Introduction
Current cancer statistics indicate that non-Hodgkin
lymphoma (NHL) has the sixth highest morbidity rate and the ninth
highest mortality rate of all cancers (1). Diffuse large B cell lymphoma (DLBCL)
is the most common type of NHL, and is an aggressive lymphoma with
heterogeneous morphology, various molecular abnormalities and
different clinical outcomes (2,3).
DLBCL has been stratified based on gene expression profiling and is
classified into two subgroups: Germinal center B cell-like (GCB)
and non-germinal center B cell-like (non-GCB). This demonstrates
the different responses to treatments and clinical outcomes
(2); however, a recent study has
reported that there was no difference in survival rate between
these two groups of treatment (4).
The standard treatment for patients with DLBCL is rituximab
monoclonal antibody combined with cyclophosphamide, doxorubicin,
vincristine and prednisone (R-CHOP) chemotherapy, which has
significantly improved the outcome (3). Although the 5-year overall survival
(OS) and the event-free survival of patients with DLBCL have
increased to 70 and 52%, respectively, one-third of patients
experience refractory disease or relapse following treatment
(5,6). Therefore, studies investigating novel
signaling pathways and potential therapeutic targets for DLBCL are
necessary.
The ubiquitin proteasome system is vital for protein
degradation and maintains normal cellular biological processes,
including cell cycle, apoptosis, differentiation, DNA repair and
signaling pathways (7–9). Ubiquitination may be reversed by
deubiquitinating enzymes (DUBs), which remove ubiquitin from
ubiquitinated proteins. Deubuiquitination by DUBs is involved in
the pathogenesis of breast cancer and inflammation (7). Ubiquitin-specific-processing protease
(USPs), the most prominent family of DUBs, serve fundamental roles
in the ubiquitin system (10), and
the role of various USPs has been examined in cancer pathogenesis.
For example, USP10 was reported to be an independent poor
prognostic factor of gastric carcinoma (11). USP14 was revealed to induce cell
proliferation and promote apoptosis in breast cancer and epithelial
ovarian cancer cells (7,12). In addition, previous studies have
demonstrated that USP22 was a poor prognostic factor in oral
squamous cell carcinoma and invasive breast cancer (8,13).
USP34 is located on chromosome 2p15 and
encodes a deubiquitinating enzyme that was previously demonstrated
to stabilize β-catenin and modulate Wnt signaling (14). USP34 was also demonstrated to
prevent constitutive activation of Toll-dependent immune signaling
(15). In addition, USP34 was
reported to negatively regulate nuclear factor (NF)-κB signaling in
T cell receptor-dependent lymphocyte activation (16). A number of recent studies have
linked USP34 with various diseases. For example, USP34 may serve a
potential role in the pathophysiology of polycystic ovary syndrome
(PCOS), although no significant correlation has been identified
between PCOS and USP34 gene polymorphisms in the Chinese
women involved in that study (17). A previous case study of 2p15-p16.1
microdeletion syndrome revealed that USP34 may affect the
fundamental developmental process of nervous system (18). In addition, a gain of USP34
due to duplication in the 2p15-p16.1 region was frequently observed
in patients with DLBCL compared with controls by qPCR (19–21),
and USP34 may participate in the transformation from follicular
lymphoma to DLBCL (19). Recent
studies using array comparative genomic hybridization,
single-nucleotide polymorphism (SNP)-chips and gene expression
profiling analyses have revealed high levels of USP34
expression in DLBCL tissue chips (18–22).
The present study aimed to examine the expression
level of USP34 in DLBCL and to explore its association with the
patient clinicopathological features and prognosis. In addition, a
dataset from The Cancer Genome Atlas (TCGA) was used to assess
genetic mutations, expression and clinical significance of USP34 in
DLBCL.
Materials and methods
Patients and tissue samples
A total of 131 patients with DLBCL and 30 patients
with reactive lymphoid hyperplasia were included in this study.
Patients with DLBCL were recruited between July 1, 2011 and
September 30, 2015, and all DLBCL diagnoses were confirmed
following 2008 WHO criteria. The median age for patients with DLBCL
at diagnosis was 54 years (range 18–86 years). The follow-up time
ranged between 0.5 and 63 months. None of the patients had a
history of immunodeficiency. The tissue used for initial diagnosis
(as detailed below) was used to measure USP34 protein expression
levels. For DLBCL, these tissues included lymph nodes (n=43),
mainly neck and inguinal lymph nodes, and extranodal lesions
(n=88), mainly in stomach, intestines, liver, spleen and brains. Of
the 131 patients with DLBCL, 78 were treated with chemotherapy,
among which 39 received R-CHOP treatment, 29 received CHOP therapy
and 10 received other chemotherapy (methotrexate or temozolomide).
Hematoxylin and eosin (H&E) staining on these tissues was
performed at room temperature in order to observe the morphology
using a light microscope (Olympus BX53; Olympus Corporation, Tokyo,
Japan). The H&E staining dyeing procedure is as follows: i) The
paraffin tissues were dewaxed with xylene scavenger for about 20
min. ii) Different concentrations of ethanol (95, 80 and 70%
ethanol) were used to remove xylene. iii) distilled water was used
for 1 min to remove alcohol. iv) Subsequently, tissues were
immersed in hematoxylin dye for 1 min, and v) washed with tap water
for 1 min. vi) Hydrochloric acid (0.5%) was used to differentiate
alcohol 2 sec and tap water for 2 sec. vii) Subsequently, tissues
were immersed in 1% lithium carbonate solution for 1 min, washed in
tap water for 1 min, distilled water for 1 min, and immersed in
0.5% eosin for 10 sec. viii) Subsequently, tissues were washed with
distilled water for 2 sec, dehydrated with different concentrations
of alcohol, and ix) xylene was used as a clearing reagent to remove
the alcohol from the tissues. Finally, tissues were sealed with
neutral balsam. Furthermore, clinical stage was assessed based on
WHO Ann Arbor Criteria (2008), and patient status was evaluated
with Eastern Cooperative Oncology Group (ECOG) and International
Prognostic Index (IPI) scores. Additionally, 131 patients with
DLBCL were utilized to analyze the corresponding clinical
information. This study is approved by Ethics Committee of the
First Affiliated Hospital of Guangxi Medical University, and
written informed consent was obtained from each patient prior to
enrollment in the study.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissues were
sectioned (4 µm) and used for immunohistochemical analysis. The
slides were baked at 65°C for 6 h, and subsequently dewaxed
(23), the slides were rehydrated
through an ethanol series. EDTA buffer PH:9.0 solution (1:50;
Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for
antigen retrieval following 3 min of high-pressure, and the
endogenous peroxidase activity was inhibited with 3% hydrogen
peroxide for 20 min at room temperature. The primary antibody
against USP34 (1:200; catalog no. ab91617; Abcam, Cambridge, UK)
was added and incubated at 4°C overnight. Tissue sections were also
incubated with primary antibodies against B-cell lymphoma (BCL)-2,
BCL6 (Fuzhou Maixin Biotech Co., Ltd., Ningde, China), CD20,
mutated melanoma-associated antigen (MUM)-1, CD10 and Ki67 (Ready
to use; OriGene Technologies, Inc., Beijing, China) were incubated
at 37°C for 1 h. Sections were subsequently incubated with
secondary antibodies (Polymerized HRP-Anti Mouse/Rabbit IgG;
Shanghai Changdao Biotechnology Co., Ltd., Shanghai, China) for 30
min at 37°C. Following incubation, secondary antibodies were
removed and slides were washed three times with PBS. Expression was
developed with 3,3′-diaminobenzidine buffer, and slides were
counterstained with hematoxylin. Following staining, sections were
dehydrated for transparency and slides were sealed with neutral
balsam. The positive control tissues were selected according to a
previous study (23) and primary
antibodies were replaced with PBS in the negative control
groups.
Evaluation of immunohistochemical
staining
From a previous study it was demonstrated that USP34
is expressed in the nucleus of DLBCL cells, and in the cytoplasm
and/or nucleus of reactive lymphoid hyperplasia cells (19). As no previously published studies
describe the detection of USP34 protein expression with
immunohistochemical method in tumors, the immunohistochemical
evaluation standards of the USP family, including USP2a (24), USP9X (25), USP10 (11), USP14 (7,12),
USP22 (13,26–29),
USP28 (30) and USP39 (31), in tumors were used, and the middle
evaluation standard value was considered as the cut-off value of
USP34. The expression level of USP34protein was scored with a
combination of intensity and percentage of positive tumor cells
(26,27,31).
