Introduction
Lung cancer is a common type malignant tumor with
the highest incidence rate worldwide compared with other types of
cancers (1), and ~80% of lung
cancer cases may be classified as non-small cell lung cancer
(NSCLC) (2). Chemotherapy has been
reported to improve the survival rate of patients with NSCLC and is
considered to be a first-line treatment following surgery (3). Cisplatin-based chemotherapy is
frequently used in the clinical treatment of cancer (4). Patients may respond well to cisplatin
at the beginning of treatment; however, the majority of patients
develop resistance to cisplatin, resulting in therapy failure
(5). Thus, revealing the molecular
mechanism underlying the development of cisplatin resistance is
required to improve the treatment outcome of patients with lung
cancer.
By binding to the 3′ untranslated region (UTR) of
target mRNAs, microRNAs (miRNA/miRs) are able to regulate gene
expression at the transcriptional and translational levels
(6). Through the downregulation of
certain proteins, miRNAs have been associated with numerous
cellular behaviors and the pathogenesis of a number of diseases
(7,8). During the initiation and development
of cancer, the dysregulation of miRNAs has been frequently observed
to lead to alterations in the expression of oncogenes or tumor
suppressors (9). In-depth studies
on the regulatory networks of miRNAs in cancer may provide novel
prognostic predictors and therapeutic approaches, beneficial for
patients with cancer (10).
miR-328 has been reported to promote the progression of various
cancer types, including breast and hepatocellular cancers (11,12).
In NSCLC, elevations in miR-328 expression levels were associated
with brain metastasis (13);
however, the role of miR-328 in the development of cisplatin
resistance in NSCLC remains unclear.
Phosphatase and tensin homolog (PTEN) is a
well-characterized phosphatase, as well as a tumor suppressor,
which may undergo mutations in a variety of cancers (14). PTEN serves its tumor suppressor
role by negatively regulating the phosphatidylinositol 3-kinase
(PI3K)-RAC-α serine/threonine-protein kinase (AKT) signaling
pathway (15). The expression of
PTEN is tightly controlled by noncoding RNAs, protein-protein
interactions and protein modifications (16). Alterations in the expression or
activity of PTEN may lead to the uncontrolled activation of the
PI3K-AKT signaling pathway, which may promote the progression of
cancer and induce drug resistance (17,18).
In the present study, miR-328 expression levels were
elevated, while the mRNA expression levels of PTEN were decreased
in tumor tissues obtained from patients with cisplatin-resistant
NSCLC, compared with patients with cisplatin-sensitive NSCLC. In
addition, there was a negative correlation between PTEN mRNA and
miR-328 expression levels in the tumor tissues of patients with
NSCLC. The present study reported an increase in miR-328 expression
levels in cisplatin-resistant A549 (A549rCDDP) cells) compared with
parental cells. Furthermore, PTEN was predicted to be a direct
target of miR-328 in NSCLC cells via computational and
dual-luciferase methods. Additionally, the present study proposed
that the exposure of miR-328-silenced A549rCDDP cells to cisplatin
may be associated with cellular apoptosis and inhibition of cell
growth, indicating that miR-328 may serve a central role in
mediating cisplatin-resistance exhibited by A549rCDDP cells. The
present study demonstrated that miR-328 may function in cisplatin
resistance in NSCLC by targeting PTEN.
Patients and methods
Patients
A total of 58 tumor tissue samples were collected
from 35 patients with cisplatin-sensitive NSCLC and 23 patients
with cisplatin-resistant NSCLC; 47 of the patients were aged
<60-years-old and 11 of the patients were aged ≥60-years old. A
total of 42 male patients and 16 female patients participated in
the present study. Patients were eligible if they had no history of
prior chemotherapy and radiotherapy. The cisplatin-sensitive
patients were defined as sensitive to cisplatin-based chemotherapy
following surgery; cisplatin-resistant patients were defined as
non-responsive to cisplatin-based chemotherapy following surgery.
The response to cisplatin-based chemotherapy was defined in
accordance with the Response Evaluation Criteria in Solid Tumors
(RECIST) criteria (19), and
patients were categorized into two different groups:
Cisplatin-sensitive and cisplatin-resistant. Patients in the
sensitive group exhibited a complete response (CR, as indicated in
the RECIST criteria). Patients in the resistant group exhibited
persistent disease [PR (Partial response), SD (Stable disease) or
PD, as indicated in the RECIST criteria]. According to the WHO
classification of lung cancer (20), patients were divided into the
well-intermediate and the poor differentiation groups.
All samples were obtained from Dezhou People's
Hospital (Dezhou, China) between April 2013 and June 2016. Written
informed consent was provided by all patients prior to surgery. All
participants did not receive radiotherapy or chemotherapy prior to
surgery. The present study was approved by the Ethics Committee of
Dezhou People's Hospital (approval no. DZPH-2013-16). Tissue
samples were immediately frozen at −80°C prior to protein and RNA
extraction.
Cell culture and agents
The human NSCLC cell line A549 was purchased from
the American Type Culture Collection (Manassas, VA, USA). The
cisplatin-resistant A549 sub-line A549rCDDP was purchased from the
Cancer Hospital of Peking Union Medical College, Chinese Academy of
Medical Sciences (Beijing, China). The cell line was cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) in an incubator at 37°C with 5%
CO2. For the A549rCDDP cells, the medium also contained
2 mg/l cisplatin (Selleck Chemicals, Houston, TX, USA). For the
analysis of cisplatin response, increasing concentrations of
cisplatin (2, 4, 6 and 8 µM) were added into the culture media of
A549 cells and A549rCDDP cells at 37°C for 48 h, which were
subsequently subjected to further experimentation.
