Introduction
Sepsis, which is caused by the maladjustment of a
host response to infection, may culminate in fatal organ
dysfunction, and has a high mortality rate. The lung is the most
vulnerable target organ among the multiple organ injuries
associated with sepsis, and acute lung injury (ALI) is also one of
the important causes of sepsis-associated mortality, due to its
early occurrence and high incidence rate (1). This has led to the requirement for a
more in-depth understanding of sepsis pathogenesis, as the majority
of therapeutic approaches have failed to decrease mortality from
severe sepsis. A previous study reported that endothelial damage
leads to neutrophil aggregation, inflammatory responses and
oxidative stress, which ultimately leads to lung injury (2). Therefore, it is essential to
understand the pathogenesis of lung injury in order for novel and
effective therapeutic targets to be identified.
Rhodopsin (Rho) is among the most well-characterized
downstream effectors of the Rho family of small GTPases. The
Rho/Rho-associated protein kinase (ROCK) signaling peptide, with
signal transduction and molecular switching effects, regulates
endothelial cell permeability via endothelial cell cytoskeleton
remodeling (3). Previous studies
have indicated that the Rho/ROCK signaling pathway serves an
important role in the pathogenesis of sepsis-induced pulmonary
epithelial permeability increases (4).
Fasudil is principally used to treat cerebral
vasospasm and improve brain microcirculation clinically. The
present study demonstrated that, as a specific Rho inhibitor,
fasudil has a wide range of pharmacological effects, including as a
vasodilator and as an anti-inflammatory, by inhibiting Rho/ROCK to
influence a variety of cellular functions. It has been reported
that fasudil inhibits systemic inflammation and guards against ALI
in septic mice (5); however, the
data were not fully conclusive and it is necessary to have an
improved understanding of how fasudil achieved these beneficial
effects through modulation of the inflammatory response in the
murine model. In the present study, a sepsis model was established
in rats using cecal ligation and puncture (CLP), and the effects of
fasudil on the Rho/ROCK signaling pathway and endothelial cell
permeability in sepsis-induced ALI were investigated.
Materials and methods
Experimental animals
Specific pathogen-free, 8-week old male Wistar rats
(220±20 g) used in the present study were obtained from the
Experimental Animal Center of the Chinese Academy of Medical
Sciences (Beijing, China). Animals received food and water ad
libitum during 1 week of acclimation. Rats were kept under a
12-h light/dark cycle at 22–25°C and 60% relative humidity. All
experimental protocols were conducted with the approval of the
Medical Research and New Technology Ethics Committee of Shengjing
Hospital affiliated to China Medical University.
Rat sepsis model
Polymicrobial sepsis in rats was induced by CLP as
described previously (6). In
brief, the rats were anesthetized using 5% chloral hydrate (300
mg/kg) via intraperitoneal injection and a 2 cm-long incision was
made along the midline of the anterior abdomen. The cecum was
gently exposed and ligated at ~50% of the total length with a 5-0
silk suture. On the antimesenteric side, the cecum was punctured
twice with an 18-gauge needle. A small amount of feces was squeezed
out from both ends of the perforation. Following this, the cecum
was repositioned and the abdomen was closed. Rats in the
sham-operated group had an identical laparotomy performed; however,
the cecum was neither ligated nor perforated.
Animal groups
A total of 60 Wistar rats were randomized into the
following three groups: A sham-operated group, a CLP (sepsis) group
and a CLP + fasudil (treatment) group, with 20 rats in each group.
The treatment groups received intraperitoneal injections of fasudil
30 mg/kg (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 1 h prior
to surgery. The sham-operated and sepsis groups were treated with
an equal amount of sterile saline by intraperitoneal injection. In
all groups, the following experiments were performed at 24 h
following the CLP procedure.
Monitoring of hemodynamics
At 24 h following surgery, the rats were again
anesthetized with 5% chloral hydrate (300 mg/kg) by intraperitoneal
injection. When the right carotid artery had been exposed, a
catheter was plunged in the right common carotid artery, and the
mean arterial pressure (MAP) was measured with an RM6240BD
multichannel physiological signal acquisition and processing system
(Chengdu Instrument Factory, Chengdu, China).
Bacterial cultures
At 24 h following CLP, blood samples were drawn from
the inferior vena cava and cultured to evaluate the bacterial
clearance. Serial logarithmic diluted blood was plated on
trypticase soy agar II (BD Biosciences, Franklin Lakes, NJ, USA)
with 5% sheep blood (BD Biosciences, Franklin Lakes, NJ, USA).
Plates were incubated under aerobic conditions at 37°C, and the
colonies were calculated after 24 h of incubation. Bacterial counts
are expressed as the number of colony-forming units
(×105) per milliliter of blood.
Detection of serum biochemical
indexes
Blood samples were collected from the inferior vena
cava (1:10 acid citrate dextrose) and centrifuged at 10,010 × g for
10 min at 4°C. The supernatant was collected and stored at −20°C.
Then, 1 ml of supernatant was taken to detect the serum alanine
aminotransferase (ALT), aspartate aminotransferase (AST), blood
urea nitrogen (BUN) and creatinine (Cr) levels in rats, and the
whole process was completed by the Roche cobas-8000 automatic
biochemical analyzer (Roche Diagnostics, Basel, Switzerland)
provided by the laboratory department of the Shengjing Hospital
affiliated to China Medical University.
Detection of endothelial cell
permeability (7)
A total of 8 rats in each group were injected with
Evans blue (EB; 20 mg/kg, Sigma-Aldrich; Merck KGaA) through the
tail vein. After 30 min, the rats were sacrificed and the left
lungs were collected. The left lung tissue was homogenized with
formamide and incubated at 37°C for 18 h, following which the
homogenate was centrifuged at 8,000 × g for 10 mins at 4°C. The
absorbance (A) of the supernatant was measured at 620 nm with a
spectrophotometer, and the amount of EB in the lung tissue was
calculated.
Determination of bronchoalveolar
lavage fluid (BALF)
The remaining rats in each group were sacrificed 24
h following CLP and a tracheal intubation was performed upon
separating the trachea and the main bronchus. The left lung was
washed three times with 0.5 ml pre-cooled PBS. The BALF was
centrifuged for 10 min at 1,500 × g at 4°C. The protein
concentrations in the BALF were detected using a protein
quantification kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to the manufacturer's protocol. The levels of TNF-α (cat.
no. ab208348), IL-1β (cat. no. ab100704) and IL-6 (cat. no.
ab100712) in the BALF were determined using ELISA kits (Abcam,
Cambridge, UK). The TNF-α, IL-1β and IL-6 content in the samples
was calculated using a standard calibration curve. The detection
ranges of the TNF-α, IL-1β and IL-6 ELISA assays were 12.5–800,
31.25–2,000 and 62.5–4,000 pg/ml, respectively. Concentrations of
samples that were initially over the limit of the standard curve
were measured following dilution.
The BALF cells were resuspended with PBS, and a
small number of the suspended cells were dropped onto a slide.
Following Wright-Giemsa staining for 10 min at room temperature,
polymorphonuclear neutrophils (PMN) were counted using an Eclipse
E200 optical microscope (Nikon Corporation, Tokyo, Japan) under a
high-power field (magnification, ×400). A total of five randomly
selected non-overlapping fields on each slide were observed.
Determination of oxidative stress in
lung tissue
Right lung tissue was collected, and the lung
homogenate was centrifuged for 15 min at 15,000 × g at 4°C.
Malondialdehyde (MDA; cat. no. A003-1) levels were detected using
the thiobarbituric acid colorimetric method, superoxide dismutase
(SOD; cat. no. A001-3) was determined by the yellow purine oxidase
method and myeloperoxidase (MPO; cat. no. A044) was determined in
the supernatant by tetramethylbenzidine method, using kits from
Nanjing Jiancheng Bioengineering Institute, Nanjing, China in
strict accordance with the manufacturer's protocol.
Wet/dry (W/D) lung weight ratios
The right upper lung was collected and weighed (W)
when the water and blood on the surface had been removed with
filter paper. The lung tissue was subsequently placed in an 80°C
dryer for 24 h, and weighed again (D). The W/D ratio of the lung
tissue was calculated.
Lung tissue histological
evaluations
Rat lung tissues were fixed in 4% paraformaldehyde
for 48 h at 4°C, and the specimens were dehydrated, embedded in
paraffin, sectioned at 5 µm and stained with hematoxylin and eosin
for 10 min at room temperature. Subsequently, the pathological
alterations in the lung tissue were examined under an optical
microscope. A scoring system was used to assess the degree of lung
injury based on the following histological features: Edema;
hyperemia and congestion; neutrophil margination and tissue
infiltration; intra-alveolar hemorrhage and debris; and cellular
hyperplasia. These characteristics were subjectively scored on a
scale between 0 and 3: 0, normal; 1, slight effect; 2, moderate
presence of that feature; and 3, severe effect. A total score was
calculated for each rat (8). A
total of 6 slides were randomly selected from each group. At least
10 high-power fields were captured per well (magnification, ×400).
