Introduction
Melanoma, as a cutaneous cancer caused by the
malignant transformation of melanocytes, is the most aggressive
form and common cause of skin cancer-associated mortality in humans
(1,2). Risk factors for melanoma include age,
sex, race, the constitutive color of the skin and the geographical
zone (1). Recently, the incidence
of melanoma has demonstrated rapid growth worldwide (3). Surgery, radiotherapy and
chemotherapies are common therapies for early-stage,
non-metastasized melanoma, with most cases of melanoma being
curable. However, once metastasized, the efficiency of treatment
was reduced significantly (2).
Chemotherapy is an extremely ineffective and unsatisfactory method
for treating malignant melanoma because of drug resistance, which
is characteristic of this disease and limits its usage (4). The development of novel agents for
melanoma treatment in the clinic is urgently needed.
Cantharidin (CTD), a terpenoid that is isolated from
Chinese blister beetles, has been used as a traditional Chinese
medicine to treat tumors for a very long time (5). Until now, cantharidin and its
derivatives have been proven to possess anticancer activities in
various types of cancer, including pancreatic cancer (6), hepatoma (7), and glioma (8). Furthermore, CTD has been reported to
suppress A375.S2 human melanoma cell migration, invasion and
apoptosis through cell cycle arrest and the induction of apoptosis
(9,10). However, there is no available
information demonstrating that CTD can inhibit the proliferation of
melanoma and clarifying its underlying mechanisms.
MicroRNAs (miRs) are short (approximately 22 nt),
endogenous non-coding RNAs that regulate gene expression by binding
to the 3′-untranslated region (3′UTRs) of target genes, suppressing
mRNA translation or degrading mRNA. Emerging evidence has
demonstrated that miRs are involved in diverse biological processes
and disease development, including a number of types of cancer.
Recently, dysregulation of miR-21 was reported in melanoma
(11,12) and selective expression alteration
of miR-21 has been one of the important mechanisms for melanoma
treatment with high-intensity focused ultrasound (13). However, whether the roles of CTD in
melanoma are associated with miR-21 remains unknown.
The present study aimed to investigate the role of
CTD in regulating the proliferation of human melanoma A375 cells
and its association with the miR-21-PTEN signaling pathway. It was
demonstrated that in A375 cells CTD could inhibit proliferation and
tumorigenesis, as well as induce apoptosis, possibly mediated by
downregulating the expression of miR-21 and upregulating the
expression of phosphatase and tensin homolog (PTEN).
Materials and methods
Chemicals and reagents
CTD and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA), fetal
calf serum (FCS) and L-glutamine were purchased from Gibco (Thermo
Fisher Scientific, Inc.). Primary antibodies against PTEN, protein
kinase B (AKT), protein kinase B (p) AKT, Bax, B-cell lymphoma-2
(bcl-2), caspase-3, anti-GAPDH and the appropriate secondary
antibodies were purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). The enhanced chemiluminescence (ECL) detection system was
obtained from Millipore (EMD Millipore, Billerica, MA, USA).
Cell culture
The A375 human melanoma cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured at 37°C in a 5% CO2 humidified
incubator in RPMI-1640 medium supplemented with 10%
heat-inactivated FCS, 2 mM glutamine, 100 U/ml penicillin and 0.1
mg/ml streptomycin.
Transfection of miR-21 agomir,
antagomir, PTEN small interfering siRNA or overexpressed PTEN
Micron™ hsa-miR-21-5p agomir
(5′-UAGCUUAUCAGACUGAUGUUGA-3′) or agomir control (sequence
unavailable), micrOFF™hsa-miR-21-5p antagomir
(5′-UCAACAUCAGUCUGAUAAGCUA-3′) or antagomir control (sequence
unavailable) were obtained from Guangzhou RiboBio Co., Ltd.,
(Guangzhou, China; 10 nM). SignalSilence® PTEN siRNA
(5′-GCATATGGAAAGCTTCATT-3′) and its negative control
oligonucleotides (5′-ACACGTCCGAACATACTAC-3′) were purchased from
Cell Signaling Technology (50 nM). Transfections were performed
using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
overexpression, the full-length PTEN sequence was cloned into the
lentiviral vector [Obio Technology (Shanghai) Corp., Ltd.,
Shanghai, China]. Following amplification, lentiviral-PTEN
(LV-PTEN) or empty vector (Vector) as a control was transduced into
A375 cells at a concentration of 5×104 transducing
units/ml using polybrene [Obio Technology (Shanghai) Corp.]
according to the manufacturer's protocol. Next, 5×105
cells were seeded into 6-well plates. A total of 48 h following
transfection, the cells were harvested for subsequent
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the A375 cell line using
Trizol (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration and purity of these RNA samples were detected. The
total RNA samples were reverse transcribed into complementary DNA
(cDNA) using PrimeScript RT Reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China). qPCR was performed using the MyiQ Real-Time
PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and the SYBR-Green I premix (Takara Biotechnology Co., Ltd.).