The staining intensity of USP34 was scored as follows: 0,
colorless; 1, light yellow; 2, yellowish brown; and 3, dark brown.
The percentage of USP34-positive DLBCL cells was scored as follows:
0, ≤5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, >75%. Overall
USP34 expression level was calculated by multiplying the percentage
score with the intensity score, such that a score <6 was
considered as low expression and a score ≥6 was defined as high
expression. BCL2, CD20 and CD10 were observed in cytomembrane of
DLBCL cells; BCL6, MUM1 and Ki67 were stained in nucleus of DLBCL
cells. The selection of antibodies and the evaluation standards
(32,33) were kept with clinical proposals
applied by the Department of Pathology, The First Affiliated
Hospital of Guangxi Medical University (Nanning, China) based on
the current clinical practice. Tissue samples with >25% stained
tumor cells were considered as positive for BCL2, CD20, CD10, BCL6
and MUM1, and >70% for Ki67. All slides were evaluated
independently by two experienced pathologists.5 fields were
selected using the Olympus BX53 and 1,000 cells were counted per
field at high magnification (×400), repeated at least three times
to evaluate the immunoreactivity.
cBioPortal analysis of TCGA
dataset
Genetic alterations of USP34 in 48 DLBCL cases from
TCGA (cancergenome.nih.gov/) (34,35)
were analyzed with ‘OncoPrint’ of cBioPortal (www.cbioportal.org) (36). The clinicopathological and survival
significances of USP34 were displayed in the ‘plot’ and ‘survival’
modules of cBioPortal, respectively. The integral clinical data of
USP34 mRNA expression in 48 DLBCL cases were downloaded from
cBioPortal, and the correlations of USP34 and clinical features of
DLBCL were analyzed by SPSS 22.0 (IBM Corp, Armonk, NY, USA), with
r>0 indicating a positive correlation and r<0 indicating a
negative correlation. In addition, the network graph of
USP34 and possible related pathogenic genes in DLBCL were
downloaded from cBioPortal.
Statistical analysis
Statistical analysis was analyzed using SPSS 22.0
(IBM Corp., Armonk, NY, USA). Categorical data was calculated by
χ2 test and Spearman's rank correlation, and data are
presented as the mean ± standard deviation. Kaplan-Meier analysis
was applied to estimate OS, progression-free survival (PFS) and
Disease-free survival (DFS), and the differences between curves
were measured with log-rank test. Multivariate analysis of
prognosis was assessed by Cox proportional hazards model. P<0.05
was considered to indicate a statistically significant
difference.
Results
Morphology and
immunohistochemistry
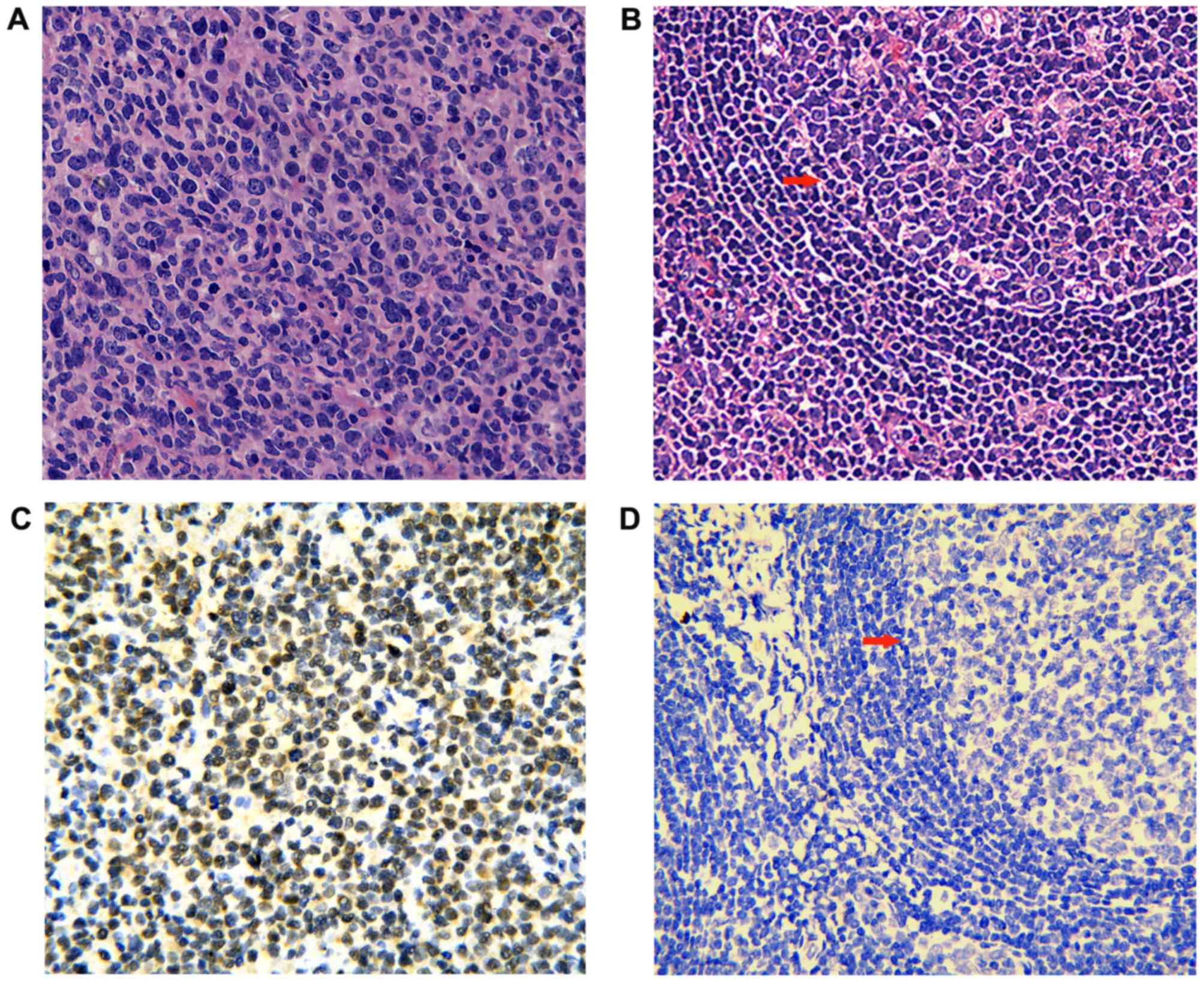
H&E stained sections of DLBCL and reactive
lymphoid hyperplasia tissues were evaluated under a microscope.
Morphologically, DLBCL is characterized with diffuse infiltration
of tumor cells with the destruction of normal architecture in lymph
nodes or extranodal tissues. Lymphoma cells had abundant cytoplasm,
vesicular chromatin and inconspicuous to distinct nucleolus. The
nucleus of tumor cells was equal to or larger than the nucleus of
background macrophages (Fig. 1A).
The main histologic changes of reactive lymphoid hyperplasia
manifested follicular hyperplasia, paracortical lymphoid
hyperplasia or sinus histiocytosis, while the normal structure
maintained (Fig. 1B).
USP34 protein expression was primarily detected in
the nucleus of DLBCL cells (Fig.
1C), whereas in lymph nodes with reactive hyperplasia, it was
mainly located in cytoplasm and nucleus of cells in germinal
center, medullary cord and medullary sinus (Fig. 1D). The number of USP34+ cells was
significantly higher in DLBCL (nucleus; 58.8%; 77/131) compared
with those cells in reactive lymphoid hyperplasia tissue (cytoplasm
and/or nucleus; 36.7%; 11/30; P=0.028).
Association of USP34 expression and
clinical features in patients with DLBCL
Correlations between USP34 protein expression and
clinicopathological parameters were assessed in 131 patients with
DLBCL (Table I). The
overexpression of USP34 was associated with older age
(χ2=4.979; P=0.026), GCB subtype (χ2=3.871;
P=0.049), multiple extranodal involvements (χ2=4.401;
P=0.036) and high IPI scores (χ2=3.897; P=0.048). By
contrast, the expression of USP34 protein was not significantly
correlated with sex, clinical stage, B symptoms (37) lactate dehydrogenase (LDH),
hemoglobin (Hb), ECOG score, BCL2 expression status or Ki67
expression status (P>0.05).
 | Table I.Correlation of USP34 protein
expression level and clinicopathological parameters of 131 DLBCL
cases. |
Table I.