Cell transfection
For the cell transfection assay, 1×106
A549 or A549rCDDP cells were treated with 50 nM miR-328 inhibitor
(5′-GACCGGGAGAGACGGGAAGGCA-3′) or miR-NC (negative control)
inhibitor (NC, 5′-GGCAAGACGAAACGAGACGACA-3′ (Shanghai GenePharma
Co., Ltd., Shanghai, China) in 1 µl Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. For the control group, cells were neither
treated with miR-NC or miR-328 inhibitor.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cells was extracted using
an miRNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) following
the manufacturer's protocol. The synthesis of first-strand cDNA was
performed with an M-MLV kit (GE Healthcare Life Sciences) following
the manufacturer's protocol. Subsequently, qPCR was performed on a
CFX96 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using
SYBR® Premix Ex Taq (Takara Bio, Inc., Otsu, Japan).
GAPDH and U6 served as internal controls for and mRNA and miRNA
measurements, respectively. The reaction conditions were as
follows: 95°C for 10 min, 40 cycles at 95°C for 15 sec and at 60°C
for 1 min. The relative expression levels of the indicated genes
were calculated using the of 2−ΔΔCq method (21). The sequences for primers were:
miR-328 forward, 5′GCTGGCCCTCTCTGCCC3′ and reverse,
5′CGTCAGATGTCCGAGTAGAGG3′; PTEN forward, 5′CGGTGTCATAATGTCTTTCAGC3′
and reverse, 5′TGAAGGCGTATACAGGAACAAT3′; GAPDH forward,
5′AGAAGGCTGGGGCTCATTTG3′ and reverse, 5′AGGGGCCATCCACAGTCTTC3′; and
U6 forward, 5′CTCGCTTCGGCAGCACA3′ and reverse,
5′AACGCTTCACGAATTTGCGT3′.
Western blot analysis
GAPDH (cat. no. 5174; 1:5,000) and PTEN antibodies
(cat. no. 9188, 1:1,000) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Secondary antibodies against
rabbit (HRP-conjugated Goat polyclonal Secondary Antibody to Rabbit
IgG; ab97080; 1:10,000) and mouse (HRP-conjugated Goat polyclonal
Secondary Antibody to Mouse IgG; ab97040; 1:10,000) were purchased
from Abcam (Cambridge, MA, USA). Total protein from A549 and
A549rCDDP cell lysates was prepared using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China). The protein lysates were quantified using Pierce BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. Then, 15 µg proteins were isolated and
then separated by 8% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were then blocked with 5% non-fat milk for 0.5 h at room
temperature, washed with Tris-buffered saline with Tween-20 (0.1%)
and incubated with the aforementioned primary antibodies at 4°C
overnight, followed by incubation with the secondary antibodies at
room temperature for 1 h. The bands were visualized using Pierce
ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.).
GAPDH served as an internal expression control.
Cell viability assay
The inhibitory effect of cisplatin was investigated
using a Cell Counting kit-8 (CCK8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Following transfection with miR-NC
inhibitor or miR-328 inhibitor or untransfected, 1,000 A549 or
A549rCDDP cells were seeded in each well of 96-well plates in the
RPMI-1640 medium containing vehicle (DMSO) or different
concentrations of cisplatin (0.25, 0.5, 1, 2, 4 or 8 µM) then
incubated for 72 h at 37°C. Following incubation for 0, 24, 48 or
72 h, 10 µl CCK8 solution was added into each well and the cells
were incubated for a further 2 h at 37°C. The absorbance of each
well at 450 nm was measured with a microplate reader (Bio-Rad
Laboratories, Inc.).
Cellular apoptosis assay
Cellular apoptosis in response to treatment with
cisplatin (1 µM) was measured using an Annexin-V/Dead Cell
Apoptosis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocols. Briefly, following
treatment, A549rCDDP cells were harvested by trypsinization and
suspended in Annexin V binding buffer; propidium iodide and Annexin
V-fluorescein isothiocyanate were then added to the cell
suspensions and incubated for 15 min at room temperature. The cells
were analyzed using a BD FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The cells positive for
Annexin V staining were considered as apoptotic cells following
analysis using FlowJo version 10.2 (FlowJo LLC, Ashland, OR,
USA).
Dual-luciferase reporter assay
Prediction of miR-328 target genes was carried out
on miRanda (http://34.236.212.39/microrna/home.do). For the
preparation of A549 cDNA, A549 RNA was extracted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol and then reverse transcribed to cDNA with
PrimeScript™ II 1st Strand cDNA Synthesis kit (Takara Bio, Inc.)
following the manufacturer's protocol. The wild type PTEN 3′UTR
(PTEN 3′UTR WT) was amplified from the cDNA of A549 cells using
TaKaRa Ex Taq® DNA Polymerase. kit (Takara Bio, Inc.).