U6 or GAPDH mRNA were used as an endogenous control. The
thermocycling conditions were as follows: 95°C for 10 min, 40
cycles of 95°C for 15 sec and 60°C for 1 min. The experiment was
repeated three times and the levels of miR were normalized for each
well to the levels of U6 using the 2−ΔΔCqmethod
(14). The forward (F) and reverse
(R) primers used in this study were as follows: miR-21, F:
5′-GGACTAGCTTATCAGACTG-3′ and R: 5′-CATCAGATGCGTTGCGTA-3′; U6 F:
5′-ATTGGAACGATACAGAGAAGAT-3′ and R, 5′-GGAACGCTTCACGAATTT-3′; PTEN
F: 5′-CGGCAGCATCAAATGTTTCAG-3′ and R:
5′-AACTGGCAGGTAGAAGGCAACTC-3′.
Western blot analysis
Cells were collected and homogenized in ice-cold
RIPA buffer (Beyotime Institute of Biotechnology, Shanghai, China).
The amounts of protein from each treatment were detected and
normalized using a bicinchoninic acid assay. Proteins (20 µg/lane)
were separated by 10% SDS-PAGE and were subsequently transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% non-fat milk at room temperature for 2 h and then
separately probed overnight at 4°C with the following primary
antibodies: Anti-GAPDH (1:5,000; cat. no. 10494-1-AP), Bcl-2
(1:1,000; cat. no. 12789-1-AP), Bax (1:1,000; cat. no. 50599-2-Ig),
caspase-3 (1:1,000, cat. no. 19677-1-AP), PTEN (1:1,000, cat. no.
22034-1-AP), AKT (1:1,000, cat. no. 10176-2-AP), p AKT (1:1,000;
cat. no. 66444-1-Ig). After washing with TBST buffer (0.1%
Tween-20), the membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin (Ig)G or goat
anti-rabbit IgG (1:5,000; cat. no. SA00001-1 or SA00001-2) for 1 h
at room temperature. Images were then captured of the membranes
using the ECL detection system (EMD Millipore). Densitometry
analysis of the bands was performed using Quantity One (version
4.5.0; Bio-Rad Laboratories, Inc.). The proteins were quantified
and expressed as their ratio to GAPDH.
Cell Counting Kit-8 (CCK-8) assay
A375 cells were seeded in 96-well plates at a
density of 1,000 cells/well in 100 µl culture medium with different
doses (0, 0.2, 1 or 5 µM) of CTD (dissolved with 0.5% dimethyl
sulfoxide; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
treatments. Next, at 24, 48, 72 and 96 h following treatment, CCK-8
solution was added to each well. The optical density of the cells
was measured using a microplate reader (Bio-Rad Laboratories, Inc.)
at 450 nm.
Colony-formation assay
A375 cells were seeded in a 6-well plate (600
cells/well) in complete medium and were allowed to grow for 24 h.
The cells were then exposed to 5 µM CTD or vehicle for 48 h. After
the drug was removed, the cells were washed with PBS and incubated
for another 14 days in complete medium. The cells were stained with
1% crystal violet solution at room temperature. Following 10 min
incubation, the excess crystal violet was washed out and the
stained colonies were counted under an inverted microscope (Nikon
Corporation, Tokyo, Japan).
Establishment of subcutaneous
xenograft tumor models
Male BALB/C Nu mice were obtained from Vital River
Laboratory Animal Technology Co., Ltd., (Beijing, China) at 3- to
4-weeks old and weighing 18–20 g. Five mice per cage were housed
together under a 12/12 h light/dark cycle in standard laboratory
specific pathogen free cages (22±2°C, 50–60% humidity), with food
and water ad libitum. A375 cells were digested using 0.25%
pancreatin and were counted. The cell concentration was adjusted to
1×107 cells/ml and a 0.1 ml cell suspension was
subcutaneously injected into the right armpit of the mice. The
volume of tumors was measured with a caliper every 3 days using the
following equation: Tumor volume=width (W)2xlength
(L)/2. When the tumor volumes reached approximately 100
mm3, the mice were randomly and equally divided into 2
groups and were treated with control or CTD (intraperitoneal
injected with 200 µl vehicle or CTD, 0.5 mg/kg/day) for 3 weeks. In
addition, certain mice in the CTD-treated group were intratumorally
injected with miR21 agomir (10 nM diluted with 50 µl of PBS, three
times per week; Guangzhou Ribobio Co., Ltd.) or agomir control
accompanied with CTD treatment (n=6 per group). A total of 30 mice
were included in the present study. All the experiments were
performed in accordance with the relevant guidelines and approved
by the Institutional Animal Care and Use Committee (IACUC) of
Taishan Medical University.
Flow cytometric analysis
Following treatment with CTD for 48 h, the cells
were harvested and reconstituted to 1–5×106/ml. Next,
100 µl cell suspension was transferred into a 5 ml flow tube,
followed by staining with 5 µl of Annexin V/fluorescein
isothiocyanate (FITC; Beijing 4A Biotech Co., Beijing, China) for 5
min at room temperature in the dark. Next, the cells were stained
with 10 µl propidium iodide (PI) and 400 µl PBS, and then collected
and detected by flow cytometry. The results were analyzed using
Flowjo software (version 9.3.2; FlowJo LLC, Ashland, OR, USA).
Statistical analysis
Data analysis was performed using SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA). All the data were presented as the
mean ± standard deviation. The data of only two groups were
analyzed using a Student's t-test. One-way analysis of variance
followed by the Student Newman Keuls test was used for the analysis
of three or more groups. P<0.05 was considered to indicate a
statistically significance difference.