Correlation of USP34 protein
expression level and clinicopathological parameters of 131 DLBCL
cases.
|
|
| USP34 |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | n | + | − | χ2 | P-value | r | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | 77 | 48 | 29 | 0.084 | 0.772 | −0.025 | 0.774 |
|
Female | 54 | 35 | 19 |
|
|
|
|
| Age |
|
|
|
|
|
|
|
|
>60 | 49 | 37 | 12 | 4.979 | 0.026a | 0.195 | 0.026a |
|
≤60 | 82 | 46 | 36 |
|
|
|
|
| Extranodal
sites |
|
|
|
|
|
|
|
| ≥2 | 39 | 30 | 9 | 4.401 | 0.036a | 0.183 | 0.036a |
|
<2 | 92 | 53 | 39 |
|
|
|
|
| Clinical stage |
|
|
|
|
|
|
|
|
III–IV | 59 | 37 | 22 | 0.019 | 0.889 | −0.012 | 0.890 |
|
I–II | 72 | 46 | 26 |
|
|
|
|
| LDH level |
|
|
|
|
|
|
|
|
High | 51 | 31 | 20 | 0.065 | 0.798 | −0.026 | 0.801 |
|
Normal | 49 | 31 | 18 |
|
|
|
|
| ECOG PS |
|
|
|
|
|
|
|
| ≥2 | 32 | 21 | 11 | 0.118 | 0.731 | 0.030 | 0.733 |
|
<2 | 98 | 61 | 37 |
|
|
|
|
| IPI score |
|
|
|
|
|
|
|
|
3–5 | 47 | 35 | 12 | 3.897 | 0.048a | 0.172 | 0.049a |
|
0–2 | 84 | 48 | 36 |
|
|
|
|
| B symptoms |
|
|
|
|
|
|
|
|
Yes | 29 | 20 | 9 | 0.556 | 0.456 | 0.065 | 0.460 |
| No | 101 | 62 | 39 |
|
|
|
|
| Hb |
|
|
|
|
|
|
|
|
Low | 64 | 40 | 24 | 0.013 | 0.908 | −0.010 | 0.909 |
|
Normal | 63 | 40 | 23 |
|
|
|
|
| Chemotherapy |
|
|
|
|
|
|
|
| CHOP +
other | 39 | 22 | 17 | 2.006 | 0.157 | 0.160 | 0.161 |
|
R-CHOP | 39 | 28 | 11 |
|
|
|
|
| Treatment
responses |
|
|
|
|
|
|
|
| CR +
PR | 43 | 30 | 13 | 1.041 | 0.307 | −0.123 | 0.315 |
| SD +
PD | 26 | 15 | 11 |
|
|
|
|
| Subtype |
|
|
|
|
|
|
|
|
non-GCB | 93 | 54 | 39 | 3.871 | 0.049a | −0.172 | 0.050 |
|
GCB | 38 | 29 | 9 |
|
|
|
|
| BCL2 |
|
|
|
|
|
|
|
| + | 105 | 64 | 41 | 1.320 | 0.251 | −0.100 | 0.254 |
| − | 26 | 19 | 7 |
|
|
|
|
| Ki67 |
|
|
|
|
|
|
|
| + | 83 | 54 | 29 | 0.282 | 0.595 | 0.046 | 0.598 |
| − | 48 | 29 | 19 |
|
|
|
|
Survival analysis of patients with
DLBCL
Of the 131 patients with DLBCL, the median follow-up
time was 27.1 months (ranging between 0.5 and 63 months). The
3-year and 5-year OS rates were 54.9% [95% confidence interval
(CI); 49.8–60.0%] and 29.4% (95% CI; 22.1–36.7%), respectively. In
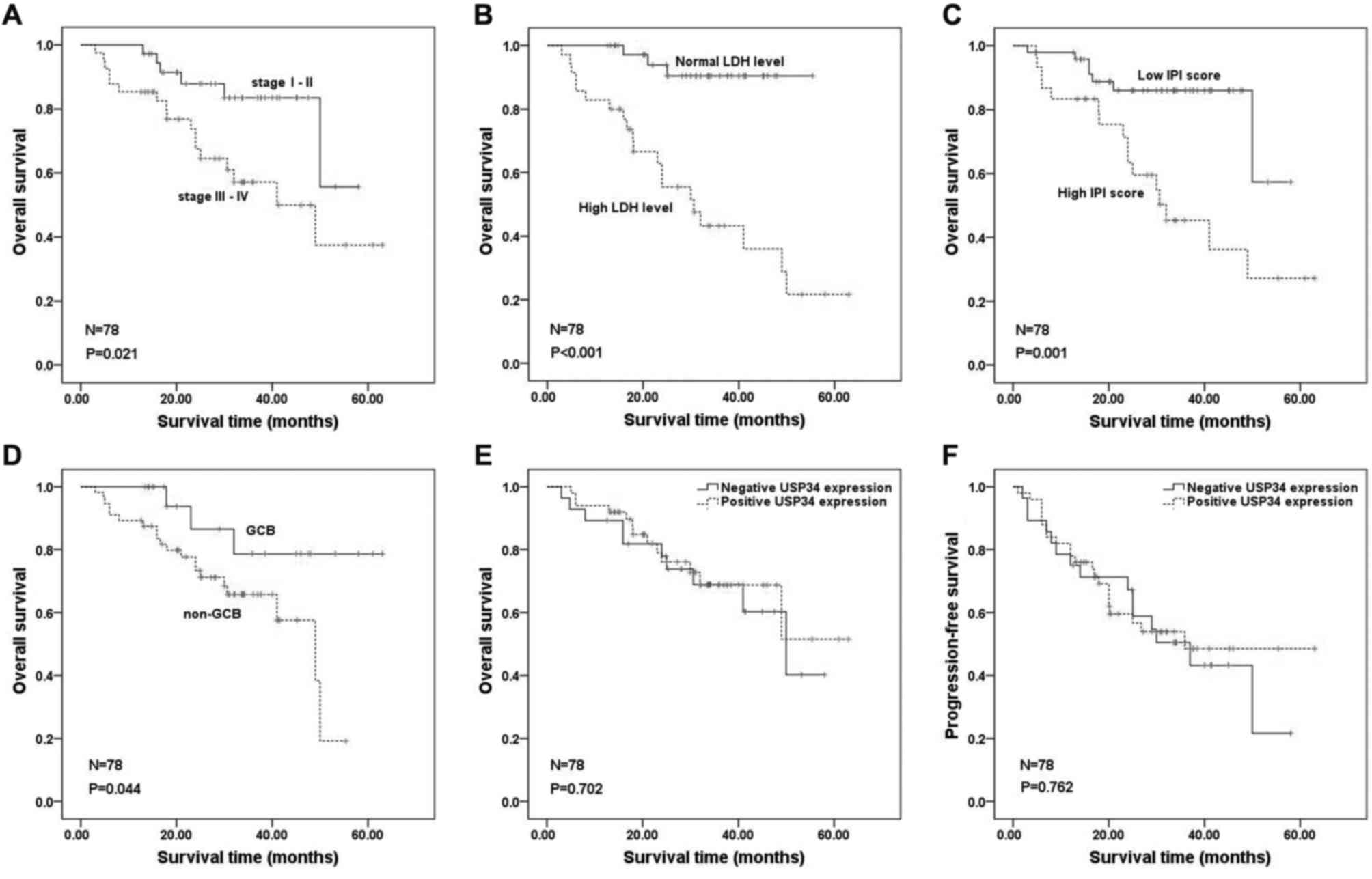
univariate analysis, unfavorable clinical variables for prognosis
were elderly age (P=0.036), advanced clinical stage III–IV
(P=0.035), high LDH level (P<0.001), high IPI scores
(P<0.001), non-GCB subtype (P=0.031) and positive BCL2
expression (P=0.028; Fig. 2A-F,
respectively; Table II), whereas
USP34 protein expression was not associated with OS or PFS
(P>0.05; Fig. 2G and H,
respectively). To eliminate the influence of non-chemotherapy on
the prognosis, survival analysis of 78 patients who received
chemotherapy were further analyzed. Patients with DLBCL that had
clinical stage III–IV (P=0.021), high LDH (P<0.001), high IPI
scores (P=0.001) or non-GCB subtype (P=0.044), had worse prognosis
(Fig. 3A-D, respectively; Table III). In addition, no significant
association was identified between the expression of USP34 and OS
or PFS (P>0.05; Fig. 3E and F,
respectively).