The thermo cycle conditions were denaturation at 98°C for 10 sec,
annealing at 55°C for 30 sec, followed by elongation at 72°C for 60
sec, repeated for 30 cycles. It was then inserted into XbaI site of
the pGL-3 vector (Promega Corporation, Madison, WI, USA). A mutated
PTEN 3′UTR (PTEN 3′UTR Mut) was created using a site-directed
mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA, USA)
according to the manufacturer's protocols. Then, 1×106
A549 cells were cotransfected with 2 µg pGL3-PTEN 3′UTR WT or
pGL3-PTEN 3′UTR Mut, 50 nM miR-328 mimics
(5′-CUGGCCCUCUCUGCCCUUCCGU-3′) or miR-NC
(5′-AUUGGAACGAUACAGAGAAGA-3′) (Shanghai GenePharma Co., Ltd.) and
internal control Renilla plasmid (Promega Corporation) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following transfection for 24 h, luciferase and
Renilla activities were detected using a Dual Luciferase
Reporter Assay kit (Promega Corporation) following the
manufacturer's protocols.
Statistical analysis
All experiments were repeated 3 times and all
statistical analyses were performed using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). All data are
presented as the mean ± standard deviation. The correlation
analysis was carried out using Pearson Correlation analysis.
Differences between two groups were compared using Student's
t-tests. Differences among >2 groups were analyzed with one-way
analysis of variance followed by Newman-Keuls analysis. Comparison
of patient characteristics was achieved using the χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-328 expression levels are elevated
in tumor tissues from patients with cisplatin-resistant NSCLC and
are negatively correlated with PTEN mRNA expression levels
The present study investigated the expression of
miR-328 and PTEN in the tissues of patients with NSCLC. As
presented in Table I, the
expression of miR-328 was not correlated with every one of the
listed characteristics: Sex (P=0.38), age (P=0.39) or tumor size
(P=0.06). In addition, no significant differences were reported in
the expression levels of PTEN between the characteristics of sex
(P=0.67), age (P=0.66) or tumor size (P=0.98). Furthermore, there