Results
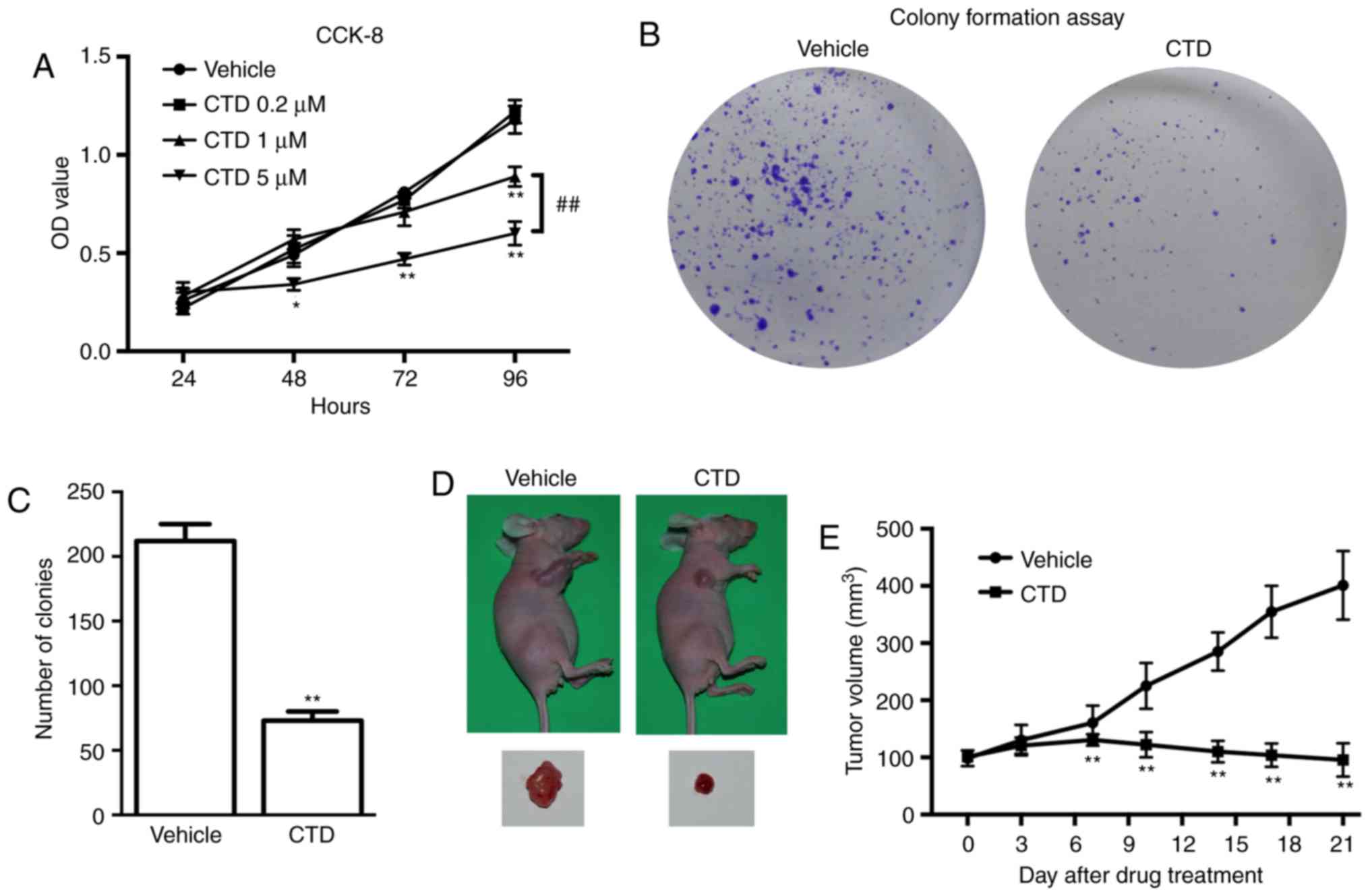
CTD inhibits the proliferation and
tumorigenesis of melanoma
The effects of different doses of CTD (0.2, 1 and 5
µM) were first evaluated on the growth of A375 cells using the
CCK-8 assay. The results demonstrated that the OD value of 5 µM
CTD-treated A375 cells was significantly decreased compared with
the vehicle control at 48, 72 and 96 h (P<0.05). Next, 1 µM CTD
significantly decreased the OD value of A375 cells only at 96 h
(P<0.01) and significantly increased compared with 5 µM CTD
(P<0.01). Additionally, 0.2 µM CTD demonstrated no effect on the
OD value (Fig. 1A). These results
suggest that CTD had an inhibitory effect on the proliferation of
A375 cells in a dose-dependent manner. Therefore, 5 µM CTD was used
in subsequent in vitro experiments. The colony formation
assay was also used to detect the roles of CTD in the growth of
A375 cells. The plates with 5 µM CTD treatment exhibited
significantly fewer cell clones compared with the control group
(P<0.01; Fig. 1B and C). All
these results indicated that CTD inhibits the proliferation of A375
cells effectively in vitro. Whether CTD had antitumor
effects on A375 cell xenografts in vivo was next
investigated. The results demonstrated that CTD retarded the growth
of the xenograft tumor significantly and the final tumor volume was
significantly smaller compared with the vehicle-treated group
(P<0.01; Fig. 1D and E),
suggesting that CTD inhibited the tumorigenesis of melanoma in
vivo.