The lung injury scores were evaluated by an independent pathologist
to objectively quantify the degree of lung injury.
Detection of caspase-3 activity in
lung tissue
Caspase-3 protease activity in the lung tissue was
determined using a caspase-3 colorimetric assay kit (Sigma-Aldrich;
Merck KGaA), in accordance with the manufacturer's protocol. In
brief, following homogenization of the whole lung tissue in cell
lysis buffer, homogenates were centrifuged for 1 min at 10,000 × g,
and the supernatant was extracted and incubated with
Asp-Glu-Val-Asp-p-nitroanilide (pNA) and reaction buffer for 90 min
at 37°C. Levels of the chromophore pNA were quantified using a
spectrophotometer at 405 nm, which reflected the caspase-3
activity. The data were normalized to the lung weight.
Activity of the Rho/ROCK signaling
pathway and expression of zonula occludens 1 (ZO-1)
Proteins were extracted from the lung tissue using a
commercial Protein Extraction kit (Nanjing Keygen Biotech Co.,
Ltd., Nanjing, China), according to the manufacturer's protocol.
Protein concentrations were quantified using a bicinchoninic acid
assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Equal amounts of protein loading per well were separated by
SDS-PAGE on 10% gels and transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skimmed milk for 1 h at room temperature, and
subsequently incubated with primary antibodies [rabbit monoclonal
anti-Rho (cat. no. ab40673; 1:500), monoclonal anti-ROCKl (cat. no.
ab4517; 1:500; both Abcam) or rabbit monoclonal anti-myosin
phosphatase targeting subunit 1 (MYPT-1; cat. no. 8574; 1:1,000),
monoclonal anti-phosphorylated (p)-MYPT-1 (cat. no. 4563; 1:1,000;
both Cell Signaling Technology, Inc., Danvers, MA, USA) or rabbit
monoclonal anti-ZO-1 (cat. no. 14-9776-82; 1:1,000; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) or β-actin (cat.
no. ARE6011; 1:1,000; Hangzhou HuaAn Biotechnology Co. Ltd.,
Hangzhou, China) at 4°C overnight. The membranes were subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (cat. no. A9298; 1:1,000; Beyotime Institute of
Biotechnology) at 37°C for 45 min. An enhanced chemiluminescence
imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to visualize the protein bands. The ratio of p-MYPT-1
expression to MYPT-1 expression reflected the level of MYPT-1
phosphorylation. Image J version, 1.51 h (National Institutes of
Health, Bethesda, MD, USA) was used for image analysis.
Cell strain
Human pulmonary microvascular endothelial cells
(HPMVEC-ST1.6R) were purchased from Clonetics™ (Lonza
Group Ltd., Basel, Switzerland). Cells were cultured in 10 cm
plates and maintained in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc.) in a 37°C humidified incubator with 5%
CO2. The medium was changed every 2 days. When the cells
grew to 80–90% confluence, they were digested and subcultured with
0.25% EDTA-trypsin, and the fourth passage of endothelial cells
were used for subsequent experimentation.
MTT assay for cell viability
The cytotoxic effects of lipopolysaccharide (LPS) on
HPMVEC-ST1.6R were assessed using an MTT assay (9). The HPMVEC-ST1.6R were inoculated in
96-well culture plates (5×104 cells/well in 100 µl
medium), and the cells were exposed to varying concentrations (0,
0.01, 0.1, 1, 10 and 100 µg/ml) of LPS and incubated for 24 h when
the cells had adhered. Each well was treated with 10 µl of 0.5
mg/ml MTT at the end of the incubation. The plate was placed in a
37°C humidified incubator for an additional 4 h, the supernatant
was discarded, and 200 µl dimethyl sulfoxide was added to each
well. The absorbance was measured at 490 nm using a BioTek MQX 680
(BioTek Instruments, Inc., Winooski VT, USA).
In order to observe the effects of fasudil on
LPS-induced HPMVEC-ST1.6R injury, the synchronized cells were
randomly divided into a control group, a 24 h LPS (10 µg/ml)
treatment group and fasudil (10, 25 and 50 mM) pretreatment groups.
Synchronized cells were pretreated with different concentrations of
fasudil for 2 h, and subsequently treated with 10 µg/ml LPS for 24
h. The determination method was the same as the MTT assay protocol
described above.
Quantitative analysis of vascular
endothelial growth factor (VEGF), intercellular adhesion molecule-1
(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) production
in endothelial cells
HPMVEC-ST1.6R were respectively pre-incubated with
fasudil at different concentrations (10, 25 and 50 µM) for 30 min,
following which 10 µg/ml LPS was added to supernatant in each well
(1×105 cells/ml) and reacted for 24 h. The quantitative
analysis of VEGF (cat. no. ab100751), ICAM-1 (cat. no. ab100688)
and VCAM-1 (cat. no. ab100750) in endothelial cell supernatants was
performed via ELISA (Abcam), according to the manufacturer's
protocol.
Effects of different concentrations of
fasudil on the HPMVEC-ST1.6R skeletal structural alterations
induced by LPS
Synchronized cells were randomly divided into a
control group, LPS (10 µg/ml) treatment group and fasudil (50 mM)
pretreatment group. Synchronized cells were pretreated with
different concentrations of fasudil for 2 h, and subsequently
treated with 10 µg/ml LPS for 24 h. The cells were fixed with 4%
paraformaldehyde for 30 min at room temperature and washed with PBS
solution three times. Subsequently, the cells were permeabilized
with 0.2% Triton X-100 for 30 min at room temperature.
Subsequently, 10% goat serum (cat. no. 50062Z; Invitrogen; Thermo
Fisher Scientific, Inc.) was used for blocking for 30 min at room
temperature following washing with PBS. The Fluorescein
isothiocyanate (FITC)-conjugated phalloidin (1:1,000) was added to
the cells at 4°C overnight. The nuclei were re-stained with DAPI
and incubated at room temperature without light for 10 min. The
different groups were detected using a Leica SP2 laser confocal
microscope (magnification, ×600; Leica Microsystems GmbH, Wetzlar,
Germany). Filamentous (F-)actin expression abundance was determined
by the intensity of cellular fluorescence.
Data analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Measurement data are expressed
as the mean ± standard deviation. One-way analysis of variance
followed by Tukey's test was used to analyze the differences among
groups. P<0.05 was considered to indicate a statistically
significant difference. The experiments were repeated independently
>3 times.
Results
Experimental model and mortality
A total of 24 h following CLP, the rats presented
with reduced activity, erect hair, shivering, fatigue, exudation
around the eyes, dyspnea and tachycardia. The rats were
unresponsive to external stimuli and exhibited hematuria, pyuria
and diarrhea. The laparotomy revealed bloody ascites with an odor,
swelling and necrosis at the cecal ligation site, in addition to
whole abdominal adhesion and severe inflation of the small
intestine, with visceral congestion and edema. Conversely, in the
fasudil group, the general health of the rats was improved. A
laparotomy revealed a reduction in the number of ascites and the
level of necrosis. Adhesions were observed only at the cecal
ligation site, and the abscess was confined to the area surrounding
the cecum, and the expansion of the small intestine was less
severe. Rats in the CLP group exhibited a survival rate of 83.3%
(10 survivals in 12 rats) at 24 h, while the survival rate of the
fasudil group was 91.7% (11 survivals in 12 rats), which was higher
compared with that of the sepsis group; however, there were no
statistically significant differences in the survival rates between
the two groups (Data not shown). Compared with the sham-operated
group, the serum levels of ALT, AST, BUN and Cr in the sepsis group
and fasudil group were all increased. Compared with the sepsis
group, the serum levels of ALT, AST, BUN and Cr in the fasudil
group were all decreased (P<0.05; n=10; Fig. 1A).