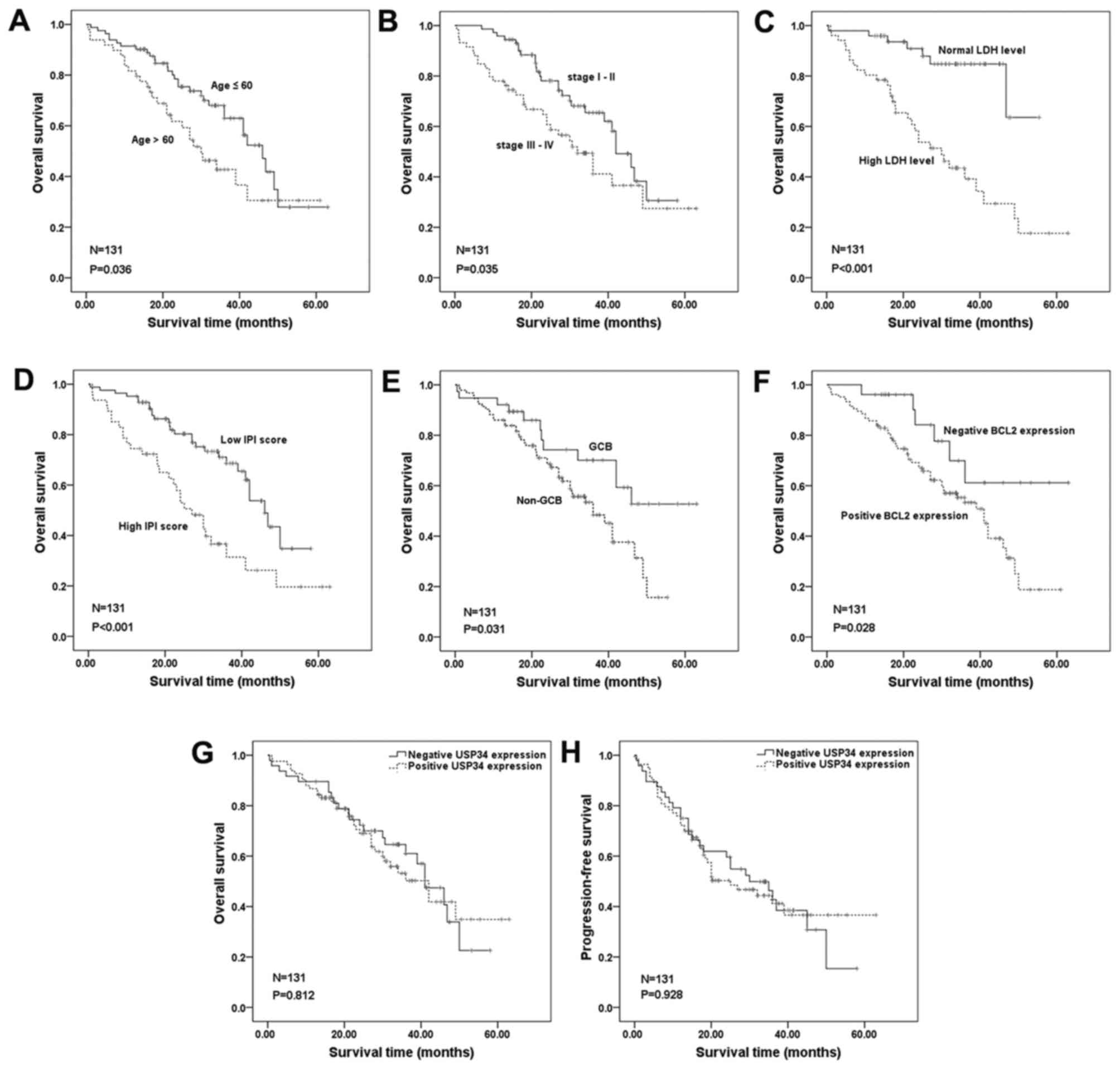 | Figure 2.Univariate survival curves of
clinical parameters and USP34 protein expression were analyzed for
131 patients with DLBCL. OS was determined for (A) age, (B)
clinical stage, (C) LDH level, (D) IPI scores, (E) GCB subtype, (F)
BCL2 expression, (G) USP34 expression and (H) progression-free
survival of USP34. BCL, B cell lymphoma; DLBCL, diffuse large
B-cell lymphoma; GCB, germinal center B cell-like; IPI,
International Prognostic Index; LDH, lactate dehydrogenase; OS,
overall survival; USP34, ubiquitin-specific-processing protease
34. |
 | Table II.Univariate prognostic analysis of
clinicopathological parameters of 131 patients with DLBCL. |
Table II.
Univariate prognostic analysis of
clinicopathological parameters of 131 patients with DLBCL.
| Risk factor | n | 3-year OS (%) | 5-year OS (%) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 77 | 59.5 | 27.6 | 0.185 |
|
Female | 54 | 47.7 | 32.5 |
|
| Age |
|
|
|
|
|
>60 | 49 | 42.7 | 30.5 | 0.036a |
|
≤60 | 82 | 68.0 | 27.9 |
|
| Extranodal
sites |
|
|
|
|
|
<2 | 92 | 58.8 | 35.1 | 0.160 |
| ≥2 | 39 | 45.9 | 15.1 |
|
| Clinical stage |
|
|
|
|
|
III–IV | 59 | 41.2 | 27.4 | 0.035a |
|
I–II | 72 | 65.5 | 30.6 |
|
| LDH level |
|
|
|
|
|
High | 51 | 39.2 | 17.6 |
<0.001a |
|
Normal | 49 | 84.8 | 63.6 |
|
| ECOG PS |
|
|
|
|
|
<2 | 98 | 55.3 | 33.1 | 0.526 |
| ≥2 | 32 | 51.9 | 26.0 |
|
| IPI score |
|
|
|
|
|
3–5 | 47 | 31.4 | 19.6 |
<0.001a |
|
0–2 | 84 | 68.6 | 34.8 |
|
| B symptoms |
|
|
|
|
|
Yes | 29 | 45.6 | 0.0 | 0.171 |
| No | 101 | 56.7 | 36.2 |
|
| Hb |
|
|
|
|
|
Low | 64 | 47.4 | 8.0 | 0.080 |
|
Normal | 63 | 58.1 | 48.2 |
|
| Subtype |
|
|
|
|
|
GCB | 38 | 70.1 | 52.7 | 0.031a |
|
non-GCB | 93 | 48.4 | 15.7 |
|
| Treatment |
|
|
|
|
| No | 53 | 37.6 | 13.7 |
<0.001a |
|
Yes | 78 | 69.1 | 46.2 |
|
| Chemotherapy |
|
|
|
|
|
R-CHOP | 39 | 74.0 | 74.0 | 0.101 |
| CHOP +
other | 39 | 64.5 | 17.9 |
|
| USP34 |
|
|
|
|
| + | 83 | 50.2 | 34.9 | 0.812 |
| − | 48 | 61.0 | 22.6 |
|
| BCL2 |
|
|
|
|
| + | 105 | 53.3 | 18.8 | 0.028a |
| − | 26 | 61.2 | 61.2 |
|
| Ki67 |
|
|
|
|
| + | 83 | 57.7 | 32.5 | 0.419 |
| − | 48 | 50.4 | 25.6 |
|
 | Table III.Univariate prognostic analysis of
clinicopathological parameters of 78 DLBCL patients'
post-chemotherapy. |
Table III.