were significant differences in the expression levels of miR-328
(P=0.00) and PTEN (P=0.00) between the well-intermediate and the
poor differentiation groups.
 | Table I.Expression of miR-328 and PTEN in
NSCLC tissues. |
Table I.
Expression of miR-328 and PTEN in
NSCLC tissues.
| Factor | No. cases | miRNA-328, mean ±
standard deviation | P-value | PTEN, mean ± standard
deviation | P-value |
|---|
| Sex |
|
| 0.38 |
| 0.67 |
| Male | 42 | 1.51±0.72 |
| 0.86±0.32 |
|
|
Female | 16 | 1.32±0.52 |
| 0.90±0.32 |
|
| Age, years |
|
| 0.39 |
| 0.66 |
|
<60 | 47 | 1.49±0.70 |
| 0.86±0.32 |
|
| ≥60 | 11 | 1.30±0.55 |
| 0.91±0.30 |
|
| Tumor size, cm |
|
| 0.06 |
| 0.98 |
| ≥5 | 21 | 1.68±0.51 |
| 0.87±0.32 |
|
|
<5 | 37 | 1.33±0.73 |
| 0.88±0.32 |
|
| Histological
grade |
|
| 0.00 |
| <0.001 |
|
Well-intermediate
differentiation | 22 | 1.10±0.26 |
| 1.05±0.28 |
|
| Poor
differentiation | 36 | 1.67±0.76 |
| 0.77±0.32 |
|
Additionally, the characteristics of patients in the
cisplatin-responsive and non-responsive groups were analyzed in the
present study. As presented in Table
II, there were no significant differences in the
characteristics of sex (P=1.00), age (P=0.74), tumor size (P=0.58)
or histological grade (P=1.00) between cisplatin-responsive and
non-responsive groups.
 | Table II.Patient characteristics in
cisplatin-responsive and non-responsive. |
Table II.
Patient characteristics in
cisplatin-responsive and non-responsive.
| Factor |
Cisplatin-responsive |
Cisplatin-nonresponsive | P-value |
|---|
| Sex |
|
| 1.00 |
|
Male | 25 | 17 |
|
|
Female | 10 | 6 |
|
| Age, years |
|
| 0.74 |
|
<60 | 29 | 18 |
|
|
≥60 | 6 | 5 |
|
| Tumor size, cm |
|
| 0.58 |
| ≥5 | 14 | 7 |
|
|
<5 | 21 | 16 |
|
| Histological
grade |
|
| 1.00 |
|
Well-intermediate
differentiation | 13 | 9 |
|
| Poor
differentiation | 22 | 14 |
|
To investigate the role of miR-328 in the
development of cisplatin resistance in NSCLC, RT-qPCR was employed
to detect whether there were differences in miR-328 expression
levels between tumor tissues from patients with cisplatin-sensitive
and cisplatin-resistant NSCLC. A significant increase in miR-382