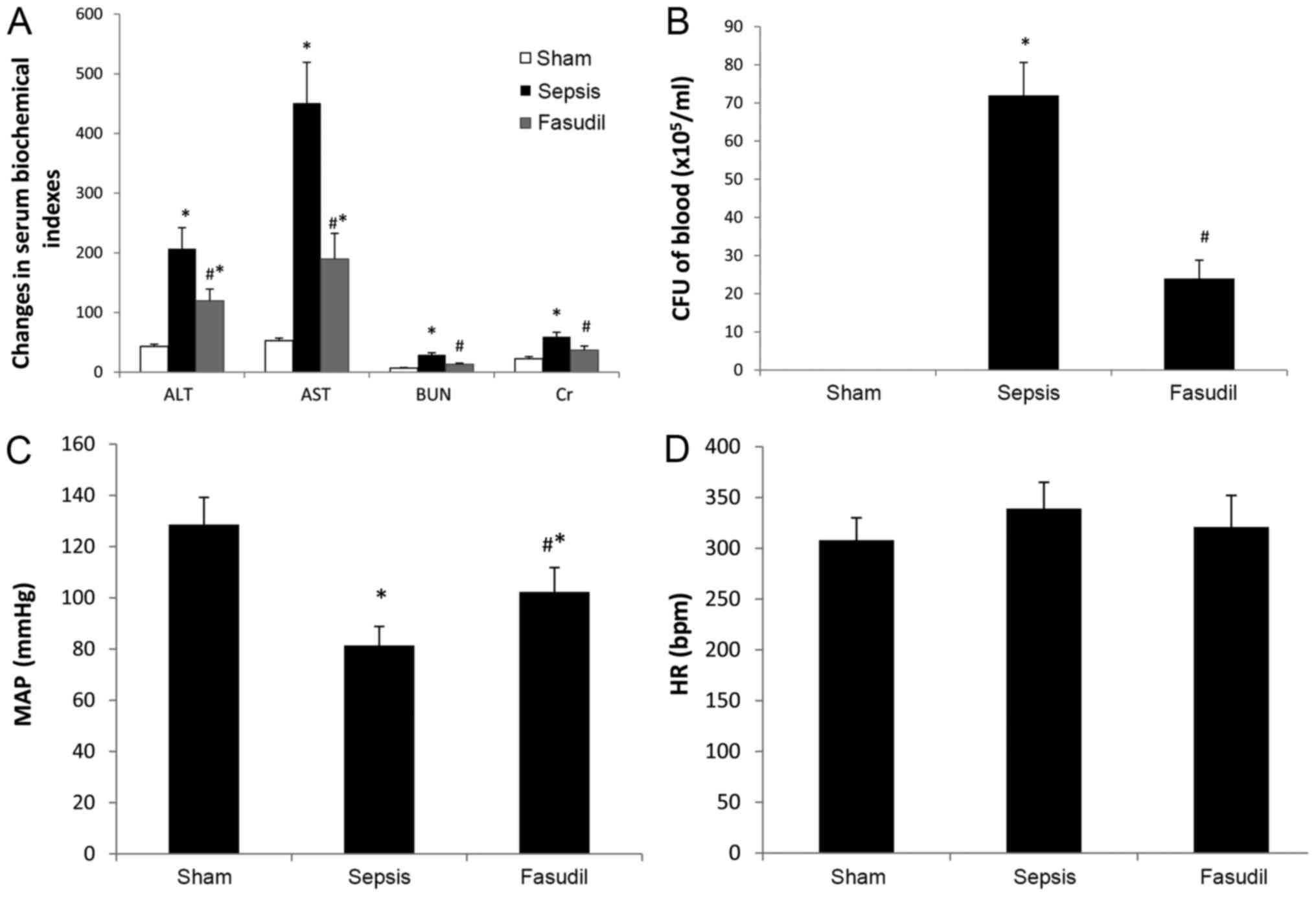 | Figure 1.Effect of fasudil on serum
biochemical indexes, systemic bacteremia and hemodynamics in rats
with sepsis-induced ALI. (A) Fasudil decreased the serum levels of
ALT, AST, BUN and Cr in rats with sepsis-induced ALI. (B) Fasudil
decreased the number of bacteria in the blood in rats with
sepsis-induced ALI. (C) Fasudil increased the MAP in rats with
sepsis-induced ALI. (D) HR in the three groups. Data are expressed
as the mean ± standard deviation. n=10. *P<0.05 vs. Sham;
#P<0.05 vs. Sepsis. ALI, acute lung injury; ALT,
alanine aminotransferase; AST, aspartate aminotransferase, BUN,
blood urea nitrogen; Cr, creatinine; CFU, colony-forming units;
MAP, mean arterial pressure; HR, heart rate. |
Systemic bacteremia
The number of bacteria in the blood from the sepsis
group was markedly higher compared with that from the sham-operated
group. However, the number of bacteria in the blood from the
fasudil group was significantly lower compared with that from the
sepsis group (P<0.05; n=10; Fig.
1B).
Hemodynamic analysis
MAP was significantly increased in the fasudil group
compared with the sepsis group (P<0.05), whereas the differences
in heart rate between the three groups were not statistically
significant (P>0.05; n=10; Fig. 1C
and D).
Permeability of lung endothelial
cells
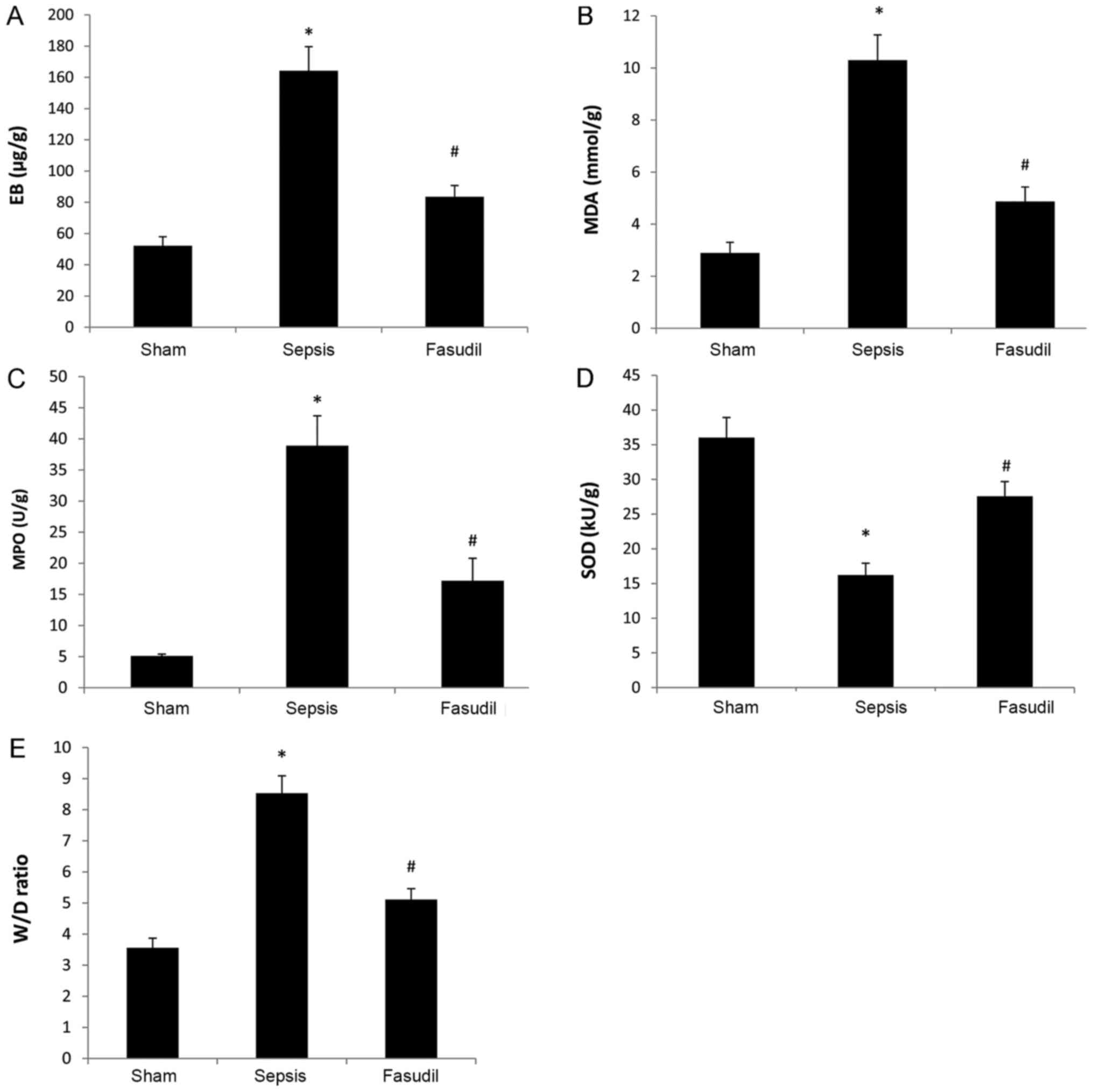
The EB content in the lung tissue from the sepsis
group was significantly increased compared with the sham-operated
group, whereas the EB content in the fasudil group was
significantly reduced compared with the sepsis group (P<0.05;
n=10; Fig. 2A).