Univariate prognostic analysis of
clinicopathological parameters of 78 DLBCL patients'
post-chemotherapy.
| Risk factor | n | 3-year OS (%) | 5-year OS (%) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 44 | 72.1 | 38.4 | 0.625 |
|
Female | 34 | 65.4 | 65.4 |
|
| Age |
|
|
|
|
|
>60 | 23 | 65.6 | 65.6 | 0.969 |
|
≤60 | 55 | 70.8 | 38.9 |
|
| Extranodal
sites |
|
|
|
|
|
<2 | 53 | 58.4 | 58.4 | 0.263 |
| ≥2 | 25 | 52.1 | 26.0 |
|
| Clinical stage |
|
|
|
|
|
III–IV | 41 | 57.1 | 37.5 | 0.021a |
|
I–II | 37 | 83.5 | 55.7 |
|
| LDH level |
|
|
|
|
|
High | 35 | 43.2 | 21.6 |
<0.001a |
|
Normal | 40 | 90.4 | 90.4 |
|
| ECOG PS |
|
|
|
|
|
<2 | 58 | 70.0 | 63.0 | 0.491 |
| ≥2 | 19 | 65.2 | 39.1 |
|
| IPI score |
|
|
|
|
|
3–5 | 30 | 45.3 | 27.2 | 0.001a |
|
0–2 | 48 | 86.0 | 57.3 |
|
| B symptoms |
|
|
|
|
|
Yes | 18 | 65.8 | 0.0 | 0.520 |
| No | 59 | 68.8 | 55.0 |
|
| Hb |
|
|
|
|
|
Low | 34 | 62.8 | 16.7 | 0.234 |
|
Normal | 41 | 71.6 | 71.6 |
|
| Subtype |
|
|
|
|
|
GCB | 22 | 78.7 | 78.7 | 0.044a |
|
non-GCB | 56 | 65.8 | 19.2 |
|
| Chemotherapy |
|
|
|
|
|
R-CHOP | 39 | 74.0 | 74.0 | 0.101 |
| CHOP +
other | 39 | 64.5 | 17.9 |
|
| USP34 |
|
|
|
|
| + | 50 | 68.8 | 51.6 | 0.702 |
| − | 28 | 68.9 | 40.2 |
|
| BCL2 |
|
|
|
|
| + | 61 | 66.7 | 30.0 | 0.076 |
| − | 17 | 77.1 | 77.1 |
|
| Ki67 |
|
|
|
|
| + | 49 | 70.5 | 47.0 | 0.700 |
| − | 29 | 67.0 | 55.9 |
|
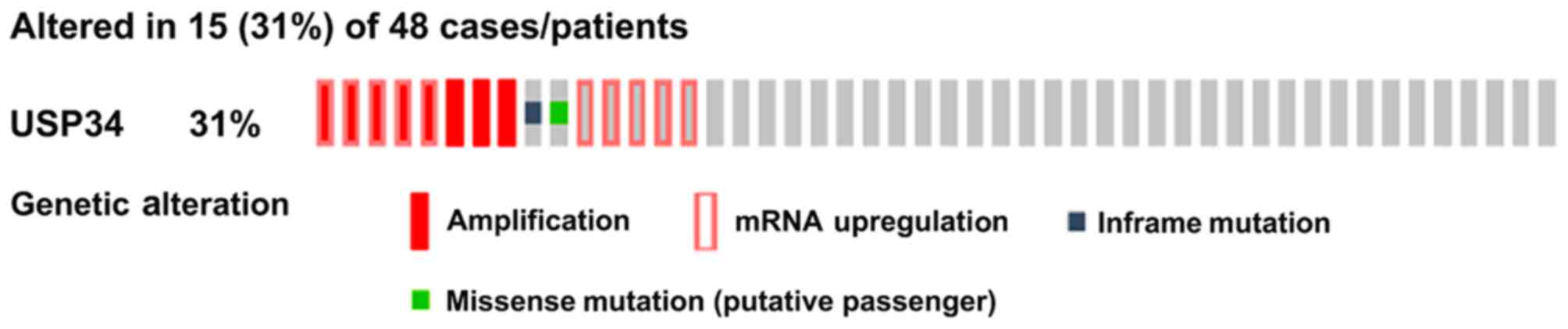
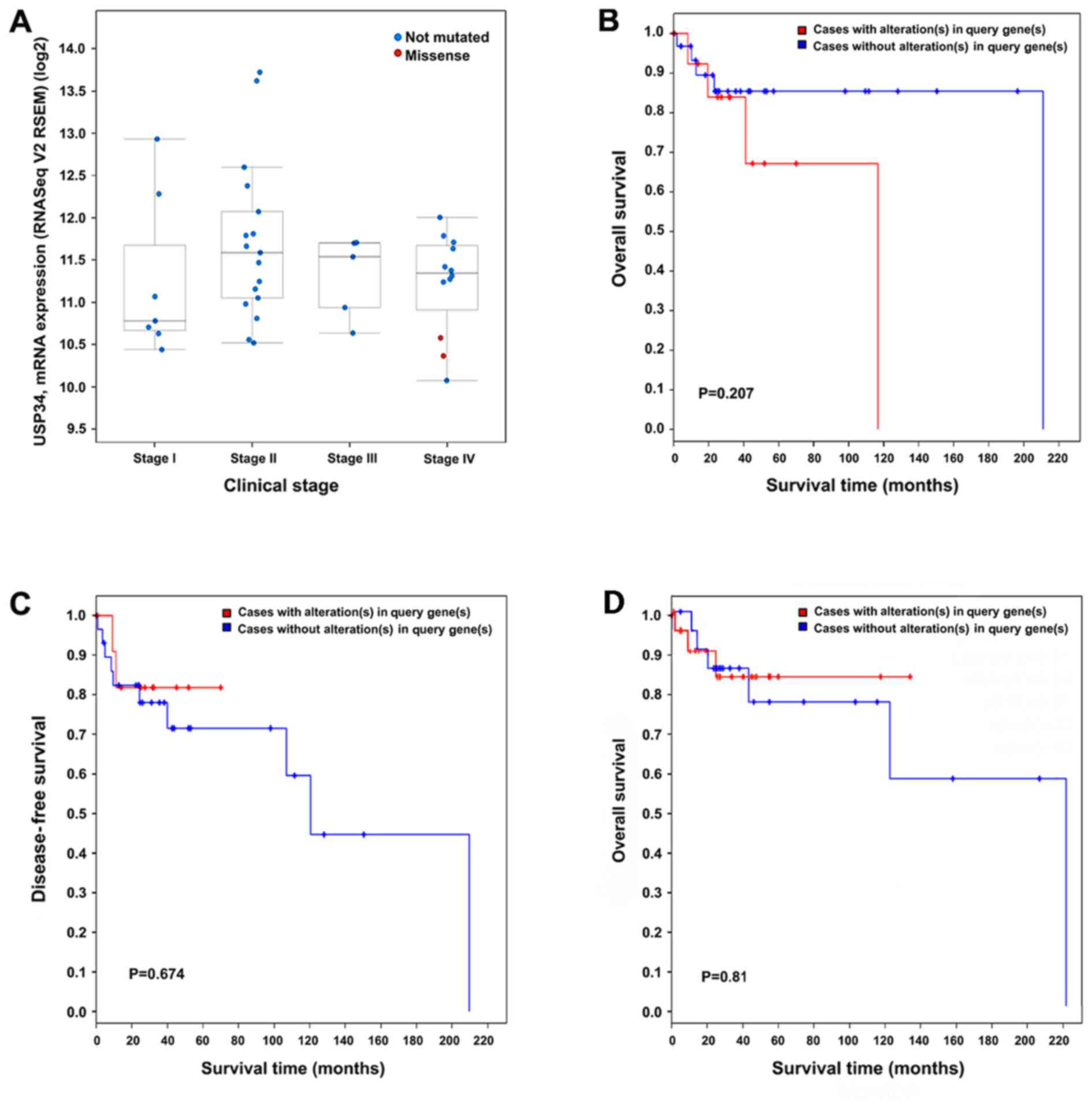
TCGA data analysis via cBioPortal
platform
Of the 48 patients with DLBCL (TCGA, provisional) in
cBioPortal, 15 (31%) had genetic alterations of USP34 in
‘OncoPrint’, which included mRNA upregulation, in-frame and
missense mutations (Fig. 4). To