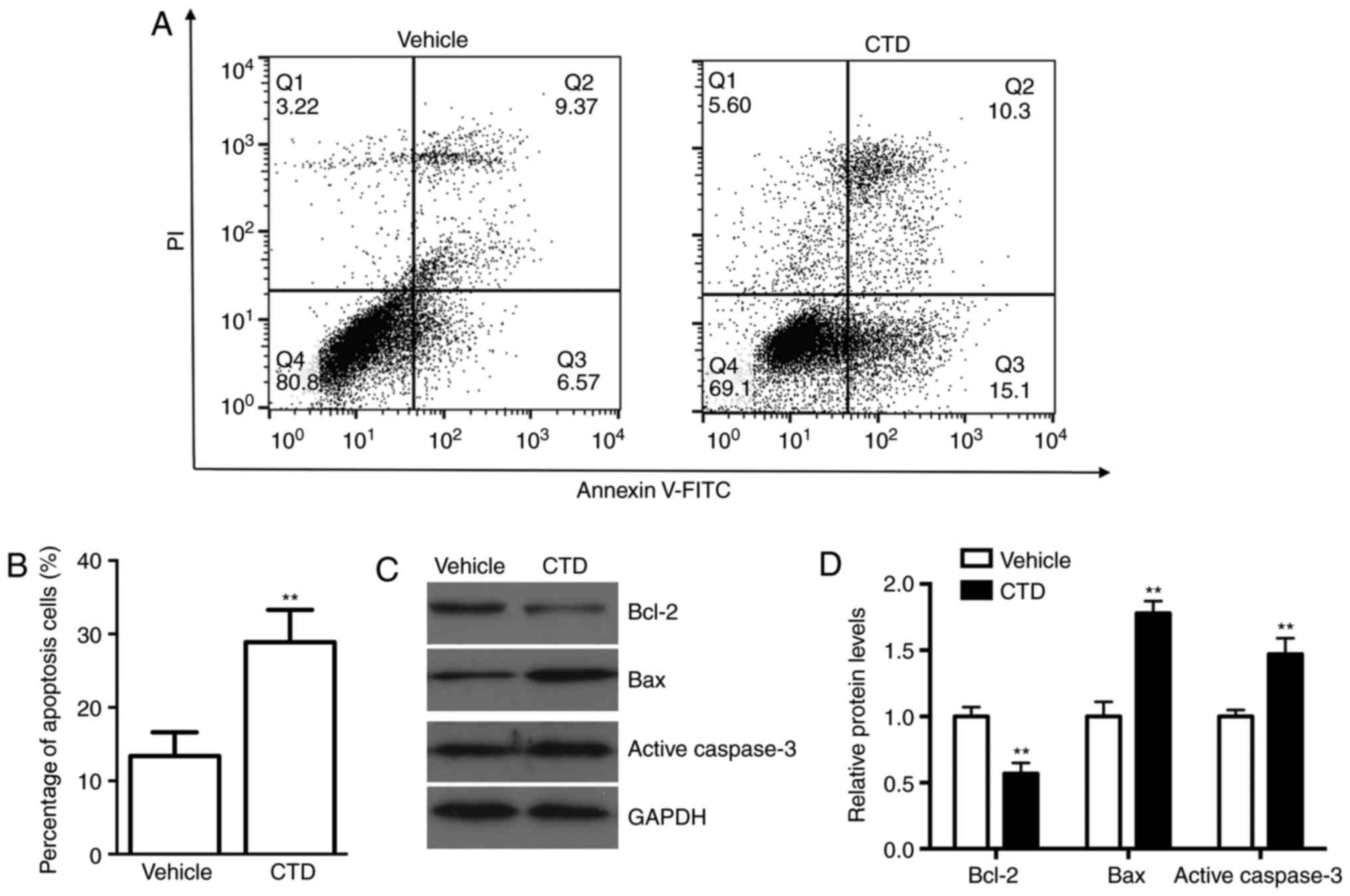
CTD promotes the apoptosis of A375
cells
To observe the effect of CTD on cell apoptosis in
A375 cells, an Annexin V-FITC/PI staining assay was conducted. As
shown in Fig. 2A and B, CTD
increased the number of apoptotic cells compared with those in the
vehicle control group (P<0.01). These data suggest that CTD may
promote human melanoma cell apoptosis. To study further the effects
of CTD on cell apoptosis western blot analysis was performed. The
expression level of Bcl-2, an anti-apoptosis protein, was
significantly decreased following CTD treatment (P<0.01), while
the expression levels of pro-apoptosis proteins Bax and active
caspase-3 were significantly increased (P<0.01; Fig. 2C and D). These results indicate
that CTD could effectively induce the apoptosis of A375 cells.