Oxidative stress indexes in lung
tissue
Levels of MDA and MPO in lung tissues from the
sepsis group were significantly increased compared with the
sham-operated group; however, the activity of SOD was significantly
reduced. Conversely, the levels of MDA and MPO in lung tissues from
the fasudil group were significantly lower compared with those from
the sepsis group, yet the activity of SOD was significantly
increased (P<0.05; n=10; Fig.
2B-D).
W/D lung weight ratios
W/D weight ratios in the sepsis group were
significantly increased compared with the sham-operated group;
however, the ratios in the fasudil group were significantly
decreased compared with the sepsis group (P<0.05; n=10; Fig. 2E).
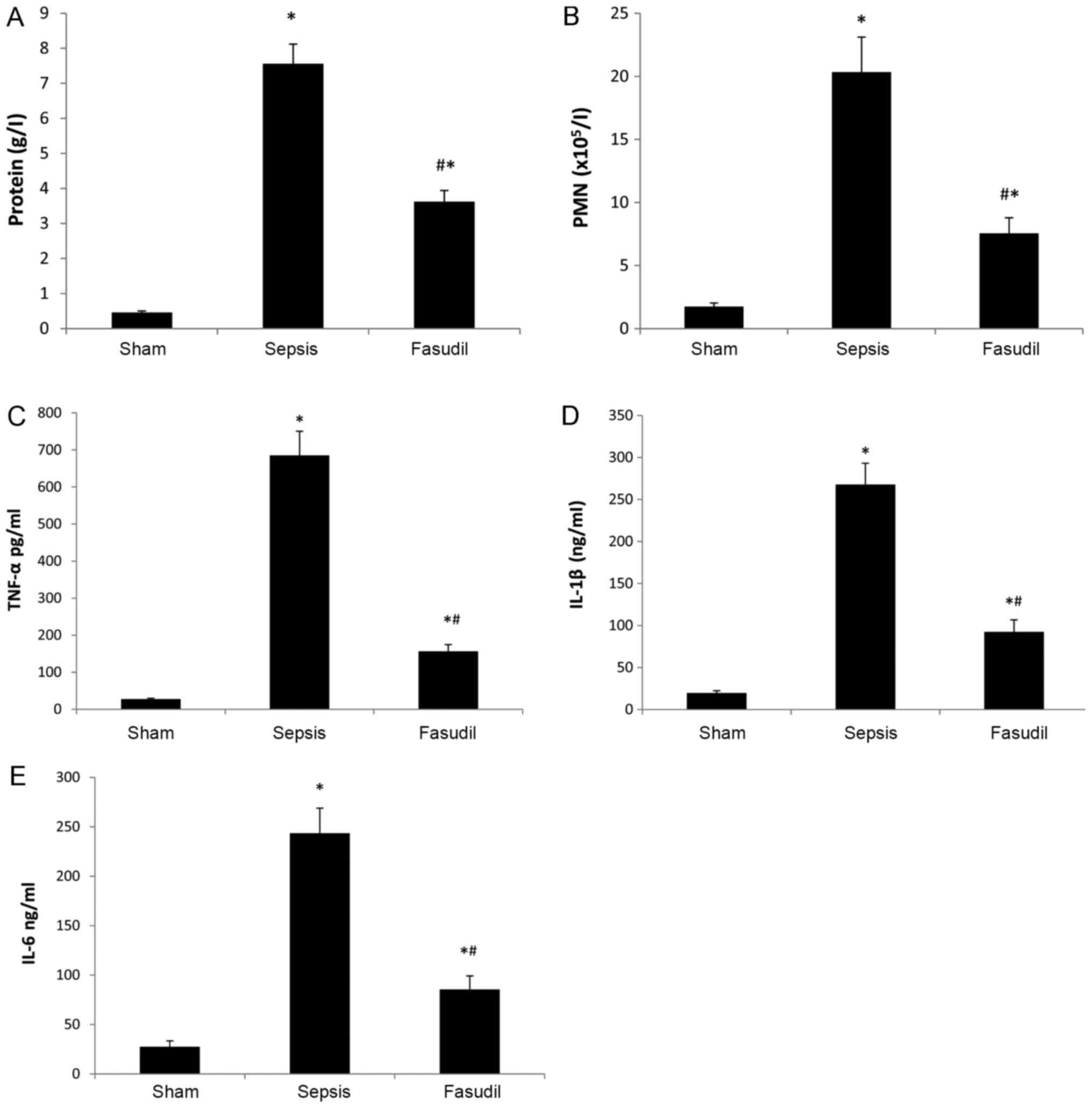
BALF indexes
Compared with the sham-operated group, PMN counts
and the total protein content, TNF-α, IL-1β and IL-6 in the BALF
were significantly increased in the sepsis group. Conversely, all
of these indices in the fasudil group were significantly reduced
compared with the sepsis group (P<0.05; n=10; Fig. 3). Furthermore, the concentrations
of TNF-α, IL-1β and IL-6 in the sepsis group were 685.3±65.3 pg/ml,
267.8±25.3 and 243.5±25.4 ng/ml, respectively; whereas, the
concentrations of TNF-α, IL-1β and IL-6 in the fasudil group were
156.4±18.3 pg/ml, 92.4±14.3 and 85.4±13.7 ng/ml. Therefore, fasudil
significantly decreased BALF concentrations of TNF-α by 77%, IL-1β
by 65% and IL-6 by 65% in CLP-induced ALI.
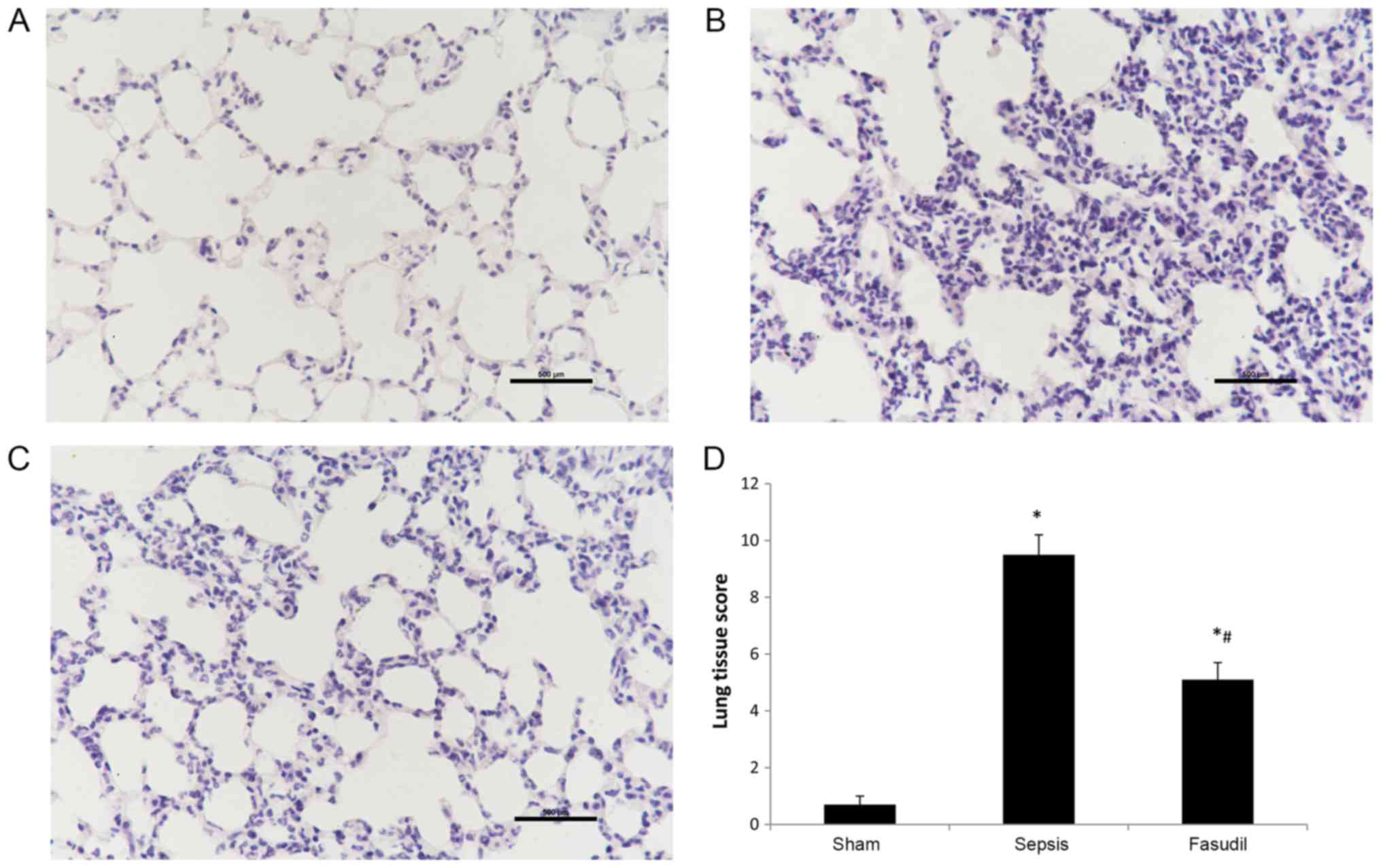
Pulmonary histopathology
When examined microscopically, the alveolar
structure appeared to be intact, the pulmonary interstitium was not
notably edematous, and there was no evidence of inflammatory cell
infiltration in the sham-operated group. Conversely, lung tissues
from the sepsis group were severely damaged. Interstitial
congestion, edema and thickening of alveolar wall, in addition to
large numbers of inflammatory cells, were observed. Lung tissue
injuries in the fasudil group were significantly less severe
compared with the sepsis group (Fig.