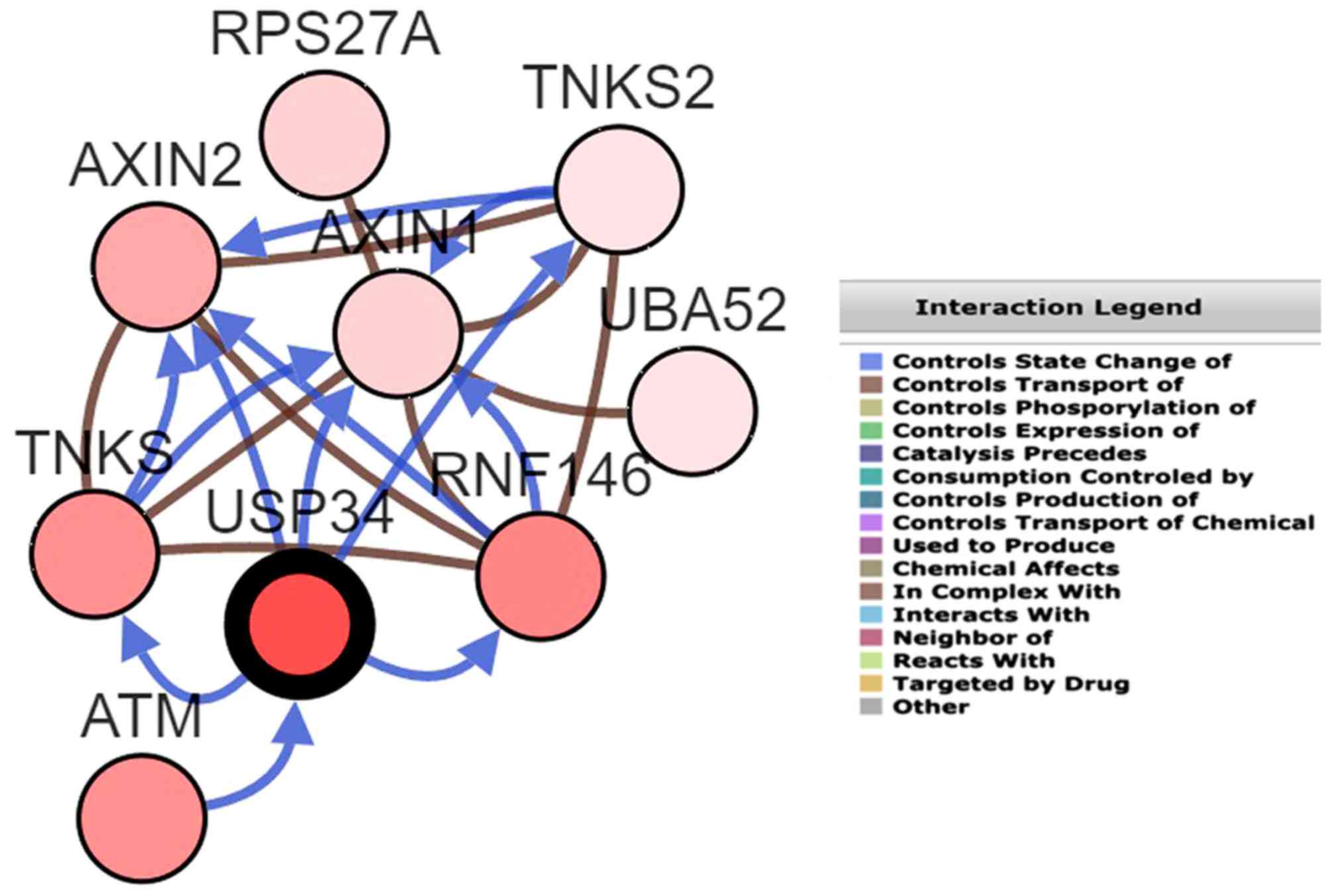
better understand the target genes associated with USP34 in NBCLB,
online predictions from cBioPortal was used, and these genes
(PSP27A, TNKS2, AXIN2, AXIN1, UBA52, TNKS, RNF146, ATM) were
regarded as the potential target genes of USP34 and an interaction
gene network of USP34 with other genes in DLBCL is provided in the
Fig. 5. No significant
correlations were identified between USP34 mRNA expression level
and clinical stages (Fig. 6A). A
total of 4 out of the 15 patients with alterations were deceased,
and the median survival time was 116.72 months; and 5 out of the 32
cases without alterations were deceased, and the median survival
time was 211.07 months. No significant association was demonstrated
between USP34 genetic alterations with OS (Fig. 6B) in the present data,
respectively, data also showed no significance with disease free
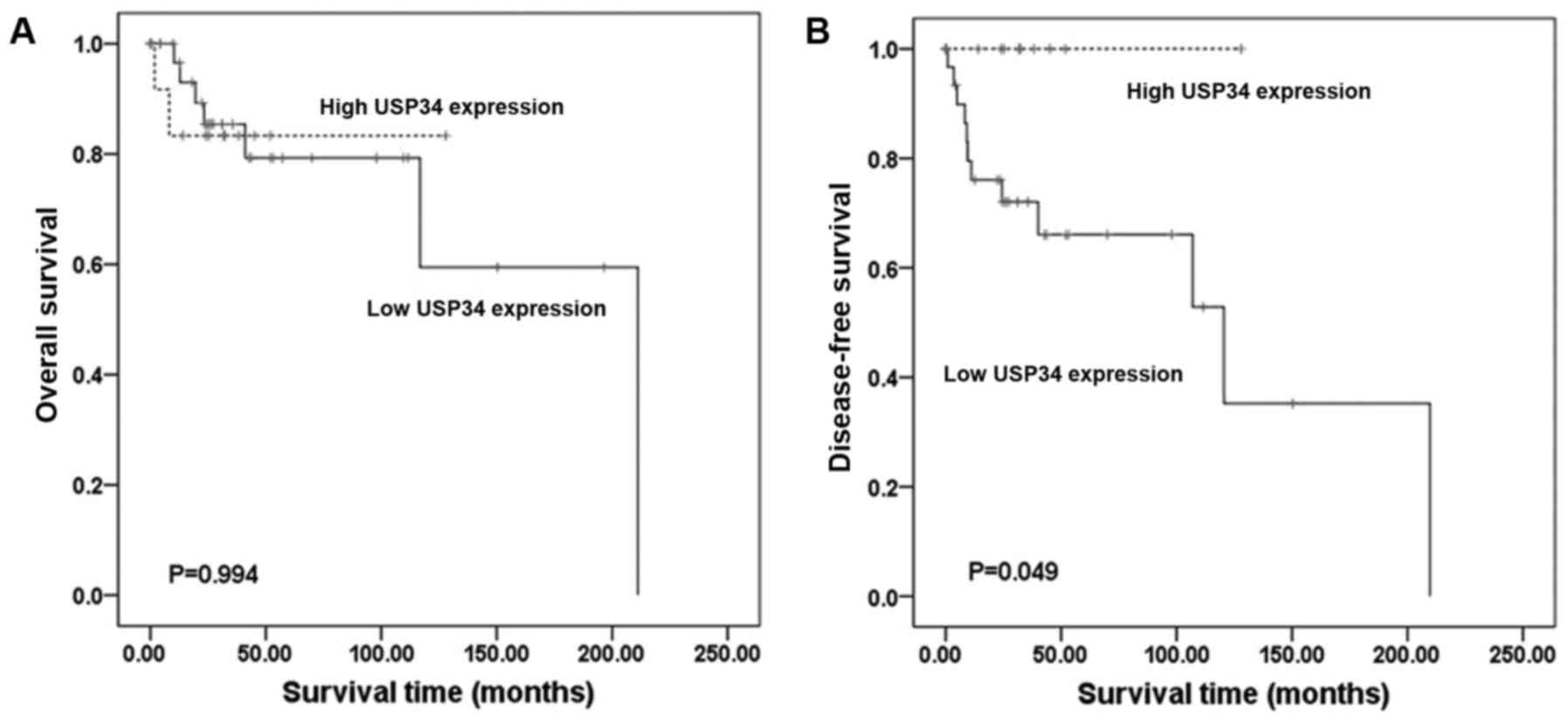
survival and OS in TCGA dataset (DFS; P>0.05; Fig. 6C. OS; P>0.05; Fig. 6D). In TCGA dataset 47 cases of
DLBCL contained adequate clinical information. The average
expression level of USP34 mRNA was regarded as the cut-off
value. No significant relationship was identified between
USP34 mRNA level and clinical parameters (P>0.05) (data
not shown). In univariate survival analysis, no clinical factor was
found to be associated with OS (P=0.994; Fig. 7A). However, patients with low USP34
expression (P=0.049; Fig. 7B),
stage III–IV (P=0.045), high ECOG score (P=0.007), advanced IPI
scores (P=0.018) or non-chemotherapy (P=0.002) were indicated to be
associated with poor DFS. Although USP34 mRNA level was
associated with DFS, the OS was not affected.