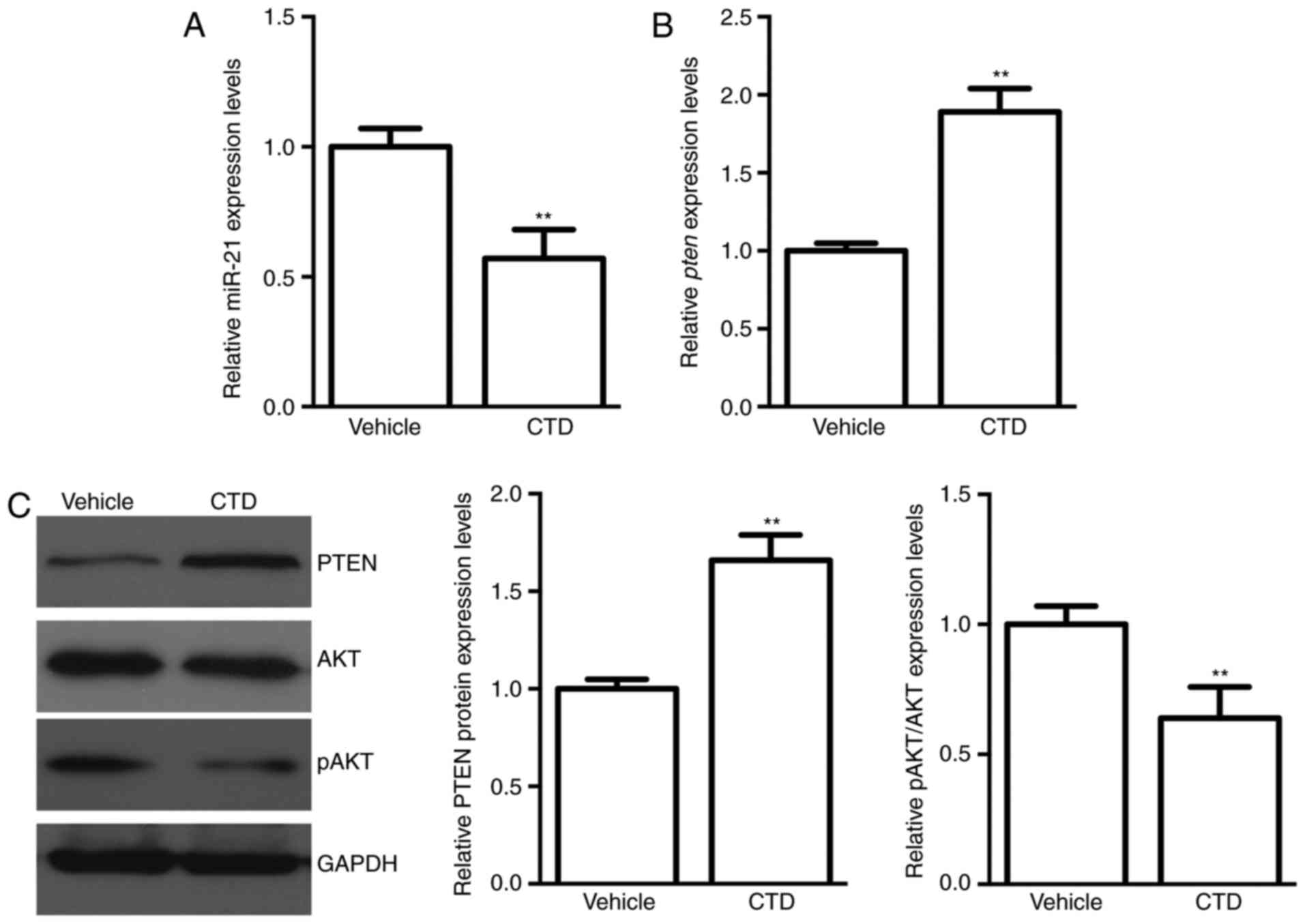
CTD suppresses miR-21 and increases
PTEN expression in A375 cells
Next, alterations in the relative expression levels
of miR-21 and its target gene PTEN following CTD
administration were measured. A higher miR-21 expression in A375
cells was decreased significantly following CTD treatment
(P<0.01; Fig. 3A). Furthermore,
PTEN, the potential downstream gene of miR-21, was
upregulated in both the mRNA and protein expression levels
following CTD treatment (P<0.01; Fig. 3B and C). These results suggested
that miR-21 and PTEN may be involved in the effects of CTD on A375
cells. The AKT kinase is the one of the important downstream
effectors of PTEN. Accompanied with the increased expression of
PTEN following CTD treatment, the expression of the active form of
AKT, pAKT, was decreased (Fig.
3C). These results proved that CTD may suppress the AKT pathway
by increasing PTEN expression.