4A-C). Furthermore, lung injury scores in the fasudil group
were significantly lower compared with the sepsis group (P<0.05;
Fig. 4D).
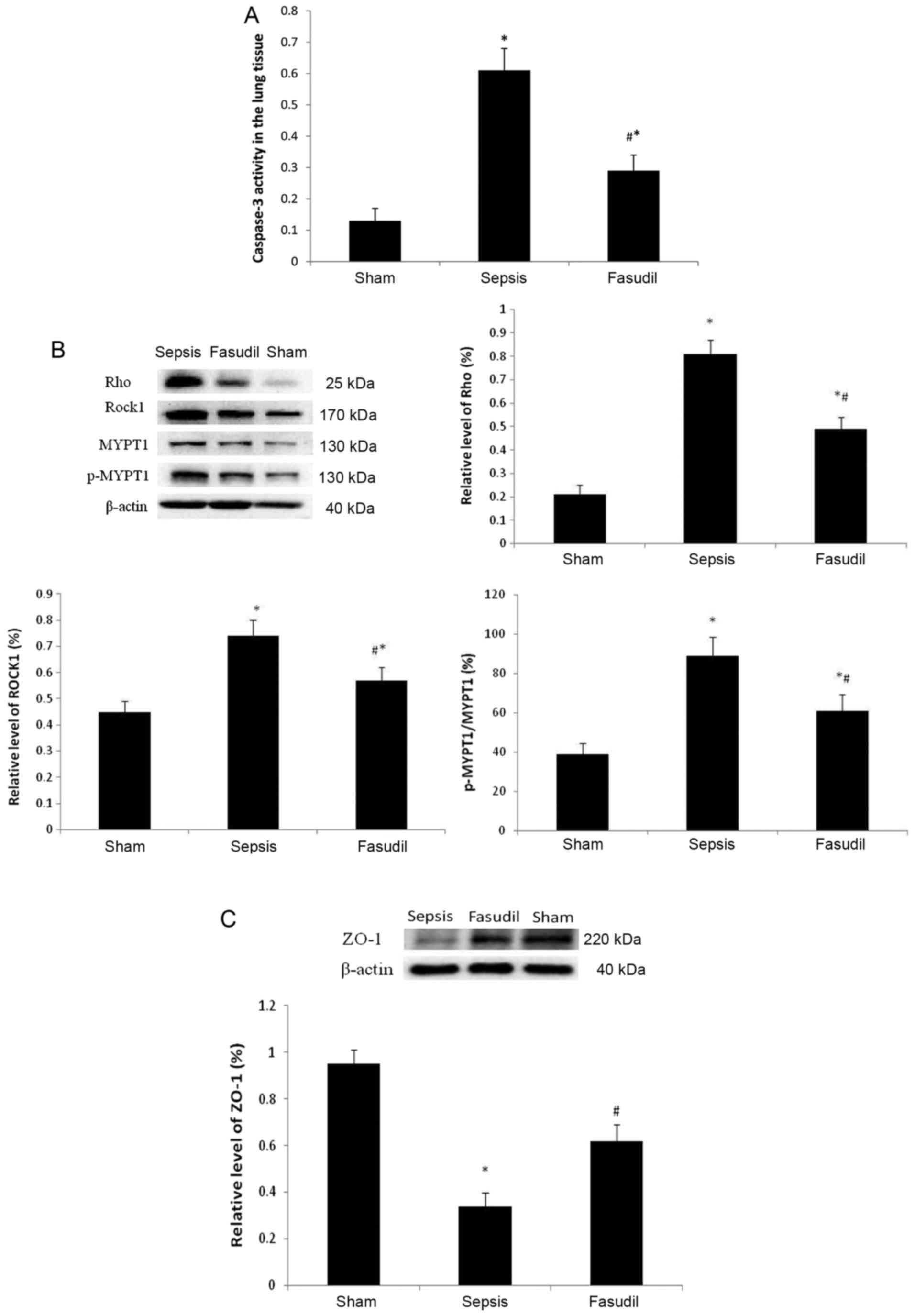
Caspase-3 activity in lung tissue
The caspase-3 activity in the lung tissue was 0.13
in the sham operation group; however, in the sepsis group, the
proportion increased to 0.61. When compared with the sepsis group,
in the fasudil group the caspase-3 activity in the lung tissue was
decreased to 0.29 (P<0.05; n=10; Fig. 5A).
Expression of Rho, ROCK1, MYPT-1 and
p-MYPT-1 in lung tissue
Compared with the sham group, the expression of Rho
and ROCKl was significantly upregulated, and the phosphorylation of
MYPT-1 in the lung tissues was significantly increased in the
sepsis group (P<0.05). However, when compared with the sepsis
group, the expression of Rho and ROCKl in lung tissues was
significantly downregulated, and the phosphorylation of MYPT-1 in
lung tissues was significantly decreased in the fasudil group
(P<0.05; Fig. 5B).
Protein expression levels of ZO-1 in
the lung tissue
Compared with the sham group, the protein expression
levels of ZO-1 were decreased in the sepsis group, whereas the
protein expression levels of ZO-1 increased in the fasudil group
(P<0.05; Fig. 5C).
Effects of LPS on the growth of
HPMVEC-ST1.6R cells and the intervention effect of fasudil
To analyze the effect of LPS on the viability of
HPMVEC-ST1.6R, the cells were exposed to different concentrations
of LPS for 24 h. The results of the MTT assay indicated that LPS
significantly reduced the viability of HPMVEC-ST1.6R in a
dose-dependent manner (Fig. 6A).
The results demonstrated that compared with the control group,
0.01, 0.1 and 1 µg/ml LPS had no apparent effect on HPMVEC-ST1.6R
growth; whereas, 10 and 100 µg/ml LPS significantly inhibited
HPMVEC-ST1.6R growth, and the OD values were reduced to 0.754±0.19
and 0.50±0.12 respectively. From the above results, it appeared
that 10 µg/ml LPS may affect the growth of HPMVEC-ST1.6R without
causing too much damage. There remained enough cells for the
subsequent experiments. Therefore, 10 µg/ml LPS was selected to
generate HPMVEC-ST1.6R damage models. The results of the present
study were similar to those of a previous study (10). Pretreatment with either 25 or 50 µM
fasudil was demonstrated to alleviate the cell damage induced by
LPS for 24 h (P<0.05 vs. LPS group; Fig. 6B).