Discussion
USP34 is a deubiquitinase that has become a topic of
interest in recent years (16,17).
Previous studies have reported that USP34 may be involved in the
pathogenesis of various diseases (14,15);
however, the role of USP34 in DLBCL remained to be determined.
Thus, it is important to investigate whether USP34 serves a role in
DLBCL pathogenesis. In the present study, the expression level and
the clinical significance of USP34 in 131 patients with DLBCL were
analyzed, and it was demonstrated that USP34 is expressed at higher
levels in DLBCL compared with reactive lymphoid hyperplasia. In
addition, increased of USP34 expression was associated with older
age, GCB subtype, multiple extranodal site involvements and high
IPI scores in patients with DLBCL (3,6). In
the present study, positive USP34 expression was more often to be
detected in patients with GCB immunophenotype. Consistent with this
finding, the increased expression of USP34 was detected by
SNP-chip in GCB subtype of DLBCL cases (20,21).
A previous study on DLBCL reported a gain of 2p15 and 2p16.1 was
more frequent in the GCB subgroup compared with the non-GCB type,
and poor prognosis was exhibited in the 2p15 amplified region
(21). The present study analyzed
the role of USP34 in GCB-subtype DLBCL, no significant association
was found between USP34 protein expression level and clinical
parameters or survival. Using a TCGA dataset, a number of cases
were identified with mRNA upregulation, in-frame and missense
mutations of USP34. However, pathogenesis of USP34 in DLBCL,
particularly in the GCB subtype, requires further
investigation.
In this study, the clinical outcome of 131 patients
with DLBCL was analyzed. In the univariate survival analysis,
clinical stage III–IV, high LDH level, high risk IPI and non-GCB
subtype were associated with poor OS of DLBCL. In addition, poor OS
was seen in patients with advanced age and positive BCL2
expression. These data are consistent with previous studies, which
reported that reduced OS was associated with older age, elevated
LDH, advanced stage, non-GCB subtype and high risk IPI score
(3,6). Although USP34 protein expression was
associated with older age and multiple site involvement, no
significant relationship was found between USP34 level and OS, PFS
or DFS. By contrast, analysis from TCGA datasets indicated that low
USP34 mRNA expression level was associated with poor DFS, but not
OS. The mechanisms mediating this discrepancy are uncertain, but
USP34 was measured at different level (mRNA vs. protein) in the
TCGA data set and in patients recruited in the present study.
Although USP34 expression was not associated with
OS, increased USP34 expression in DLBCL, compared with reactive
lymphoid hyperplasia, and its association with adverse
clinicopathological features indicated that USP34 may serve a
potential role in DLBCL pathogenesis. The potential roles of USP34
in cancer are multifocal. USP34 is closely linked to the Wnt
signaling activation through the stabilization of β-catenin
(14). AXIN, a key component of
cellular machinery, induces phosphorylation of β-catenin. The
present study identified a potential network connection of
USP34 with AXIN1 and AXIN2 in TCGA, but this
needs to be verified. In DLBCL, potentiated Wnt/β-catenin signal
transduction was previously reported, along with forkhead box P1
overexpression, and Wnt-targeted therapy was examined in DLBCL
(38). In addition, USP34 was
reported to be a DNA damage-responsive protein and regarded as the
downstream target of ataxia telangiectasia-mutated in DNA damage
(9), and the interaction between
them was identified in TCGA. A link between DSP34 and the NF-κB
pathway is also described (39).
NF-κB and B cell-receptor signaling may be activated by the E3
ubiquitin ligases cellular inhibitor of apoptosis 1 and 2 in
activated B cell-like DLBCL. USP34 was reported to be distributed
in cytosol and to negatively regulate NF-κB signaling pathway via
the downstream CARMA1/BCL10/MALT1-inhibitor of NF-κB kinase complex
in Tlymphocytes (16). In
addition, USP34 was reported to prevent Toll pathway and immune
deficiency-dependent immune signal in Drosophila (15). In a recent study, E3
ubiquitin-protein ligase pellino 1, which serves a pivotal role in
the activation of NF-κB pathway and Toll-like receptor signaling,
was demonstrated to be associated with a poor prognosis of patients
with DLBCL (40). The research of
USP34 in DLBCL is limited, and future studies to explore its
function are needed.
In conclusion, high USP34 protein expression was
identified in patients with DLBCL, and was associated with older
age, GCB subtype, multiple extranodal extension and high risk IPI.
Future studies will focus on exploring the role of USP34 in the
pathogenesis of DLBCL.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Key Programs of
University Scientific Research of Guangxi Education Agency (grant
no. ZD2014033), The Key Programs of Guangxi Natural Science Fund
(grant no. 2015GXNSFDA139028) and The Basic Ability Improvement
Project for Young and Middle-aged Teachers in Guangxi Universities
(grant no. 2017KY0091).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
CL and LH collected data from clinical and public
dataset, performed statistical analysis and drafted the manuscript.
CL, HL and QZ carried out the clinical samples gathering and
in-house immunoreactivity. GC and WW participated in the design of
the study and language modification. YG and ZP evaluated the
clinical dataset, morphology and immunoreactivity. ZF conceived of
the study and helped to edit the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
the First Affiliated Hospital of Guangxi Medical University.
Written informed consents were signed by all the patients involved
to ensure their approval of the data used in this research.
Patient consent for publication
All patients signed to consent for publishing their
individual clinical data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seo S, Hong JY, Yoon S, Yoo C, Park JH,
Lee JB, Park CS, Huh J, Lee Y, Kim KW, et al: Prognostic
significance of serum beta-2 microglobulin in patients with diffuse
large B-cell lymphoma in the rituximab era. Oncotarget.
7:76934–76943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nowakowski GS and Vitolo U: Recent
advances in clinical studies and the evolving role of subtyping for
patients with diffuse large B-cell lymphoma. Future Oncol.
13:859–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oki Y, Ewer MS, Lenihan DJ, Fisch MJ,
Hagemeister FB, Fanale M, Romaguera J, Pro B, Fowler N, Younes A,
et al: Pegylated liposomal doxorubicin replacing conventional
doxorubicin in standard R-CHOP chemotherapy for elderly patients
with diffuse large B-cell lymphoma: An open label, single arm,
phase II trial. Clin Lymphoma Myeloma Leuk. 15:152–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedberg JW: Relapsed/refractory diffuse
large B-cell lymphoma. Hematol Am Soc Hematol Educ Program.
2011:498–505. 2011.
|
|
7
|
Zhu L, Yang S, He S, Qiang F, Cai J, Liu
R, Gu C, Guo Z, Wang C, Zhang W, et al: Downregulation of
ubiquitin-specific protease 14 (USP14) inhibits breast cancer cell
proliferation and metastasis, but promotes apoptosis. J Mol Histol.
47:69–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piao S, Liu Y, Hu J, Guo F, Ma J, Sun Y
and Zhang B: USP22 is useful as a novel molecular marker for
predicting disease progression and patient prognosis of oral
squamous cell carcinoma. PLoS One. 7:e425402012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sy SM, Jiang JO WS, Deng Y and Huen MS:
The ubiquitin specific protease USP34 promotes ubiquitin signaling
at DNA double-strand breaks. Nucleic Acids Res. 41:8572–8580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phillips AH, Zhang Y, Cunningham CN, Zhou
L, Forrest WF, Liu PS, Steffek M, Lee J, Tam C, Helgason E, et al:
Conformational dynamics control ubiquitin-deubiquitinase
interactions and influence in vivo signaling. Proc Natl Acad Sci
USA. 110:11379–11384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS,
Yang GF, Wang P and Fu GH: Prognostic significance of USP10 as a
tumor-associated marker in gastric carcinoma. Tumour Biol.