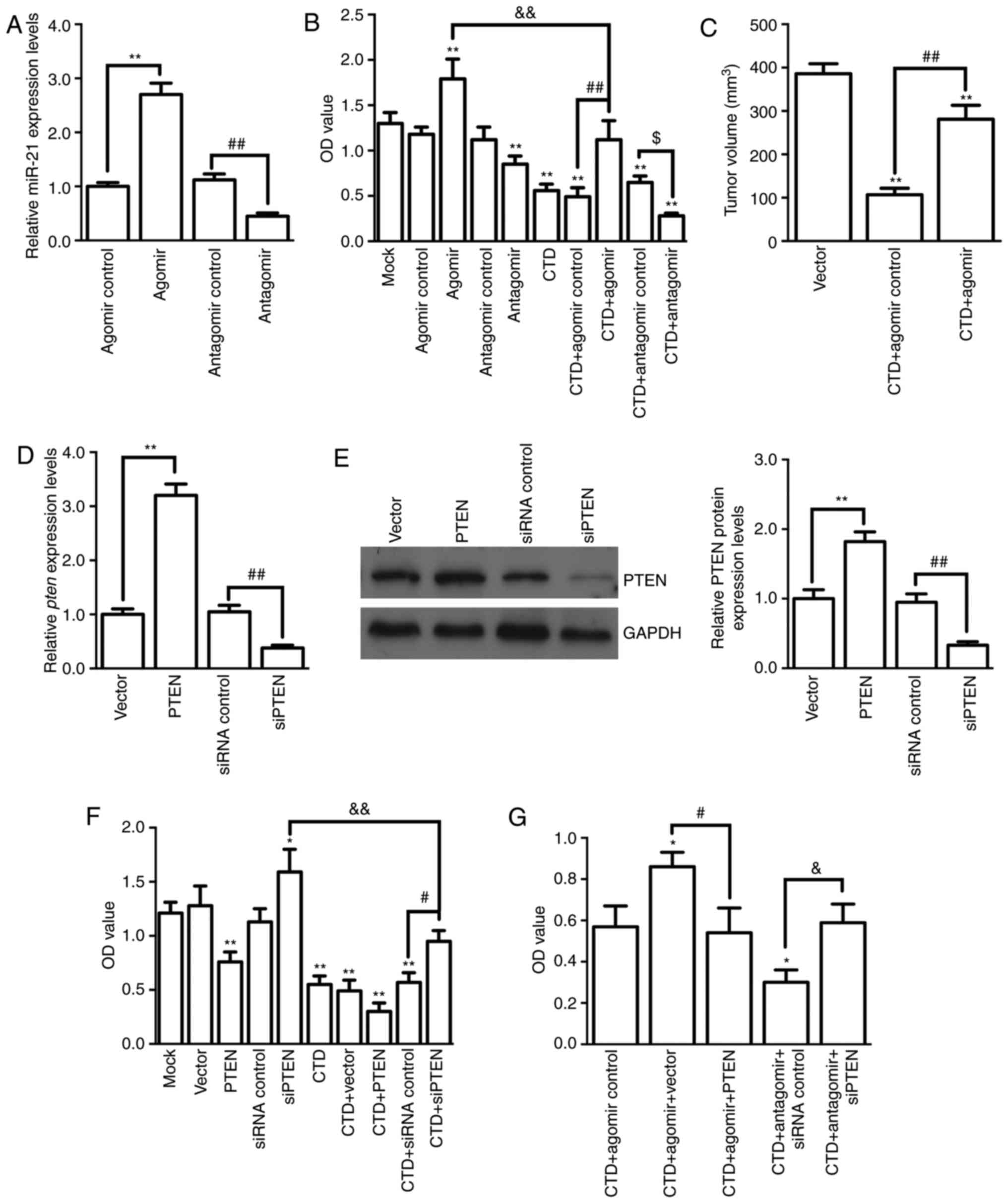
Antitumor effects of CTD via
attenuating miR-21-mediated PTEN suppression
To further verify the association between miR-21
downregulation and CTD-induced antitumor effects, miR-21 agomir and
antagomir were used to observe the CTD effects on A375
proliferation and tumorigenesis. The results demonstrated that the
miR-21 agomir or antagomir could significantly increase or
decrease, respectively, the expression of miR-21 in A375 cells
(both P<0.01; Fig. 4A).
Compared with the CTD+antagomir/agomir control, the miR-21 agomir
(P<0.01) or antagomir (P<0.05) impeded or promoted the effect
of CTD on the OD values, respectively (Fig. 4B). Furthermore, it was demonstrated
that miR-21 agomir administration could significantly reverse the
decrease in the xenograft tumor volumes caused by CTD treatment on
the 21st day (P<0.01; Fig. 4C).
These results suggest that miR-21 is involved in the antitumor
effect of CTD both in vitro and in vivo. Next, the
roles of PTEN in the antitumor effect of CTD were evaluated. First,
the effectiveness of the tools used to regulate PTEN expression was
confirmed (Fig. 4D and E).
Similarly, it was observed that PTEN overexpression or siRNA PTEN
treatment could significantly enhance or impede the roles of CTD in
A375 cell viability, respectively (P<0.01; Fig. 4F). Finally, it was demonstrated
that miR-21 agomir or antagomir administration blocked or enhanced
the decreased effect of CTD on OD values, respectively, whereas a
combination with PTEN overexpression or knockdown could,
respectively, reverse these results (all P<0.05; Fig. 4G). These results indicate that the
antitumor effects of CTD occurred via attenuating miR-21-mediated
PTEN suppression.
Discussion
In the present study, the effects and underlying
mechanisms of CTD on melanoma proliferation were evaluated. CTD
could effectively inhibit the viability and colony formation of
A375 cells, decrease the size of xenograft tumors and induce A375
cell apoptosis, all of which were regulated via attenuating
miR-21-mediated PTEN suppression.
Previous findings have shown that CTD exerts general
anticancer effects on the proliferation and metastasis of a number
of types of human cancer (5). It
was also indicated that CTD inhibited human melanoma cell migration
and invasion effectively (9). In
addition to the inhibited roles in melanoma metastasis, in the
present study, it was demonstrated that CTD could suppress the
proliferation of melanoma both in vitro and in vivo.
Furthermore, the melanoma cell apoptosis increased following CTD
treatment, which may be associated with the inhibition of AKT
signaling pathway. These results are in agreement with results of
previous studies (9,10) and indicates that CTD or its
derivatives could be candidates for melanoma treatment.
However, because of the toxicity, CTD has a very
narrow therapeutic window in clinical use (5,15).