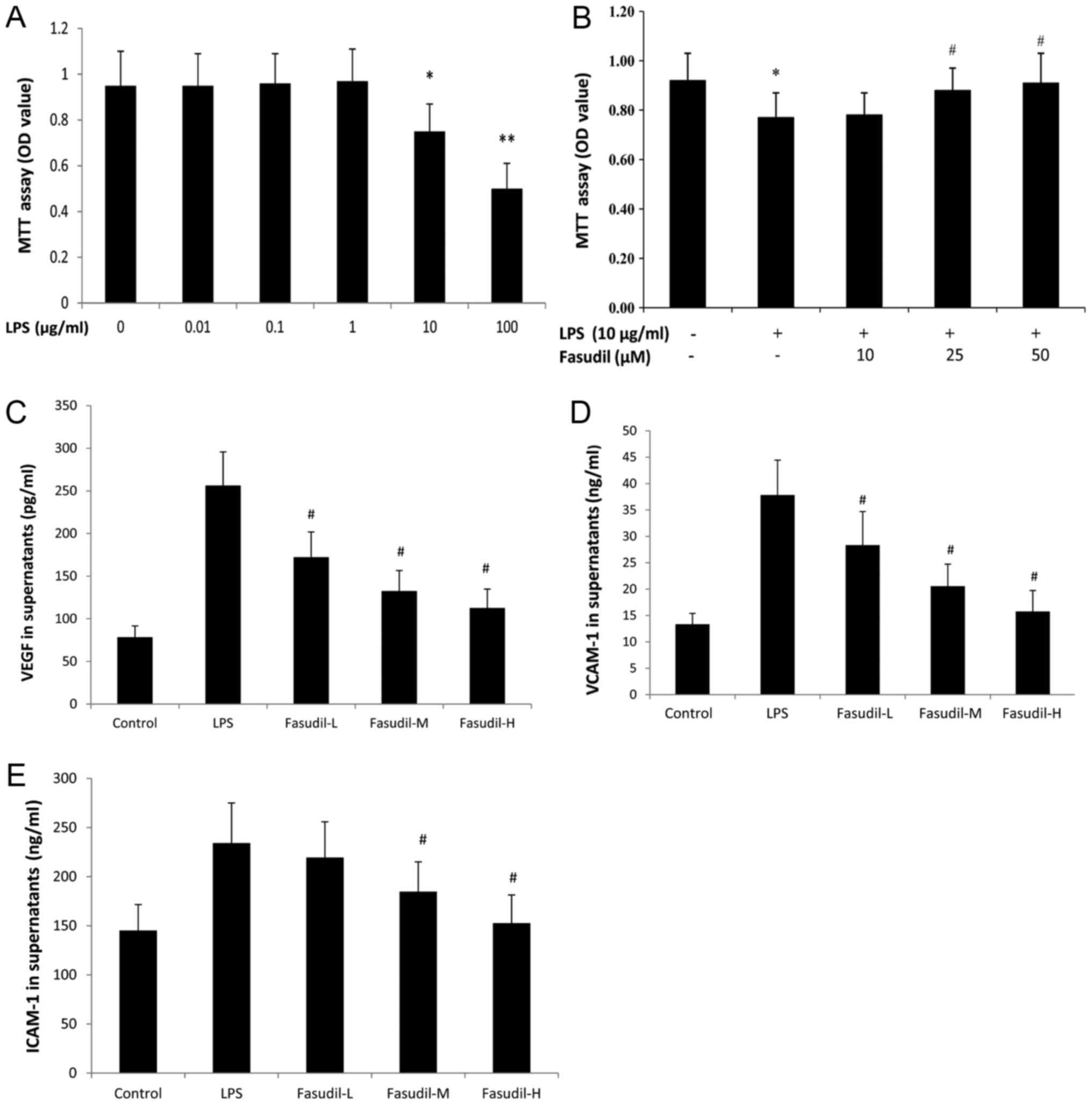 | Figure 6.Effect of fasudil on the viability
and the secretion of inflammatory cytokines from HPMVEC-ST1.6R
treated with LPS. (A) The effect of LPS on the viability of
HPMVEC-ST1.6R was examined by MTT assay. n=6. *P<0.05,
**P<0.01, vs. control. (B) Fasudil relieved the cytotoxic
effects of LPS on rat HPMVEC- ST1.6R examined by MTT assay. Data
are expressed as the mean ± standard deviation. n=6. *P<0.05 vs.
control group; #P<0.05 vs. LPS-only group. Fasudil
inhibited LPS-induced (C) VEGF, (D) ICAM-1 and (E) VCAM-1 secretion
from HPMVEC-ST1.6R. Fasudil (10, 25 and 50 µM) was separately
preincubated with HPMVEC-ST1.6R 30 min prior to LPS exposure.
Supernatants were detected using ELISA for VEGF, ICAM-1 and VCAM-1.
Fasudil-L, fasudil at a concentration of 10 µM; fasudil-M, fasudil
at a concentration of 25 µM; fasudil-H, fasudil at a concentration
of 50 µM. Data are expressed as the mean ± standard deviation. n=6.
#P<0.05 vs. LPS group. LPS, lipopolysaccharide, VEGF,
vascular endothelial growth factor, ICAM-1, intracellular cell
adhesion molecule-1; VCAM-1, vascular cell adhesion molecule 1; OD,
optical density; HPMVEC, human pulmonary microvascular endothelial
cells. |
VEGF, ICAM-1 and VCAM-1 from
HPMVEC-ST1.6R treated with LPS are weakened by fasudil
To confirm whether fasudil acted on the secretion of
VEGF, ICAM-1 and VCAM-1 from HPMVEC-ST1.6R, HPMVEC-ST1.6R exposed
to LPS were pretreated with different concentrations of fasudil.
Fasudil at high concentrations (25 and 50 µM) significantly reduced
VEGF, ICAM-1 and VCAM-1 levels in the supernatant of HPMVEC-ST1.6R
in a dose-dependent manner (Fig.
6C-E).
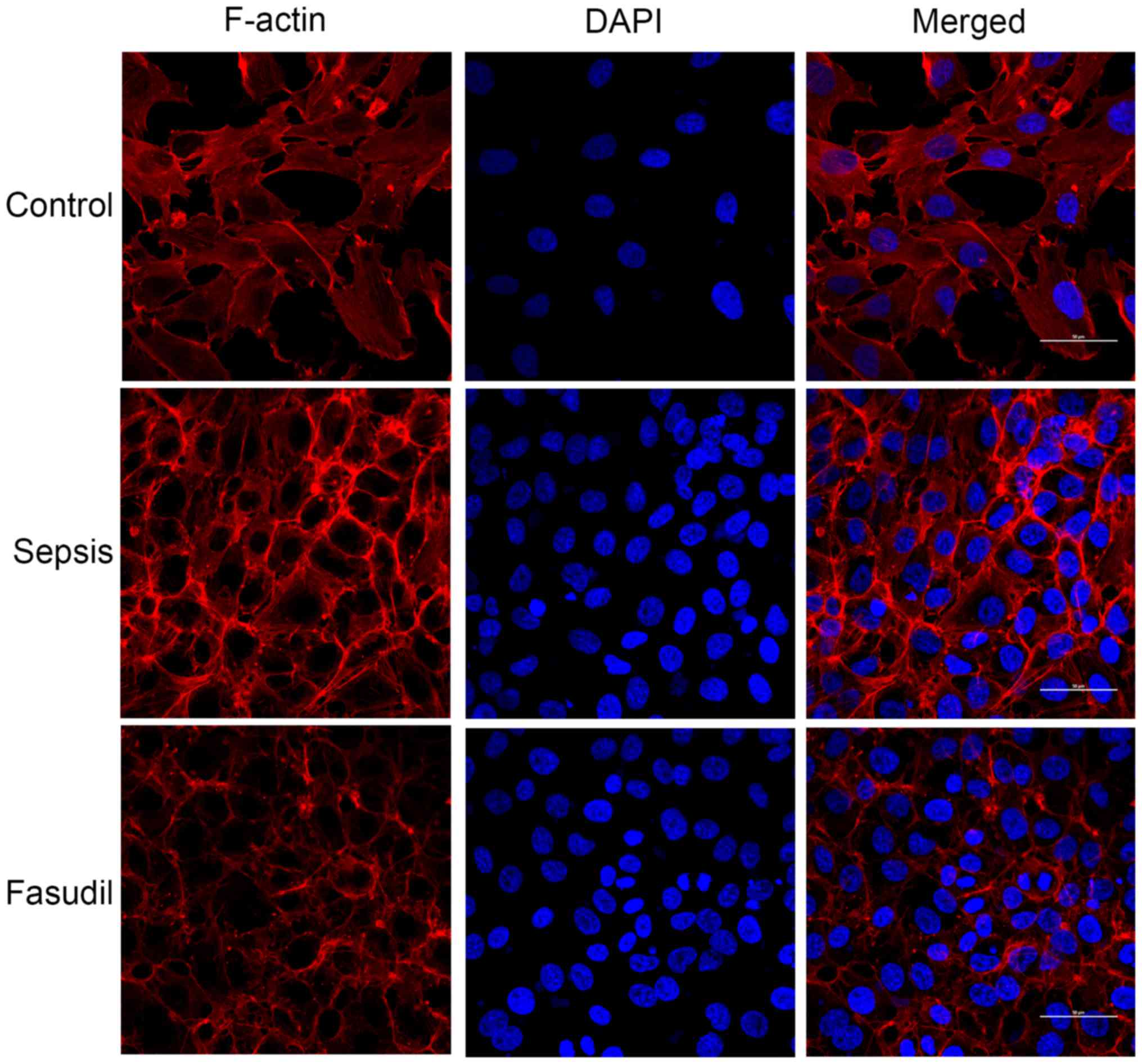
Effect of fasudil on the distribution
of F-actin in HPMVEC-ST1.6R skeletal actin
In the control group, a small amount of F-actin was
observed and was predominantly distributed around the cells with a
clear border. It was demonstrated that the F-actin around the cells
gradually decreased following 24 h of treatment with 10 µg/ml LPS.
Densely-bunched stress fibers were observed in the cytoplasm.