expression levels was detected in the cisplatin-resistant tumor
tissues compared with the cisplatin-sensitive tumor tissues
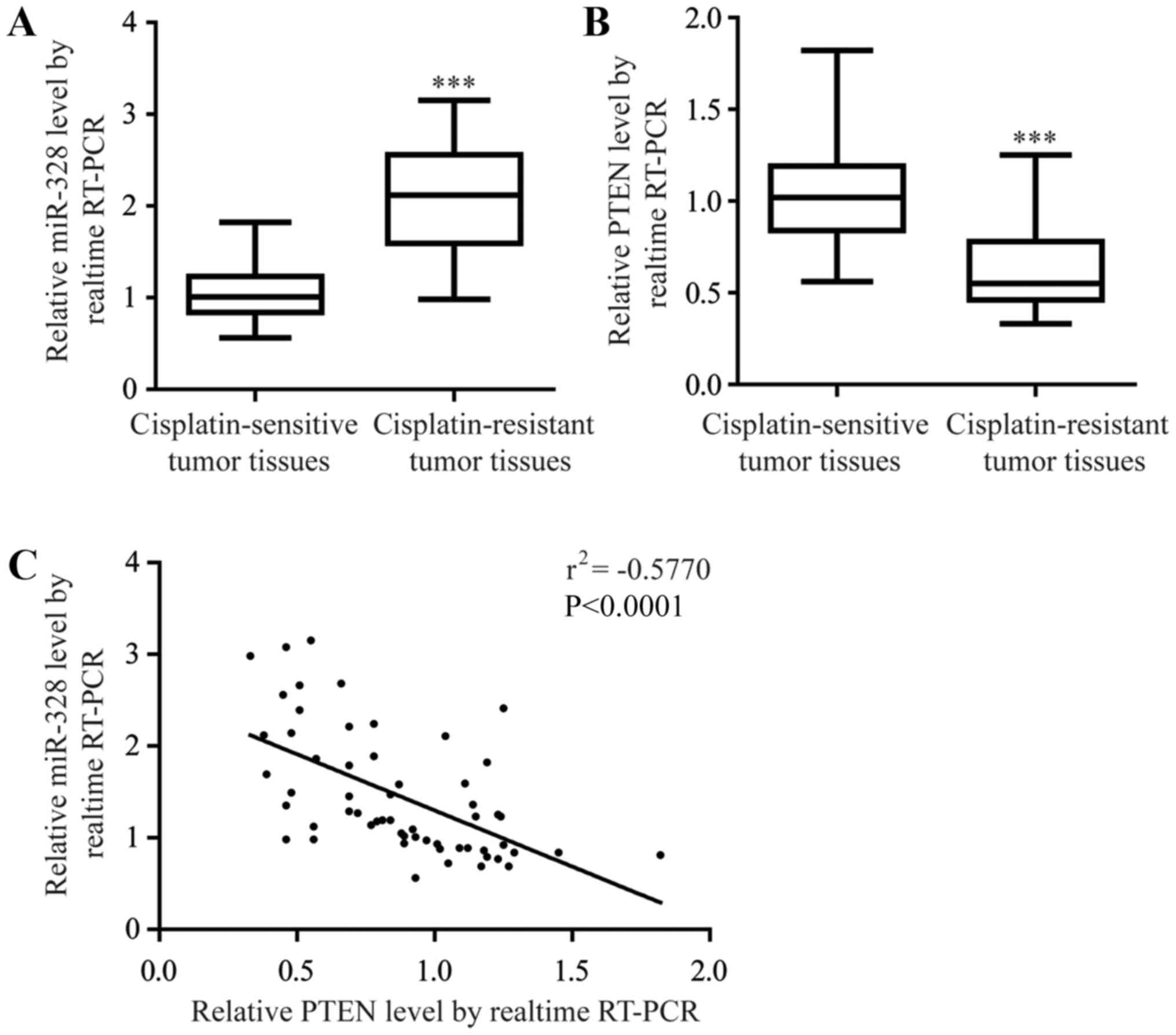
(P<0.0001; Fig. 1A). Loss of
PTEN has frequently been reported to contribute to chemoresistance,
particularly cisplatin-resistance in NSCLC (22). As expected, the present study
observed a significant decrease in PTEN mRNA expression levels
within the tumor tissues from patients with cisplatin-resistant
NSCLC compared with the cisplatin-sensitive tumor tissues
(P<0.0001; Fig. 1B). In
addition, there was a significant negative correlation between
miR-328 and PTEN mRNA expression levels in the NSCLC tumor tissues
(Fig. 1C), suggesting an
underlying regulatory association between miR-328 and PTEN in
NSCLC.
Dysregulation of miR-328 and PTEN
expression is involved in the development of cisplatin resistance
in a cell model
In order to investigate the role of miR-328 in
cisplatin resistance, the A549 cell line and its
cisplatin-resistant sub-line A549rCDDP were employed in the present
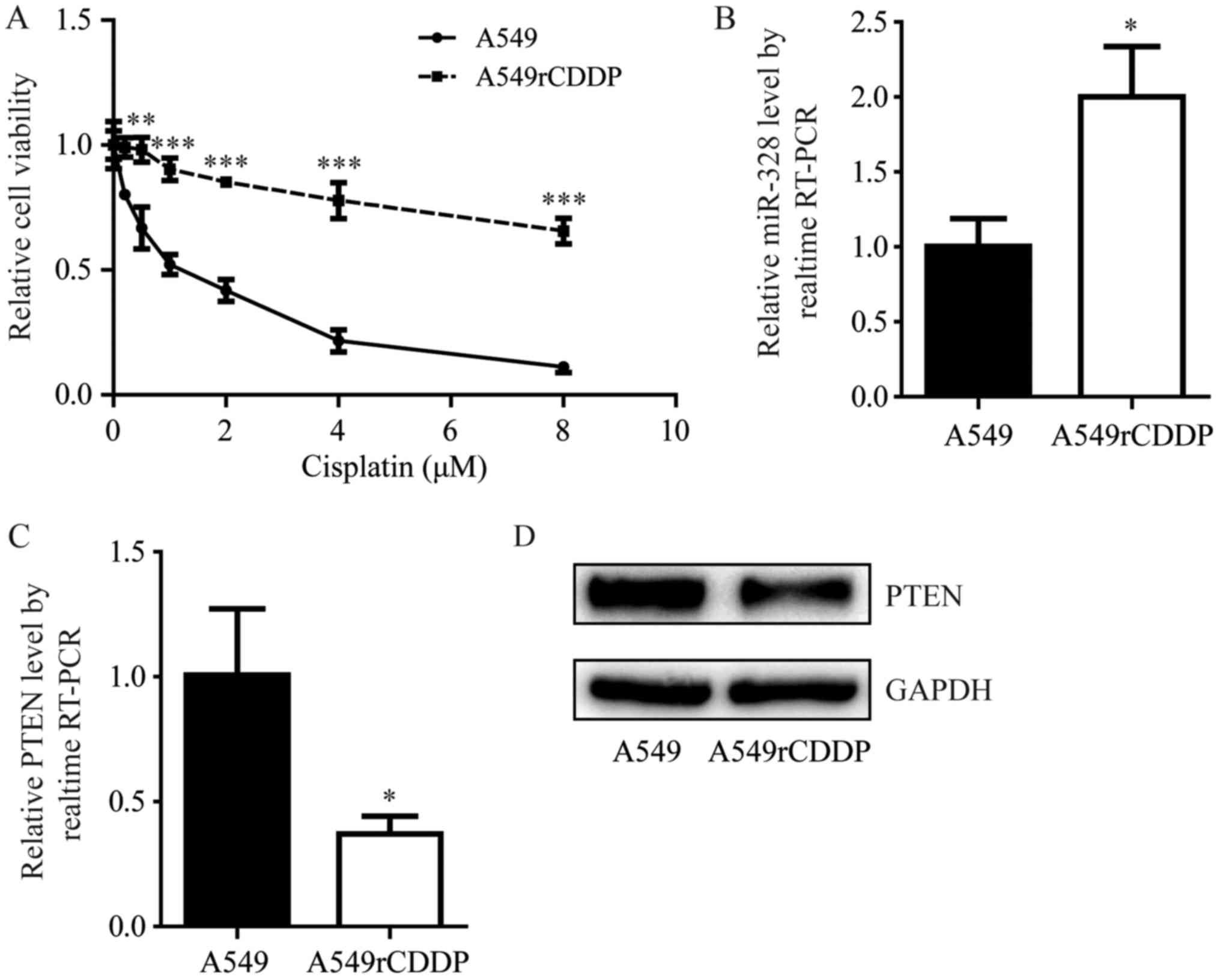
study. Compared with A549 cells, A549rCDDP cells were relatively
insensitive to increasing concentrations of cisplatin (2, 4, 6 and
8 µM; P<0.01 and P<0.0001; Fig.
2A). RT-qPCR revealed an increase in the miR-328 expression
levels within A549rCDDP cells compared with their parental cells
(P<0.05; Fig. 2B). In addition,
the significant elevation of miR-328 expression levels within
A549rCDDP cells was accompanied by a decrease in PTEN at the mRNA
and protein expression levels compared with A549 cells (P<0.05;
Fig. 2C and D). These data
indicated that the A549rCDDP cell line may be a suitable model to
study the effects of miR-328 and PTEN in the development of
cisplatin resistance in NSCLC.
PTEN is regulated by miR-328 in
A549rCDDP cells
The present study aimed to determine the association
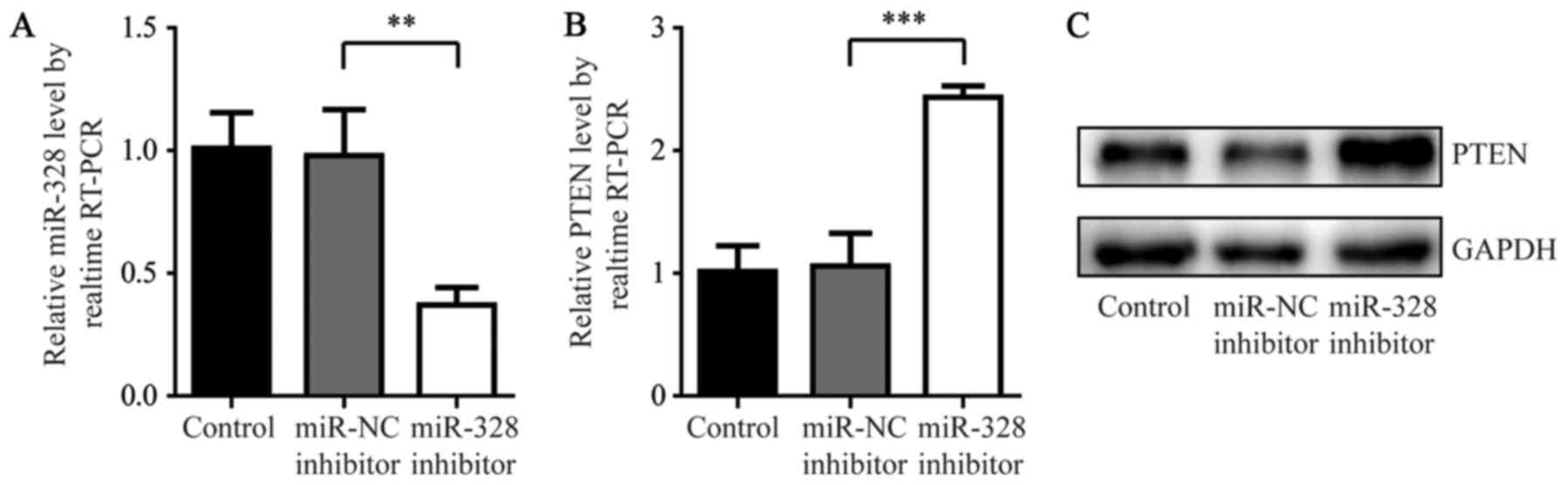
between miR-328 and PTEN using a miR-328 inhibitor. As presented in
Fig. 3A, miR-328 inhibitor induced
a significant decrease in miR-328 expression levels in A549rCDDP
cells compared with the miR-NC inhibitor groups (P<0.01).