35:3845–3853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Wang J, Zhong J, Deng Y, Xi Q, He
S, Yang S, Jiang L, Huang M, Tang C and Liu R: Ubiquitin-specific
protease 14 (USP14) regulates cellular proliferation and apoptosis
in epithelial ovarian cancer. Med Oncol. 32:3792015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Yao L, Zhang X, Ji H, Wang L, Sun
S and Pang D: Elevated expression of USP22 in correlation with poor
prognosis in patients with invasive breast cancer. J Cancer Res
Clin Oncol. 137:1245–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lui TT, Lacroix C, Ahmed SM, Goldenberg
SJ, Leach CA, Daulat AM and Angers S: The ubiquitin-specific
protease USP34 regulates axin stability and Wnt/β-catenin
signaling. Mol Cell Biol. 31:2053–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engel E, Viargues P, Mortier M,
Taillebourg E, Couté Y, Thevenon D and Fauvarque MO: Identifying
USPs regulating immune signals in Drosophila: USP2 deubiquitinates
Imd and promotes its degradation by interacting with the
proteasome. Cell Commun Signal. 12:412014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poalas K, Hatchi EM, Cordeiro N, Dubois
SM, Leclair HM, Leveau C, Alexia C, Gavard J, Vazquez A and Bidère
N: Negative regulation of NF-κB signaling in T lymphocytes by the
ubiquitin-specific protease USP34. Cell Commun Signal. 11:252013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao S, Tian Y, Zhang W, Xing X, Li T, Liu
H, Huang T, Ning Y, Zhao H and Chen ZJ: An association study
between USP34 and polycystic ovary syndrome. J Ovarian Res.
8:302015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mimouni-Bloch A, Yeshaya J, Kahana S, Maya
I and Basel-Vanagaite L: A de-novo interstitial microduplication
involving 2p16.1-p15 and mirroring 2p16.1-p15 microdeletion
syndrome: Clinical and molecular analysis. Eur J Paediatr Neurol.
19:711–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwiecinska A, Ichimura K, Berglund M,
Dinets A, Sulaiman L, Collins VP, Larsson C, Porwit A and
Lagercrantz SB: Amplification of 2p as a genomic marker for
transformation in lymphoma. Genes Chromosomes Cancer. 53:750–768.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scholtysik R, Kreuz M, Hummel M,
Rosolowski M, Szczepanowski M, Klapper W, Loeffler M, Trümper L,
Siebert R and Küppers R; Molecular Mechanisms in Malignant
Lymphomas Network Project of the Deutsche Krebshilfe, :
Characterization of genomic imbalances in diffuse large B-cell
lymphoma by detailed SNP-chip analysis. Int J Cancer.
136:1033–1042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taskinen M, Louhimo R, Koivula S, Chen P,
Rantanen V, Holte H, Delabie J, Karjalainen-Lindsberg ML, Björkholm
M, Fluge Ø, et al: Deregulation of COMMD1 is associated with poor
prognosis in diffuse large B-cell lymphoma. PLoS One. 9:e910312014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trifonov V, Pasqualucci L, Dalla Favera R
and Rabadan R: MutComFocal: An integrative approach to identifying
recurrent and focal genomic alterations in tumor samples. BMC Syst
Biol. 7:252013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mo CH, Gao L, Zhu XF, Wei KL, Zeng JJ,
Chen G and Feng ZB: The clinicopathological significance of UBE2C
in breast cancer: A study based on immunohistochemistry, microarray
and RNA-sequencing data. Cancer Cell Int. 17:832017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boustani MR, Khoshnood RJ, Nikpasand F,
Taleshi Z, Ahmadi K, Yahaghi E and Goudarzi PK: Overexpression of
ubiquitin-specific protease 2a (USP2a) and nuclear factor erythroid
2-related factor 2 (Nrf2) in human gliomas. J Neurol Sci.
363:249–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Liu Y, Yang B, Cao H, Yang CX,
Ouyang W, Zhang SM, Yang GF, Zhou FX, Zhou YF and Xie CH: Elevated
expression of USP9X correlates with poor prognosis in human
non-small cell lung cancer. J Thorac Dis. 7:672–679.
2015.PubMed/NCBI
|
|
26
|
Wang Z, Zhu L, Guo T, Wang Y and Yang J:
Decreased H2B monoubiquitination and overexpression of
ubiquitin-specific protease enzyme 22 in malignant colon carcinoma.
Hum Pathol. 46:1006–1014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang B, Tang F, Li B, Yuan S, Xu Q,
Tomlinson S, Jin J, Hu W and He S: High USP22 expression indicates
poor prognosis in hepatocellular carcinoma. Oncotarget.
6:12654–12667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ning Z, Wang A, Liang J, Xie Y, Liu J,
Feng L, Yan Q and Wang Z: USP22 promotes the G1/S phase transition
by upregulating FoxM1 expression via β-catenin nuclear localization
and is associated with poor prognosis in stage II pancreatic ductal
adenocarcinoma. Int J Oncol. 45:1594–1608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ao N and Liu Y, Bian X, Feng H and Liu Y:
Ubiquitin-specific peptidase 22 inhibits colon cancer cell invasion
by suppressing the signal transducer and activator of transcription
3/matrix metalloproteinase 9 pathway. Mol Med Rep. 12:2107–2113.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Xu B, Qiang Y, Huang H, Wang C,
Li D and Qian J: Overexpression of deubiquitinating enzyme USP28
promoted non-small cell lung cancer growth. J Cell Mol Med.
19:799–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Zhang B, Lei Y, Sun J, Zhang Y,
Yang S and Zhang X: Knockdown of USP39 induces cell cycle arrest
and apoptosis in melanoma. Tumour Biol. 37:13167–13176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Colomo L, Lopez-Guillermo A, Perales M,
Rives S, Martínez A, Bosch F, Colomer D, Falini B, Montserrat E and
Campo E: Clinical impact of the differentiation profile assessed by
immunophenotyping in patients with diffuse large B-cell lymphoma.
Blood. 101:78–84. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao W: Different expression status of
apoptosis related proteins Fas, FasL, Bcl-2 and survivin in GCB and
non-GCB immuno-subtypes of diffuse large B-cell lymphoma in
Guangxi, China. unpublished PhD thesisGuangxi Medical University;
2012
|
|
34
|
Gan TQ, Xie ZC, Tang RX, Zhang TT, Li DY,
Li ZY and Chen G: Clinical value of miR-145-5p in NSCLC and
potential molecular mechanism exploration: A retrospective study
based on GEO, qRT-PCR and TCGA data. Tumour Biol.
39:10104283176916832017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Ye ZH, Liang HW, Ren FH, Li P,
Dang YW and Chen G: Down-regulation of miR-146a-5p and its
potential targets in hepatocellular carcinoma validated by a TCGA-
and GEO-based study. FEBS Open Bio. 7:504–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei DM, Chen ZX, He RQ, Shi L, Zhou S, Li
W, Chen G, Peng Z, Dang Y and Luo D: Genomic alterations andprotein
expression of STAT4 in pancreatic cancer: A study of bioinformatics
based on public data and immunohistochemistry validation with 241
tissue samples. Int J Clin Exp Pathol. 9:9761–9774. 2016.
|
|
37
|
Kubuschok B, Held G and Pfreundschuh M:
Management of diffuse large B-cell lymphoma (DLBCL). Cancer Treat
Res. 165:271–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walker MP, Stopford CM, Cederlund M, Fang
F, Jahn C, Rabinowitz AD, Goldfarb D, Graham DM, Yan F, Deal AM, et
al: FOXP1 potentiates Wnt/β-catenin signaling in diffuse large B
cell lymphoma. Sci Signal. 8:ra122015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Kelly P, Shaffer AL III, Schmitz
R, Yoo HM, Liu X, Huang DW, Webster D, Young RM, Nakagawa M, et al:
Targeting non-proteolytic protein ubiquitination for the treatment
of diffuse large B cell lymphoma. Cancer Cell. 29:494–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choe JY, Park M, Yun JY, Na HY, Go H, Kim
HJ, Oh S and Kim JE: PELI1 expression is correlated with MYC and
BCL6 expression and associated with poor prognosis in diffuse large
B-cell lymphoma. Mod Pathol. 29:1313–1323. 2016. View Article : Google Scholar : PubMed/NCBI
|