F-actin appeared to exhibit a scattered distribution, and the
normal junctions between cells were lost. However, in the cells
pretreated with the ROCK inhibitor fasudil (50 µM), the formation
of HPMVEC stress fibers and alterations in the cytoskeletal
morphology were partially inhibited. The fluorescence intensity and
the expression of F-actin were also markedly decreased (Fig. 7), indicating that fasudil may
inhibit LPS-induced morphological and cytoskeletal alterations in
HPMVECs.
Discussion
Sepsis may lead to multiple organ dysfunction
syndrome and multiple organ failure, among which ALI is the most
common organ injury (11).
Alveolar endothelial cell damage is a principal mechanism of ALI.
Once damaged, endothelial cells exhibit trans-endothelial fluid
transport, taking advantage of disordered cell-gap formation,
causing inflammatory cell infiltration and resulting in pulmonary
edema and lung parenchymal injury, which are associated with
pulmonary dysfunction (12). Early
identification and early treatment of sepsis/septic shock may
improve the prognosis and reduce the mortality associated with
sepsis (13). Certain patients
with appendicitis, with a history of abdominal pain for less than
24 h, abdominal local pressure pain and hemodynamic stability, may
be treated non-surgically (14).
However, others with appendicitis are advised to undergo surgery
within 24 h of symptom onset so as to reduce the risk of
complications (15). With or
without shock, patients with severe sepsis required treatment as
early as possible (16). Based on
these considerations, fasudil was administered 1 h prior to CLP in
the present study. In addition, the dose of fasudil (30 mg/kg) was
determined based on previous research (17,18),
in addition to pharmacological and toxicological profile of
fasudil. The present study aimed to investigate the pharmacological
effects of fasudil on ALI in septic rats. The results of the
present study demonstrated that the EB content and the protein
levels in the BALF increased in CLP-induced septic rats, suggesting
that the permeability of the endothelial cells had increased. The
levels of bacteria in the blood increased, in addition to the
concentrations of TNF-α, IL-1β and IL-6 in the BALF, accompanied by
a large number of PMN and an augmentation of MDA and MPO levels.
However, a decrease in SOD activity was observed. The expression of
activated caspase-3 was upregulated in septic rats. These results
indicated that the lung tissue exhibited a marked inflammatory
reaction, inducing oxidative stress and apoptosis, leading to
pulmonary edema and lung parenchymal injury. Treatment with fasudil
inhibited the increase in lung endothelial cell permeability,
inflammatory response, oxidative stress and apoptosis, thereby
reducing pulmonary edema and lung parenchymal injury, demonstrating
that fasudil had a protective effect against lung injury in septic
rats with ALI.
Rho is a G protein that cycles between an inactive
GDP-bound and an active GTP-bound state, and serves an important
role in cell adhesion, contraction, movement and division by
combining with its downstream target molecules (19). ROCK is a major downstream signaling
molecule of Rho with two subtypes, ROCKl and ROCK2. ROCK is
distributed in tissues throughout the body. ROCK1 is principally
expressed in non-neural tissues, including the liver, lungs, spleen
and testes; however, ROCK2 exhibits increased expression in the
brain, heart and muscles. MYPT-1 acts as the principal downstream
effector protein of ROCK, whose phosphorylation level may
indirectly reflect the activity of ROCK (20). Rho may be activated by histamine,
thrombin, VEGF, LPS and mechanical action. Activated Rho binds to
ROCK and may increase calmodulin formation, upregulate the
concentration of intracellular Ca2+, and activate and
induce the phosphorylation of MYPT-l, which inhibits the activity
of myosin light chain phosphatase, causing myosin light chain
phosphorylation. The phosphorylation of myosin light chain results
in myosin contraction, cytoskeletal remodeling, and the abnormal
expression and distribution of intercellular tight junction
proteins including ZO-1, which eventually leads to endothelial cell
permeability enhancement and a deficiency in barrier function
(21–23). Caspase-3 is the most important
terminal cleavage enzyme in the process of apoptosis. Caspase-3 can
induce a conformational change in ROCK-1 during apoptosis, which
leads to a persistent activation state of ROCK-1 (24,25).
The results of the present study demonstrated that the expression
of Rho and ROCK1 was downregulated and the phosphorylation level of
MYPT-1 in the lung tissue was decreased in the group treated with
fasudil. Furthermore, as an indicator of endothelial integrity and
ROCK-targeting, the expression of ZO-1 was upregulated in the group
treated with fasudil. Fasudil also prevented the LPS-induced
reorganization of actin filaments in vitro. This suggested
that the mechanism of action of fasudil in alleviating oxidative
stress, and reducing the inflammatory response and apoptosis in
septic rats with ALI, was associated with inhibition of the
Rho/ROCK pathway. Rho/ROCK pathway targeted therapy has been used
in clinical disease and has achieved positive results (26–28).
PMVECs, important parenchymal cells in lung tissue,
are an important target of inflammation and a source of
inflammatory reactions, which may be activated to produce a number
of inflammatory mediators, including IL-6, IL-8, TNF-α and cellular
chemokines, including ICAM-1 and VCAM-1. These inflammatory
mediators are able to directly move into the blood vessels and
alveolar cavity or indirectly move through the recruitment of other
inflammatory cells, thereby increasing the endothelial cells and
the surrounding tissue damage induced by pathological alterations
in ALI (29). Therefore, damage to
pulmonary endothelial cells, particularly microvascular endothelial
cells, has been regarded as one of the characteristics of ALI. As a
strong inflammatory response promoter, LPS is the key initiator of
endothelial dysfunction in sepsis (30). LPS may directly act on PMVECs,
resulting in apoptosis, cytoskeletal rearrangement, permeability
enhancement and inflammatory cytokine release (31). Therefore, the present study aimed
to examine the effect of fasudil on the expression of HPMVE
inflammatory factors in an LPS-induced HPMVEC injury model.
VEGF is known as the most active substance in
promoting vascular permeability in vivo, and is primarily
expressed by alveolar type II epithelial cells. When the epithelial
barrier of the lung is damaged, VEGF may increase vascular
permeability, which eventually leads to pulmonary edema. The
expression of VEGF protein in the lung tissue of rats with sepsis
was significantly increased (32).
ICAM-1 and VCAM-1 belong to the adhesion molecule immunoglobulin
superfamily. A small amount of ICAM-1 was expressed in pulmonary
vascular endothelial cells in a normal physiological environment,
while it was overexpressed in sepsis. The necrosis and apoptosis of
pulmonary vascular endothelial cells in rats is associated with the
overexpression of ICAM-1 (33).
VCAM-1 is a neutrophil surface adhesion
molecule-integrin ligand, which serves an important role in the
adhesion and migration of neutrophils to endothelial cells
(34). According to the present
results, the levels of VEGF, ICAM-1 and VCAM-1 secreted by HPMVECs
were significantly increased following LPS stimulation. Following
intervention with fasudil, the levels of VEGF, ICAM-1 and VCAM-1
secreted by HPMVECs were significantly decreased in a
dose-dependent manner. In vitro experiments also confirmed
that fasudil downregulated the expression of associated adhesion
molecules and improved pulmonary capillary permeability induced by
CLP.
Fasudil dilates blood vessels by inhibiting the
final stage of smooth muscle contraction via the phosphorylation of
myosin light chains (35), causing
hypotension and reflex tachycardia. According to the results of the
present study, MAP was increased in the fasudil group compared with
the sepsis group, whereas the difference in heart rate between the
three groups was not statistically significant. It was hypothesized
that fasudil has a protective effect on lung injury in sepsis, and
also is not associated with any serious adverse reactions. The
present study provided a theoretical basis for the use of fasudil
in the clinical treatment of sepsis. However, only a single dose of
fasudil was given at one time point in the present study, which may
limit the possibility for clinical extrapolation.
A previous study demonstrated that pretreatment with
the ROCK inhibitor Y-27632 markedly reduced the LPS-induced
expression of IL-1β and IL-6, and the activation of nuclear factor
(NF)-κB. Furthermore, ROCK inhibitor treatment antagonized the
expression of tissue factor and plasminogen activator inhibitor-1
in lung tissue and HPMVECs. These results suggested the ROCK
inhibition protects against endotoxin-induced pulmonary
inflammation and coagulation via NF-κB pathway modulation (36).
Fasudil, an inhibitor of ROCK which is a commonly
used drug in clinical practice, has a wide range of pharmacological
effects, and may potentially serve an invaluable role in the
prevention and treatment of cardiovascular and cerebrovascular
diseases. The clinical application of fasudil in sepsis remains to
be further investigated. With an in-depth study of the mechanism of
action of ALI, fasudil may become a candidate drug for future ALI
treatment.
In summary, the present study demonstrated that
treatment with fasudil had a protective effect on lung injury in
septic rats. The mechanism may involve fasudil contributing to
inhibition of the activity of the Rho/ROCK signaling pathway in
lung tissues. Fasudil improved endothelial permeability and reduced
lung inflammation, oxidative stress and apoptosis, thereby reducing
lung injury.