Additionally, the inhibition of miR-328 significantly increased
PTEN mRNA expression and markedly increased the protein expression
levels compared with miR-NC inhibitor groups (P<0.0001; Fig. 3B and C). These results indicated
that miR-328 may negatively regulate PTEN expression in A549rCDDP
cells.
miR-328 directly targets the PTEN
3′UTR
Using the online tool MiRanda, the present study
predicted that miR-328 may directly target the 3′UTR of PTEN mRNA
(Fig. 4A). A dual-luciferase assay
revealed that miR-328 mimics may significantly decrease the
luciferase activity of cells transfected with PTEN 3′UTR-WT,
although not PTEN 3′UTR-Mut (P<0.01; Fig. 4B). These results indicated that
miR-328 may directly target PTEN and reduce the expression of PTEN
by binding to its 3′UTR.
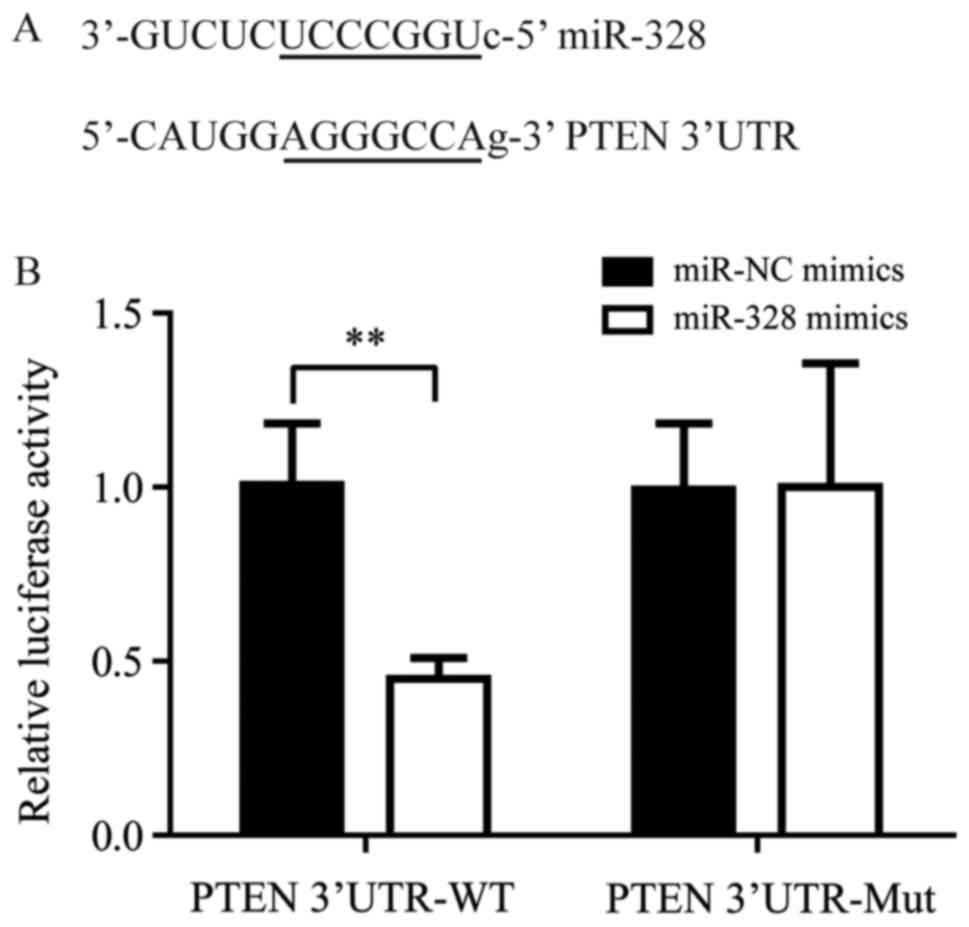 | Figure 4.miR-328 directly targets the PTEN
3′UTR. (A) Sequence alignment of miR-328 and the PTEN 3′UTR. (B) In
cells transfected with PTEN 3′UTR-WT, co-transfection of miR-328
mimics significantly decreased the luciferase activity compared
with cells co-transfected with miR-NC mimics; within cells
transfected with PTEN 3′UTR-Mut, no significant difference in
luciferase activity was observed between the miR-328 mimics and
miR-NC mimics groups. **P<0.01, miR-328 mimics vs. miR-NC
mimics. miR, microRNA; Mut, mutated; Nc, negative control; PTEN,
phosphatase and tensin homolog; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; UTR,
untranslated region; WT, wild-type. |
Downregulation of miR-328 reverses
cisplatin resistance in A549rCDDP cells
To further confirm the function of miR-328 in the
development of cisplatin resistance, A549rCDDP cells were