In addition, CTD can accumulate in certain healthy organs and
impair its normal physiological function (5). Therefore, to fully exploit CTD in the
future, it is important to clarify the underlying mechanisms and
identify selective downstream targets of CTD during cancer
treatment. CTD has been demonstrated to be the inhibitor of protein
phosphatases (PP) type 1 and type 2A (PP2A), which serve key roles
in regulating the signal transfer of the cell cycle, mitosis and
apoptosis (16). However, the
detailed underlying mechanisms of CTD remain unclear. Recently,
miRs were demonstrated to serve important roles in cancer
chemotherapy (17–19), but little is known about the
involvement of miRs in CTD treatment. Abnormal miR-21 expression
was demonstrated in melanoma tumor tissue samples and cell lines
(11,12,20).
Furthermore, proliferation, invasion, migration and apoptosis are
partly regulated by miR-21 in melanoma tumorigenesis (13,20,21).
Therefore, the selective inhibition of miR-21 is a promising
therapy for melanoma. In the present study, it was demonstrated
that the high expression of miR-21 in A375 cells was inhibited by
CTD. Overexpression or downregulation of miR-21 in A375 cells could
impair or enhance the effect of CTD on cell proliferation,
metastasis and apoptosis. These results suggest that miR-21 was one
of the important targets of CTD for melanoma treatment, a result
that, to the best of our knowledge, has not been previously
reported.
miR-21 can regulate certain target genes and is
involved in melanoma (20,22). PTEN, a famous tumor
suppressor, is one of the miR-21 target genes reported recently
(23). Previous studies have
demonstrated that PTEN can promote the host immune response against
cancer cells by suppressing the intracellular levels of certain
immunosuppressive factors, including interleukin (IL)-6, IL-10,
vascular endothelial growth factor, and programmed cell death 1
ligand, by negatively regulating the AKT/protein kinase B signaling
pathway (24–27). In addition, PTEN is associated with
melanoma aggressiveness and a worse prognosis in patients (28). Recently, a number of studies have
demonstrated that various drugs or compounds promote PTEN mRNA and
protein expression to repress tumor formation and progression
(29,30). Therefore, whether the roles of CTD
in A375 cells are associated with miR-21-regulated PTEN was further
investigated in the present study. The putative relationship
between the PTEN/AKT pathway and effects of CTD on A375 cell
proliferation were investigated, which were mediated by miR-21.
Certainly, miR-21 has other potential targets such as programmed
cell death 4 and p53, in addition to PTEN (23,31).
Consequently, further studies are necessary to investigate whether
other miR-21 target genes are involved in the CTD-induced
repression of melanoma proliferation.
In conclusion, results of the present study provide
evidence that CTD has anti-proliferative effects on human melanoma
and underlying mechanisms are via miR-21 downregulation, increased
PTEN expression, and decreased AKT activity. The identification of
the miR-21/PTEN/AKT pathway in CTD treatment may be useful in the
development of more efficacious and less toxic CTD analogs for
melanoma chemotherapy in the future.
Acknowledgements
Not applicable.
Funding
This study was supported by the Projects of Medical
and Health Technology Development Program in Shandong Province
(grant no. 2017WS254).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZM and QS designed the study. ZM performed all the
experiments and analyzed the data. ZM and QS wrote and critically
revised the manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the relevant guidelines and approved by the Institutional Animal
Care and Use Committee (IACUC) of Taishan Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Apalla Z, Lallas A, Sotiriou E, Lazaridou
E and Ioannides D: Epidemiological trends in skin cancer. Dermatol
Pract Concept. 7:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalal BS, Upadhya D and Pai VR:
Chemotherapy resistance mechanisms in advanced skin cancer. Oncol
Rev. 11:3262017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng LP, Dong J, Cai H and Wang W:
Cantharidin as an antitumor agent: A retrospective review. Curr Med
Chem. 20:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen M, Wu MY, Chen LP, Zhi Q, Gong FR,
Chen K, Li DM, Wu Y, Tao M and Li W: Cantharidin represses invasion
of pancreatic cancer cells through accelerated degradation of MMP2
mRNA. Sci Rep. 5:118362015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng LH, Bao YL, Wu Y, Yu CL, Meng X and
Li YX: Cantharidin reverses multidrug resistance of human hepatoma
HepG2/ADM cells via down-regulation of P-glycoprotein expression.
Cancer Lett. 272:102–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie D, Xie J, Wan Y, Ma L, Qi X, Wang K
and Yang S: Norcantharidin blocks Wnt/β-catenin signaling via
promoter demethylation of WIF-1 in glioma. Oncol Rep. 35:2191–2197.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji BC, Hsiao YP, Tsai CH, Chang SJ, Hsu
SC, Liu HC, Huang YP, Lien JC and Chung JG: Cantharidin impairs
cell migration and invasion of A375.S2 human melanoma cells by
suppressing MMP-2 and −9 through PI3K/NF-κB signaling pathways.
Anticancer Res. 35:729–738. 2015.PubMed/NCBI
|
|
10
|
Hsiao YP, Tsai CH, Wu PP, Hsu SC, Liu HC,
Huang YP, Yang JH and Chung JG: Cantharidin induces G2/M phase
arrest by inhibition of Cdc25c and Cyclin A and triggers apoptosis
through reactive oxygen species and the mitochondria-dependent
pathways of A375.S2 human melanoma cells. Int J Oncol.
