J, Razavi SA, Kardar GA and Khorramizadeh MR:
Study of curcumin immunomodulatory effects on reactive astrocyte
cell function. Int Immunopharmacol. 22:230–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fahn S: Description of Parkinson's disease
as a clinical syndrome. Ann N Y Acad Sci. 991:1–14. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH,
Rho S, Hwang D, Masliah E and Lee SJ: Direct transfer of
alpha-synuclein from neuron to astroglia causes inflammatory
responses in synucleinopathies. J Biol Chem. 285:9262–9272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Snow DM, Lemmon V, Carrino DA, Caplan AI
and Silver J: Sulfated proteoglycans in astroglial barriers inhibit
neurite outgrowth in vitro. Exp Neurol. 109:111–130. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han J and Sun P: The pathways to tumor
suppression via route p38. Trends Biochem Sci. 32:364–371. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun A, Liu M, Nguyen XV and Bing G: P38
MAP kinase is activated at early stages in Alzheimer's disease
brain. Exp Neurol. 183:394–405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gürsel DB, Banu MA, Berry N, Marongiu R,
Burkhardt JK, Kobylarz K, Kaplitt MG, Rafii S and Boockvar JA:
Tight regulation between cell survival and programmed cell death in
GBM stem-like cells by EGFR/GSK3b/PP2A signaling. J Neurooncol.
121:19–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonzalez Mde L, Malemud CJ and Silver J:
Role of astroglial extracellular matrix in the formation of rat
olfactory bulb glomeruli. Exp Neurol. 123:91–105. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Juraszek B and Nałęcz KA: Protein
phosphatase PP2A-a novel interacting partner of carnitine
transporter OCTN2 (SLC22A5) in rat astrocytes. J Neurochem.
139:537–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mauch DH, Nägler K, Schumacher S, Göritz
C, Müller EC, Otto A and Pfrieger FW: CNS synaptogenesis promoted
by glia-derived cholesterol. Science. 294:1354–1357. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011, https://www.ncbi.nlm.nih.gov/books/NBK54050/PubMed/NCBI
|
|
17
|
Kaech S and Banker G: Culturing
hippocampal neurons. Nat Protoc. 1:2406–2415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sofroniew MV and Vinters HV: Astrocytes:
Biology and pathology. Acta Neuropathol. 119:7–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CC, Yang CC, Chen YW, Hsiao LD and
Yang CM: Arachidonic acid induces ARE/Nrf2-dependent heme
oxygenase-1 transcription in rat brain astrocytes. Mol Neurobiol.
55:3328–3343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quincozes-Santos A, Bobermin LD, Latini A,
Wajner M, Souza DO, Gonçalves CA and Gottfried C: Resveratrol
protects C6 astrocyte cell line against hydrogen peroxide-induced
oxidative stress through heme oxygenase 1. PLoS One. 8:e643722013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuse Y, Tsuruma K, Mizoguchi T, Shimazawa
M and Hara H: Progranulin deficiency causes the retinal ganglion
cell loss during development. Sci Rep. 7:16792017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Degos V, Charpentier TL, Chhor V, Brissaud
O, Lebon S, Schwendimann L, Bednareck N, Passemard S, Mantz J and
Gressens P: Neuroprotective effects of dexmedetomidine against
glutamate agonist-induced neuronal cell death are related to
increased astrocyte brain-derived neurotrophic factor expression.
Anesthesiology. 118:1123–1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medeiros R, Castello NA, Cheng D, Kitazawa
M, Baglietto-Vargas D, Green KN, Esbenshade TA, Bitner RS, Decker
MW and LaFerla FM: α7 Nicotinic receptor agonist enhances cognition
in aged 3×Tg-AD mice with robust plaques and tangles. Am J Pathol.
184:520–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petty EL, Lafon A, Tomlinson SL,
Mendelsohn BA and Pillus L: Promotion of cell viability and histone
gene expression by the acetyltransferase Gcn5 and the protein
phosphatase PP2A in saccharomyces cerevisiae. Genetics.
203:1693–1707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chica N, Rozalén AE, Pérez-Hidalgo L,
Rubio A, Novak B and Moreno S: Nutritional control of cell size by
the greatwall-endosulfine-PP2A·B55 pathway. Curr Biol. 26:319–330.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Merrill RA, Slupe AM and Strack S:
N-terminal phosphorylation of protein phosphatase 2A/Bβ2 regulates
translocation to mitochondria, dynamin-related protein 1
dephosphorylation, and neuronal survival. FEBS J. 280:662–673.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faulkner JR, Herrmann JE, Woo MJ, Tansey
KE, Doan NB and Sofroniew MV: Reactive astrocytes protect tissue
and preserve function after spinal cord injury. J Neurosci.
24:2143–2155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berns EJ, Sur S, Pan L, Goldberger JE,
Suresh S, Zhang S, Kessler JA and Stupp SI: Aligned neurite
outgrowth and directed cell migration in self-assembled monodomain
gels. Biomaterials. 35:185–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang K, Cao F, Sheikh AM, Malik M, Wen G,
Wei H, Ted Brown W and Li X: Up-regulation of Ras/Raf/ERK1/2
signaling impairs cultured neuronal cell migration, neurogenesis,
synapse formation, and dendritic spine development. Brain Struct
Funct. 218:669–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dayer C and Stamenkovic I: Recruitment of
matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface
by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-β
(TGF-β) activation and fibroblast differentiation. J Biol Chem.
290:13763–13778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ward MP and Spiers JP: Protein phosphatase
2A regulation of markers of extracellular matrix remodelling in
hepatocellular carcinoma cells: Functional consequences for tumour
invasion. Br J Pharmacol. 174:1116–1130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghosh S, Kumar A, Tripathi RP and Chandna
S: Connexin-43 regulates p38-mediated cell migration and invasion
induced selectively in tumour cells by low doses of γ-radiation in
an ERK-1/2-independent manner. Carcinogenesis. 35:383–395. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kocić J, Santibañez JF, Krstić A,
Mojsilović S, Ilić V and Bugarski D: Interleukin-17 modulates
myoblast cell migration by inhibiting urokinase type plasminogen
activator expression through p38 mitogen-activated protein kinase.
Int J Biochem Cell Biol. 45:464–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernet JD, Doles JD, Hall JK, Kelly Tanaka
K, Carter TA and Olwin BB: p38 MAPK signaling underlies a
cell-autonomous loss of stem cell self-renewal in skeletal muscle
of aged mice. Nat Med. 20:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Xia Y, Kuang D, Duan Y and Wang G:
PP2A regulates SCF-induced cardiac stem cell migration through
interaction with p38 MAPK. Life Sci. 191:59–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng HY, Shen FJ, Tong YQ and Li Y: PP2A
inhibits cervical cancer cell migration by dephosphorylation of
p-JNK, p-p38 and the p-ERK/MAPK signaling pathway. Curr Med Sci.
38:115–123. 2018. View Article : Google Scholar : PubMed/NCBI
|