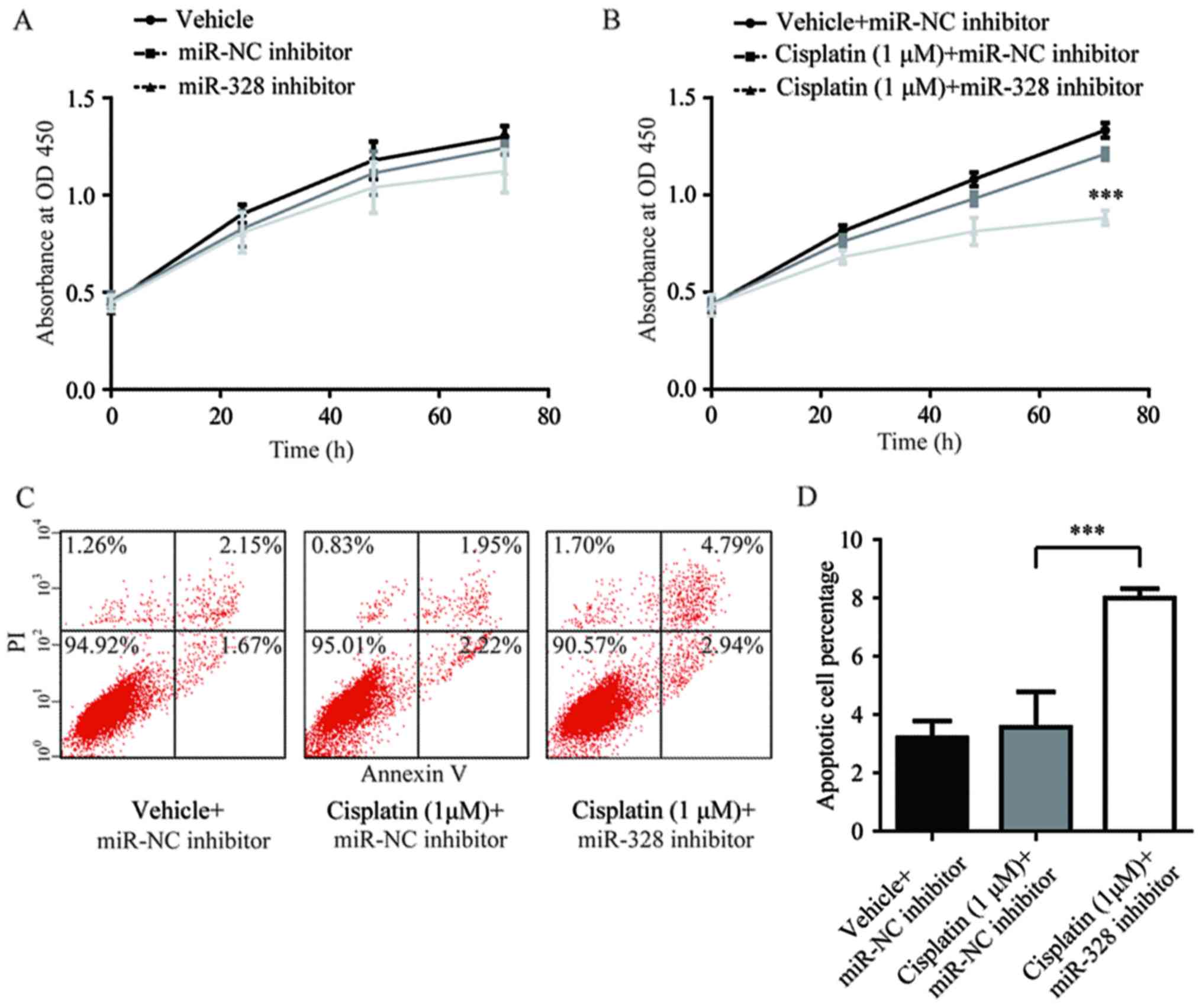
transfected with miR-328 inhibitor in the present study. The
antagonist effects of miR-328 did not alter the cell proliferation
of A549rCDDP cells compared with the vehicle and miR-NC inhibitor
groups (Fig. 5A). However,
A549rCDDP cell proliferation was significantly inhibited in the
miR-328 inhibitor-transfected group when cells were treated with 1
µM cisplatin, compared with that of the vehicle- and cisplatin +
miR-NC inhibitor groups. This indicated that miR-328 may serve a
pivotal role in cisplatin resistance and not in cell proliferation
(P<0.001; Fig. 5B).
Additionally, in the presence of cisplatin, miR-328 inhibitor,
although not miR-NC inhibitor, induced an elevation in cellular
apoptosis; there was no significant difference in the extent of
cellular apoptosis between the vehicle + miR-NC inhibitor and
cisplatin + miR-NC inhibitor groups (Fig. 5C and D). Collectively, the results
of the present study suggested that the upregulation of miR-328
contributed to cisplatin resistance in A549rCDDP cells.
Discussion
As cisplatin is a first-line drug for the treatment
of NSCLC, cisplatin resistance has been considered to be a major
issue affecting treatment efficacy for patients with NSCLC
(23). The present study
identified abnormal overexpression of miR-328 in
cisplatin-resistant cells and tumor tissues from patients with
cisplatin-resistant NSCLC, suggesting a novel mechanism underlying
cisplatin-resistance in NSCLC associated with miR-328.
In addition to its role in the suppression of cell
proliferation and migration, PTEN is well known for its function in
modulating cancer cell responses to drug treatment (24,25).