45:2393–2402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wandler A, Riber-Hansen R, Hager H,
Hamilton-Dutoit SJ, Schmidt H, Nielsen BS, Stougaard M and
Steiniche T: Quantification of microRNA-21 and microRNA-125b in
melanoma tissue. Melanoma Res. 27:417–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Latchana N, Ganju A, Howard JH and Carson
WE III: MicroRNA dysregulation in melanoma. Surg Oncol. 25:184–189.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Yuan SM, Yang M, Zha H, Li XR, Sun
H, Duan L, Gu Y, Li AF, Weng YG, et al: High intensity focused
ultrasound inhibits melanoma cell migration and metastasis through
attenuating microRNA-21-mediated PTEN suppression. Oncotarget.
7:50450–50460. 2016.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Lin C, Lu A, Lin G, Chen H, Liu
Q, Yang Z and Zhang H: Liposomes equipped with cell penetrating
peptide BR2 enhances chemotherapeutic effects of cantharidin
against hepatocellular carcinoma. Drug Deliv. 24:986–998. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y,
Xu Z and Han X: Cantharidin, a potent and selective PP2A inhibitor,
induces an oxidative stress-independent growth inhibition of
pancreatic cancer cells through G2/M cell-cycle arrest and
apoptosis. Cancer Sci. 101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garajová I, Ferracin M, Porcellini E,
Palloni A, Abbati F, Biasco G and Brandi G: Non-Coding RNAs as
predictive biomarkers to current treatment in metastatic colorectal
cancer. Int J Mol Sci. 18(pii): E15472017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Beijnum JR, Giovannetti E, Poel D,
Nowak-Sliwinska P and Griffioen AW: miRNAs: Micro-managers of
anticancer combination therapies. Angiogenesis. 20:269–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng J, Wang Y, Lei J, Lei W and Xiong JP:
Insights into the involvement of noncoding RNAs in 5-fluorouracil
drug resistance. Tumour Biol. 39:10104283176975532017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mao XH, Chen M, Wang Y, Cui PG, Liu SB and
Xu ZY: MicroRNA-21 regulates the ERK/NF-κB signaling pathway to
affect the proliferation, migration, and apoptosis of human
melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol
Carcinog. 56:886–894. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin del Campo SE, Latchana N, Levine
KM, Grignol VP, Fairchild ET, Jaime-Ramirez AC, Dao TV, Karpa VI,
Carson M, Ganju A, et al: MiR-21 enhances melanoma invasiveness via
inhibition of tissue inhibitor of metalloproteinases 3 expression:
In vivo effects of MiR-21 inhibitor. PLoS One. 10:e01159192015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Wu R, Liu Z, Fan J and Yang H:
Enforced expression of microRNA-21 influences the replication of
varicella-zoster virus by triggering signal transducer and
activator of transcription 3. Exp Ther Med. 7:1291–1296. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu X, He Y, Wang X, Peng D, Chen X, Li X
and Wang Q: Overexpression of miR-21 in stem cells improves ovarian
structure and function in rats with chemotherapy-induced ovarian
damage by targeting PDCD4 and PTEN to inhibit granulosa cell
apoptosis. Stem Cell Res Ther. 8:1872017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bu LL, Yu GT, Wu L, Mao L, Deng WW, Liu
JF, Kulkarni AB, Zhang WF, Zhang L and Sun ZJ: STAT3 Induces
Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J Dent Res.
96:1027–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
George S, Miao D, Demetri GD, Adeegbe D,
Rodig SJ, Shukla S, Lipschitz M, Amin-Mansour A, Raut CP, Carter
SL, et al: Loss of PTEN Is associated with resistance to anti-PD-1
checkpoint blockade therapy in metastatic uterine leiomyosarcoma.
Immunity. 46:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou K, Zhong Q, Wang YC, Xiong XY, Meng
ZY, Zhao T, Zhu WY, Liao MF, Wu LR, Yang YR, et al: Regulatory T
cells ameliorate intracerebral hemorrhage-induced inflammatory
injury by modulating microglia/macrophage polarization through the
IL-10/GSK3β/PTEN axis. J Cereb Blood Flow Metab. 37:967–979. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mondal S, Ghosh-Roy S, Loison F, Li Y, Jia
Y, Harris C, Williams DA and Luo HR: PTEN negatively regulates
engulfment of apoptotic cells by modulating activation of Rac
GTPase. J Immunol. 187:5783–5794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conde-Perez A and Larue L: PTEN and
melanomagenesis. Future Oncol. 8:1109–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li WX, Chen X, Yang Y, Huang HM, Li HD,
Huang C, Meng XM and Li J: Hesperitin derivative-11 suppress
hepatic stellate cell activation and proliferation by targeting
PTEN/AKT pathway. Toxicology. 381:75–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He Z, Chen AY, Rojanasakul Y, Rankin GO
and Chen YC: Gallic acid, a phenolic compound, exerts
anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling
pathway in ovarian cancer cells. Oncol Rep. 35:291–297. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo YB, Ji TF, Zhou HW and Yu JL: Effects
of microRNA-21 on nerve cell regeneration and neural function
recovery in diabetes mellitus combined with cerebral infarction
rats by targeting PDCD4. Mol Neurobiol. 55:2494–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|