Acknowledgements
Not applicable.
Funding
The present study was funded in part by Liaoning
Science and Technology Project of China (grant no.
17-230-9-58).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
YW contributed to conceiving and designing the
experiment, analysis and interpretation of data, manuscript
preparation and critical evaluation. XW contributed to experimental
studies, data interpretation, statistical analysis and manuscript
preparation. WL and LZ contributed to experimental studies, data
interpretation and statistical analysis. All authors reviewed the
manuscript.
Ethics approval and consent to
participate
All experimental protocols were conducted with the
approval of the Medical Research and New Technology Ethics
Committee of Shengjing Hospital affiliated to China Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bastarache JA, Ware LB and Bernard GR: The
role of the coagulation cascade in the continuum of sepsis and
acute lung injury and acute respiratory distress syndrome. Semin
Respir Crit Care Med. 27:365–376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eiznhamer DA, Flavin MT, Jesmok GJ, Borgia
JF, Nelson DJ, Burhop KE and Xu ZQ: Effective attenuation of
endotoxin-induced acute lung injury by 2,3-diacetyloxybenzoic acid
in two independent animal models. Pulm Pharmacol Ther. 17:105–110.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin E, Dubey BN, Zhang SC, Gremer L,
Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV
and Ahmadian MR: Rho-kinase: Regulation, (dys)function, and
inhibition. Biol Chem. 394:1399–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cinel I, Ark M, Dellinger P, Karabacak T,
Tamer L, Cinel L, Michael P, Hussein S, Parrillo JE and Kumar A and
Kumar A: Involvement of Rho kinase (ROCK) in sepsis-induced acute
lung injury. J Thorac Dis. 4:30–39. 2012.PubMed/NCBI
|
|
5
|
Ding RY, Zhao DM, Zhang ZD, Guo RX and Ma
XC: Pretreatment of Rho kinase inhibitor inhibits systemic
inflammation and prevents endotoxin-induced acute lung injury in
mice. J Surg Res. 171:e209–e214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han J, Ding R, Zhao D, Zhang Z and Ma X:
Unfractionated heparin attenuates lung vascular leak in a mouse
model of sepsis: Role of RhoA/Rho kinase pathway. Thromb Res.
132:e42–e47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu R, Dong W, Zhou M, Zhang F, Marini CP,
Ravikumar TS and Wang P: Ghrelin attenuates sepsis-induced acute
lung injury and mortality in rats. Am J Respir Crit Care Med.
176:805–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim I, Kim HG, So JN, Kim JH, Kwak HJ and
Koh GY: Angiopoietin-1 regulates endothelial cell survival through
the phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Circ Res. 86:24–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Ma D, Chen M, Zhang L, Zhang L,
Zhang J, Qu X and Wang C: Ulinastatin attenuates LPS-induced human
endothelial cells oxidative damage through suppressing JNK/c-Jun
signaling pathway. Biochem Biophys Res Commun. 474:572–578. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tasaka S, Koh H, Yamada W, Shimizu M,
Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM
and Ishizaka A: Attenuation of endotoxin-induced acute lung injury
by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell
Mol Biol. 32:504–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore LJ and Moore FA: Early diagnosis and
evidence-based care of surgical sepsis. J Intensive Care Med.
28:107–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abeş M, Petik B and Kazil S: Nonoperative
treatment of acute appendicitis in children. J Pediatr Surg.
42:1439–1442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim M, Kim SJ and Cho HJ: Effect of
surgical timing and outcomes for appendicitis severity. Ann Surg
Treat Res. 91:85–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrer R, Martin-Loeches I, Phillips G,
Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C and Levy
MM: Empiric antibiotic treatment reduces mortality in severe sepsis
and septic shock from the first hour: Results from a
guideline-based performance improvement program. Crit Care Med.
42:1749–1755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thorlacius K, Slotta JE, Laschke MW, Wang
Y, Menger MD, Jeppsson B and Thorlacius H: Proective effect of
fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte
recruitment, and hepatocellular apoptosis in septic liver injury. J
Leukoc Biol. 79:923–931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Preau S, Delguste F, Yu Y, Remy-Jouet I,
Richard V, Saulnier F, Boulanger E and Neviere R: Endotoxemia
engages the RhoA kinase pathway to impair cardiac function by
altering cytoskeleton, mitochondrial fission and autophagy.
Antioxid Redox Signal. 24:529–542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wojciak-Stothard B and Ridley AJ: Rho
GTPases and the regulation of endothelial permeability. Vascul
Pharmaeol. 39:187–199. 2002. View Article : Google Scholar
|
|
20
|
Ma T, Liu L, Wang P and Xue Y: Evidence
for involvement of ROCK signaling in bradykinin-induced increase in
murine blood-tumor barrier permeability. J Neurooncol. 106:291–301.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen SC, Liu CC, Huang SY and Chiou SJ:
Vascular hyperpermeability in response to inflammatory mustard oil
is mediated by Rho kinase in mice systemically exposed to arsenic.
Microvasc Res. 82:182–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Qin J, Liu M, Ruan Q, Li Y and Zhang
Z: Role of Rho kinase in lysophosphatidic acid-induced altering of
blood-brain barrier permeability. Int J Mol Med. 33:661–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bogatcheva NV, Zemskova MA, Poirier C,
Mirzapoiazova T, Kolosova I, Bresnick AR and Verin AD: The
suppression of myosin light chain (MLC) phosphorylation during the
response to lipopolysaccharide (LPS): Beneficial or detrimental to
endothelial barrier. J Cell Physiol. 226:3132–3146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen LW, Chen W, Hu ZQ, Bian JL, Ying L,
Hong GL, Qiu QM, Zhao GJ and Lu ZQ: Protective effects of growth
arrest-specific protein 6 (Gas6) on sepsis-induced acute kidney
injury. Inflammation. 39:575–582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bessho M, Aki T, Funakoshi T, Unuma K,
Noritake K, Kato C and Uemura K: Rho-kinase inhibitor Y-27632
attenuates arsenic trioxide toxicity in H9c2 cardiomyoblastoma
cells. Cardiovasc Toxicol. 13:267–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nozaki Y, Kinoshita K, Hino S, Yano T,
Niki K, Hirooka Y, Kishimoto K, Funauchi M and Matsumura I:
Signaling Rho-kinase mediates inflammation and apoptosis in T cells
and renal tubules in cisplatin nephrotoxicity. Am J Physiol Renal
Physiol. 308:F899–F909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kohno M, Watanabe M, Goto T, Kamiyama I,
Ohtsuka T, Tasaka S and Sawafuji M: Attenuation of lung
ischemia-reperfusion injury by rho-associated kinase inhibition in
a rat model of lung transplantation. Ann Thorac Cardiovasc Surg.
20:359–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen W, Wang L, Pi R, Li Z and Rikang
Wang: L-F001, a multifunctional ROCK inhibitor prevents
paraquat-induced cell death through attenuating ER stress and
mitochondrial dysfunction in PC12 cells. Biochem Biophys Res
Commun. 464:794–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Li Q, Deng Z, Zhang Z, Xu J, Qian
G and Wang G: Protection from lipopolysaccharide-induced pulmonary
microvascular endothelial cell injury by activation of hedgehog
signaling pathway. Mol Biol Rep. 38:3615–3622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizumura K, Gon Y, Kumasawa F, Onose A,
Maruoka S, Matsumoto K, Hayashi S, Kobayashi T and Hashimoto S:
Apoptosis signal-regulating kinase 1-mediated signaling pathway
regulates lipopolysaccharide-induced tissue factor expression in
pulmonary microvasculature. Int Immunopharmacol. 10:1062–1067.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Ren JG, Cooper WL, Hawkins CE,
Cowan MR and Tong PY: Identification of the antivasopermeability
effect of pigment epithelium-derived factor and its active site.
Proc Natl Acad Sci USA. 101:6605–6610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS and
Koh GY: Vascular endothelial growth factor expression of
intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion
molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B
activation in endothelial cells. J Biol Chem. 276:7614–7620. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jersmann HP, Hii CS, Ferrante JV and
Ferrante A: Bacterial lipopolysaccharide and tumor necrosis factor
alpha synergistically increase expression of human endothelial
adhesion molecules through activation of NF-kappaB and p38
mitogen-activated protein kinase signaling pathways. Infect Immun.
69:1273–1279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosinII: Modulated by G
proteins, kinases, and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding R, Zhao D, Li X, Liu B and Ma X:
Rho-kinase inhibitor treatment prevents pulmonary inflammation and
coagulation in lipopolysaccharide-induced lung injury. Thromb Res.
150:59–64. 2017. View Article : Google Scholar : PubMed/NCBI
|