In NSCLC, promoter methylation was initially reported to account
for PTEN silencing, with ~35% of PTEN-negative NSCLC patients
exhibiting high levels of promoter methylation (26). In recent years, numerous miRNAs
including miR-10a, miR-205 and miR-21 have been reported to
negatively regulate PTEN expression at the post-transcriptional
level in NSCLC (27–29). Additionally, overexpression of
miR-328 was correlated with brain metastasis in patients with NSCLC
(13). In vitro studies
additionally indicated that miR-328 had the ability to promote A549
cell invasion and migration (30).
The present study observed an upregulation in miR-328 and a
downregulation in PTEN expression levels within cisplatin-resistant
NSCLC cells and tumor tissues from cisplatin-resistant NSCLC
patients. Furthermore, PTEN may be negatively regulated by miR-328
in NSCLC cells; miR-328 inhibition may reverse cisplatin resistance
in NSCLC cells as observed in the present study. Thus, miR-328 may
be considered to be an inducer of cisplatin resistance in NSCLC
cells via PTEN targeting.
In conclusion, the present study demonstrated that
dysregulation of miR-328 was associated with cisplatin resistance
in NSCLC. Mechanistically, miR-328 may directly bind to the 3′UTR
of PTEN to negatively regulate PTEN expression in NSCLC cells. The
results of the present study proposed miR-328 as a novel prognostic
marker and therapeutic target in patients with NSCLC exhibiting
cisplatin resistance.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, SW and FM participated in the design and
performance of the experiments; WZ and CW contributed to the
collection of samples and clinical data; and WZ supervised and
wrote the manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were approved by the Ethics Committee
of Dezhou People's Hospital.
Patient consent for publication
Written informed consent for the publication of any
associated data and accompanying images was provided by all
patients prior to surgery.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan XL, Moyer AM, Fridley BL, Schaid DJ,
Niu N, Batzler AJ, Jenkins GD, Abo RP, Li L, Cunningham JM, et al:
Genetic variation predicting cisplatin cytotoxicity associated with
overall survival in lung cancer patients receiving platinum-based
chemotherapy. Clin Cancer Res. 17:5801–5811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology, : Comparison of four chemotherapy regimens
for advanced non-small-cell lung cancer. N Engl J Med. 346:92–98.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hesse M and Arenz C: MicroRNA maturation
and human disease. Methods Mol Biol. 1095:11–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gommans WM and Berezikov E: Controlling
miRNA regulation in disease. Methods Mol Biol. 822:1–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero-Cordoba SL, Salido-Guadarrama I,
Rodriguez-Dorantes M and Hidalgo-Miranda A: miRNA biogenesis:
Biological impact in the development of cancer. Cancer Biol Ther.
15:1444–1455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin VY and Chu KM: MiRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saberi A, Danyaei A, Neisi N, Dastoorpoor
M and Tahmasbi Birgani MJ: MiR-328 may be considered as an oncogene
in human invasive breast carcinoma. Iran Red Crescent Med J.
18:e423602016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo X, Yang S, Zhou C, Pan F, Li Q and Ma
S: MicroRNA-328 enhances cellular motility through
posttranscriptional regulation of PTPRJ in human hepatocellular
carcinoma. Onco Targets Ther. 8:3159–3167. 2015.PubMed/NCBI
|
|
13
|
Arora S, Ranade AR, Tran NL, Nasser S,
Sridhar S, Korn RL, Ross JT, Dhruv H, Foss KM, Sibenaller Z, et al:
MicroRNA-328 is associated with (non-small) cell lung cancer
(NSCLC) brain metastasis and mediates NSCLC migration. Int J
Cancer. 129:2621–2631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S,
Malaponte G, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade
inhibitors: How mutations can result in therapy resistance and how
to overcome resistance. Oncotarget. 3:1068–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, National cancer
institute of the United States, National cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health Organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao L, Chen J, Ou B, Liu C, Zou Y and Chen
Q: GAS5 knockdown reduces the chemo-sensitivity of non-small cell
lung cancer (NSCLC) cell to cisplatin (DDP) through regulating
miR-21/PTEN axis. Biomed Pharmacother. 93:570–579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gschwantler-Kaulich D, Tan YY, Fuchs EM,
Hudelist G, Köstler WJ, Reiner A, Leser C, Salama M, Attems J,
Deutschmann C, et al: PTEN expression as a predictor for the
response to trastuzumab-based therapy in Her-2 overexpressing
metastatic breast cancer. PLoS One. 12:e01729112017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soria JC, Lee HY, Lee JI, Wang L, Issa JP,
Kemp BL, Liu DD, Kurie JM, Mao L and Khuri FR: Lack of PTEN
expression in non-small cell lung cancer could be related to
promoter methylation. Clin Cancer Res. 8:1178–1184. 2002.PubMed/NCBI
|
|
27
|
Yu T, Liu L, Li J, Yan M, Lin H, Liu Y,
Chu D, Tu H, Gu A and Yao M: MiRNA-10a is upregulated in NSCLC and
may promote cancer by targeting PTEN. Oncotarget. 6:30239–30250.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lei L, Huang Y and Gong W: miR-205
promotes the growth, metastasis and chemoresistance of NSCLC cells
by targeting PTEN. Oncol Rep. 30:2897–2902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du C, Zheng J, Lu X and Wang Y:
Downregulation of miR-18a or miR-328 inhibits the invasion and
migration of lung adenocarcinoma A549 cells. Xi Bao Yu Fen Zi Mian
Yi Xue Za Zhi. 32:1051–1054. 2016.(In Chinese). PubMed/NCBI
|